Abstract
Pseudoxanthoma elasticum (PXE), which demonstrates progressive build‐up of calcium phosphate and proteoglycan deposits in skin, eye, and arteries, has been associated with myocardial infarctions, stroke, and blindness. In a mouse model of PXE, a magnesium‐enriched diet prevents mineralization of the vibrissae capsule, an early biomarker for PXE. However, biomarkers for therapeutic responses in PXE have not been identified in humans. Because PXE patients have an increased carotid intima‐media thickness (CIMT), a risk factor for cardiovascular disease and stroke, we analyzed the feasibility of CIMT as a treatment endpoint before and after magnesium supplementation in a mouse model of PXE (Abcc6 −/−). CIMT was measured in 1‐year‐old Abcc6 −/− and Abcc6+/+ mice fed either standard rodent diet with or without magnesium oxide supplementation for 2 months. Baseline CIMT in Abcc6 −/− versus Abcc6+/+ mice was increased (p value = 0.009), whereas CIMT in magnesium‐treated versus untreated Abcc6−/− mice was reduced (p value = 0.024). CIMT is a novel treatment endpoint in this mouse model and may serve as a predictive biomarker of therapeutic response in PXE patients. In that context, magnesium oxide significantly reduced CIMT in PXE mice, and may be useful for disease prevention in PXE patients. Clin Trans Sci 2012; Volume #: 1–6
Keywords: magnesium, carotid intima‐media thickness, biomarker in pseudoxanthoma elasticum, translational biomarker in mouse model, PXE knockout mouse, ABCC6 gene, mineralizing disorder, reducing cardiovascular risk factors
Introduction
Pseudoxanthoma elasticum (PXE), caused by mutations in the ABCC6 gene, is a relatively rare genetic disorder that affects approximately 1 in 70,000 to 1 in 100,000 persons worldwide. 1 Progressive build‐up of calcium phosphate deposits in tissues causes the symptoms associated with this disease, but the severity of the disease can vary significantly between patients even in the same family.
PXE patients develop calcification in the dermis, in the Bruch’s membrane in the eye, as well as in the intima and media in arteries associated with aberrant elastic fibers. These manifest clinically with sagging skin, 2 loss of visual acuity, as well as intermittent claudication, and hypertension (HTN), 3 , 4 occasionally resulting in early myocardial infarction and gastric artery bleeding. It is postulated that alterations observed in skin, i.e., elastin fragmentation, calcium deposition, and increased proteoglycan content 5 , 6 , 7 may also occur in arteries of PXE patients, underlying early development of HTN and arterial stiffness in these patients. 7 , 8 It has been suggested that before calcification, an increased influx of proteoglycans takes place in the vessels. 9
PXE patients under the age of 65 have a 3.6‐fold risk of ischemic stroke compared with the general population. 10 They develop arterial stiffness or reduced aortic compliance, 6 , 7 which is also a risk factor for cardiovascular disease. 11 It has been established that among various risk factors, increased carotid intima‐media thickness (CIMT) is an independent predictor of stroke and coronary heart disease. 12
In a mouse model of PXE, a diet relatively high in magnesium (5×) prevented mineralization of the connective tissue capsule surrounding the vibrissae (an early murine biomarker for PXE), without causing osteoporosis. 13 Dietary magnesium produced a 10‐fold increase in calcium and decrease in phosphorus in urine, without changing their serum concentrations or parathyroid hormone levels. 13 Unfortunately a reliable, safe, and practical biomarker of therapeutic response for this disease does not exist in humans. We chose CIMT because of its strong correlation with morbidity and mortality (cardiovascular disease and stroke) 12 , 14 and ease of measurement in humans (with carotid artery ultrasound). Moreover, case reports have noted that PXE patients have an increased CIMT, an established risk factor for cardiovascular disease and stroke in the PXE population. 6 , 7
In this study, CIMT measurement was analyzed as a treatment endpoint after the administration of magnesium in a mouse model for PXE. Feeding regular rodent chow with no added magnesium, 1‐year‐old Abcc6−/− mice have an approximately 30% statistically significant increase in CIMT (p= 0.009) versus wild‐type mice matched for age. This correlates with established human data. Compared to normotensive, age‐matched, healthy patients, individuals with PXE were noted to have a statistically significant 36% increase in CIMT (0.56 mm in control vs. 0.76 mm in PXE patients). 7 Until now, it is not known if magnesium reduces CIMT in a PXE mouse model and, potentially, in PXE patients. If magnesium lowers CIMT in Abcc6−/− mice, CIMT may serve as a novel treatment biomarker in humans with PXE.
