Abstract
Background
It has been estimated that approximately 12% of women consume alcohol at some time during their pregnancy, and as many as 5% of children born in the United States are impacted by prenatal alcohol exposure (PAE). The range of physical, behavioral, emotional, and social dysfunctions that are associated with PAE are collectively termed fetal alcohol spectrum disorder (FASD).
Methods
Using a saccharin-sweetened ethanol solution, we developed a limited access model of PAE. C57BL/6J mice were provided access to a solution of either 10% (w/v) ethanol and 0.066% (w/v) saccharin or 0.066% (w/v) saccharin (control) for 4 h/d. After establishing consistent drinking, mice were mated and continued drinking during gestation. Following parturition, solutions were decreased to 0% in a stepwise fashion over a period of 6 days. Characterization of the model included measurements of maternal consumption patterns, blood ethanol levels, litter size, pup weight, maternal care, and the effects of PAE on fear-conditioned and spatial learning, and locomotor activity.
Results
Mothers had mean daily ethanol intake of 7.17 ± 0.17 g ethanol/kg body weight per day, with average blood ethanol concentrations of 68.5 ± 9.2 mg/dl after 2 hours of drinking and 88.3 ± 11.5 mg/dl after 4 hours of drinking. Food and water consumption, maternal weight gain, litter size, pup weight, pup retrieval times, and time on nest did not differ between the alcohol-exposed and control animals. Compared with control offspring, mice that were exposed to ethanol prenatally displayed no difference in spontaneous locomotor activity but demonstrated learning deficits in 3 hippocampal-dependent tasks: delay fear conditioning, trace fear conditioning, and the delay nonmatch to place radial-arm maze task.
Conclusions
These results indicate that this model appropriately mimics the human condition of PAE and will be a useful tool in studying the learning deficits seen in FASD.
Keywords: Prenatal Ethanol Exposure, Learning, Memory, Hippocampus, Dentate Gyrus
In both humans and laboratory animal models, exposure to alcohol during development has been shown to cause a multitude of dose-dependent effects on the structure and function of the central nervous system, with the extent of damage being dependent on the pattern and chronicity of the exposure and the stage(s) of development during which it occurs (Riley and McGee, 2005). The effects of prenatal alcohol exposure (PAE) on the brain display regional variability (Guerri, 1998; Willford et al., 2004), with the hippocampus being particularly sensitive to the damaging effects of the exposure (Berman and Hannigan, 2000). The dysfunctions that are associated with PAE are collectively termed fetal alcohol spectrum disorder (FASD) (Streissguth and O’Malley, 2000). It is estimated that the incidence of the use of any alcohol during pregnancy is about 12% (Floyd et al., 2009), with the incidence of newborns displaying prenatal alcohol-related damage as high as 5% in the United States (May et al., 2009). While heavy and binge (5 or more drinks in a single episode) patterns of drinking during pregnancy are associated with the development of fetal alcohol syndrome (Clarren and Smith, 1978; Jones and Smith, 1973), extensive research shows that even moderate maternal drinking (1 to 2 drinks per day) is, in some individuals, associated with cognitive and behavioral deficits, often revealed only under stressful or challenging conditions (Streissguth et al., 1999).
Existing rodent models for PAE employ various lengths and routes of administration, as well as a range of ethanol doses (Costa et al., 2000; Cudd, 2005). Although liquid diets and intragastric intubation are capable of producing moderate-to-high blood ethanol concentrations (BECs), they often induce maternal stress and malnutrition, which may confound the results (Slone and Redei, 2002; Ward and Wainwright, 1989). Voluntary oral consumption can reduce stress and malnutrition issues, but it may be difficult to reach moderate-to-high BECs with this procedure (Finn et al., 2005). However, limiting access to ethanol in voluntary consumption paradigms has been shown to increase self-administration (Finn et al., 2005; Rhodes et al., 2005, 2007). A recent study (Boehm et al., 2008) employed this method to develop a PAE model using C57BL/6J mice, with pregnant dams being given access to 20% ethanol for 2 hours during the dark cycle. These mice reached BECs ranging from 100 to 200 mg/dl, and the offspring exhibited motor deficits at postnatal day (PD) 33.
