Summary
Although sensory processing in V1 has been extensively characterized, the role of GABAergic inhibition is still not well understood. Advances in molecular biology have now removed significant barriers to the direct investigation of inhibitory processes in vivo. Recent studies have provided important insights into the influence of GABAergic inhibition on cortical processing at both the single cell level, where inhibition helps to shape cortical receptive fields, and at the network level, where inhibition is critical for generating cortical oscillations and setting network state.
Introduction
The importance of GABAergic inhibition in neural processing is evident. Inhibition is critical in a wide range of brain processes (e.g., network oscillations, synaptic plasticity, and response gain control), and abnormal inhibitory circuits have been implicated in a number of neurological disorders such as epilepsy, schizophrenia, anxiety, and Alzheimer's disease [1]. While sensory processing in the primary visual cortex (V1) is one of the most researched topics in systems neuroscience, until recently the role of GABAergic inhibition has remained obscure due to technological limitations. We are just beginning to understand the response properties of GABAergic neurons and how they influence visual processing in the cortex.
The focus of this review is recent advances in our understanding of the role of inhibition in V1. We will begin with the analyses at the level of individual neurons: the influence of inhibition on visual cortical receptive fields (RFs) as well as the RF properties of GABAergic interneurons themselves. We will end with a discussion on recent insights into the powerful influence of inhibition on cortical network dynamics.
Influence of Inhibitory Inputs on Orientation Tuning
Ever since Hubel and Wiesel first proposed the simple and elegant model in which excitatory, feed forward connections (LGN→ layer IV simple cells → layer II/III complex cells) give rise to the orientation-selective RFs in V1 [2], researchers have attempted to determine the role of inhibition in shaping cortical RFs. Due to the technical difficulties in directly measuring inhibitory processes in vivo, they were often inferred from extracellular recordings. The preponderance of evidence from these experiments indicates that in layer IV, the primary recipient of thalamic input, the basic RF structure and orientation selectivity of neurons are established primarily by the spatial arrangement of excitatory inputs from the LGN [3] (but see [4]). However, inhibitory input, either orthogonal to a neuron's excitatory input or weakly tuned for orientation, was thought to be necessary for the suppression of neuronal responses by the superimposition of a grating perpendicular to the preferred orientation (cross-orientation suppression) [5-6]. Furthermore, computational models suggested that such inhibition is necessary for sharpening cortical orientation tuning and for preserving the tuning width across multiple stimulus intensities (contrast-invariant orientation tuning) [7-8].
To characterize orientation tuning of the excitatory and inhibitory inputs to cortical neurons, whole-cell recordings have been used to measure each type of synaptic conductance. These experiments indicate that excitatory and inhibitory inputs to most cortical neurons are tuned to similar orientations (iso-orientation inhibition) [9-10]. To reconcile the difference between the response properties measured extracellularly and synaptic conductances measured with whole-cell recordings, it was recently proposed that cross-orientation suppression of cortical spiking is mediated by a reduction of thalamic excitation rather than an increase of intracortical inhibition [11]. Indeed, intracellular measurement of synaptic inputs showed that the net reduction of spiking during cross-orientation suppression is accompanied by a reduction in both synaptic inhibition and excitation [12]. To assess the capability of thalamic excitation to generate cross-orientation suppression, a computational model was created to simulate the integration of physiologically measured LGN spike trains by a layer IV simple cell. The combination of nonlinear LGN response properties and a biologically realistic, contrast-dependent cortical spike threshold mechanism was found to be sufficient to explain cross-orientation suppression and contrast-invariant orientation tuning without the influence of cortical inhibition [11,13].
Of course, although this nonlinear, excitatory model provides the simplest explanation for cross-orientation suppression, it does not rule out the involvement of additional intracortical processes. A key prediction of the model is that cross-orientation suppression should only be seen at high contrasts, where the rectification and saturation of LGN responses are prominent. Contrary to this prediction, cross-orientation suppression was observed at very low contrasts in tree shrew visual cortex [14]. Although these data only provide an indirect contradiction, the nonlinear, excitatory model should be tested under low-contrast conditions to resolve the discrepancy.
