Introduction
The estimated prevalence of chronic idiopathic constipation in the US ranges from 4–28%.[1] Constipation can have a significant impact on overall health-related quality of life (HRQoL). Significantly lower scores on the Psychological General Well-Being index have been reported for patients with idiopathic constipation compared with a matched healthy cohort. [3] A recent systematic review of 8 studies using the Short Form-36 (SF-36) to assess HRQoL found that both the mental (MCS) and physical (PCS) component scores were substantially lower in those with constipation than in healthy controls and the general US population. [4] Notably, these studies failed to adjust for other physical and mental health problems.
There is a lack of data concerning the impact of constipation in black Americans. One systematic review estimated that the prevalence of constipation was 1.13–2.89 higher in nonwhite compared with white citizens.[1] The 3 largest studies assessing the effect of constipation on HRQoL do not provide enough demographic details to determine the racial composition of the study groups.[5–7] Moreover, under-recruitment of blacks in clinical trials studying new constipation treatments has been demonstrated.[8,9] The objective of this study was to assess the impact of constipation on HRQoL in black Americans.
Subjects and Methods
This study was approved by our institutional review board and all subjects provided informed consent.
Subjects
Self-described black Americans who were aged 40–70 years, English-speaking with at least a sixth-grade reading level, and referred to our hospital for colon cancer screening were included as controls. Controls could not meet the Rome III criteria for Functional Constipation (FC). Additionally, subjects must have had a Bristol Stool Score of 3–5 for >75% of bowel movements over the previous 6 months. Exclusion criteria included: use of prescription or over-the-counter medicines for any problem referable to the gastrointestinal tract ≥2 times per month for the previous 6 months; use of medications for constipation within the previous 6 months; a healthcare visit for any problem referable to the gastrointestinal tract within the previous 2 years.
Subjects with language and reading level requirements similar to control subjects and fulfilling the Rome III criteria for FC [11] were recruited through a local newspaper advertisement. Loose stools were to be rarely present without associated laxative use in these subjects. These criteria were to be fulfilled for 3 months within symptom onset and ≥6 months before enrollment. Subjects with irritable bowel syndrome, a known constipation-associated disorder, and those regularly using narcotics, anticholinergics, calcium channel blockers, psychotropics, or bile acid binders were excluded.
Questionnaires
Subjects completed a questionnaire surveying demographics and medical and medication history. Information on the existence of ≥1 of the following comorbid conditions was collected: congestive heart failure; chronic obstructive pulmonary disease; coronary artery disease; degenerative arthritis; chronic headaches; chronic low back pain; depression. The standard form of the Medical Outcomes Study SF-36 version 2 (Quality Metric Health Outcomes Solutions, Lincoln, RI) was used to assess HRQoL.[13]
Statistical Analysis
To control for age and gender, FC subjects were enrolled after controls using frequency matching (age stratified into 10 year blocks). Unpaired t-tests were used for univariate comparisons of continuous data between groups. Results were presented as means ± standard deviation. For categorical variables, Fisher’s Exact test was utilized. Paired t-tests were used to compare continuous data within groups. For ordinal and rank scale results not including the SF-36, the Mann-Whitney test was used. Factorial ANOVA was utilized to adjust PCS and MCS summary scores for comorbid conditions. Comorbidity data was aggregated into the categorical variable “comorbidity present” or “comorbidity absent”. SPSS v. 18.0 (IBM, Chicago, Illinois) was used for analysis. A two-tailed P-value <0.05 was considered significant.
Study participants’ PCS and MCS scores were compared with those of a healthy US general population and an age-standardized group of 116 healthy black Americans after adjusting for age, gender, and comorbid conditions.
SF-36 Data Quality Evaluation
The SF-36 underwent data quality evaluation and scoring by Quality Metric (Lincoln, RI). Norm-based scores were standardized by performing a T-score transformation using a mean (50) and standard deviation (10) derived from the general US population.[15]
Power Analysis
To detect a ≥3-point (ie, clinically meaningful)[16, 17] between-group difference in MCS and PCS scores, assuming equality of variances and α=0.05, 99 patients per group were required to provide a β=0.20.
