Abstract
Acute and chronic solid organ failures are costly disease processes with high mortality rates. Inflammation plays a central role in both acute and chronic organ failure, including heart, lung and kidney. In this regard, new therapies for these disorders have focused on inhibiting the mediators of inflammation, including cytokines and free radicals, with little or no success in clinical studies. Recent novel treatment strategies have been directed to cell-based rather than mediator-based approaches, designed to immunomodulate the deleterious effects of inflammation on organ function. One approach, cell therapy, replaces cells that were damaged in the acute or chronic disease process with stem/progenitor technology, to rebalance excessive inflammatory states. As an example of this approach, the use of an immunomodulatory role of renal epithelial progenitor cells to treat acute renal failure (ARF) and multiorgan failure arising from acute kidney injury is reviewed. A second therapeutic pathway, cell processing, does not incorporate stem/progenitor cells in the device, but rather biomimetic materials that remove and modulate the primary cellular components, which promote the worsening organ tissue injury associated with inflammation. The use of an immunomodulating leukocyte selective cytopheretic inhibitory device is also reviewed as an example of this cell processing approach. Both of these unconventional strategies have shown early clinical efficacy in pilot clinical trials and may transform the therapeutic approach to organ failure disorders.
Keywords: acute kidney injury (AKI), cell therapy, systemic inflammation, stem/progenitor cells, selective cytopheretic inhibitory device (SCD)
Introduction
Inflammation plays a central role in the development of acute and chronic solid organ failure, including heart, kidney and lung. Loss of immunoregulation results in a propensity to develop systemic inflammatory response syndrome (SIRS), sepsis, multiple organ failure (MOF) and has a high risk of death because of systemic immunologic or inflammatory imbalance. In acute kidney injury (AKI), activation and release of inflammatory proteins from circulating activated leukocytes and imbalance between pro- and anti-inflammatory proteins are provoked and aggravated by kidney cell injury. These conditions play a central role in the proinflammatory state in AKI with SIRS and/or MOF. SIRS is a catastrophic consequence of a variety of clinical insults and is usually present with AKI. The inflammatory response appears to underlie the MOF syndrome, as there are now data linking patient outcomes to initial plasma levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and other proinflammatory cytokines [1–3]. Septic shock is an acute syndrome that is characterized by hypotension, coagulopathy and eventual MOF primarily due to ischemic tissue injury. This disorder is associated with dramatic elevations in inflammatory cytokines, including TNF-α, IL-1β, IL-6, IL-8 and interferon-γ [4]. This reactive and uncontrolled inflammatory response results in the adverse hemodynamic and metabolic disturbances in septic shock. If this balance between pro- and anti-inflammatory mediators is lost, the patient may proceed to cardiovascular collapse if proinflammatory processes are excessive, or develop profound immunosuppression with increased risk of recurrent or continuing infection if the anti-inflammatory cascade overreacts [2, 3].
As a paradigm case of organ failure due to inflammation, consider the sepsis syndrome. Despite prompt treatment with antibiotics, fluid resuscitation and artificial organ function support, mortality rates still exceed 30% [5, 6]. As sepsis progresses in severity, the patient develops cardiovascular instability with hypotension, lung dysfunction and renal function deterioration [7, 8]. These major clinical manifestations of sepsis, however, are not caused directly by the invading microbes but are results of dysregulation of the patient's own inflammatory response [9], as leukocytes exacerbate tissue damage [10, 11]. Prior investigation and therapeutic approaches to these disorders have focused on extracorporeal devices designed to interrupt the excessive levels of inflammatory cytokines (cytokine storm) or the activation of the coagulation system during sepsis, with little or modest effects on this disease process when tested clinically [12].
Part of the reason that treatment strategies have resulted in limited therapeutic success is the nature of cytokine function in the mediation of inflammation. Simply stated, cytokines play a complex regulatory role that is upstream from the final effector of the inflammatory response: the activated neutrophil [8, 13]. The neutrophil is a short-lived circulating phagocyte which, when activated, binds to the microvascular endothelium and extravasates into local tissue spaces to degrade injured tissue or kill ingested pathogens with a variety of stored proteolytic enzymes and rapid production of reactive oxygen species [8, 13]. Its essential role in sepsis is demonstrated by the recurrence of life-threatening infections in patients with neutropenia or with leukocyte defects [14, 15].