Push-Pull Inhibition
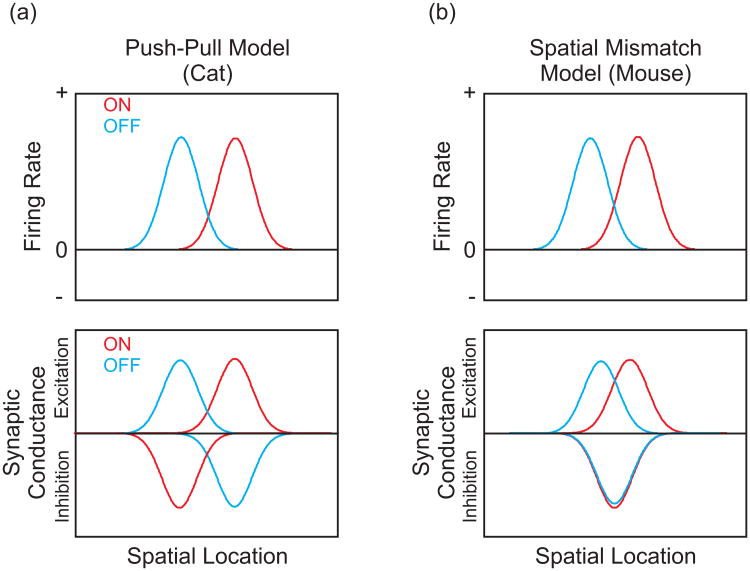
A prominent feature of layer IV RFs is the spatially segregated ON and OFF subfields and the push-pull interaction within each subfield (i.e., stimuli of opposite contrast polarities evoke responses of opposite signs) [15]. In cat visual cortex, intracellular current-clamp recordings indicate that cortical inhibition is critical for this “push-pull” RF structure. The RFs of excitatory and inhibitory conductances are aligned to the same orientation, but are 180° out of phase with each other [9-10] (Figure 1a). Thus, increases in luminance over an ON-subregion evoke excitatory conductances (“push”), while luminance decreases in the same region evoke inhibitory conductances (“pull”). This push-pull interaction is believed to be important for spatial and temporal frequency selectivity as well as network stability [7].
Figure 1.
RF structure of simple cells in (a) cat and (b) mouse visual cortices. (a, Top) In cat visual cortex, simple cell RFs defined by spike rate have spatially segregated ON and OFF subregions (red and blue traces, respectively). (Bottom) This spatial segregation is also present in the RFs of excitatory and inhibitory inputs. The phase difference between the excitatory and inhibitory RFs gives rise to the classic “push-pull” antagonism. Increases in luminance over an ON subregion evoke an excitatory conductance, while the same stimulus over an OFF subregion evokes an inhibitory conductance. The opposite stimulus-response pattern is found for decreases in luminance. (b, Top) In mouse visual cortex, layer II/III simple cells also have spatially segregated ON and OFF subregions. (Bottom) This spatial segregation, however, is caused by a spatial mismatch between partially segregated ON and OFF excitatory subregions and centrally located, overlapping ON and OFF inhibitory subregions.
Recently, the RF structures of synaptic inputs have been examined with voltage-clamp recordings in mouse visual cortex [*16]. The arrangement of the inhibitory and excitatory RFs does not seem to support the push-pull model. While the excitatory inputs to simple cells displayed spatially segregated ON- and OFF-subregions, the ON- and OFF-subregions of inhibitory inputs were in nearly perfect register, centered between the excitatory subregions (Figure 1b). The spatial mismatch between the excitatory and inhibitory conductances was shown to increase the spatial separation of the ON- and OFF-subregions measured by membrane depolarization in current-clamp mode [*16], and this effect could explain the loss of ON- and OFF subfield segregation after GABAergic conductances are blocked in cat visual cortex [17].
While the mouse visual system lacks the spatial acuity and organizational complexity of primates and felines, the cortical neurons have simple and complex cell RFs with orientation selectivity similar to that in larger mammals [*18]. With the recent advances in transgenic technology, the mouse is becoming an increasingly important animal model for the study of visual processing. Thus, it is important to resolve the discrepancy between the results from the cat and mouse. The disagreement could be methodological in nature; data from the cat visual cortex were collected using current-clamp recording, while data from the mouse were collected under voltage-clamp. It could also be caused by circuitry differences across species. In cat visual cortex, simple cells dominate layer IV while complex cells are mainly reported in other layers [15,19]. In mouse visual cortex, however, simple and complex cells are both found in layer II/III, while layer IV primarily consists of cells with unipolar RFs [*16]. Given this difference in the laminar organization, simple cells in the cat and mouse could have different underlying synaptic mechanisms.
