Abstract
Dendritic cells (DC) play a pivotal role in regulating immunity, establishing immunologically privileged tissue micro-environments and maintaining homeostasis. It is becoming increasingly clear that one key mechanism that mediates many DC functions is production of the immunomodulatory enzyme indoleamine 2,3-dioxygenase (IDO). For pathogens that cause chronic infection, exploitation of host DCs is a solution to establish and persist within a host. Leishmania parasites cause a range of clinical manifestations, all involving chronic infection, and are proficient at avoiding immune responses. We demonstrate here that infection of human myeloid-derived DC with L. major and L. donovani induces IDO expression using a mechanism that involves autocrine or paracrine stimulation with a DC secreted factor. Leishmania-induced IDO suppresses allogeneic and tetanus toxoid-specific lymphocyte proliferation; an inhibition that is reversed with the IDO inhibitor, 1-Methyl Tryptophan (1-MT). Furthermore, IDO expression by human DC does not require live Leishmania infection, as parasite lysates also up-regulate IDO mRNA production. Our data suggest that one mechanism Leishmania parasites utilize to circumvent immune clearance may be to promote the induction of IDO among host DC within the infection micro-environment.
Keywords: Leishmania, Indoleamine 2, 3-dioxygenase, dendritic cell
Introduction
Chronicity is a hallmark of parasitic infections. As these pathogens often have complex life-cycles involving multiple host species, they elicit a variety of different immune-evasion strategies to persist, increasing the probability of transmission to their vector hosts. All the major protozoan and metazoan pathogens associated with human disease have evolved multiple sophisticated strategies to avoid immune destruction (1, 2); complexities that, to date, have placed efficacious vaccines outside the reach of development. Leishmania parasites employ a variety of different mechanisms to establish and maintain infection in their vertebrate hosts (3, 4), and despite substantial efforts, an effective vaccine remains elusive.
Leishmaniasis is endemic in 88 countries with approximately 12 million individuals infected and 350 million more at risk (5). A spectrum of disease manifestations is associated with Leishmania infection, ranging from localized cutaneous lesions to fatal visceral disease. Although host genetics are no doubt important, these varied clinical outcomes generally are attributed to differences in the Leishmania species initiating the infections (6–9). For example, the old world parasite L. major induces cutaneous lesions that heal spontaneously; resolution of this disease usually corresponds with immunity, however, substantial scarring occurs along with recurrent infections in immunocompromised individuals (10). The most debilitating form of infection is visceral leishmaniasis, or kala-azar. This disease is most often associated with infection with L. donovani and L. infantum. Visceral leishmaniasis is a severe systemic disease, characterized by a lack of cutaneous pathology, parasite dissemination, and uncontrolled growth in the liver, spleen, and bone-marrow. If the symptoms are left untreated, this disease is generally fatal.
Leishmania parasites are notably adept at circumventing immunity. These parasites reside within cells of the mononuclear lineage, primarily macrophages (MP) and dendritic cells (DC). Intracellular survival in these cells requires these organisms be able to escape the host cell anti-microbicidal mechanisms. Leishmania avoid intracellular elimination in MP by failing to elicit an oxidative burst upon entry (11, 12), delaying phagosome maturation (13), and inhibiting IFN-γ signaling in infected MP (3). While these pathogens enter naïve MP silently, they are destroyed by IFN-γ primed MP in an NO-dependent manner (14), necessitating that they also suppress or delay the production of activating cytokines.
Leishmania primarily reside in MP, although several cell types can harbor these pathogens including DC, neutrophils, monocytes and even fibroblasts. Given the central role of DC in regulating immune responses, exploiting these cells for immune-mediation provides an evolutionary survival strategy to suppress host cell-mediated immunity. One mechanism by which DC exert their regulatory ability is through regulation of the tryptophan catabolism pathway wherein indoleamine 2,3-dioxygenase (IDO) converts tryptophan to kynurenine (15). IDO-producing DC deplete local tryptophan levels during polyclonal T-cell responses (16), supporting the idea that IDO-producing DC inhibit T-cell proliferation. Once T-cell proliferation has been halted by a lack of tryptophan, T-cell death is promoted through exposure of anergic T-cells to toxic kynurenines, exerting differential effects on the Th1/Th2 balance (17–20) and skewing the balance toward a Th2 bias.
The importance of IDO during infectious disease events is only beginning to emerge. Previous studies on Toxoplasma spp. and β-Streptococci revealed that IDO is able to deprive an invading organism of tryptophan, thereby halting their replication (21–23). It has also been shown that IDO is induced as a result of infection with HIV (24), influenza (25), hepatitis C (26), and Plasmodium yoelii (27). More recently, it has been reported that L. major induced IDO expression reduces T-cell proliferation and attenuates pro-inflammatory responses in a murine model system (28). Here, we demonstrate that Leishmania infection induces IDO production in human DC and that inhibition of IDO restores the ability of infected DC to activate a proliferative response. Our results suggest that IDO induction is involved in the immunosuppression associated with Leishmania infection in humans.