Materials and Methods
Mice and diets
The Abcc6 −/− mouse model was developed by targeted ablation of the ABCC6 gene. 15 All mice used were from C57BL/6J genetic background and confirmation of genotyping of tail tip with polymerase chain reaction (PCR) was done before beginning the experiments. Two groups of mice were tested. Panel A: Four 1‐year‐old Abcc6 −/− males, one 1‐year‐old Abcc6 −/− female, and five 1‐year‐old Abcc6+/+ males, all fed standard rodent diet (Lab Diet 5010). Panel B: Two 1‐year‐old Abcc6−/− males, four 1‐year‐old Abcc6−/− females, and four 1‐year‐old Abcc6+/+ males, and four 1‐year‐old Abcc6+/+ female mice were fed the same diet as in Panel A but with fivefold magnesium oxide added (1.85 g/100 g food), for 2 months. Each mouse in Panel B ate about 5 g of food/day and of those 5 g, each mouse consumed about 92.5 mg elemental magnesium per day, or 55.5 mEq magnesium/day. Approval for this study was obtained by the Institutional Animal Care and Use Committee of Thomas Jefferson University before the start of these experiments.
Ultrasound
Mice from Panel A were anesthetized and depilated in the neck area, and carotid arteries were visualized with Vevo 770, 30 Hz (VisualSonics, Toronto, ON, Canada) ultrasound machine.
CIMT measurement and histology
Carotid arteries were harvested from each panel and embedded in paraffin. Specific areas on each carotid artery were measured (starting from the bifurcation) to be designated for H&E staining (at 1,000, 3,000, and 5,000 μm), von Kossa staining (calcium phosphate) (at 2,500 μm), alcian blue (pH 2.5; for total proteoglycan) staining (at 1,500, 3,500, or 4000 μm), and alizarin red (calcium) staining (at 2,000 μm). The carotid arteries were submitted on numbered cassettes, prepared by a collaborating technician, and the corresponding numbered cassettes to mouse were kept on a separate document (blinded). Slides were labeled according to the cassettes to allow for blinding at the time of interpretation and reading. Slides were read using 40× magnification on a Nikon Eclipse TE 2000‐U microscope (Nikon Instruments Inc., Melville, NY, USA) and interpreted with calibrated Image‐Pro Plus software (version 6.1.0.346 for Windows 2000/XP professional, Media Cybernetics, Inc., Bethesda, MD, USA). The mean of the measurements done at the 1,000, 3,000, and 5000 μm mark distal to the bifurcation were used in the statistical analyses.
Vibrissae histology
Muzzle skin containing vibrissae from each knockout and wild‐type mouse in both panels was stained with H&E and with alcian blue (pH 2.5) for proteoglycans, von Kossa, and with alizarin red for comparison. Panels A and B are shown in Figure 4 .
Figure 4.