Inhibitory Surround Influences
Visual stimulation beyond a neuron's contrast-dependent summation field typically suppresses its spiking response [20]; however, see [21] (Figure 2a). Such surround suppression is tuned to the preferred orientation of the neuron and it helps to sharpen the neuronal orientation selectivity [22]. Surround suppression was thought to be mediated by horizontal cortical connections acting through polysynaptic inhibition [23-24] and has traditionally been modeled by a difference of Gaussians (DOG) function [25]. Recently, however, both of these notions have been challenged.
Figure 2.
Neural mechanisms of spatial summation and surround suppression. (a) The spatial summation properties of cortical neurons can be quantified by plotting the response as a function of stimulus diameter. This function is dependent on stimulus contrast: the high-contrast summation field (HSF, xx trace) peaks at a smaller diameter (red arrow, preferred size) relative to the low-contrast summation field (LSF, xx trace). Surround suppression is defined as the decrease in response when the stimulus exceeds the preferred size. (b) The HSF and LSF are approximately equal in spatial extent to the spread of feed forward excitation from the LGN and the spread of intralaminar horizontal projections, respectively. The surround suppression is more consistent with cortical feedback via disynaptic inhibition. (c) The classic DOG model of surround suppression (top) predicts increased excitatory and inhibitory conductances as stimulus size is increased (bottom). (d) Experimental data, however, show that surround suppression is associated with decreases in both excitatory and inhibitory conductances.
First, quantitative measurement of the spatiotemporal properties of surround suppression indicates that they are inconsistent with the properties of polysynaptic inhibition from horizontal projections. The conduction velocities of unmyelinated horizontal axons in the visual cortex are not fast enough to account for the short onset latency of surround suppression, and their arborization patterns are not expansive enough to account for the spatial extent of surround suppression [26]. The area of the suppressive surround is estimated to be 5x the high-contrast summation field (HSF, Figure 2a), while the spatial extent of horizontal projections is more comparable to the summation field size measured under low contrast (Figure 2b). Unlike contrast invariant orientation tuning and cross-orientation suppression, cortical surround suppression cannot be explained by subcortical mechanisms, due to the weak strength, lack of orientation selectivity, and small spatial extent of LGN surround suppression [26-**27]. Instead, the large spatial extent and short onset latency of surround suppression are more consistent with signals from extrastriate cortex, conveyed by myelinated feedback axons [28-29].
Second, the difference of Gaussians (DOG) model with a strong but localized excitatory center and a weaker, expensive inhibitory surround predicts an increase in both synaptic excitation and inhibition as the size of a visual stimulus is increased [30] (Figure 2c). However, intracellular recordings showed that increases in visual stimulation beyond the neuron's preferred size caused decreases in both excitation and inhibition [**27] (Figure 2d). Computationally, these observed decreases can be explained by an inhibition-stabilized network (ISN), in which increases in the external excitatory drive onto inhibitory cells can result in a net decrease in their firing rate. This type of networks has been reported in both the hippocampus and neocortex, and it is consistent with the general finding of balanced excitation and inhibition across changes in overall network load [31-34].
Receptive Field Properties of Inhibitory Interneurons
Uncovering the RF properties of GABAergic interneurons is essential for understanding how inhibition influences visual cortical processing. Although the RF properties of cortical neurons have been extensively characterized, historically it has been difficult to ascertain whether the recorded neuron is excitatory or inhibitory, and whether the different subpopulations exhibit different RF properties.
One of the oldest approaches to cell type classification is based on the shape of individual action potentials and the temporal characteristics of spike trains: fast-spiking (FS) neurons are thought to be inhibitory and regular-spiking (RS) cells excitatory. In cat visual cortex, FS neurons measured with intracellular recordings have been reported to have a wide range of orientation tuning properties, from highly selective to completely unselective, while in mouse visual cortex, FS neurons were generally found to be unselective [35]; Cardin, 2007 #37} [18]. An important limitation of this approach is that although a majority of FS cells are parvalbumin positive (PV+) basket cells, they do not represent a single homogenous population of neurons [36]. Conversely, FS neurons only account for less than 50% of GABAergic neurons in V1 [36-38], thus the inhibitory neurons with RS characteristics would be mis-identified as excitatory neurons by this method.
The introduction of two-photon (2P) microscopy and transgenic labeling of specific neuronal subtypes has greatly enhanced our ability to investigate RF properties of GABAergic neurons. For example, using 2P Ca2+ imaging to measure the visual responses of mouse cortical neurons, transgenically labeled GABAergic neurons (GAD67-GFP) in layer II/III were found to be unselective for stimulus orientation [39]. Additional experiments are currently conducted in several laboratories to characterize the RF properties of specific subtypes of GABAergic neurons.