Materials and Methods
Dendritic Cell Generation
Human monocytes (CD14+) were isolated from peripheral blood samples of healthy adult donors (Indiana Blood Distribution) using the Ficoll-Paque (MediaTech) method followed by selection using anti-CD14 magnetic microbeads (Miltenyi). The studies described herein were exempt from Ethics Board Review, according to US National Institutes of Health Guidelines (Exemption 45 CFR § 46.101(b)(4)). DC were differentiated from monocytes by IL-4 and GMCSF supplementation, as previously described (29). For mixed lymphocyte reactions (MLR), the same differentiation procedure was utilized except that the media used was serum-free X-vivo media (BioWhittaker).
Parasite Infection Assays
Leishmania major NIH Friedlin V1 strain and (MHOM/IL/80/FN), Leishmania donovani 1S strain (MHOM/SD/62/1S), were cultured in 1X M199 media supplemented with 20% FBS, 100mg/mL Penicillin/Streptomycin, and 2mM L-glutamine, and incubated at 26°C. Metacyclic promastigotes were isolated as previously described (30). Leishmania spp. were complement opsonized with 5% normal human serum before infecting at a concentration of 10 parasites: 1 DC; infection rates were determined by Diff-Quick staining and were between 63 and 82% with infections between species not differing by more than 12%. For transwell experiments, equal numbers of DC were plated in (lower) wells and (upper) 0.4 micron inserts (Becton Dickinson Laboratories). Lysates were generated by three successive and rapid freeze-thaw cycles (−80°C and 37°C) with brief waterbath sonication of parasite samples equivalent to the 10:1 infection concentrations noted earlier.
Plasmodium falciparum strain 3D7 was maintained as described by Trager and Jensen (31). Schizonts were magnetically selected prior to exposure to human DC. Briefly, infected red blood cell (RBC) cultures were washed in RPMI-1640 and applied to an LS column (Miltenyi) in a Vario Macs magnet. The column was washed, removed from the magnet, and mature schizonts were eluted.
Proliferation Assays
For MLR, 2.0×104 non-adherent DC mixed with 4.0×105 allogeneic lymphocytes (CD14− fraction from monocyte separation) and Leishmania (10 parasites:1 DC) were cultured for 5 days in a tryptophan defined media (32). 1-methyl tryptophan (1-MT) (Sigma-Aldrich) was added to wells at day 0 at a concentration of 200μM, as previously reported (16) to inhibit IDO activity. Proliferation was assessed by the addition of 1μCi/ml 3H- thymidine (Perkin Elmer) the last 18 hrs of culture. Antigen-specific proliferation was assessed as described for MLR, except DC were pulsed with 100 μg/ml tetanus toxoid (Astarte Biologicals) and autologous responder cells were utilized. Statistical significance was determined using a Student’s T-Test with a confidence level of 95%.
Quantitative RT-PCR and Analysis
RNA was isolated using an RNeasy Mini kit (Qiagen) and cDNA was generated using SuperScript III (Invitrogen) following manufacturer protocols. Quantitative real-time PCR (qRT-PCR) reactions were performed using SYBR Green Mastermix (Applied Biosystems) was utilized as previously described (33), with 25ng cDNA and 300nM Forward and Reverse primers (IDT) per reaction. Human primers for HPRT1, IDO1, IFNA1, IFNB1, and IFNG mRNA transcripts were used (see Supplemental Table 1 for primer sequences). Fold change over uninfected gene expression levels were calculated using the delta delta Ct method normalized to HPRT. Significance levels were determined with the Mann-Whitney test or one-way repeated measures ANOVA with Bonferroni post-tests using GraphPad Prism 5.0 software.
Flow Cytometry
Samples fixed with 2% paraformaldehyde were washed 3 times with 0.1% BSA – PBS. Cells were blocked in PBS supplemented with normal mouse serum (Jackson Immunoresearch) and BSA for 1hr and subsequently stained with a panel of antibodies: isotypes, anti-CD40-PE (5C3), anti-CD80-PE (L307.4), anti-CD83-PE (HB15e), anti-CD86-PE (FUN-1), and anti-HLA-DR-PE (L243) (BD Pharmingen). All samples were washed and then analyzed using an MCL500 flow cytometer (Beckman Coulter). Data was expressed relative to uninfected controls.
Western Blot Analysis
DC were lysedfor 20 min with ice-cold lysis buffer (34). Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. Blots were probed with primary antibodies against IDO (Chemicon) and GAPDH (Biogenesis), incubated with a secondaryHRP-conjugated anti-mouse Ig antibody (BD Biosciences)in PBST plus 5% BSA. Antibodies were detected usingSuperSignal West Pico and Femto ECL reagents (Pierce).
Results and Discussion
IDO Expression in Human Dendritic Cells
Our initial observations of IDO expression in Leishmania infected DC were made by re-mining previously published microarray data (35). These microarray data represent pooled monocyte-derived DC generated from blood samples of seven healthy adult human donors that were subsequently infected in vitro with either L. major or L. donovani. RNA was harvested 16 hours post-infection, pooled, and hybridized to an Affymetrix HU95A microarray (35). Our data analysis revealed that IDO is induced in human DC 10.35 and 1.55 fold in response to L. major and L. donovani infection respectively compared with uninfected DC (data not shown).