Similarly, inflammation is a key player in chronic organ dysfunction, including disease states such as congestive heart failure (CHF) and chronic kidney disease (CKD). The clinical ramifications of this dysregulated immune state in CHF and CKD have profound implications. For CKD, the leukocyte dysfunction translates into the fact that infection is the second most common cause of death in hemodialysis patients, approaching 25% of the annual mortality rate [16]. This rate of infection complication is not diminished with higher dialysis dose or high-flux membrane utilization [17]. Mortality due to sepsis occurs ∼250-fold more commonly in these patients compared with the general population [18]. In terms of clinical ramifications for CHF, there is a clear correlation between chronic inflammation and accelerated atherosclerotic process [19].
Because leukocytes are major contributors to the pathogenesis and progression of many high-impact clinical inflammatory disorders, new strategies to directly effect this dysregulated leukocyte population make good potential therapies The purpose of this review is to describe novel strategies of cell-based approaches to mitigating inflammation: cell processing and cell therapy.
The selective cytopheretic device: a cell processing approach
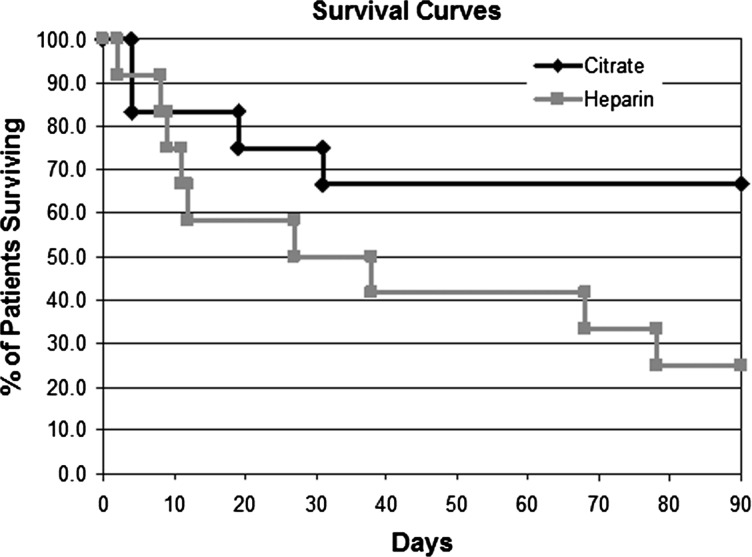
A novel approach to the treatment of organ failure or dysfunction developing from excessive dysregulated inflammation utilizes a new therapeutic device, referred to as a selective cytopheretic device (SCD), which immunomodulates activated circulating leukocytes, including neutrophils and monocytes. The development of this potential paradigm-shifting therapeutic approach to the SIRS arose from the clinical evaluation of a tissue-engineered renal assist device (RAD) [20, 21] containing adult human renal progenitor epithelial cells as a component of a bioartificial kidney (BAK). The RAD cartridge was based on a standard hollow fiber hemofiltration cartridge and contained up to 108 renal epithelial cells (RECs) grown along the lumen of the hollow fiber membranes. In a number of preclinical large animal studies, cell function and viability within the RAD was confirmed both prior to, during and after therapeutic application [20, 22]. For clinical application, RAD membranes were well suited, as they provided both a tubular scaffold and immunoprotection from the blood perfusing through the extracapillary space of the cartridge. In a randomized control, blinded multicenter clinical study in intensive care unit (ICU) patients with acute renal failure (ARF) secondary to AKI undergoing continuous renal replacement therapy (CRRT), subsets of patients were treated with a cell containing RAD or a sham (noncell containing) RAD cartridge [23]. The RAD was connected in series to a conventional hemofilter in an extracorporeal blood circuit, and maintained with either systemic heparin or regional citrate anticoagulation with anticoagulant citrate dextrose solution formula A (ACD-A). The clinical study was suspended after an interim analysis due to an unanticipated high survival rate of the sham device arm of the study. In the subsequent retrospective analysis of the sham control groups, the improved survival rate was demonstrated in the presence of regional citrate anticoagulation when compared with systemic heparin anticoagulation, Figure 1 [23].
Fig. 1.
Survival of citrate vs. heparin in SCD in the control subset of the RAD trial.