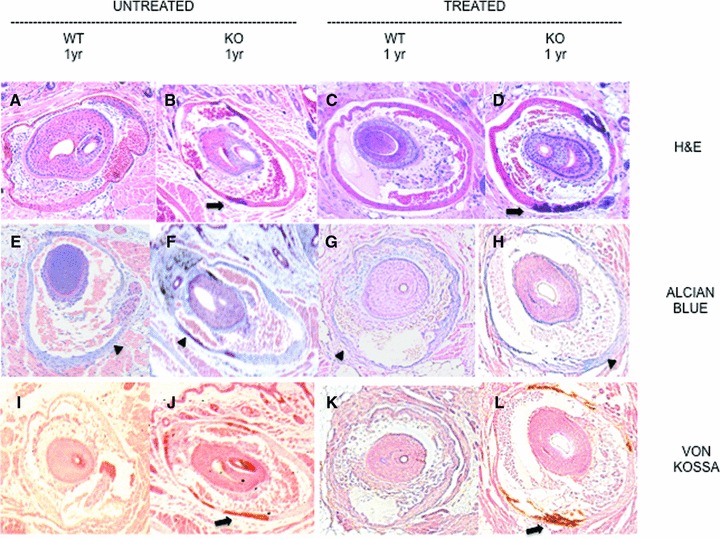
Stained vibrissae in untreated versus treated mice. Frames (A) through (D): H&E stains. Frames (A) and (B) are untreated wild‐type and knockout mouse vibrissae capsules. Frames (C) and (D) show treated wild‐type and knockout vibrissae capsule sections. Arrows show mineralization of the connective tissue capsule surrounding the vibrissae. The second row shows staining of the vibrissae with alcian blue (pH 2.5). Frames (E) and (F) are from are from untreated wild‐type and knockout mouse sections. Frames (G) and (H) are treated wild‐type and knockout mouse sections. Arrowheads in the alcian blue row (E through H) show the total proteoglycan deposits in the connective tissue capsule surrounding the vibrissae. The last row (J through L) are vibrissae sections of untreated (wild‐type and knockout) followed by treated (wild‐type and knockout) mice stained with von Kossa. Arrows show mineralization of the connective tissue capsule surrounding the vibrissae. Mineralization of the connective tissue capsule surrounding the vibrissae can be best viewed in the knock out columns of untreated or treated groups in the H∓E and von Kossa rows.
Statistical analysis
Using 40× magnification, with calibrated software, the CIMT measurements were measured in triplicate at the 1,000, 3,000, and 5000 μm mark on each carotid vessel, right and left, totaling 18 measurements per mouse. These measurements were then averaged to represent the final CIMT measurement for each mouse. Then, each mouse’s CIMT was averaged to represent the final CIMT measurement from either knockout (Abcc6−/−) or wild‐type (Abcc6+/+) group. This was calculated separately for Panel A and Panel B groups. Values from both Panel A and Panel B were analyzed for normal distribution using Hamilton’s test. To determine the statistical significance between the untreated and treated groups of mice, a two‐tailed Student’s t‐test was used.
Results
Poor resolution ultrasound not helpful for CIMT in the mouse
The Vevo 770, 30 Hz (VisualSonics), ultrasound machine captured the carotid vessel of each mice from Panel A, however, the resolution was relatively poor with this device, and accurate CIMT measurement was not possible. The carotid arteries from the remaining panels were therefore not measured using this modality ( Figure 1 ).
Figure 1.
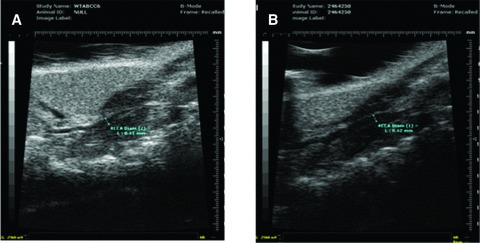
Carotid artery ultrasounds with the Vevo 770, 30 Hz (VisualSonics). (A): Abcc6 +/+ (wild‐type), and (B): Abcc6 −/− (knockout) mice.