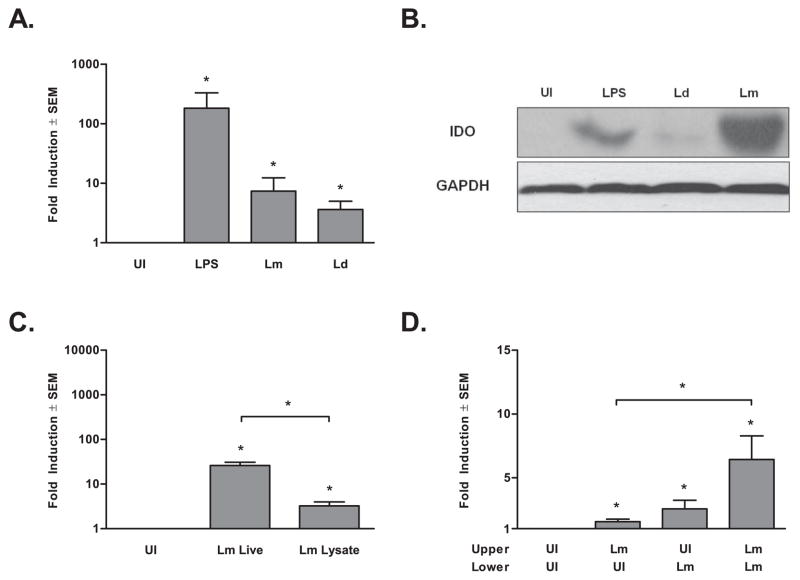
In order to confirm these results, human monocyte-derived DC from six additional healthy human blood donors were infected in vitro with either L. major or L. donovani. Following 16 hours of infection, RNA was harvested, and quantitative RT-PCR (qRT-PCR) was utilized to measure IDO expression from each donor individually. IDO mRNA was significantly induced by infection with L. major or L. donovani (p ≤ 0.05, Fig. 1 A). Although not statistically significant, the levels induced by L. donovani were generally lower than that induced by L. major. DC from another six donors were utilized to determine the translational state of IDO during the infections detailed above (Fig. 1B). IDO protein also was induced by Leishmania infection, and similar to IDO mRNA expression, expressed at a lower level in L. donovani-infected DC as compared to L. major infected DC. To determine if IDO induction requires live Leishmania infection we stimulated DC with L. major lysate (Fig. 1C). Parasite lysates induced significant IDO mRNA expression, though to a lower level than during live L. major infections. To distinguish between the effect of phagocytosis versus infection with Leishmania parasites, we exposed DC to uninfected and P. falciparum-infected allogeneic RBC and assessed the induction of IDO by qRT-PCR (Suppl. Fig. 1A). IDO expression was not induced by uninfected RBC uptake, suggesting that the effect observed in response to Leishmania infection is not solely due to phagocytosis. Interestingly, IDO mRNA is up-regulated in response to P. falciparum-infected RBC in human DC, a response that results in detectable protein (data not shown).
Figure 1. IDO is Induced in Human DC in Response to Leishmania Infection.
A)IDO mRNA transcript expression as measured by qRT-PCR using equal concentrations of cDNA from six healthy adult human monocyte-derived DC and normalized to HPRT. Cells were left uninfected (UI) or infected in vitro with L. major (Lm) or L. donovani (Ld) or exposed to 1μg/mL of LPS (LPS) for 16 hrs. B) Western blot analysis of human monocyte-derived DC infected with L. major, L. donovani, or stimulated with LPS (1μg/mL) for 48 hrs. Blots were probed for IDO and GAPDH as a loading control. Data from one of six representative donors is presented. C) qRT-PCR of IDO mRNA from human monocyte-derived DC, infected in vitro with live L. major or stimulated with an equivalent amount of parasite lysate for 16 hrs. D) IDO mRNA detected from uninfected or L. major infected DC from the lower chamber of a transwell system. Infection in upper wells as indicated, n = nine donors. Data is expressed as mean ± standard error of th mean (SEM) fold induction over uninfected controls, *p ≤ 0.05 by Mann-Whitney Test.
An early pro-IDO signal is likely mediated through pathogen interaction with pattern recognition receptors, such as toll like receptors (TLR) on the DC surface, as many TLR have been shown to induce IDO (36). Interestingly, Leishmania species have been shown to interact with TLR2 and TLR3 (37, 38) and effective control of L. major infection in vivo is partially dependent on TLR4 (39). IDO also is induced by cytokines, in particular interferons (40). To investigate if autocrine or paracrine regulation is involved in Leishmania induction of IDO, we utilized a transwell system whereby infected DC in the upper chambers could produce soluble mediators to affect IDO production in uninfected DC in the lower chambers (Fig. 1D). We infected cells in the lower chamber with L. major parasites, where these cells expressed IDO as expected. This induction appears to be mediated partially by a soluble factor(s) as IDO expression was up-regulated in uninfected DC exposed only to infection via the physically segregated upper transwell. Infection in both chambers, however, resulted in synergistic induction of IDO. Two possible explanations can account for this latter result. First, increased IDO expression may only result from the additive effect of more soluble mediators being present due to increased L. major infection (L. major in both wells). Alternatively, the increased expression may be the result of two signals, one that requires direct contact and one resulting from an autocrine mechanism.