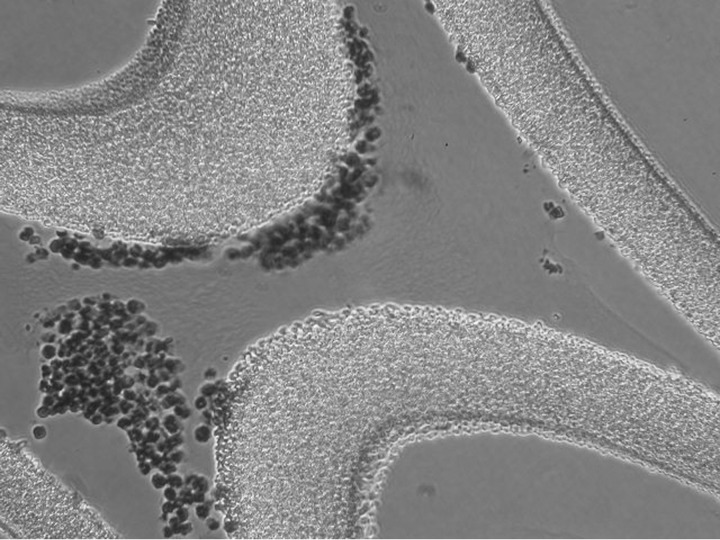
To understand the mechanism of action of this combination, a device (RAD without cells) and a pharmacological agent (citrate) on a profoundly difficult clinical disorder, a series of investigations were initiated. Starting with the knowledge that other groups had previously shown that regional citrate anticoagulation during CVVH conferred no additional benefit over systemically heparinized CVVH, for example during severe sepsis [24], and that AKI results in an acute inflammatory response state resulting in microvascular dysfunction in multiple organs [25], the initial evaluation of the mechanism of action focused on the inflammatory response in the patients treated in the sham control arm. Accordingly, the initial step in understanding the potential mechanism of this serendipitous unexpected clinical result was the histologic evaluation of the sham RAD (Figure 2). Immunofluorescence microscopy of the sham cartridges after patient treatment demonstrated adherent leukocytes on the outer surface of the membranes of the cartridge along the blood-flow path within the extracorporeal circuit [23]. The sequestered leukocytes were dominated with neutrophils (Figure 2). The ability of leukocytes to bind to the outer walls of the hollow fiber membranes rather than the inner walls, which is the conventional blood-flow path, was recognized to be due to the shear force of blood flow. The sheer stress (SS) of blood along the outer wall of the membrane was near capillary SS of <1 dyne/cm2 compared with the SS of near 100 dyne/cm2 of blood when flowing along the inner conventional surfaces of the hollow fiber membranes. The role of citrate infusion (ACD-A infused at a rate of 1:40 of the blood-flow rate) in this device blood circuit related to the effect of citrate to lower the ionized calcium (iCa) levels of blood to below 0.4 mM, a level that inhibits the coagulation system of blood. This concentration of citrate causing lower blood iCa also has an inhibitory effect on neutrophil activation [25], resulting in a simultaneous combination effect to sequester activated circulating leukocytes and alter the activity of the bound leukocytes. Further studies now suggest that the bound leukocytes were subsequently released back to the systemic circulation in an altered apoptotic state. Consequently, the membrane cartridge is referred to as an SCD, and in the presence of citrate anticoagulation, an immunomodulatory membrane device.
Fig. 2.
Microscopy of the sham cartridges after patient treatment demonstrated adherent leukocytes on the outer surface of the membranes of the cartridge along the blood flow path within the extracorporeal circuit.