Results
The survey results met all benchmarks for quality. Missing response rate was 0.01%. All items demonstrated substantial correlation (r>0.40) with their hypothesized scales. Internal consistency reliability estimates exceeded the minimum standard for group level comparisons (>0.7) for all 8 scales. Age and gender frequency matching was successful. The FC and control groups were well-matched on body size, education level, income, and medical care access (Table 1). FC subjects had significantly fewer daily spontaneous bowel movements and harder stools according to the Bristol Stool Scale, thereby establishing criterion validity.
Table 1.
Characteristics of the Control Group and those with Functional Constipation
| Variable | Control | Functional Constipation | P Value |
|---|---|---|---|
| n = 100 | n = 102 | ||
| Age, mean (±SD) | 56.8 (6.2) | 55.3 (8.3) | 0.15 |
| Males, % | 46.0 | 46.1 | 0.99 |
| Body Mass Index, kg/m2 (±SD) | 31.5 (1.9) | 31.1 (2.1) | 0.64 |
| Spontaneous Bowel | |||
| Movement/d (median) | 1.0 | 0.5 | < 0.001 |
| Bristol Stool Type (median) | 3.0 | 2.0 | < 0.001 |
| Without Health Insurance (%) | 7.0 | 10.8 | 0.11 |
| Highest Education Level (%)* | 0.38 | ||
| < 12th grade | 28.3 | 20.6 | |
| High School Grad | 57.6 | 58.8 | |
| College Grad | 14.1 | 19.6 | |
| Income, dollar/yr (%) | 0.07 | ||
| < 20,000 | 57.0 | 64.7 | |
| 20–50,000 | 33.0 | 19.6 | |
| >50,000 | 10.0 | 15.7 | |
| Marital Status (%) | 0.006 | ||
| Separated | 19.0 | 6.9 | |
| Married | 32.0 | 22.5 | |
| Never Married | 38.0 | 49.0 | |
| Divorced | 11.0 | 21.6 | |
| Exercise, d/wk (median) | 3.0 | 2.0 | 0.13 |
| Co-morbid Condition (%)** | 23.0 | 56.9 | <0.001 |
| Frequency, n | |||
| congestive heart failure | 2 | 4 | |
| COPD | 5 | 2 | |
| coronary artery disease | 1 | 1 | |
| chronic headaches | 0 | 5 | |
| degenerative arthritis | 12 | 31 | |
| chronic low back pain | 8 | 28 | |
| depression | 3 | 18 |
1 subject in control group did not report education level.
Percent does not equal sum of co-morbidities divided by total in group as some subjects with >1 comorbidity.
Effect of FC on HRQoL
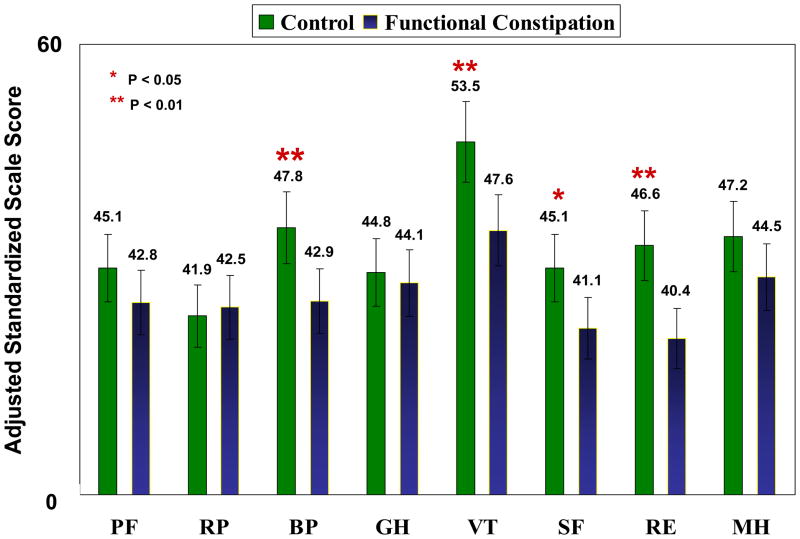
In 4 of 8 SF-36 scales, adjusted scores were clinically and statistically lower for the FC group compared with the control group (Figure 1). The largest difference was in the Vitality scale; Bodily Pain, Social Functioning, and Role-Emotional scales showed smaller but significant differences.