Although 2P Ca2+ imaging provides a powerful tool for studying cortical RF properties, the results of these experiments should be interpreted cautiously. While changes in intracellular Ca2+ concentration have been shown to be linearly related to changes in activity at low spike rates [40-41], this relationship has not been studied in GABAergic neurons that generally exhibit higher firing rate. Given that GABAergic neurons also have different Ca2+ buffering mechanisms [42], the relationship between the spiking activity and the changes in fluorescence may be different and thus need to be verified experimentally.
In addition to Ca2+ imaging, 2P imaging has been used to guide the patch electrodes toward individual neurons [43]. The two-photon targeted patching (TPTP) technique, which is unaffected by potential nonlinear transformations between neural activity and Ca2+ signals and not limited by the slow sampling rates associated with raster scanning, has been employed to determine the RF structure of inhibitory neurons transgenically labeled with GFP in mouse visual cortex [**44]. The results were consistent with the original finding from 2P Ca2+ imaging that the inhibitory neurons in layer II/III are much less orientation selective [39]. In addition, detailed mapping of the RF structure showed that the majority of inhibitory cells had overlapping ON- and OFF-subregions and thus were characterized as complex cells. Similar GABAergic complex cells have been reported in layer IV of cat V1 [45].
GABAergic Neurons and Brain States
Beyond the view of sensory processing from the perspective of individual neurons, the network state is likely to have significant influences on information processing in the visual cortex [46-48]. Different cortical states are associated with a variety of dominant frequencies of network activity. In general, periods of active sensory processing with elevated attention and arousal are dominated by low-amplitude, high-frequency (e.g., gamma) activity; periods of inactive quiescence and sleep are dominated by high-amplitude, low-frequency oscillations [49].
It has long been proposed that GABAergic neurons are critical for the generation of gamma oscillations [50-51]. In two recent studies using transgenic mice, channelrhodopsin and/or halorhodopsin expressed in PV+ GABAergic neurons allowed rapid and selective modulation of their activity. Inactivation of PV+ neurons caused a marked reduction of gamma activity, while activation of these neurons caused a significant increase in gamma oscillations in the neocortex [**52-**53]. The increase in gamma activity in turn increased the precision of spiking activity and the amount of information carried by the spike trains.
Brain states are long known to be regulated by various neuromodulators [54], and these effects are likely mediated at least in part by GABAergic neurons. For example, cholinergic inputs to the neocortex from the basal forebrain can cause a dramatic decrease in correlated firing between neurons and an increase in the reliability of each neuron in response to visual stimuli [*55], enhance the gain of thalamic input [56], and contribute to attentional modulation in the visual cortex [57]. These cholinergic inputs are known to target specific subpopulations of GABAergic neurons [36,58]. Muscarinic acetylcholine receptors are found on PV+, calretinin+, and calbindin+ GABAergic neurons, while nicotinic receptors are expressed on CCK+/VIP+ GABAergic neurons [51,59]. In response to both muscarinic and nicotinic acetylcholine agonists, somatostatin+ GABAergic neurons oscillate synchronously at 3-12 Hz (theta band activity) [*60]. Interestingly, theta oscillations interact with gamma oscillations in the hippocampus during spatial navigation. Given the recently developed techniques that allow selective manipulation of specific subtypes of interneurons, we are now beginning to understand how these neurons mediate neuomodulation of cortical network dynamics.
Conclusions
Much remains to be learned about the role of inhibition in sensory processing. Recent experiments have revealed important properties of inhibition in the neocortex, but we have only uncovered the tip of a very large iceberg. Advancements in molecular techniques have made it possible to address questions that were unapproachable just a decade ago. In vision research, inhibition has traditionally been studied as a single homogeneous process. As new methods of selectively targeting specific subpopulations of inhibitory interneurons are perfected, we will be able to incorporate subtype specific populations of GABAergic neurons into models of visual processing. This includes understanding how GABAergic neurons interact with the large diversity of neuromodulators to influence visual RF properties and network dynamics.
Acknowledgments
This work was supported by NIH grant EY18861.