We sought to expand upon the model developed by Boehm and colleagues (2008) to determine its impact on hippocampal-dependent learning. Significant deficits in both delay- and trace-conditioned learning and a delayed nonmatching to place radial-arm maze (RAM) task in adult PAE mice were observed. The model will likely be an important tool in the study of molecular mechanisms underlying cognitive dysfunction associated with PAE.
MATERIALS AND METHODS
Animals
All of the procedures used in the current studies were approved by the University of New Mexico Health Sciences Center Institutional Animal Care and Use Committee.
Sixty-day-old C57BL/6J male and female mice (Jackson Laboratory, Bar Harbor, ME) were acclimated for 1 week in the vivarium at 22°C on a reverse 12-hour dark/12-hour light schedule (lights on at 20:00 hours) in group-housed cages. After this acclimation period, animals were individually housed for 1 week before the start of the alcohol (or control) drinking procedure. Standard chow was available ad libitum in all cages. Water was available at all times except during the 4-hour drinking period. Offspring were group-housed in same-sex, littermate cages with free access to tap water and chow, and maintained on a reverse light–dark cycle, as described earlier. All behavioral measurements were conducted during the dark period between 09:00 and 14:00 in a behavioral room lit with red lighting.
PAE Procedure
Prenatal exposure of mice to ethanol was performed using a modification of our previously published method (Allan et al., 2003). Two hours into the dark cycle, female mice were offered access to either 0.066% (w/v) saccharin (Sigma-Aldrich, St. Louis, MO) or an ethanol (DLI, King of Prussia, PA) solution sweetened with 0.066% (w/v) saccharin for 4 hours (from 10:00 to 14:00 hours). Research has shown that saccharin may increase ethanol intake without affecting blood alcohol concentrations (Czachowski et al., 1999; Roberts et al., 1999). The concentration (w/v) of ethanol was increased in two 2-day steps: 0 → 5%, then 5 → 10%. After 1 week of drinking 10% ethanol or the saccharin control (SAC) solution, individual females were placed into the cage of a singly housed male for 2 hours immediately after the drinking period (from 14:00 to 16:00 hours). Water and food were available ad libitum in the males’ cages. Females continued to consume ethanol and saccharin solutions throughout the 5-day mating period. Five days was chosen for the mating period based on the average estrous cycle length of 4 to 5 days in C57BL/6J mice (Jemiolo et al., 1986; Walf et al., 2009). Pregnancy was positively determined by monitoring weight gain, measured every 3 to 4 days. The length of gestation in C57BL/6J mice is approximately 19.5 days and is not affected by maternal consumption of alcohol (Kleiber et al., 2011; Murray et al., 2010). Thus, we identified gestational day 1 as 20 days prior to the day of parturition. Food and water consumptions were determined both before mating and during the last week of pregnancy. Within 1 day of birth, the alcohol and saccharin concentrations were halved every 2 days, until the animals were drinking only water.
BEC Measurements
Maternal BECs were determined during the last week of pregnancy, based on a method described previously (Smolen et al., 1987). Briefly, blood samples (40 µl) were collected from the submandibular vein at both the 2-hour (12:00 hours) and 4-hour (14:00 hours) time points, with only a single sample being collected from an animal. The samples were treated with 2 ml of 3.5% (v/v; 0.58 M) perchloric acid (Mallinckrodt Baker, Inc., Phillipsburg, NJ) and centrifuged (300×g, 10 minutes, 4°C) to obtain serum. Eighty microliters of serum, or known ethanol standard ranging from 0 to 400 mg/dl, was incubated in 2 ml of reaction buffer (10 units alcohol dehydrogenase [Sigma-Aldrich], 2.0 mM NAD [Sigma-Aldrich], 0.5 M Tris–HCl, pH 8.8) for 15 minutes at 30°C. The optical density of the sample was measured at 340 nm (Beckman DU 380 Spectrophotometer; Indianapolis, IN). Sample blood ethanol values were determined by regression analysis. Blood alcohol was determined for 3 separate breeding rounds. The offspring of the dams used for determination of BECs were not used in this study.
Litter Size and Offspring Weight
Litters were left undisturbed for 7 days (except for those used for pup retrieval test) at which time litter size was determined. Litters were not culled. Offspring were weighed on PDs 7, 14, and at weaning (approximately PD 23). Weights of the entire litter were collected, and average pup weight was determined by dividing the total weight of the litter by the number of pups present in each litter.