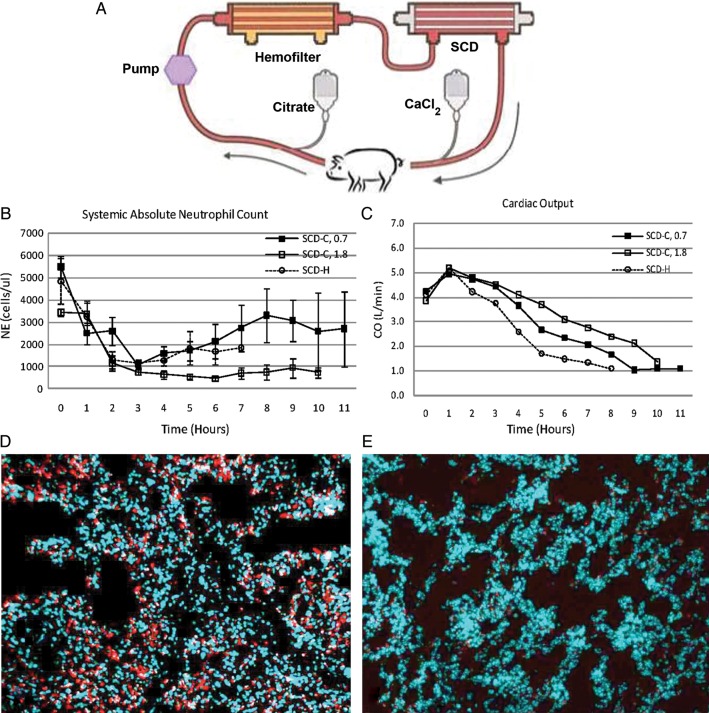
This clinical observation was further evaluated using the SCD with citrate anticoagulation in a well-established porcine model of Escherichia coli-induced septic shock [22, 25]. These studies demonstrated an ability of the SCD with citrate to lower systemic neutrophil activation, diminish aggregation of activated leukocytes in the lungs, decrease systemic capillary leak, preserve cardiac output, ameliorate renal dysfunction and prolong survival time compared with various control groups (Figure 3). Improved cardiovascular and pulmonary functions were not merely due to thermodynamic changes, as thermal parameters for the heparin only control was nearly identical to the regionally citrate SCD therapy; however, the heparin-only group did not confer any cardiovascular or pulmonary improvements. Further experiments have suggested that the ‘catch and release’ of activated neutrophils within the SCD promoted the activated neutrophil, which is in a delayed apoptotic state and has a longer life span, to revert back to a normal time to apoptosis and a normal life span despite the presence of a SIRS state. This observation is consistent with previous work that suggests that blocking calcium entry into a neutrophil activates the apoptotic pathway to programmed cell death [26].
Fig. 3.
SCD-C circuit (A) depicting locations of regional citrate anticoagulation administration and calcium infusion. Porcine sepsis model treated with SCD-C and SCD-H show differences in absolute NE counts over the duration of therapy (B), cardiac output (C) and NE infiltration in lung: SCD-H (D), SCD-C (E).
With these favorable preclinical results, the evaluation of the SCD with citrate anticoagulation has been completed in three exploratory pilot clinical trials in ICU patients with ARF and multiorgan failure. This patient group was chosen due to the ease of incorporating this device into the standard CRRT blood circuit during treatment of these critically ill patients. These early exploratory clinical trials have demonstrated an excellent safety profile and compelling efficacy impact [23, 27, 28]. Leukopenia and sustained thrombocytopenia were not observed in these clinical studies. Accelerated renal recovery with CRRT discontinuation and a ∼50% or greater relative improvement in survival rates have been observed.
The first clinical results, as briefly referred to earlier, were derived from a retrospective subgroup analysis of a randomized, blinded multicenter controlled clinical study [23]. This study was evaluated in an FDA/IND trial to evaluate renal cell therapy in ICU patients utilizing a sham noncell device as a control. Twenty-four patients were randomly assigned to a noncell cartridge group that received either systemic heparin (n = 12) or regional citrate (n = 12) anticoagulation (Figure 1). Patient baseline demographics and acuity of illness by SOFA scores at baseline were similar between the two groups. The mortality rate in the heparin patient group was 50 versus 25% in the citrate-treated group at 28 days (though not significant, demonstrates a trend toward greater citrate-treated survival) and 75 versus 33% at 90 days (χ2<0.05). The subgroups were comparable with similar SOFA scores, organ failure number and incidence of sepsis (58% in both groups). Treatment with noncell/cartridge and regional citrate (later referred to as SCD) was well tolerated, without measurable effects on hematological parameters, including neutrophil and platelet counts, with an adverse event profile expected for a seriously ill population in the ICU with AKI. The blood-flow patency of the double-cartridge circuit was comparable to single-cartridge CRRT modalities.