IDO Transcript Expression Follows a Model of Interferon Expression Over Time
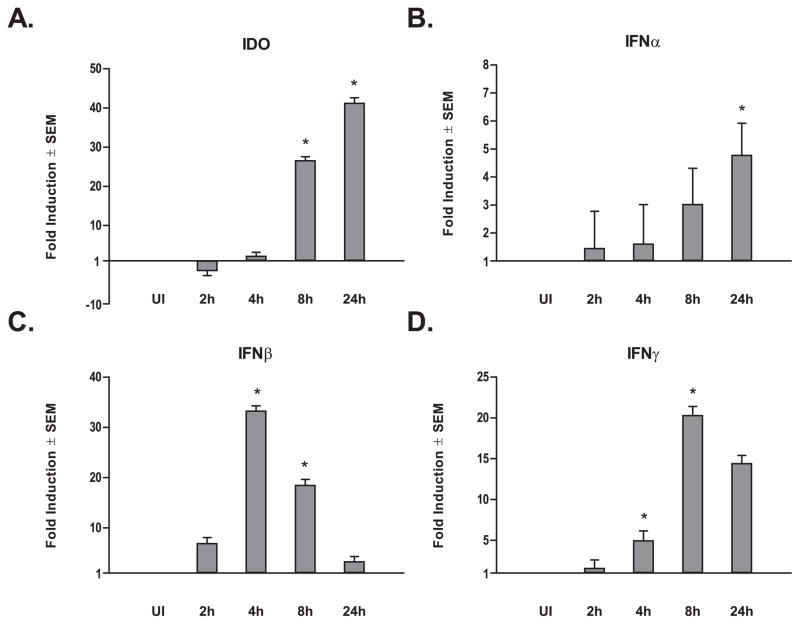
To assess gene transcript regulation during the early infection period, we used qRT-PCR to monitor the expression of IDO in DC from five healthy adult human blood donors at 2, 4, 8, and 24 hours post-L. major infection (Fig. 2A). The fold induction of IDO among infected DC compared with that of uninfected DC increased significantly after 8 hours and continued through to 24 hours. These data agree with our earlier qRT-PCR observations at 16 hours post-infection and suggest a progressive IDO induction as a function of Leishmania presence and engagement of host cells, within the cellular micro-environment.
Figure 2. Leishmania Infection Affects Differential Indoleamine and Interferon Transcript Expression Patterns over Time.
qRT-PCR for A) IDO, B) IFNα, C) IFNβ,and D) IFNγ normalized to HPRT transcript expression levels using equal concentrations of cDNA from five healthy adult human monocyte-derived DC. Cells were either uninfected or infected in vitro with L. major and samples extracted after 2, 4, 8, and 24 hours post-infection. Data is expressed as mean transcript expression fold change over uninfected controls normalized to HPRT ± SEM. *p ≤ 0.05 compared with uninfected (UI) gene expression levels by one-way repeated measures ANOVA and Bonferroni multiple comparisons tests.
The INDO (gene encoding IDO) promoter contains several interferon response elements (40), suggesting that the soluble mediator(s) described in either of the earlier explanations for the transwell assay results could be an interferon. Of interest is that L. major infection activates interferon regulator factors 1 and 8, as well as STAT1 in human DC—three factors that are able to bind and activate interferon response elements (41). qRT-PCR was again utilized with DC samples of the five healthy blood donors used in time course infection assays tracking expression of the three primary types of interferons (IFNα, β, and γ). IFNα mRNA levels displayed a modest up-regulatory trend over time (Fig. 2B), achieving significance compared with uninfected samples only after 24 hours post-infection. Relatively lower levels of IFNα mRNA suggests this interferon is not responsible for IDO induction, as would be expected considering the pathological role IFNα has been shown to play in aberrant IDO promotion (42). Like IDO, IFNγ was significantly up-regulated 8 hours post-infection. However, unlike IDO, IFNγ transcript levels began to decrease at 24 hours post-infection (Fig. 2D). Interestingly, IDO expression levels do not drop at 24 hours post-L. major infection, but instead continue to rise after IFNγ begins to fall (Fig. 2A). IFNβ expression peaked at approximately 4 hours post-infection, but progressively dropped back to insignificant levels by 24 hours post-infection (Fig. 2C). There is evidence supporting a role of IFNβ positively influencing secretion of autocrine-acting IFNγ by DCs (43); and it has also been shown that IFNγR engagement stimulates the translocation of IRF1 and IRF8 (ICSBP) to the DC nucleus, promoting immune molecules’ expression in a conditional manner (44). In concordance with these reports, the interferon expression profiles following L. major infection suggest a possible stepwise model of earlier IFNβ expression, its subsequent promotion of IFNγ expression followed by IDO induction. Alternatively, IFNβ may directly mediate IDO up-regulation as has been reported for human trophoblast cells (45).