Characterization of Maternal Care
The percentage of time spent on nest in a 1-hour period was evaluated at PD 5 or 6 between 10:00 and 14:00 hours. Pup retrieval time was performed in the home cage on PD 8. A pup was removed from the nest and placed in the farthest corner away from the nest (approximately 30 cm away), and latency to retrieve the pup was measured.
Locomotor Activity
Male offspring of 90 to 150 days of age were tested for their spontaneous locomotor activity, as described previously (Caldwell et al., 2008). Briefly, mice were placed in a dim red light-illuminated open field apparatus (102.5 × 102.5 × 47.5 cm; Opto-Varimex; Columbus Instruments, Columbus, OH) for 15 minutes on 2 consecutive days. The first day assessed locomotor activity in a novel environment, and the second day examined locomotor activity in a familiar environment. Openfield locomotor (horizontal) activity was measured as the number of photobeam interruptions recorded on both days. All equipment was cleaned with 70% (v/v) isopropanol between uses.
Delay Fear Conditioning
Delay fear conditioning was conducted with 90- to 150-day-old male offspring using a procedure previously described (Allan et al., 2003). Briefly, animals were placed into a Coulbourn Instruments (Allentown, PA) Habitest® System for 90 seconds of habituation. Subsequently, they received the conditioned stimulus (CS), an 80 dB, 6 Hz clicker, for 30 seconds. The unconditioned stimulus (US), an electric foot shock (0.7 mA), was delivered during the last 2 seconds of the CS. Ninety seconds later a second CS–US pairing was initiated. Thirty seconds after this second pairing, the subject was returned to its home cage. Approximately 24 hours later, the subject was returned to the training context, and its behavior was videotaped. Freezing (absence of movements other than those necessary for respiration) during the first 2 minutes in the context was measured and expressed as a percentage of the total time. Neither the CS nor the US was delivered during this period. The subject’s behavior was scored by 2 individuals, one of whom was blind to the animal’s history.
Trace Fear Conditioning
Trace conditioning was conducted with 90- to 150-day-old male offspring in the same apparatus used for delay conditioning. The trace conditioning procedure was a modification of a procedure described previously (Huerta et al., 2000). Briefly, after 90 seconds of habituation, subjects experienced 7 trials each consisting of the CS (10 seconds, 80 dB 6 Hz clicker), a 30 second trace, the US (1 second, 0.8 mA scrambled foot shock), and a 210 second intertrial interval; the subject was removed from the chamber 60 seconds following the delivery of the last US. The equipment was cleaned with 70% (v/v) isopropanol between uses. A separate “untrained” group of mice was exposed to all of the same conditions except for delivery of the US.
Approximately 24 hours later, freezing to the CS in a novel context (a standard, clean mouse cage with minimal bedding) was assessed. Five minutes after being placed in the novel environment, the 10-second CS was sounded; 4 minutes later another 10-second CS was delivered. Three minutes later the subject was returned to its home cage. The animal’s behavior was videotaped, and the amount of time spent freezing during the tone and 30 seconds after the tone (40 seconds total duration) was scored for each CS. The average for the 2 measures was calculated and expressed as a percentage of time spent freezing. Untrained animals (cage mates from the same litter) also experienced the CS in the novel context, and the percentage of time spent freezing was similarly determined.
Delay Nonmatch to Place Spatial Memory
Delay nonmatch to place (DNMP) spatial memory test was performed with 90- to 150-day-old male offspring based on a published method with modification (Clelland et al., 2009) using a RAM purchased from Coulbourn Instruments. The maze consisted of an octagonal-shaped center arena (28 cm diameter, 25.5 cm height) with 8 arms (68.5 cm length, 9.5 cm width, 12.1 cm height), each made of clear Plexiglas with a wire grid floor. Mice were on a caloric-restricted diet (80 to 85% of starting body weight) throughout the testing period.