CIMT is a useful treatment biomarker in the 1‐year‐old Abcc6−/− mouse model
Photographs of the H&E carotid sections from Panel A (untreated) and B (treated) were taken at 40× magnification and the CIMT was measured with calibrated software (Image‐Pro Plus). Figure 2 displays CIMT in untreated, wild‐type and knockout (first two columns) and treated, wild‐type and knockout (last two columns) mice. The first row (A through D) shows H&E staining with CIMT measurements. The CIMT measurements were averaged for each panel (as described earlier) and are compared in Figure 3 . The differences between the 1‐year‐old Abcc6−/− and 1‐year‐old Abcc6+/+ mice in Panel A were statistically significant, p value = 0.0091 (Student’s t‐test).
Figure 2.
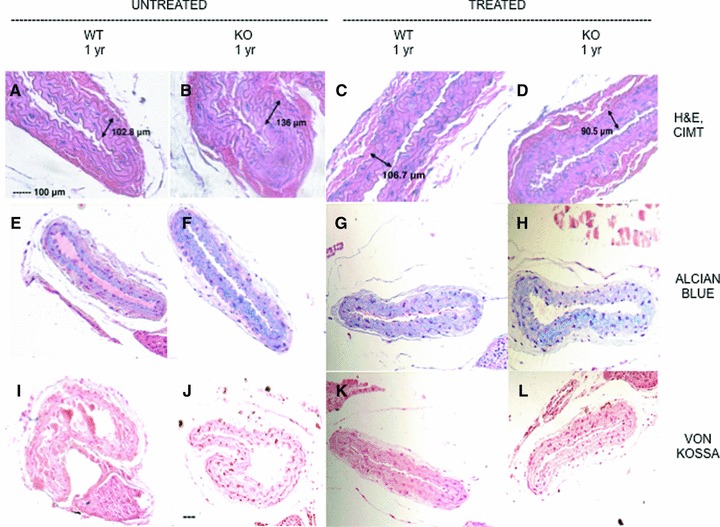
Carotid artery staining and CIMT measurement. Frames (A) through (D): H&E stains and CIMT measurement. Frames (A) and (B) are from untreated wild‐type and knockout mouse carotid sections. Frames (C) and (D) show treated wild‐type and knockout mouse carotid sections. The second row shows staining of carotid arteries with alcian blue (pH 2.5). Frames (E) and (F) are from untreated wild‐type and knockout mouse carotid sections. Frames (G) and (H) are treated wild‐type and knockout mouse carotid sections. The last row (J through L) are sections of untreated (wild‐type and knockout) followed by treated (wild‐type and knockout) mice stained with von Kossa. All frames have the same magnifi cation bar, 100 μm.
Figure 3.
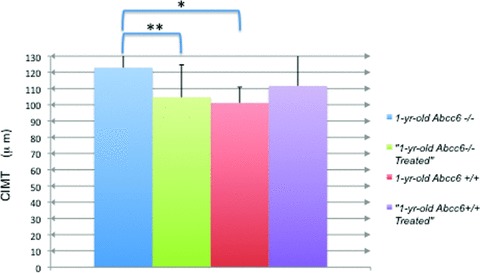
CIMT in 1‐year‐old wild‐type and treated versus knockout and treated mice. Using 40 × magnifi cation and Image‐Pro Plus software, measurements of intima to media were taken after calibration and averaged for each panel. The averages, with standard deviation, of the five untreated knockout mice (blue), six treated knockout mice (green), five untreated wild‐type mice (red), and eight treated wild‐type mice (purple) were calculated. The difference between untreated wild‐type and knockout CIMT is statistically signifi cant, p value = 0.009*. There is a statistically signifi cant difference between the untreated versus treated knockout groups (p = 0.02)** but not between the untreated versus treated wild‐type.
Magnesium oxide lowers CIMT in 1‐year‐old Abcc6−/− (knockout) mice
Compared to the untreated 1‐year‐old Abcc6−/− group, the treated 1‐year‐old Abcc6−/− group showed approximately a 20% reduction in CIMT (p= 0.02, Student’s t‐test). There was no significant reduction in CIMT in the treated versus untreated Abcc6+/+ mice. ( Figure 3 ).