Infection-Induced IDO is Active and Inhibits a Mixed Lymphocyte Reaction
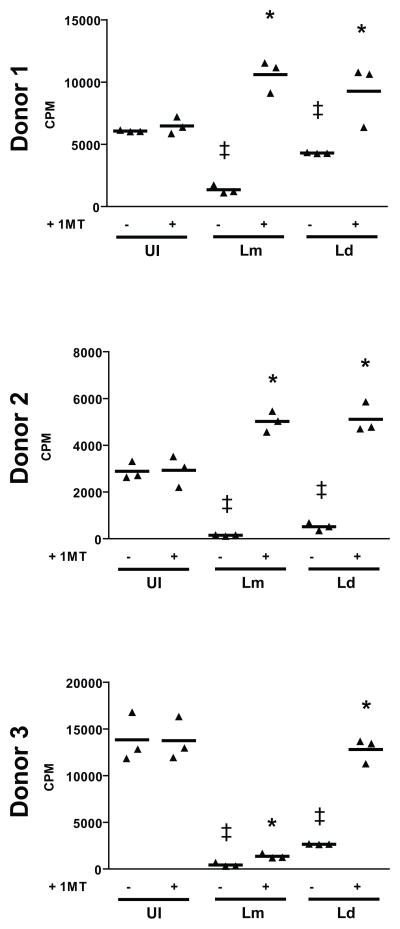
In some systems IDO mRNA and protein is produced but the protein is not functional (16, 46); to ensure that IDO induced by Leishmania infection (Fig. 1B) was functional, mixed lymphocyte reactions (MLR) were utilized. Active IDO inhibits T-cell polyclonal activation associated with MLR, and the addition of 1-Methyl Tryptophan (1-MT) reverses this inhibition (47). Our MLR results indicated that IDO is active as conditions that include Leishmania infection exhibit significantly reduced levels of proliferation relative to uninfected allogeneic conditions (Fig. 3). This infection-induced inhibition is reversed by the addition of the IDO inhibitor, 1-MT. To further demonstrate that IDO induced by L. donovani infection is active, we supplemented the MLR media with L-tryptophan (Suppl. Fig. 2). Addition of exogenous tryptophan significantly reversed the proliferation inhibition induced by L. donovani infection of DC.
Figure 3. Leishmania-Infection Induced IDO is Active and Inhibits MLR.
DC from nine healthy donors were either left uninfected (UI) or infected with either L. major (Lm) or L. donovani (Ld) and then mixed in auto- and allogeneic conditions at a ratio of 20 responders to 1 DC and incubated for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as β-particle counts per minute (CPM) with conditions listed with or without 1-MT. ‡ p ≤ 0.05 compared to uninfected control; * p ≤ 0.05 compared to same infection lacking 1-MT as determined by Student’s T-Test. Data from three representative donors out of nine are shown.
To assess if Leishmania-infection induced IDO also is active against antigen-specific cell activation, a tetanus toxoid-based proliferation assay was utilized. DC from healthy human blood monocyte donors were infected and co-incubated with autologous responder cells with or without 1-MT. Patterns in antigen-specific proliferation were similar to those observed with MLR, as infection with either L. major or L. donovani inhibits proliferation and the addition of 1-MT circumvents the infection-based inhibition (Fig. 4). Conversely, the IDO induced in response to uptake of P. falciparum-infected RBC does not inhibit antigeneic-specific cell proliferation (Suppl. Fig. 1B).
Figure 4. Leishmania-Infection Induced IDO is Active and Inhibits Antigen-Specific Proliferation.
Uninfected DC (UI) or DC infected with either L. major (Lm) or L. donovani (Ld), autologous responders, and 0.10 ng/mL tetanus toxoid were cultured for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as counts per minute (CPM) with conditions listed with or without 1-MT. ‡ p ≤ 0.05 compared to uninfected control; * p ≤ 0.05 compared to same infection lacking 1-MT as determined by Student’s T Test.
Even though previous studies have demonstrated that Leishmania infection has little impact on co-stimulatory markers (29, 48), to rule out the role of decreased surface expression in inhibition of proliferation, surface level expression of CD40, CD80, CD86, and HLA-DR was assessed by flow cytometric analysis (Suppl. Fig. 3). No significant differences were observed in Leishmania-infected DC compared to uninfected controls. While previous literature demonstrates a connection between immunosuppression and Leishmania infection (49, 50), no direct mechanism influencing this phenomenon has been defined in humans. Our studies imply a role for IDO in immunosuppression associated with leishmaniasis.
A recent murine study demonstrates that IDO contributes to the L. major parasite burden and footpad swelling indicating that IDO expression is advantageous for pathogen survival (28). A few novel studies have investigated the potential of Leishmania infection induced immunosuppression to alter a host’s response to other organisms or vaccines. In BALB/c mice, L. major infection ablates the cellular mediated response induced by a previous vaccination against the HIV-1 gag gene (51). In a clinical case report, an individual presented with classical co-infections associated with HIV (i.e., tuberculosis, pneumoncystosis, and strongyloidiasis). Those infections persisted despite their specific treatments and finding that the patient was HIV negative. However, he was later found to be infected with L. infantum chagasi as a co-morbidity. Treatment with antimony-based compounds led to the patient making a full recovery, with clearance of all other pathogens after the L. i. chagasi-induced immunosuppression was alleviated (52). No study has provided a definitive mechanism for this noted Leishmania-induced immunosuppression, although the interaction of CTLA-4 with B7 (53) has been implicated. Interestingly, this same interaction is a potent inducer of IDO (16).