On the first day, mice were placed into 1 of the maze arms that was baited with food; the central exit of the arm was blocked, and the animal was left for 30 minutes prior to return to its home cage. On the second through sixth days, a DNMP procedure was employed: days 2 to 3 for training (self-correction permitted) and days 4 to 6 for testing. Mice experienced 2 trials in the morning and 2 trials in the afternoon; each trial consisted of a sample phase and choice phase. Between each trial, mice were returned to their home cage, and all other mice were tested before the next trial began.
In the sample phase, only the start and food-baited sample arms were accessible to the mouse. Mice were removed from the maze and placed in a holding cage if they had either spent 60 seconds in the sample arm or exited the sample arm. To reduce the possibility that odor cues were being utilized, the maze was rotated between sample and choice presentations, with the spatial location for the start arm remaining constant. The rotation took approximately 15 seconds. During the choice phase, a third arm was open and baited with food, while the start and sample arms were open but not baited. The choice arm varied in distance from the sample arm by either 2 (separation 2 or Sep 2) or 4 (separation 4 or Sep 4) arms. Mice that entered the choice arm were scored as correct. Failure to make a selection in 3 minutes was scored as incorrect. The percentage of correct arm entries for days 4 to 6 were determined for Sep 2 and Sep 4.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 (San Diego, CA). All studies used at least 2 different breeding rounds. For maternal measures, the reported n corresponds to the number of dams, while for offspring measures, the reported n represents the number of litters. All measures except those listed below were analyzed using a Student’s 2-tailed t-test. BECs were calculated using linear regression and Pearson’s correlation analysis was used to determine significance. Trace fear conditioning and DNMP data were analyzed using a 2-way analysis of variance (ANOVA) followed by Student’s t-test with Bonferroni correction. For the delay fear conditioning task and DNMP, 2 animals from a litter were averaged and used as a single data point. For the trace fear conditioning task, 2 animals were used from a litter, 1 trained and 1 untrained. In all cases, data are presented as mean ± SEM.
RESULTS
Ethanol Consumption and BEC Levels
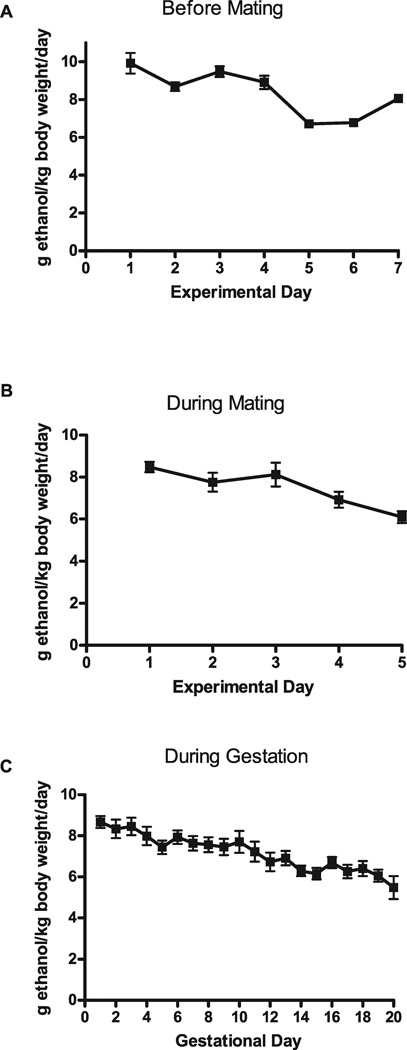
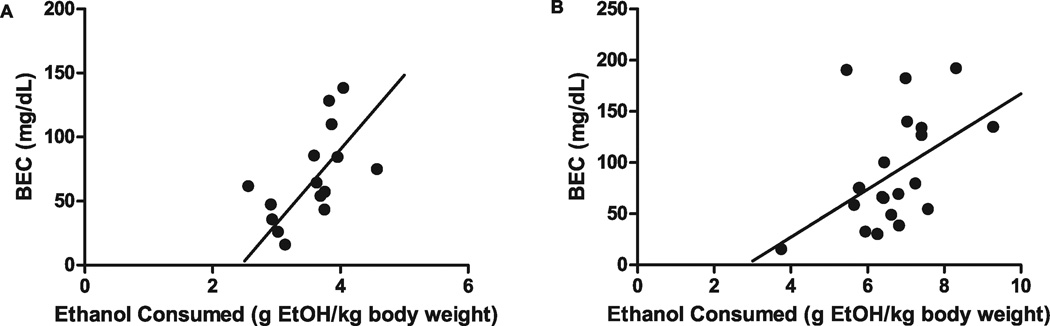
Ethanol consumption was monitored before and during mating, as well as throughout gestation, from 3 different breeding rounds (Fig. 1). Ethanol intake reached moderate-to-high levels before mating (8.36 ± 0.47 g ethanol/kg body weight per day) and continued throughout mating (7.47 ± 0.43 g ethanol/kg body weight per day). During gestation, ethanol consumption remained relatively constant, averaging 7.17 ± 0.17 g ethanol/kg body weight per day. After 2 and 4 hours drinking, BECs were 68.5 ± 9.2 mg/dl and 88.3 ± 11.5 mg/dl, respectively. Consumption of ethanol over both a 2-hour period and a 4-hour period significantly predicted BEC levels (2-hour period: Pearson’s r = 0.57, p < 0.05, 4-hour period: Pearson’s r = 0.48, p < 0.05; Fig. 2A,B).