Figure 1.
Standardized mean marginal scores of the eight SF-36 scales stratified by group after adjustment for co-morbidities using factorial ANOVA.
RP – role physical; PF – physical functioning; BP – bodily pain; GH – general health; VT – vitality; SF – social functioning; RE – role emotional; MH – mental health. For the 8 scales, differences of ≥4 are considered clinically meaningful.(16, 17)
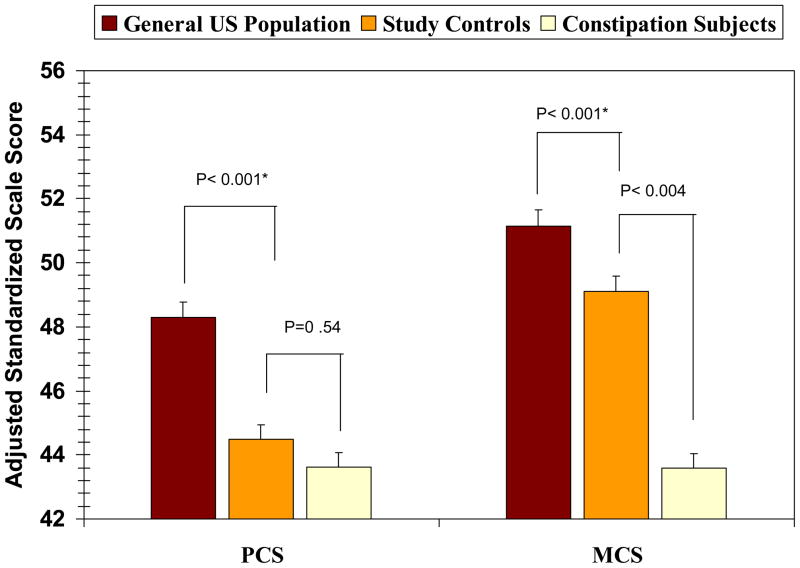
Unadjusted PCS and MCS scores were significantly higher in control than in FC subjects (47.1 ± 10.6 vs 43.3 ± 8.6, P=0.005 and 50.6 ± 12.4 vs 43.4 ± 11.8; P< 0.001, respectively). After adjustment for comorbidities, only MCS differences remained significant (49.1 ± 1.4 vs 43.6 ± 1.2; P=0.004) (Figure 2). In both analyses, the presence of a comorbidity was independently associated with overall PCS (P<0.001) and MCS (P=0.02) results. PCS and MCS scores for the healthy black American reference group were 52.9 ± 0.9 and 56.4 ± 1.8 respectively, significantly higher than the PCS and MCS scores for both the control and FC subjects (all P<0.001).
Figure 2.
Comparison of standardized Physical Component Summary (PCS) and Mental Component Summary (MCS) scores for the constipation and control groups (adjusted for comorbidities) compared with summary results for age and gender matched controls from a healthy reference population representing the US general population.[13]
For the PCS and MCS, differences between groups of ≥3 are considered clinically meaningful.(16, 17)
* Indicates level of significance applies to both controls and FC groups.