Concluding Remarks
Our report demonstrates that active IDO is induced in human DC in response to infection with L. major or L. donovani, leading to inhibition of both polyclonal and antigen-specific proliferative responses. Together, these data indicate that one of the mechanisms behind the establishment of Leishmania spp. infection is through the induction of DC-expressed IDO.
Supplementary Material
A) IDO mRNA transcript expression as measured by qRT-PCR using equal concentrations of cDNA from six healthy adult human monocyte-derived DC and normalized to HPRT. Cells were left uninfected (UI) or infected in vitro with L. major (Lm) or L. donovani (Ld) or exposed to uninfected (uRBC) or P. falciparum-infected RBC (PfRBC) for 16 hrs. Data is expressed as mean ± SEM fold induction over uninfected controls, *p ≤ 0.05 by Mann-Whitney Test. B) Uninfected DC (UI) or DC infected with either L. major (Lm) or L. donovani (Ld) or exposed to uninfected (uRBC) or P. falciparum-infected RBC (PfRBC), autologous responders, and 0.10 ng/ml tetanus toxoid were cultured for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as counts per minute (CPM) with conditions listed with or without 1-MT. ‡ p ≤ 0.05 compared to uninfected control; * p ≤ 0.05 compared to same infection lacking 1-MT as determined by Student’s T-Test. Note: donors are the same as those presented in Fig. 4.
DC from six healthy donors were infected with L. donovani (Ld) and then mixed in allogeneic conditions at a ratio of 20 responders to 1 DC and incubated for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as β-particle counts per minute (CPM) with conditions listed with or without 0.05μM L-tryptophan supplementation. * p ≤ 0.05 as determined by Wilcoxon matched pairs signed rank test.
Adult human monocyte-derived DC from five healthy donors were infected with either L. major (Lm) or L. donovani (Ld) and incubated for 16 hours. Cells were stained for CD40 (A), CD80 (B), CD86 (C), and HLA-DR (C) and analyzed by flow cytometry. Error bars are representative of the SEM * p<0.05 by Student’s T Test. n≥4 donors per stain.
Supplemental Table 1. Primers Utilized in qRT-PCR
Acknowledgments
This work was supported by National Institutes of Health grant 5RO1AI056242.
ABBREVIATIONS
- IDO
Indoleamine 2,3-dioxygenase
- 1-MT
1-Methyl Tryptophan
Footnotes
DISCLOSURES: This work was supported by National Institutes of Health grant 5RO1AI056242 to MAM.
References
- 1.Maizels R. Regulation of the immune system in metazoan parasite infections. Novartis Found Symp. 2007;281:192–204. doi: 10.1002/9780470062128.ch16. discussion -9. [DOI] [PubMed] [Google Scholar]
- 2.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002 Nov;3(11):1041–7. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 3.McDowell MA, Sacks DL. Inhibition of host cell signal transduction by Leishmania: observations relevant to the selective impairment of IL-12 responses. Curr Opin Microbiol. 1999;2(4):438–43. doi: 10.1016/S1369-5274(99)80077-0. [DOI] [PubMed] [Google Scholar]
- 4.Peters N, Sacks D. Immune privilege in sites of chronic infection: Leishmania and regulatory T cells. Immunol Rev. 2006 Oct;213:159–79. doi: 10.1111/j.1600-065X.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 5.Choi CM, Lerner EA. Leishmaniasis as an emerging infection. J Investig Dermatol Symp Proc. 2001 Dec;6(3):175–82. doi: 10.1046/j.0022-202x.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- 6.Antinori S, Schifanella L, Corbellino M. Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis. 2011 Feb;31(2):109–18. doi: 10.1007/s10096-011-1276-0. [DOI] [PubMed] [Google Scholar]
- 7.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011 Aug;9(8):604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 8.Liese J, Schleicher U, Bogdan C. The innate immune response against Leishmania parasites. Immunobiology. 2008;213(3–4):377–87. doi: 10.1016/j.imbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Mougneau E, Bihl F, Glaichenhaus N. Cell biology and immunology of Leishmania. Immunol Rev. 2011 Mar;240(1):286–96. doi: 10.1111/j.1600-065X.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 10.Pearson DD, Wilson ME. Host defenses against portotypical intracellular protozoans, the Leishmania. In: Genta PDWRM, editor. Parasitic Infections in the Compromised Host. New York and Basel: Marcel Dekker, Inc; 1989. [Google Scholar]
- 11.Gantt KR, Schultz-Cherry S, Rodriguez N, Jeronimo SM, Nascimento ET, Goldman TL, et al. Activation of TGF-beta by Leishmania chagasi: importance for parasite survival in macrophages. J Immunol. 2003 Mar 1;170(5):2613–20. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- 12.Passwell JH, Shor R, Smolen J, Jaffe CL. Infection of human monocytes by Leishmania results in a defective oxidative burst. Int J Exp Pathol. 1994;75(4):277–84. [PMC free article] [PubMed] [Google Scholar]
- 13.Lodge R, Descoteaux A. Modulation of phagolysosome biogenesis by the lipophosphoglycan of Leishmania. Clin Immunol. 2005 Mar;114(3):256–65. doi: 10.1016/j.clim.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Green SJ, Meltzer MS, Hibbs JB, Jr, Nacy CA. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990;144(1):278–83. [PubMed] [Google Scholar]