Fig. 1.
Average ethanol consumption (g ethanol/kg body weight per day) for C57BL/6J mice before mating (A; n = 23), during mating (B; n = 21), and during gestation (C; n = 30).
Fig. 2.
Correlation of blood alcohol concentrations (BECs) to the amount of ethanol (EtOH) consumed by female mice. (A) BEC levels significantly correlate with the amount of EtOH consumed at 2 hours (n = 15; r = 0.57, p < 0.05). (B) BEC levels significantly correlate with the amount of EtOH consumed at 4 hours (n = 22; r = 0.48, p < 0.05). BEC levels were determined from blood taken in a separate cohort of female mice after 2 or 4 hours of drinking, using an enzymatic method described in Materials and Methods.
Food and Water Consumption
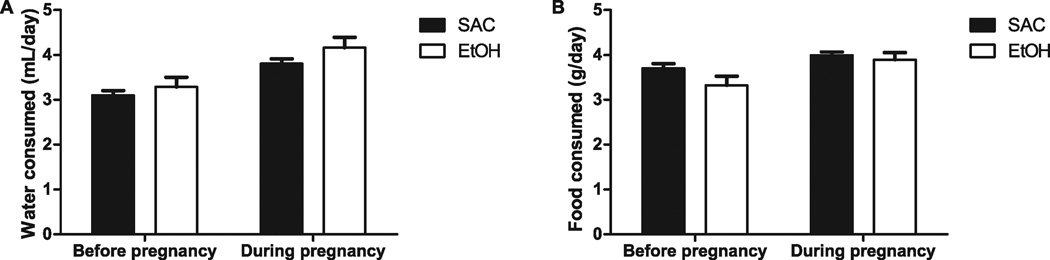
We measured intake of food and water both before mating and during the last week of pregnancy for both groups in 3 separate rounds of breeding (Fig. 3). Food and water consumption before mating did not differ significantly between the saccharin and the ethanol groups (Water—saccharin 3.28 ± 0.22 ml/d, ethanol 3.10 ± 0.11 g/d, t[17] = 0.74, n.s.; Food—saccharin 3.32 ± 0.2 g/d, ethanol 3.70 ± 0.12 g/d, t[18] = 1.66, n.s.; Fig. 3A,B). Similarly, there was not a significant difference in food and water consumption between groups in the last week of pregnancy (Water—saccharin 3.81 ± 0.11 ml/d, ethanol 4.16 ± 0.23 ml/d, t[14] = 1.47, n.s.; Food—saccharin 3.99 ± 0.07 g/d, ethanol 3.89 ± 0.16 g/d, t[18] = 1.66, n.s.; Fig. 3A,B). Average caloric intake during the last week of pregnancy did not differ significantly between groups (saccharin 13.98 ± 0.25 kcal/d, ethanol 15.21 ± 0.53 kcal/d; t[12] = 2.10, n.s.), and the calorie equivalent of ethanol consumed was about 10% of the daily diet intake. Additionally, there was no significant difference between groups for the change of maternal weight that occurred during pregnancy (Table 1).
Fig. 3.