Discussion
This is the first study to examine the impact of constipation on the HRQoL in middle-aged black Americans using standardized scoring and adjusting for physical and mental comorbidities. FC substantially impacted HRQoL in our selected group of black Americans and this effect was significant relative to a gender-, age-, and demographically-matched subject group and a gender-and age-matched sample of healthy US citizens. On adjustment for comorbidities, significantly lower standardized SF-36 Vitality, Social Functioning, Bodily Pain, and Role-Emotional scores were observed for FC subjects. Furthermore, a clinically meaningful (≥3-point) difference in MCS scores between control and FC groups remained after adjustment for comorbidities. [16, 17] The observed significant difference in PCS scores between the control and FC groups on univariate analysis did not remain after adjustment for comorbidities, likely because both groups’ adjusted PCS scores indicated significant physical functioning impairment. We hypothesized that the observed differences between our reference US population (PCS: 48.30 ± 1.35; MCS: 51.13 ± 0.79) and control group were overestimates as black Americans appear to have a poorer HRQoL versus the US population;[18] however, we were not able to validate this hypothesis. Instead, our healthy black American reference group had higher PCS and MCS scores than our study subjects and the general healthy US population. This may represent Type I error due to the limited sample size (n=116) or changes in the operational definition of “healthy”, which has undergone refinement at Quality Metrics.
Whereas most previous studies that have used the SF-36 to evaluate the effect of chronic constipation on HRQoL do not allow for study cross-comparisons as they did not standardize raw score results, these studies have demonstrated a negative impact of dyssynergic defecation or constipation on HRQoL. [5–7, 19–22] Two previous studies have published standardized scores for FC. A nationwide Canadian survey of all Rome II functional gastrointestinal disorders revealed significantly lower MCS (48.8 vs 51.0) and PCS (49.9 vs 47.3) scores for a subset of FC patients versus those without FC (P<0.05 for both comparisons). [5] A multinational survey revealed mean standardized PCS and MCS values for both constipated and healthy US participants to be >49, indicating minimal HRQoL impairment. Standardized, unadjusted scores in our study differed substantially between the FC subjects and controls, showing markedly impaired physical and mental well-being in FC patients.
One strength of our study is the use of factorial ANOVA to adjust for the confounding effects of 7 common comorbid conditions on HRQoL. We found that this group of comorbid disorders was independently associated with HRQoL and therefore suggest the standard inclusion of relevant comorbidities for future functional bowel disease studies. Another study strength is the inclusion of a power analysis. We determined that ≥99 healthy and FC subjects respectively were needed to detect a clinically significant (≥3-point) between-group difference in MCS and PCS scores. This procedure follows the Strobe recommendations for high-quality observational research.[23]
There are several limitations to our study. The results are confined only to middle-aged black Americans. Another limitation is that we used a generic instrument to assess HRQoL rather than a disease specific instrument. A final limitation is that we may not have adequately controlled for comorbid conditions using our methodology. Rather than categorical assessment of comorbidities, further assessment of disease severity may have been helpful to account for differences in SF-36 scores.
In conclusion, we found that black Americans meeting the Rome III definition of FC demonstrated a significantly poorer HRQoL relative to a matched control group and the healthy US population, signifying their need for access to FC treatments. As such, efforts should be made to include black Americans in clinical trials.
Acknowledgments
This study was funded, in part, by Takeda Pharmaceuticals America, Deerfield, IL. Additional funding was provided by the National Institute of Health (grant 1K24DK083268)
Abbreviations
- HRQoL
Health-related Quality of Life
- PCS
Physical Component Summary
- MCS
Mental Component Summary
- SF-36
Short Form-36
- FC
Functional Constipation
- STC
Slow-Transit Constipation
- DD
Dyssynergic Defecation
- GERD
Gastroesophageal Reflux Disease
References