The second clinical study was a prospective, single-arm, single-center study to evaluate safety and efficacy of the SCD in a dose-ranging study in ICU patients with ARF and multiorgan failure [27]. This study was approved by the local IRB as a nonsignificant risk designation. The dose-ranging study included increasing the effective outer membrane surface area from 1.0 to 1.4 m2 and by increasing SCD treatment from 72 h up to 7 days with SCD replacement every 24 h. Nine patients were enrolled in the trial and were compared with historical case-matched controls with respect to age and SOFA score utilizing the PICARD/NIH database [29]. The mortality rate for the case-matched controls was 78%, whereas the mortality in the SCD treatment group was 22% (P = 0.027). Multiple regression analysis identified treatment with SCD as the only significant variable affecting mortality among age, SOFA score. This encouraging result was suggestive of a survival advantage; however, it should be noted that contemporaneous control groups were not tested. Patients receiving SCD treatment exhibited improved renal function, as mean total urine output increased from a baseline of ∼500 mL/day to more than 2000 mL/day by Day 7 of treatment. In fact, the five patients receiving therapy utilizing an SCD with the larger membrane surface area (1.4 m2), improved renal function and urine output in 72 h so that no dialytic treatment was necessary after 3 days. Once again, treatment with SCD was well tolerated, without significant effects on hematological parameters and with an adverse event profile expected for a seriously ill population in the ICU with AKI. The blood-flow patency of SCD was comparable with single-cartridge CRRT modalities. These results suggested a higher dose with a larger membrane SA and longer treatment time was safe and potentially more effective.
The third clinical study was a US multicenter pilot study to assess the safety and efficacy of the SCD in ICU patients with ARF and multiorgan failure [28]. This study was undertaken with an FDA-approved Investigational Device Exemption (IDE) (G090189). A total of 35 patients at six clinical sites were enrolled (Clinical Trials.gov., ID#NCT01072682). Those 35 patients had an average age of 56 years, an average SOFA score of 11.5 and 28/35 (80%) patients were septic. Ninety percent (31/35) of the patients were on mechanical ventilation. For the entire patient group, 28-day all-cause mortality was 20% at 28 days and 31% at 60 days. For the septic patients, 28-day and 60-day all-cause mortality were similar to that of the entire patient group outcomes. This compared favorably to historical conventional renal replacement therapy mortality rates exceeding 50%. Once again, this encouraging result was suggestive of a survival advantage with SCD treatment; however, it should be noted that contemporaneous control groups were not tested. There were no SCD treated patients requiring dialysis at 60 days versus the comparative control group of 8%.
The clinical results also demonstrated that respiratory improvement occurred in ventilator patients, as measured by FiO2 requirements, within 24 h; vasopressor requirements were reduced within 48 h and renal recovery, as assessed by urine volume, improved within 72 h. These time frames are significantly shorter than conventional therapy. The need for SCD was, on average, required for 4.3 days due to rapid improvement of the patient and discontinuation of the need for CRRT, in contrast to historical patient groups, requiring >7 days of CRRT treatment. Owing to these favorable results in three separate trials in 19 different clinical sites and 56 patients, a pivotal controlled, randomized, multicenter clinical trial has begun in September 2011 under an FDA-approved IDE (G0901895) clinical trial.gov ID# NCT01400893. The FDA also accepted and encouraged a respiratory 28-day ventilator-free survival rate and days of vasopressor requirements as important indices of multiorgan recovery. The aforementioned early clinical data are summarized in Table 1.
Table 1.
Summary of mortality rates from SCD-c clinical trials
| Trial | Mortality of SCD-c group (%) | Comparative control group | Mortality of control group (%) |
|---|---|---|---|
| Retrospective analysis of acellular RAD citrate anticoagulation (n = 12) [23] | 33 | Acellular RAD with heparin anticoagulation (n = 12) [23] | 75 |
| Prospective, single-arm, single-center trial to evaluate safety and efficacy (n = 9, 1 site) [27, 29] | 33 | Historical, case-matched controls, PICARD/NIH [27, 29] | 78 |
| US Multicenter safety and efficacy trial (n = 35, 6 sites) [28] | 31 | Historical, case-matched control, PICARD/NIH [28] | 63 |
Although critical in host defense, the activation of circulating neutrophils and the microvascular endothelium in systemic infections is the basis for the progression to multiorgan dysfunction in severe sepsis and SIRS from other etiologies, such as trauma, burns and surgery. The interaction of activated neutrophils and endothelium leads to increased vascular permeability with fluid leakage from the intravascular space to tissue interstitium with resulting hypovolemia, hypotension and cardiovascular instability. Sequestration and aggregation of neutrophils in the peritubular capillaries of the kidney promotes AKI and, if substantive, ARF. Sequestration and infiltration in lung tissue progresses to diminish pulmonary gas exchange and, if severe, adult respiratory distress syndrome [30].