- 15.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004 Oct;4(10):762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004 Apr 1;172(7):4100–10. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 17.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002 Oct;9(10):1069–77. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 18.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002 Aug 19;196(4):459–68. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frumento G, Rotondo R, Tonetti M, Ferrara GB. T cell proliferation is blocked by indoleamine 2,3-dioxygenase. Transplant Proc. 2001 Feb-Mar;33(1–2):428–30. doi: 10.1016/s0041-1345(00)02078-9. [DOI] [PubMed] [Google Scholar]
- 20.Terness P, Bauer TM, Rose L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002 Aug 19;196(4):447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sibley LD, Messina M, Niesman IR. Stable DNA transformation in the obligate intracellular parasite Toxoplasma gondii by complementation of tryptophan auxotrophy. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5508–12. doi: 10.1073/pnas.91.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie CR, Willberg CB, Daubener W. Inhibition of group B streptococcal growth by IFN gamma-activated human glioblastoma cells. J Neuroimmunol. 1998 Aug 14;89(1–2):191–7. doi: 10.1016/s0165-5728(98)00138-6. [DOI] [PubMed] [Google Scholar]
- 23.MacKenzie CR, Hadding U, Daubener W. Interferon-gamma-induced activation of indoleamine 2,3-dioxygenase in cord blood monocyte-derived macrophages inhibits the growth of group B streptococci. J Infect Dis. 1998 Sep;178(3):875–8. doi: 10.1086/515347. [DOI] [PubMed] [Google Scholar]
- 24.Boasso A, Herbeuval JP, Hardy AW, Anderson SA, Dolan MJ, Fuchs D, et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007 Apr 15;109(8):3351–9. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Sluijs KF, Nijhuis M, Levels JH, Florquin S, Mellor AL, Jansen HM, et al. Influenza-induced expression of indoleamine 2,3-dioxygenase enhances interleukin-10 production and bacterial outgrowth during secondary pneumococcal pneumonia. J Infect Dis. 2006 Jan 15;193(2):214–22. doi: 10.1086/498911. [DOI] [PubMed] [Google Scholar]
- 26.Larrea E, Riezu-Boj JI, Gil-Guerrero L, Casares N, Aldabe R, Sarobe P, et al. Upregulation of indoleamine 2,3-dioxygenase in hepatitis C virus infection. J Virol. 2007 Apr;81(7):3662–6. doi: 10.1128/JVI.02248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetsutani K, To H, Torii M, Hisaeda H, Himeno K. Malaria parasite induces tryptophan-related immune suppression in mice. Parasitology. 2007 Jul;134(Pt 7):923–30. doi: 10.1017/S0031182007002326. [DOI] [PubMed] [Google Scholar]
- 28.Makala LH, Baban B, Lemos H, El-Awady AR, Chandler PR, Hou DY, et al. Leishmania major attenuates host immunity by stimulating local indoleamine 2,3-dioxygenase expression. J Infect Dis [Research Support, NIH, Extramural Research Support, NIH, Intramural] 2011 Mar 1;203(5):715–25. doi: 10.1093/infdis/jiq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 Ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002;70(8):3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99(2):97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 31.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science (New York, NY. 1976 Aug 20;193(4254):673–5. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 32.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999 May 3;189(9):1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donovan MJ, Messmore AS, Scrafford DA, Sacks DL, Kamhawi S, McDowell MA. Uninfected mosquito bites confer protection against infection with malaria parasites. Infect Immun. 2007 May;75(5):2523–30. doi: 10.1128/IAI.01928-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yadav M, Clark L, Schorey JS. Macrophage’s proinflammatory response to a mycobacterial infection is dependent on sphingosine kinase-mediated activation of phosphatidylinositol phospholipase C, protein kinase C, ERK1/2, and phosphatidylinositol 3-kinase. J Immunol. 2006 May 1;176(9):5494–503. doi: 10.4049/jimmunol.176.9.5494. [DOI] [PubMed] [Google Scholar]
- 35.Chaussabel D, Tolouei Semnani R, McDowell MA, Sacks D, Sher A, Nutman TB. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102(2):672–81. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 36.Mahanonda R, Sa-Ard-Iam N, Montreekachon P, Pimkhaokham A, Yongvanichit K, Fukuda MM, et al. IL-8 and IDO expression by human gingival fibroblasts via TLRs. J Immunol. 2007 Jan 15;178(2):1151–7. doi: 10.4049/jimmunol.178.2.1151. [DOI] [PubMed] [Google Scholar]
- 37.Becker I, Salaiza N, Aguirre M, Delgado J, Carrillo-Carrasco N, Kobeh LG, et al. Leishmania lipophosphoglycan (LPG) activates NK cells through toll-like receptor-2. Mol Biochem Parasitol. 2003 Aug 31;130(2):65–74. doi: 10.1016/s0166-6851(03)00160-9. [DOI] [PubMed] [Google Scholar]
- 38.Flandin JF, Chano F, Descoteaux A. RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol. 2006 Feb;36(2):411–20. doi: 10.1002/eji.200535079. [DOI] [PubMed] [Google Scholar]