- 1.Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–9. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- 2.Peppas G, Alexiou VG, Mourtzoukou E, Falagas ME. Epidemiology of constipation in Europe and Oceania: a systematic review. BMC Gastroenterol. 2008;8:5. doi: 10.1186/1471-230X-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glia A, Lindberg G. Quality of life in patients with different types of functional constipation. Scand J Gastroenterol. 1997;32(11):1083–9. doi: 10.3109/00365529709002985. [DOI] [PubMed] [Google Scholar]
- 4.Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment Pharmacol Ther. 2010;31(9):938–49. doi: 10.1111/j.1365-2036.2010.04273.x. [DOI] [PubMed] [Google Scholar]
- 5.Irvine EJ, Ferrazzi S, Pare P, Thompson WG, Rance L. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97(8):1986–93. doi: 10.1111/j.1572-0241.2002.05843.x. [DOI] [PubMed] [Google Scholar]
- 6.Tuteja AK, Talley NJ, Joos SK, Woehl JV, Hickam DH. Is constipation associated with decreased physical activity in normally active subjects? Am J Gastroenterol. 2005;100(1):124–9. doi: 10.1111/j.1572-0241.2005.40516.x. [DOI] [PubMed] [Google Scholar]
- 7.Wald A, Scarpignato C, Kamm MA, Mueller-Lissner S, Helfrich I, Schuijt C, et al. The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther. 2007;26(2):227–36. doi: 10.1111/j.1365-2036.2007.03376.x. [DOI] [PubMed] [Google Scholar]
- 8.Quigley EM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009;29(3):315–28. doi: 10.1111/j.1365-2036.2008.03884.x. [DOI] [PubMed] [Google Scholar]
- 9.Arias E, Rostron BL, Tejada-Vera B. United States life tables, 2005. Natl Vital Stat Rep. 2010;58(10):1–132. [PubMed] [Google Scholar]
- 10.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920–4. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 11.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 12.DeForge BR, Stewart DL, DeVoe-Weston M, Graham L, Charleston J. The relationship between health status and blood pressure in urban African Americans. J Natl Med Assoc. 1998;90(11):658–64. [PMC free article] [PubMed] [Google Scholar]
- 13.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Schlenk EA, Erlen JA, Dunbar-Jacob J, McDowell J, Engberg S, Sereika SM, et al. Health-related quality of life in chronic disorders: a comparison across studies using the MOS SF-36. Qual Life Res. 1998;7(1):57–65. doi: 10.1023/a:1008836922089. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C. Comparison of UK and US methods for weighting and scoring the SF-36 summary measures. J Public Health Med. 1999;21(4):372–6. doi: 10.1093/pubmed/21.4.372. [DOI] [PubMed] [Google Scholar]
- 16.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43(7):1478–87. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Wolinsky FD, Miller DK, Andresen EM, Malmstrom TK, Miller JP. Health-related quality of life in middle-aged African Americans. J Gerontol B Psychol Sci Soc Sci. 2004;59(2):S118–23. doi: 10.1093/geronb/59.2.s118. [DOI] [PubMed] [Google Scholar]
- 19.Chan AO, Cheng C, Hui WM, Hu WH, Wong NY, Lam KF, et al. Differing coping mechanisms, stress level and anorectal physiology in patients with functional constipation. World J Gastroenterol. 2005;11(34):5362–6. doi: 10.3748/wjg.v11.i34.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason HJ, Serrano-Ikkos E, Kamm MA. Psychological state and quality of life in patients having behavioral treatment (biofeedback) for intractable constipation. Am J Gastroenterol. 2002;97(12):3154–9. doi: 10.1111/j.1572-0241.2002.07113.x. [DOI] [PubMed] [Google Scholar]
- 21.O’Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50(4):M184–9. doi: 10.1093/gerona/50a.4.m184. [DOI] [PubMed] [Google Scholar]
- 22.Rao SS, Seaton K, Miller MJ, Schulze K, Brown CK, Paulson J, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosom Res. 2007;63(4):441–9. doi: 10.1016/j.jpsychores.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Knottnerus A, Tugwell P. STROBE--a checklist to Strengthen the Reporting of Observational Studies in Epidemiology. J Clin Epidemiol. 2008;61(4):323. doi: 10.1016/j.jclinepi.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Lippmann QK, Crockett SD, Dellon ES, Shaheen NJ. Quality of life in GERD and Barrett’s esophagus is related to gender and manifestation of disease. Am J Gastroenterol. 2009;104(11):2695–703. doi: 10.1038/ajg.2009.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe F, Michaud K, Li T, Katz RS. EQ-5D and SF-36 quality of life measures in systemic lupus erythematosus: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, and fibromyalgia. J Rheumatol. 2010;37(2):296–304. doi: 10.3899/jrheum.090778. [DOI] [PubMed] [Google Scholar]