Bioartificial renal epithelial cell system: a cell therapy approach
The clinical efficacy of the cell-based, multifactorial approach of the RAD, which leveraged the metabolic and synthetic functions of stem/progenitor cells of the kidney directed toward a renal tubule cell fate is well established [21, 24, 31]. Cell sourcing and storage/distribution issues encountered during RAD manufacturing have now been addressed through the development of a new cell-based device called the Bioartificial Renal Epithelial Cell System (BRECS). The BRECS was based on the same cell therapy concept as the RAD, with the BRECS utilizing a similar renal progenitor cell population directed toward an REC fate. However, the RAD utilized polysulfone fibers as a cell substrate, which could not be cryopreserved, and therefore hindered mass fabrication and distribution. The BRECS was designed to be fully cryopreservable, and therefore enable mass fabrication, storage and distribution.
In brief, the BRECS is a perfusion bioreactor that utilizes primary RECs isolated from the kidney, and expanded during in vitro culture. Cells are seeded on porous cell-carrying disks, which are placed within a media flow path within the BRECS. For a more detailed description and pictures of the BRECS design, see Buffington et al. [31]. Renal progenitor cells are directed toward a renal tubule cell fate and maintained in perfusion culture prior to therapeutic application. In vitro cell viability and metabolic activity are confirmed in the BRECS by measuring lactate production and oxygen consumption. Both metrics of metabolism have proven to be consistent throughout the duration of perfusion culture, with an estimated total cell number of >108 cells [31, 32]. Oxygen consumption rates are similar to previously reported values for metabolically active cells [33].
REC in BRECS maintain renal differentiated phenotypic characteristics over time in perfusion culture, human REC-seeded disks from BRECS units, upon immunohistochemical analysis, have displayed selected renal cell-differentiated markers, including acetylated tubulin (AT-1) and zona occludens (ZO-1). AT-1, a marker for apical central cilia of proximal tubule cells, exhibits regular staining in central regions of cells grown on disks. ZO-1, a marker for epithelial tight junctions, displays strong expression along the surface of the cells. ZO-1-positive tight junctions and punctate AT-1-positive central cilia are indicative of polarized epithelium and have been evident in all disks tested [31, 32].
To assess renal cell-specific function of the BRECS, a nondestructive glutathione (GSH) degradation assay was established as one measure of catabolic function of the cells in the BRECS over time. Exogenous glutathione is supplemented in the BRECS perfusion media, and metabolic degradation of GSH is measured over a 60-min period. GSH degradation rates in BRECS remain stable, ranging between 600 and 1200 nmol/h/BRECS over a 90-day period, indicative of sustained differentiated renal cell function in the device [31, 32].
Cryopreservation and thawing of REC in BRECS is accomplished using a commercially available and FDA-approved cryopreservation media, CryoStor 10. To optimize immediate postcryopreservation cell retention HTS-Purge solution (#637112, Biolife Solutions, Bothell, WA), a hypothermic solution used to prepare cells for the extreme conditions of cryopreservation, is utilized as a precryopreservation rinse buffer, in addition to using a controlled rate freezer during the cryopreservation process. Average cell retention of >80% was achieved, and furthermore, this cryopreservation procedure yielded an average cell viability of >90% [31, 32].
The extracorporeal application approach of the BRECS allows for cell therapy application while maintaining immunoisolation using a series of filters. Critical to the application of the BRECS for acute indications, the BRECS has been designed to enable cryopreservation of cells within the device in a therapeutically ready state and use an allogenic cell source, which enables mass fabrication, cryopreservation for storage and distribution for on-demand clinical need.