Abbreviation
- V1
Primary Visual Cortex
- RF
Receptive Field
- LGN
Lateral Geniculate Nucleus
- PV+
Parvalbumin-expressing GABAergic neurons
- HSF
High-Contrast Summation Field
- LSF
Low-Contrast Summation Field
- DOG
Difference of Gaussians
- FS
Fast-spiking cells
- RS
Regular-spiking cells
- 2P
Two-Photon Microscopy
References
- 1.Moult PR. Neuronal glutamate and GABAA receptor function in health and disease. Biochem Soc Trans. 2009;37:1317–1322. doi: 10.1042/BST0371317. [DOI] [PubMed] [Google Scholar]
- 2.Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441–471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- 4.Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 2003;38:689–699. doi: 10.1016/s0896-6273(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 5.DeAngelis GC, Robson JG, Ohzawa I, Freeman RD. Organization of suppression in receptive fields of neurons in cat visual cortex. J Neurophysiol. 1992;68:144–163. doi: 10.1152/jn.1992.68.1.144. [DOI] [PubMed] [Google Scholar]
- 6.Morrone MC, Burr DC, Maffei L. Functional implications of cross-orientation inhibition of cortical visual cells I Neurophysiological evidence. Proc R Soc Lond B Biol Sci. 1982;216:335–354. doi: 10.1098/rspb.1982.0078. [DOI] [PubMed] [Google Scholar]
- 7.Lauritzen TZ, Miller KD. Different roles for simple-cell and complex-cell inhibition in V1. J Neurosci. 2003;23:10201–10213. doi: 10.1523/JNEUROSCI.23-32-10201.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sompolinsky H, Shapley R. New perspectives on the mechanisms for orientation selectivity. Curr Opin Neurobiol. 1997;7:514–522. doi: 10.1016/s0959-4388(97)80031-1. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. J Neurophysiol. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch JA, Alonso JM, Reid RC, Martinez LM. Synaptic integration in striate cortical simple cells. J Neurosci. 1998;18:9517–9528. doi: 10.1523/JNEUROSCI.18-22-09517.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. This review provides an excellent historical overview of past experiments and a thorough explanation of the role of subcortical processes and spike threshold in cortical response properties. [DOI] [PubMed] [Google Scholar]
- 12.Priebe NJ, Ferster D. Mechanisms underlying cross-orientation suppression in cat visual cortex. Nat Neurosci. 2006;9:552–561. doi: 10.1038/nn1660. [DOI] [PubMed] [Google Scholar]
- 13.Finn IM, Priebe NJ, Ferster D. The emergence of contrast-invariant orientation tuning in simple cells of cat visual cortex. Neuron. 2007;54:137–152. doi: 10.1016/j.neuron.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacEvoy SP, Tucker TR, Fitzpatrick D. A precise form of divisive suppression supports population coding in the primary visual cortex. Nat Neurosci. 2009;12:637–645. doi: 10.1038/nn.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.Liu BH, Li P, Sun YJ, Li YT, Zhang LI, Tao HW. Intervening inhibition underlies simple-cell receptive field structure in visual cortex. Nat Neurosci. 2010;13:89–96. doi: 10.1038/nn.2443. Employing voltage clamp recordings, these authors provide detailed characterization of spatial receptive field maps of simple and complex cells in mouse visual cortex. Their results indicate that although the inhibitory input shows overlapped ON and OFF subregions, it plays an important role in establishing segregated ON and OFF subregions in simple cell receptive fields. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science. 1994;265:774–777. doi: 10.1126/science.8047882. [DOI] [PubMed] [Google Scholar]
- *18.Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch JA, Martinez LM. Laminar processing in the visual cortical column. Curr Opin Neurobiol. 2006;16:377–384. doi: 10.1016/j.conb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. J Neurophysiol. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- 21.Ichida JM, Schwabe L, Bressloff PC, Angelucci A. Response facilitation from the “suppressive” receptive field surround of macaque V1 neurons. J Neurophysiol. 2007;98:2168–2181. doi: 10.1152/jn.00298.2007. [DOI] [PubMed] [Google Scholar]
- 22.Okamoto M, Naito T, Sadakane O, Osaki H, Sato H. Surround suppression sharpens orientation tuning in the cat primary visual cortex. Eur J Neurosci. 2009;29:1035–1046. doi: 10.1111/j.1460-9568.2009.06645.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert CD. Horizontal integration and cortical dynamics. Neuron. 1992;9:1–13. doi: 10.1016/0896-6273(92)90215-y. [DOI] [PubMed] [Google Scholar]
- 24.Grinvald A, Lieke EE, Frostig RD, Hildesheim R. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J Neurosci. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast's effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- 26.Angelucci A, Bressloff PC. Contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–120. doi: 10.1016/S0079-6123(06)54005-1. [DOI] [PubMed] [Google Scholar]