Food and water consumption by saccharin (SAC) control group and ethanol (EtOH) drinking group before and during pregnancy. (A) Amount of water consumed (ml) per day (20-hour access) before pregnancy (n = 10) and during the last week of pregnancy (n = 7). (B) Amount of food consumed (g) per day before pregnancy (n = 9) and during last week of pregnancy (n = 9 for SAC, n = 7 for EtOH). No significant difference was found between SAC and EtOH groups at any time point for either food and water consumption.
Table 1.
Maternal Care and Offspring Data from 2 to 4 Different Breeding Rounds
| Change in maternal weight (g) |
Pup weight PD 7 (g) |
Pup weight PD 14 (g) |
Pup weight PD 23 (g) |
Litter size (pups per litter) |
Pup retrieval time (s) |
Time on nest (% of 1 hour) |
|
|---|---|---|---|---|---|---|---|
| 0.066% Saccharin solution | 7.10 ± 0.23 (n = 32) |
4.10 ± 0.17 (n = 7) |
6.90 ± 0.34 (n = 7) |
12.39 ± 0.29 (n = 25) |
6.8 ± 0.3 (n = 32) |
8.96 ± 0.76 (n = 11) |
69.71 ± 7.59 (n = 7) |
| 10% EtOH with 0.066% saccharin solution | 7.62 ± 0.23 (n = 32) |
4.01 ± 0.13 (n = 6) |
7.03 ± 0.22 (n = 6) |
13.29 ± 0.47 (n = 24) |
7.3 ± 0.3 (n = 32) |
9.80 ± 1.00 (n = 10) |
70.56 ± 8.02 (n = 7) |
Values are mean ± SEM.
EtOH, ethanol; PD, postnatal day.
Offspring Weight and Litter Size
Litter size was determined for 4 separate breeding rounds at PD 7. The average litter size between groups was not significantly different (t[62] = 1.473, n.s.; Table 1). Offspring weight was also determined at PDs 7, 14, and at weaning (approximately PD 23) (Table 1). There was no significant difference between groups at any time point (PD 7: t[11] = 0.4517, n.s.; PD14: t[11] = 0.3054, n.s.; PD 23: t[47] = 1.641, n.s.; Table 1).
Maternal Care
Although pup weights and early postnatal growth are good indicators of maternal care, we also directly assessed maternal care. There was no significant difference in the amount of time taken for pup retrieval between saccharin-drinking and ethanol-drinking mothers (t[19] = 0.6804, n.s.; Table 1), nor was there a difference in the amount of time mothers spent on the nest (t[12] = 0.07633, n.s.; Table 1).
Locomotor Activity
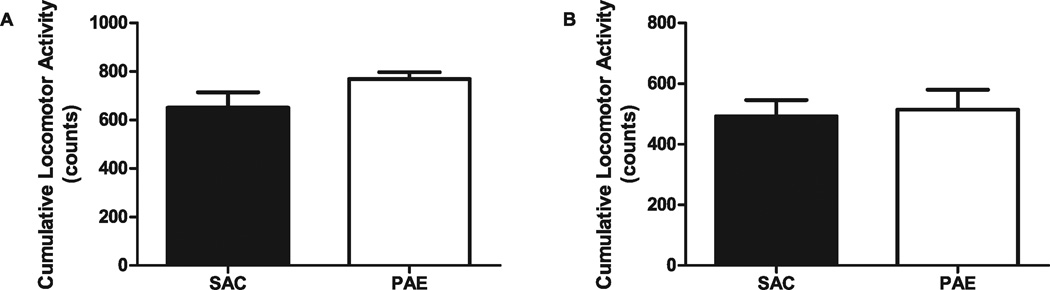
Because locomotor activity could influence the level of activity of the mice, and therefore performance in the learning tasks we used, we determined the basal level of locomotor activity in PAE and SAC control mice. In either a novel environment (day 1) or a familiar environment (day 2), PAE mice did not display differences in locomotor activity (measured as counts of beam interruptions) compared with SAC offspring (day 1—SAC 651.4 ± 63.02, PAE 768.7 ± 28.77; t[10] = 1.480, n.s.; day 2—SAC 492.1 ± 53.27, PAE 514.6 ± 64.83; t[10] = 0.2681, n.s.; Fig. 4).
Fig. 4.