Summarizing, the key differences between the BRECS and the RAD are (i) the BRECS supports cells on porous disks and the RAD supported cells on hollow fibers; (ii) the BRECS can be cryopreserved, whereas the RAD did not have this capability due to polysulfone hollow fiber fracture during the freeze-thawing process and (iii) the BRECS utilizes both an immunoisolation prefilter to generate UF and a separate postfilter after the BRECS to retain cell debris, whereas the RAD utilized an immunoisolation prefilter to generate UF, but did not require a separate postfilter, as the hollow fiber-based RAD acted as its own postfilter to retain cell debris. Other salient features of RAD therapy remain intact for BRECS treatment, which include (i) both cell-based devices provide supplementary metabolic and secretory renal functions, (ii) both utilize an extracorporeal circuit as a platform for therapy, (iii) small solute clearance is afforded by hemofiltration and (iv) reabsorption/reclamation is based on hydraulic forces generated by pumps, and not active transport.
Conclusion
New therapies directed toward treating sepsis have, in the past, focused on interrupting the excessive levels of inflammatory cytokines (cytokine storm) or the activation of the coagulation system during sepsis, with little or modest effects on this disease process when tested clinically. As activated leukocytes are central to the pathogenesis and progression of sepsis and other clinical inflammatory disorders, new therapeutic approaches are being considered to limit the deleterious clinical effect of activated leukocytes that result from a dysregulated immune response to sepsis [10]. The SCD is a synthetic, biomimetic membrane that binds and sequesters activated leukocytes from the systemic circulation along an extracorporeal blood circuit. The SCD incorporates a low-velocity, low-shear force blood-flow path around a bundled collection of biocompatible membranes to reproduce capillary shear to create a condition to bind activated leukocytes during a systemic inflammatory disease state. To further minimize the systemic effects of activated leukocytes, the blood is anticoagulated with regional citrate infusion to lower blood ionized calcium (iCa) levels to 0.2–0.5 mM, levels which inhibit the coagulation system of the blood. This lowering of blood iCa also has an inhibitory effect on neutrophil activation, thereby simultaneously combining the SCD effect to sequester activated circulating leukocytes and limits the potential activation of leukocytes entering the SCD and the low-iCa environment. This treatment approach changes systemic neutrophil kinetics and release of neutrophils from stored sites. Other novel, device-based therapies for systemic inflammation include: the BRECS, a cell bioreactor, which leverages metabolic and synthetic functions of stem/progenitor cells of the kidney directed toward a renal tubule cell fate in a multifactorial approach to the treatment of immune dysregulation and organ failure. Cell processing and cell therapy for the treatment of systemic inflammation are clinically promising approaches to combat sepsis and multiorgan failure.
Conflict of interest statement
HDH is a shareholder of Innovative BioTherapies, Inc., and CytoPherx, Inc. C.J.P., A.J.W., P.L.S. and D.A.B. are employees of Innovative BioTherapies, Inc. A.S.Y. is a consultant for CytoPherx, Inc.
Acknowledgements
We acknowledge the provision of human kidneys, used to isolate renal epithelial cells, by the National Disease Research Interchange (NDRI) with support by grant number 5 U42 RR006042 from NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH. This work was supported by the U.S. Army Medical Research and Materiel Command, contract W81XWH-05-2-0010 and W81XWH-10-2-0137, and the Small Business Innovation Research program of the National Institutes of Health, Grants NIH R44 DK074289, NIH R43 DK080529 and NIH R43 DK082050.