Magnesium oxide changed total proteoglycan content but did not change calcium phosphate deposition
The carotid artery sections from Abcc6+/+ and Abcc6−/− groups in all four panels were also stained for total proteoglycan with alcian blue ( Figure 2 E through H) and for calcium phosphate deposition with von Kossa ( Figure 2I through L) and alizarin red (data not shown). Comparing Panel A and B, there was a respective decrease in total proteoglycan in the vessel walls because CIMT was reduced in Panel B. There was no apparent calcium phosphate deposition in the carotid artery wall of either Abcc6+/+ or Abcc6−/− groups (both panels) at baseline or after treatment with magnesium as demonstrated by absence of von Kossa staining and negative alizarin red staining (data not shown). Images at 10× magnification were captured for the vibrissae for each group and were placed side by side for staining comparison ( Figure 4 ) of Panels A and B. The Abcc6+/+ group had no mineralization of the connective tissue capsule surrounding the vibrissae noted by H&E, von Kossa, and alizarin red staining (data not shown); as anticipated, this effect remains the same despite treatment with magnesium ( Figure 4 A and C, and J and K). As reported previously, 13 magnesium did not reverse the calcium phosphate deposited in the connective tissue capsule surrounding the vibrissae in these 1‐year‐old Abcc6−/− mice ( Figure 4B and D, and I and L).
Discussion
We proposed to determine if there is a difference in the CIMTs in Abcc6 −/− mice compared to Abcc6+/+ matched for age. Mice at 1 year of age were selected because by this time they strongly express the murine phenotype for the disease: early mineralization of the connective tissue capsule surrounding the vibrissae. In addition, because CIMT is a progressive condition, mice at this age are expected to show the progression of a thicker CIMT, which is what we found in this case.
Although carotid artery mineralization was not evident in this study (absence of von Kossa and alizarin red staining), the early biomarker, i.e., mineralization of the connective tissue capsule surrounding the vibrissae, for the PXE mouse model was noted ( Figure 4D ). In humans with PXE and in the PXE mouse model, mineralization has been demonstrated in the aorta and medium sized blood vessels of the upper and lower extremities. 2 , 15 In fact, a negative von Kossa stain is possible in certain arteries because this method stains for phosphate or carbonate radicals. 2 A histological examination of splenic, thyroid, and uterine arteries from PXE patients showed a negative von Kossa and positive Prussian blue stain for iron. 2 It was postulated that the iron must have been bound to another anion. Because an abundance of calcium phosphate in the carotid arteries has been demonstrated in autopsies of PXE patients 7 yet no calcium phosphate is seen in the mouse model for PXE, it is possible that the PXE‐associated arteriosclerosis of these blood vessels in humans is not only due to the mutation of ABCC6, but may be influenced by other genes, diet, and/or environment. 15 Abcc6−/− mice do not have mineralization in their coronary or gastric arteries, whereas humans with PXE do. 15 To our knowledge, this is the first study that has examined calcium deposition in the carotid arteries of PXE mice.
CIMT in Panel B changed significantly with administration of magnesium. CIMT is a novel treatment endpoint in this mouse model for PXE because it can be translated to human PXE data. Several recent studies 5 , 6 , 7 have observed that patients with PXE have higher CIMTs than nonaffected individuals, adjusted for risk factors, and that PXE patients have HTN and decreased aortic compliance that cannot be explained by traditional risk factors. 8
Distorted arterial elastin, 3 elastin fragmentation, and the abundance of proteoglycans and calcium phosphate 7 are found in the carotid arteries, especially in the media, of patients with PXE and can be a possible etiologic or contributing factor for the vascular complications seen in this disease. We did observe a decreased total proteoglycan deposition throughout the vessels of the knockout mice in Panel B that corresponded to decreased CIMT ( Figure 2I through L). Further work is necessary to determine the type(s) of proteoglycans that are represented in normal versus diseased vessels. It is postulated that the defect in the ABCC6 gene alters the metabolism of polysaccharides, 16 but exactly how it leads to the excess deposition of proteoglycans seen in the vessels and the identification of the proteoglycan types remain to be studied.