- *27.Ozeki H, Finn IM, Schaffer ES, Miller KD, Ferster D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron. 2009;62:578–592. doi: 10.1016/j.neuron.2009.03.028. This study shows that previous models of visual cortical surround suppression do not capture the synaptic dynamics of the phenomenon. Instead, a new model was proposed based on balanced inhibition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MA, Bair W, Movshon JA. Dynamics of suppression in macaque primary visual cortex. J Neurosci. 2006;26:4826–4834. doi: 10.1523/JNEUROSCI.5542-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwabe L, Obermayer K, Angelucci A, Bressloff PC. The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model. J Neurosci. 2006;26:9117–9129. doi: 10.1523/JNEUROSCI.1253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sceniak MP, Hawken MJ, Shapley R. Visual spatial characterization of macaque V1 neurons. J Neurophysiol. 2001;85:1873–1887. doi: 10.1152/jn.2001.85.5.1873. [DOI] [PubMed] [Google Scholar]
- 31.Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Paradoxical effects of external modulation of inhibitory interneurons. J Neurosci. 1997;17:4382–4388. doi: 10.1523/JNEUROSCI.17-11-04382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- 34.Shu Y, Hasenstaub A, McCormick DA. Turning on and off recurrent balanced cortical activity. Nature. 2003;423:288–293. doi: 10.1038/nature01616. [DOI] [PubMed] [Google Scholar]
- 35.Nowak LG, Azouz R, Sanchez-Vives MV, Gray CM, McCormick DA. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol. 2003;89:1541–1566. doi: 10.1152/jn.00580.2002. [DOI] [PubMed] [Google Scholar]
- 36.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 37.Gonchar Y, Wang Q, Burkhalter A. Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat. 2008;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr JN, Greenberg D, Helmchen F. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci U S A. 2005;102:14063–14068. doi: 10.1073/pnas.0506029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11:749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, Rosenmund C, Schwaller B, Neher E. Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol. 2000;525 Pt 2:405–418. doi: 10.1111/j.1469-7793.2000.t01-3-00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitamura K, Judkewitz B, Kano M, Denk W, Hausser M. Targeted patch-clamp recordings and single-cell electroporation of unlabeled neurons in vivo. Nat Methods. 2008;5:61–67. doi: 10.1038/nmeth1150. [DOI] [PubMed] [Google Scholar]
- **44.Liu BH, Li P, Li YT, Sun YJ, Yanagawa Y, Obata K, Zhang LI, Tao HW. Visual receptive field structure of cortical inhibitory neurons revealed by two-photon imaging guided recording. J Neurosci. 2009;29:10520–10532. doi: 10.1523/JNEUROSCI.1915-09.2009. Using 2-photon targeted patch recordings, these authors characterized spatial receptive field maps of GABAergic interneurons in mouse visual cortex. Their results indicate that GABAergic neurons in visual cortex are primarily non-orientation selective complex cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirsch JA, Martinez LM, Pillai C, Alonso JM, Wang Q, Sommer FT. Functionally distinct inhibitory neurons at the first stage of visual cortical processing. Nat Neurosci. 2003;6:1300–1308. doi: 10.1038/nn1152. [DOI] [PubMed] [Google Scholar]
- 46.Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ringach DL. Spontaneous and driven cortical activity: implications for computation. Curr Opin Neurobiol. 2009;19:439–444. doi: 10.1016/j.conb.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431:573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 49.Steriade M. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- 50.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26:489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- **52.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **53.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. Using optogenetic tools, these authors demonstrate that the activation of parvalbumin interneurons is sufficient to increase gamma oscillations. Furthermore, these gamma oscillations enhanced the transmission of information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steriade MM, R W. Brainstem control of wakefulness and sleep. New York, New York: Plenum Press; 1990. [Google Scholar]
- *55.Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. Using electrical stimulation of basal forebrain and multielectrode recording from visual cortex, it is shown that cholinergic input desynchronizes the cortex and enhances reliability of cortical neurons in response to visual stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci. 2008;31:317–327. doi: 10.1016/j.tins.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol. 2008;507:1748–1762. doi: 10.1002/cne.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *60.Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. This study reports the intrinsic and network properties of somatostatin interneurons. Cholinergic receptor agonists can cause synchronous low frequency (3-10 Hz) oscillations amongst gap-junction coupled somatostatin interneurons. [DOI] [PMC free article] [PubMed] [Google Scholar]