Effect of prenatal ethanol exposure on locomotor activity. Locomotor activity, measured as counts (beam interruptions), over two 15-minute periods approximately 24 hours apart, was determined in saccharin control (SAC) and prenatal alcohol exposure (PAE) mice, as described in Materials and Methods. (A) Locomotor activity for SAC (n = 7) and PAE (n = 5) mice on day 1. (B) Locomotor activity for SAC (n = 6) and PAE (n = 6) mice on day 2. No significant difference was found between SAC and PAE mice at either time point.
Delay Fear Conditioning
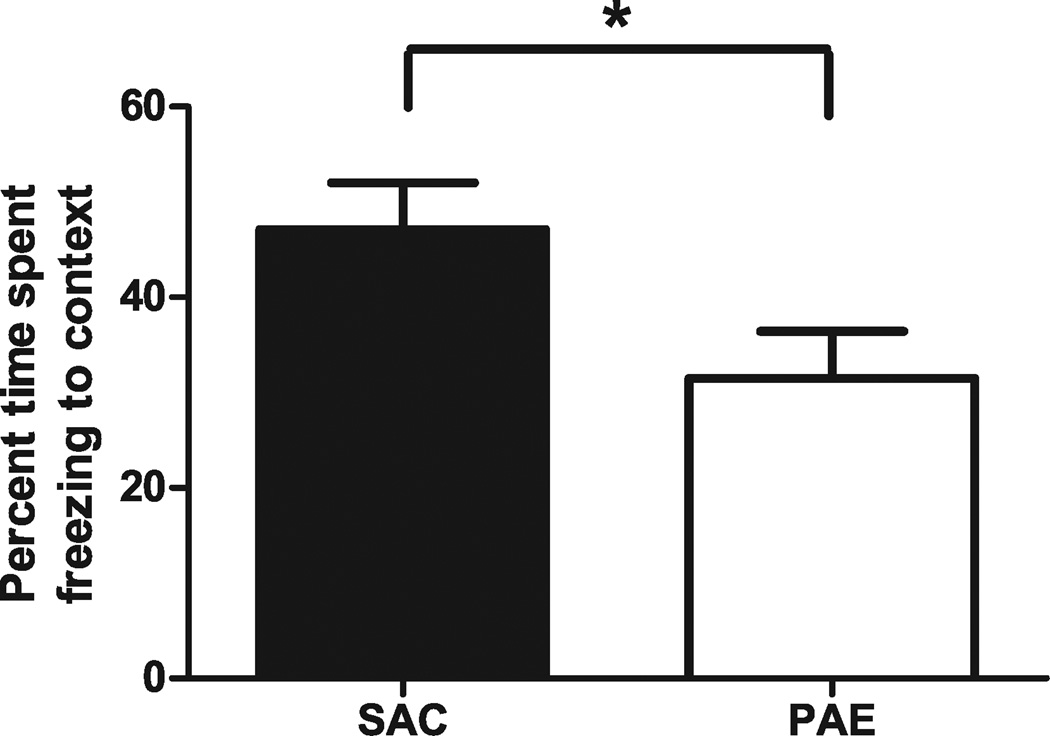
To determine whether our model produced deficits in hippocampal-dependent learning, we analyzed delay fear-conditioned learning and memory in adult offspring from both groups. Twenty-four hours following training, mice were assessed for contextual conditioning by measuring freezing following reintroduction into the training context (Fig. 5). PAE mice froze significantly less than did SAC controls (SAC 47.12 ± 4.89%, PAE 31.50 ± 4.94%; t[17] = 2.243, p < 0.05).
Fig. 5.
Effect of prenatal ethanol exposure on contextual fear learning and memory. Adult male saccharin control (SAC) and prenatal alcohol exposure (PAE) mice were trained in a delay fear conditioning paradigm as described in Materials and Methods. Approximately 24 hours later, the amount of time spent freezing in a 2-minute period after reexposure to the training context was measured and expressed as a percentage (n = 10 for SAC, n = 9 for PAE;). Unpaired Student’s t-test found a significant difference in percent freezing to the context between SAC and PAE offspring, t(17) = 2.243, *p < 0.05.