In human and these animal studies, magnesium administration has been shown to reduce CIMT. 17 Some of the possible indirect and direct mechanisms of action of magnesium on CIMT can be from a relaxation of vascular smooth muscle by magnesium’s effects on reducing angiotensin‐induced aldosterone synthesis, and by acting as a mild physiologic calcium blocker (inhibiting calcium depolarizing effects and calcium‐related excitation–contraction coupling). 18 In fact, magnesium is often used in obstetrics as a tocolytic. In this study, the decrease in CIMT and total proteoglycan can simply be secondary to magnesium’s direct effects on the myocyte: myocyte relaxation, as described earlier, can lead to a reduced myocyte proliferation, and thus, reduced proteoglycan production. Finally, magnesium has been shown to inhibit hydroxyl‐methyl‐glutaryl‐coenzyme A reductase (HMGCoA reductase), which is the same enzyme inhibited with statin administration. 18 Use of statins have been proven to reduce CIMT in humans. 19 , 20 , 21 To our knowledge this is the first study to show that magnesium reduces CIMT in a mouse model of PXE.
Although we were limited in the technology of the ultrasound machine used in this experiment ( Figure 1 ), it is possible that with a high‐resolution ultrasound device, the CIMT of PXE mice can be measured in the mouse model. 22 In a study where the Vevo 770, 30 Hz (VisualSonics), was used 23 the plaque inside the vessel was able to be viewed but the measurement of CIMT was done histologically. We feel that there is potential future use of the ultrasound to measure CIMT.
The data generated from the carotid histology (H&E) ( Figure 3 ) showed that there was a statistically significant difference in CIMT in PXE mice untreated versus treated. A review of the literature suggests that CIMT measurements in normal C57BL/6 mice are within the 30–40 μm range, however, the mice in that study were 8 weeks old and the section that was measured was very close to the bifurcation. 24 Our baseline wild‐type values were higher (average of 101.5 μm) possibly due to the fact our design calculated the average from each vessel’s CIMT measurement at 1000, 3000, and 5000 μm from the bifurcation and our mice were 1 year old.
Because CIMT has direct implications on morbidity and mortality in humans, 14 , 25 its measurement in the mouse model for PXE can be an endpoint and can have future implications in determining treatment efficacy in this disease. This study examined the reduction of the CIMT measurements after treatment with magnesium oxide in Abcc6 −/− mice. Although humans with PXE have complications of arteriosclerosis and subsequent myocardial infarction and stroke, the PXE mice, even at 22 months, were relatively healthy with some sporadic deaths due to cardiovascular complications. 15 As is often the case, the etiology of cardiovascular and cerebrovascular disease may not only be due to genetics (in this case, the mutation of the ABCC6 gene), indicating that the environment or diet may also play a role in the comorbidities seen in humans with PXE. 15 Further studies will determine the impact of CIMT reduction in PXE mice in terms of morbidity and mortality.
Dietary magnesium is already being administered to PXE patients to determine drug efficacy. According to one study, 17 the CIMT of patients on hemodialysis who were treated with 610 mg of magnesium citrate every other day for 2 months significantly improved compared to controls. In a recent study, 26 demonstrated a clinical improvement of PXE patients when randomized to sevelamer hydrochloride or placebo (both contained magnesium stearate). This study demonstrates that a diet with magnesium oxide reduces CIMT in PXE mice of 1 year in age, which may be comparable to older PXE patients; CIMT is a progressive condition and older patients with PXE demonstrated higher CIMTs compared to younger patients with PXE. 7 Although administration of magnesium did not reverse mineralization in the biomarker in these older mice ( Figure 4 ) 13 it did reverse the CIMT. This shows that magnesium may provide some benefit to reduce the morbidity and mortality associated with PXE and provides a platform to establish a therapeutic biomarker that is easy to measure in humans (with carotid artery ultrasound). We feel that, via the measurement of CIMT in PXE we will be able to ascertain the efficacy of treatment with magnesium, or with other antimineralization compounds, in PXE patients in the future.