- 39.Kropf P, Freudenberg MA, Modolell M, Price HP, Herath S, Antoniazi S, et al. Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun. 2004 Apr;72(4):1920–8. doi: 10.1128/IAI.72.4.1920-1928.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puccetti P. On watching the watchers: IDO and type I/II IFN. Eur J Immunol. 2007 Apr;37(4):876–9. doi: 10.1002/eji.200737184. [DOI] [PubMed] [Google Scholar]
- 41.Jayakumar A, Donovan MJ, Tripathi V, Ramalho-Ortigao M, McDowell MA. Leishmania major infection activates NF-kappaB and interferon regulatory factors 1 and 8 in human dendritic cells. Infect Immun. 2008 May;76(5):2138–48. doi: 10.1128/IAI.01252-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metabolic brain disease [Review] 2009 Mar;24(1):55–68. doi: 10.1007/s11011-008-9114-5. [DOI] [PubMed] [Google Scholar]
- 43.Nagai T, Devergne O, van Seventer GA, van Seventer JM. Interferon-beta mediates opposing effects on interferon-gamma-dependent Interleukin-12 p70 secretion by human monocyte-derived dendritic cells. Scandinavian journal of immunology [Research Support, NIH, Extramural] 2007 Feb;65(2):107–17. doi: 10.1111/j.1365-3083.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nature reviews Immunology [Review] 2003 Feb;3(2):133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 45.Wang B, Koga K, Osuga Y, Cardenas I, Izumi G, Takamura M, et al. Toll-like receptor-3 ligation-induced indoleamine 2, 3-dioxygenase expression in human trophoblasts. Endocrinology. 2011 Dec;152(12):4984–92. doi: 10.1210/en.2011-0278. [DOI] [PubMed] [Google Scholar]
- 46.Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol. 2002 Jan;14(1):65–8. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 47.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer research [Research Support, NIH, Extramural Research Support, Non-US Gov’t Research Support, US Gov’t, Non-PHS] 2007 Jan 15;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 48.Marovich MA, McDowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164(11):5858–65. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 49.Carvalho EM, Teixeira RS, Johnson WD., Jr Cell-mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981 Aug;33(2):498–500. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickol AD, Bonventre PF. Immunosuppression associated with visceral leishmaniasis of hamsters. Parasite Immunol. 1985 Jul;7(4):439–49. doi: 10.1111/j.1365-3024.1985.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 51.Robinson TM, Nelson R, Artis D, Scott P, Boyer JD. Experimental Leishmania major infection suppresses HIV-1 DNA vaccine induced cellular immune response. Cells Tissues Organs. 2004;177(3):185–8. doi: 10.1159/000079992. [DOI] [PubMed] [Google Scholar]
- 52.Toledo AC, Jr, de Castro MR. Pneumocystis carinii pneumonia, pulmonary tuberculosis and visceral leishmaniasis in an adult HIV negative patient. Braz J Infect Dis. 2001 Jun;5(3):154–7. doi: 10.1590/s1413-86702001000300008. [DOI] [PubMed] [Google Scholar]
- 53.Gomes NA, Barreto-de-Souza V, Wilson ME, DosReis GA. Unresponsive CD4+ T lymphocytes from Leishmania chagasi-infected mice increase cytokine production and mediate parasite killing after blockade of B7-1/CTLA-4 molecular pathway. J Infect Dis. 1998 Dec;178(6):1847–51. doi: 10.1086/314520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) IDO mRNA transcript expression as measured by qRT-PCR using equal concentrations of cDNA from six healthy adult human monocyte-derived DC and normalized to HPRT. Cells were left uninfected (UI) or infected in vitro with L. major (Lm) or L. donovani (Ld) or exposed to uninfected (uRBC) or P. falciparum-infected RBC (PfRBC) for 16 hrs. Data is expressed as mean ± SEM fold induction over uninfected controls, *p ≤ 0.05 by Mann-Whitney Test. B) Uninfected DC (UI) or DC infected with either L. major (Lm) or L. donovani (Ld) or exposed to uninfected (uRBC) or P. falciparum-infected RBC (PfRBC), autologous responders, and 0.10 ng/ml tetanus toxoid were cultured for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as counts per minute (CPM) with conditions listed with or without 1-MT. ‡ p ≤ 0.05 compared to uninfected control; * p ≤ 0.05 compared to same infection lacking 1-MT as determined by Student’s T-Test. Note: donors are the same as those presented in Fig. 4.
DC from six healthy donors were infected with L. donovani (Ld) and then mixed in allogeneic conditions at a ratio of 20 responders to 1 DC and incubated for 5 days. Proliferation was assessed by 3H-thymidine incorporation the last 18 hrs of culture. Data is expressed as β-particle counts per minute (CPM) with conditions listed with or without 0.05μM L-tryptophan supplementation. * p ≤ 0.05 as determined by Wilcoxon matched pairs signed rank test.
Adult human monocyte-derived DC from five healthy donors were infected with either L. major (Lm) or L. donovani (Ld) and incubated for 16 hours. Cells were stained for CD40 (A), CD80 (B), CD86 (C), and HLA-DR (C) and analyzed by flow cytometry. Error bars are representative of the SEM * p<0.05 by Student’s T Test. n≥4 donors per stain.
Supplemental Table 1. Primers Utilized in qRT-PCR