References
- 1.Pinsky MR, Vincent JL, Deviere J, et al. Serum cytokine levels in human septic shock. Relation to multiple-system organ failure and mortality. Chest. 1993;103:565–575. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 2.Munoz C, Carlet J, Fitting C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astiz M, Saha D, Lustbader D, et al. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;128:594–600. doi: 10.1016/s0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 4.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257(4 Pt 1):L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 5.Beale R, Reinhart K, Brunkhorst FM, et al. Promoting Global Research Excellence in Severe Sepsis (PROGRESS): lessons from an international sepsis registry. Infection. 2009;37:222–232. doi: 10.1007/s15010-008-8203-z. [DOI] [PubMed] [Google Scholar]
- 6.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 8.Brown KA, Brain SD, Pearson JD, et al. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 9.Schouten M, Wiersinga WJ, Levi M, et al. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 10.Kaneider NC, Leger AJ, Kuliopulos A. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS J. 2006;273:4416–4424. doi: 10.1111/j.1742-4658.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 11.Maroszynska I, Fiedor P. Leukocytes and endothelium interaction as rate limiting step in the inflammatory response and a key factor in the ischemia-reperfusion injury. Ann Transplant. 2000;5:5–11. [PubMed] [Google Scholar]
- 12.Atan R, Crosbie D, Bellomo R. Techniques of extracorporeal cytokine removal: a systematic review of the literature. Blood Purif. 2012;33:88–100. doi: 10.1159/000333845. [DOI] [PubMed] [Google Scholar]
- 13.Marshall JC. Neutrophils in the pathogenesis of sepsis. Crit Care Med. 2005;33(12 Suppl):S502–S505. doi: 10.1097/01.ccm.0000186266.34541.5f. [DOI] [PubMed] [Google Scholar]
- 14.Boxer LA. Neutrophil abnormalities. Pediatr Rev. 2003;24:52–62. doi: 10.1542/pir.24-2-52. [DOI] [PubMed] [Google Scholar]
- 15.Hughes WT, Armstrong D, Bodey GP, et al. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis. 1997;25:551–573. doi: 10.1086/513764. [DOI] [PubMed] [Google Scholar]
- 16.Bloembergen WE, Stannard DC, Port FK, et al. Relationship of dose of hemodialysis and cause-specific mortality. Kidney Int. 1996;50:557–565. doi: 10.1038/ki.1996.349. [DOI] [PubMed] [Google Scholar]
- 17.Allon M, Depner TA, Radeva M, et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol. 2003;14:1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58:1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 20.Humes HD, Buffington DA, MacKay SM, et al. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol. 1999;17:451–455. doi: 10.1038/8626. [DOI] [PubMed] [Google Scholar]
- 21.Tumlin J, Wali R, Williams W, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–1040. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humes HD, Buffington DA, Lou L, et al. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Crit Care Med. 2003;31:2421–2428. doi: 10.1097/01.CCM.0000089644.70597.C1. [DOI] [PubMed] [Google Scholar]
- 23.Humes HD, Sobota JT, Ding F, et al. A selective cytopheretic inhibitory device to treat the immunological dysregulation of acute and chronic renal failure. Blood Purif. 2010;29:183–190. doi: 10.1159/000245645. [DOI] [PubMed] [Google Scholar]
- 24.Mariano F, Tedesch L, Morselli M, et al. Normal citratemia and metabolic tolerance of citrate anticoagulation for hemodiafiltration in severe septic shock burn patients. Intensive Care Med. 2010;36:1735–1743. doi: 10.1007/s00134-010-1909-2. [DOI] [PubMed] [Google Scholar]
- 25.Ding F, Song JH, Jung JY, et al. A biomimetic membrane device that modulates the excessive inflammatory response to sepsis. PLoS One. 2011;6:e18584. doi: 10.1371/journal.pone.0018584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayub K, Hallett MB. Ca2+ influx shutdown during neutrophil apoptosis: importance and possible mechanism. Immunology. 2004;111:8–12. doi: 10.1111/j.1365-2567.2003.01766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding F, Yevzlin AS, Xu ZY, et al. The effects of a novel therapeutic device on acute kidney injury outcomes in the intensive care unit: a pilot study. ASAIO J. 2011;57:426–432. doi: 10.1097/MAT.0b013e31820a1494. [DOI] [PubMed] [Google Scholar]
- 28.Tumlin J, Chawla L, Tolwani AJ, et al. The effect of the selective cytopheretic device on acute kidney injury outcomes in the intensive care unit: a multi-center pilot study. Semin Dial. 2012 doi: 10.1111/sdi.12032. 25(6) DOI: 10.1111/sdi.12032. (accepted for publication) [DOI] [PubMed] [Google Scholar]
- 29.Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 30.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buffington D, Pino CJ, Chen L, et al. Bioartificial renal epithelial cell system (BRECS): a compact, cryopreservable extracorporeal renal replacement device. Cell Med. 2012;4:33–43. doi: 10.3727/215517912X653328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pino CJ, Humes HD. Bioartificial Kidney. Comprehensive Biomaterials. Vol. 6. Oxford, UK: Elsevier; 2011. [Google Scholar]
- 33.Nikolovski J, Gulari E, Humes HD. Design engineering of a bioartificial renal tubule cell therapy device. Cell Transplant. 1999;8:351–364. doi: 10.1177/096368979900800403. [DOI] [PubMed] [Google Scholar]