Conflict of Interest
The authors report no conflict of interest.
Acknowledgments
Assistance of the following individuals, all at Thomas Jefferson University, is greatly acknowledged: Dr. Scott Waldman, Department of Pharmacology and Experimental Therapeutics; Dr. Barry Goldberg and Dan Merton, Department of Radiology; Drs. John Farber and Stephen Peiper, Department of Pathology; Drs. Sergio Jimenez and Ulrich Rodeck, Department of Dermatology and Cutaneous Biology.
This work was supported by NIH grants T32 GM008562 and R01AR28450.
References
- 1. Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol. 2010; 130(3): 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol. 1988; 6(1): 151–159. [DOI] [PubMed] [Google Scholar]
- 3. Mendelsohn G, Bulkley BH, Hutchins GM. Cardiovascular manifestations of pseudoxanthoma elasticum. Arch Pathol Lab Med. 1978; 102(6): 298–302. [PubMed] [Google Scholar]
- 4. Kobolos G, Andrikovics H, Prohaszka Z, Tordai A, Varadi A, Aranyl T. The R1141X loss‐of‐function mutation of the ABCC6 gene is a strong genetic risk factor for coronary artery disease. Genet Test Mol Biomarkers. 2010; 14(1): 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boutouyrie P, Germain DP, Tropeano AI, Laloux B, Carenzi F, Zidi M, Jeunemaitre X, Laurent S. Compressibility of the carotid artery in patients with pseudoxanthoma elasticum. Hypertension. 2001; 38: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 6. Germain D, Boutouyrie P, Laloux B, Laurent S. Arterial remodeling and stiffness in patients with pseudoxanthoma elasticum. Arterioscler Thromb Vasc Biol. 2003; 23: 836–841. [DOI] [PubMed] [Google Scholar]
- 7. Kornet L, Bergen AAB, Hoeks APG, Cleutjens JP, Oostra RJ, Daemen MJ, van Soest S, Reneman RS. In patients with pseudoxanthoma elasticum a thicker and more elastic carotid artery is associated with elastin fragmentation and proteoglycans accumulation. Ultrasound Med Biol. 2004; 30(8): 1041–1048. [DOI] [PubMed] [Google Scholar]
- 8. Saban‐Ruiz J, Fuente RF, Sanchez‐Largo Uceda E, Fuente MF. Pseudoxanthoma elasticum: case report with arterial stiffness evaluated by a research cardiovascular profiling system. Eur J Dermatol. 2010; 20(6): 785–787. [DOI] [PubMed] [Google Scholar]
- 9. Rosenzweig BP, Guarneri E, Kronzon I. Echocardiographic manifestations in a patient with pseudoxanthoma elasticum. Ann Int Med. 1993; 119(6): 487–491. [DOI] [PubMed] [Google Scholar]
- 10. van den Berg JSP, Hennekam RCM, Cruysberg JRM, Steijlen PM, Swart J, Tijmes N, Limberg M. Prevalence of symptomatic intracranial aneurysm and ischaemic stroke in pseudoxanthoma elasticum. Cerebrovasc Dis. 2000; 10: 315–319. [DOI] [PubMed] [Google Scholar]
- 11. Nunez F, Martinez‐Costa C, Sanchez‐Zahonero J, Morata J, Chorro FJ, Brines J. Carotid artery stiffness as an early marker of vascular lesions in children and adolescents with cardiovascular risk factors. Rev Esp Cardiol. 2010; 63(11): 1253–1260. [DOI] [PubMed] [Google Scholar]
- 12. Simon A, Megnien JL, Chironi G. The value of carotid intima‐media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol. 2010; 30: 182–185. [DOI] [PubMed] [Google Scholar]
- 13. Li Q, LaRusso J, Grand‐Pierre AE, Uitto J. Magnesium carbonate‐containing phosphate binders prevents connective tissue mineralization in Abcc6‐/‐ mice‐ potential treatment for pseudoxanthoma elasticum. Clin Transl Sci. 2009; 2(6): 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Leary D, Bots ML. Imaging of atherosclerosis: carotid intima‐media thickness. Eur Heart J. 2010; 31: 1682–1689. [DOI] [PubMed] [Google Scholar]
- 15. Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol. 2005; 25(18): 8299–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maccari F, Gheduzzi D, Volpi N. Anomalous structure of urinary glycosaminoglycans in patients with pseudoxanthoma elasticum. Clin Chem. 2003; 49(3): 380–388. [DOI] [PubMed] [Google Scholar]
- 17. Turgut F, Kanbay M, Metin MR, Uz E, Akay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008; 40(4): 1075–1082. [DOI] [PubMed] [Google Scholar]
- 18. Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity, and diabetes. Curr Opin Lipidol. 2008; 19:50–56. [DOI] [PubMed] [Google Scholar]
- 19. Peters SA, den Ruijter HM, Bots ML. Attenuation of rate of change in carotid intima‐media thickness by lipid‐modyfying drugs: impact on clinical outcomes. Am J Cardiovasc Drugs. 2011; 11: 253–263. [DOI] [PubMed] [Google Scholar]
- 20. Sivapalarathnam S, van Loendersloot LL, Hutten BA, Kastelein JJ, Trip MD, de Groot E. Long‐term LCL‐c lowering in heterozygous familial hypercholesterolemia normalizes carotid intima‐media thickness. Atherosclerosis. 2010; 212(2): 571–574. [DOI] [PubMed] [Google Scholar]
- 21. Riccioni G, Vitulano N, Mancini B, Zanasi A, D’Orazio N. One‐year treatment with rosucastatin reduces intima‐media thickness in 45 hypercholesterolemic subjects with asymptomatic carotid artery disease. Pharmacology. 2010; 85(2): 63–67. [DOI] [PubMed] [Google Scholar]
- 22. Pistner A, Belmonte S, Coulthard T, Blaxall B. Murine echocardiography and ultrasound images. J Vis Exp. 2010; 8(42). Available at:http://www.jove.com/Details.stp?ID=2100 Accessed July 16, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li L, Cai XJ, Feng M, Rong YY, Zhan Y, Zhang M. Effect of adiponectin overexpression on stability of preexisting plaques by inducing prolyl‐4‐hydroxylase expression. Circulation. 2010; 74: 552–559. [Google Scholar]
- 24. van den Berg BM, Spaan JAE, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima‐media ratios at murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol. 2006; 290: H915–H920. [DOI] [PubMed] [Google Scholar]
- 25. Yamagishi T, Kato M, Koiwa Y, Omata K, Hasegawa H, Kanai H. Evaluation of plaque stabilization by fluvastatin with carotid intima‐medial elasticity measured by a transcutaneous ultrasonic‐based tissue characterization system. J Atheroscler Thromb. 2009; 16: 662–673. [DOI] [PubMed] [Google Scholar]
- 26. Yoo Jane Y, Blum Robin R, Singer Giselle K, Stern Dana K., Emanuel Patrick O, Fuchs Wayne, Phelps Robert G, Terry Sharon F, Lebwohl Mark G. A randomized controlled trial of oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2011; 65: 341–348. [DOI] [PubMed] [Google Scholar]