Trace Fear Conditioning
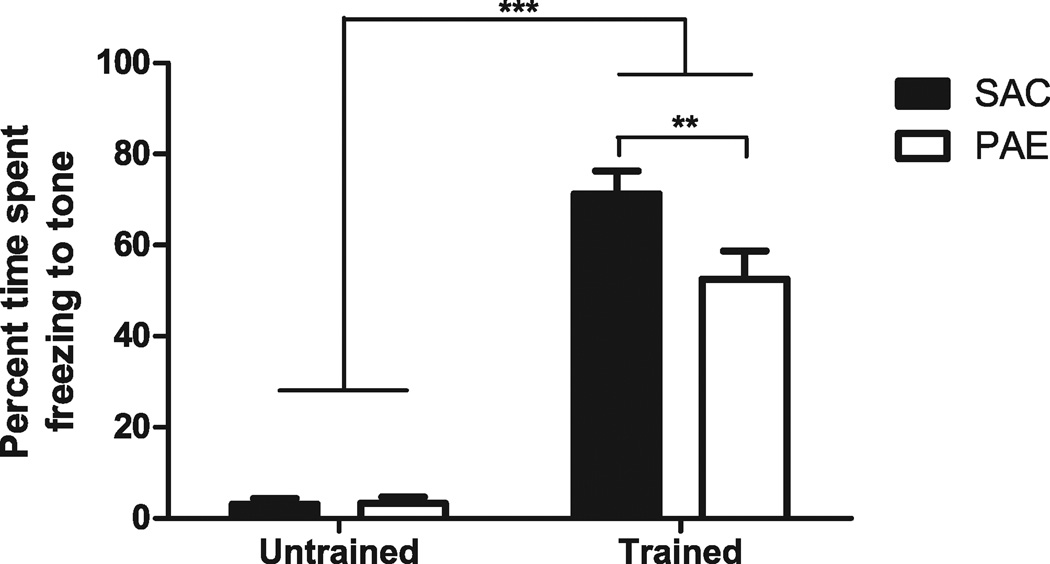
Because we were unaware of any reports examining the effects of PAE on trace fear conditioning, a hippocampal-dependent task that is temporally dependent (McEchron et al., 1998), in a murine model, we determined whether our model exhibited a deficit in this task. Twenty-four hours following training, mice were assessed for their responses to the CS by measuring freezing behavior (Fig. 6). In addition, a group of untrained mice were similarly assessed for their response to the tone. Two-way ANOVA indicated a significant main effect of training, F(1, 25) = 186.3, p < 0.001, and prenatal exposure, F(1, 25) = 4.670, p < 0.05, as well as an interaction of these factors, F(1, 25) = 4.878, p < 0.05. Post hoc analysis using a Student’s t-test with Bonferroni correction revealed a significant deficit in freezing in the trace-conditioned PAE animals compared with control animals (SAC 71.29 ± 4.973, PAE 52.51 ± 6.197; t[13] = 3.141, p < 0.01), but no difference between PAE and SAC offspring in the untrained group.
Fig. 6.
Effect of prenatal ethanol exposure paradigm on trace fear learning and memory. Adult male saccharin control (SAC) and prenatal alcohol exposure (PAE) mice were trained in a trace fear conditioning paradigm as described in Materials and Methods. Approximately 24 hours later, mice were placed in a novel context and the amount of time spent freezing during the 40 seconds after initiation of a 10-second tone, corresponding to the tone and trace periods in the training procedure, was measured and expressed as a percentage (mean ± SEM, n = 7 for SAC, n = 8 for PAE). A second (untrained) set of animals was exposed to all of the same conditions as the trained animals except for delivery of the unconditioned stimulus. Approximately 24 hours later, their freezing during the 40 seconds after initiation of the 10-second tone was recorded, similar to the trained animals. Two-way analysis of variance indicated a significant main effect of training, F(1, 25) = 186.3, ***p < 0.001, and prenatal exposure, F(1, 25) = 4.670, p < 0.05, as well as an interaction of these factors F(1, 25) = 4.878, p < 0.05. Post hoc Student’s t-test with Bonferroni correction revealed no significant difference between the untrained SAC and untrained PAE groups but a significant difference between the SAC and PAE animals that had undergone trace conditioning, t(13) = 3.141, **p < 0.01.
Delay Nonmatch to Place
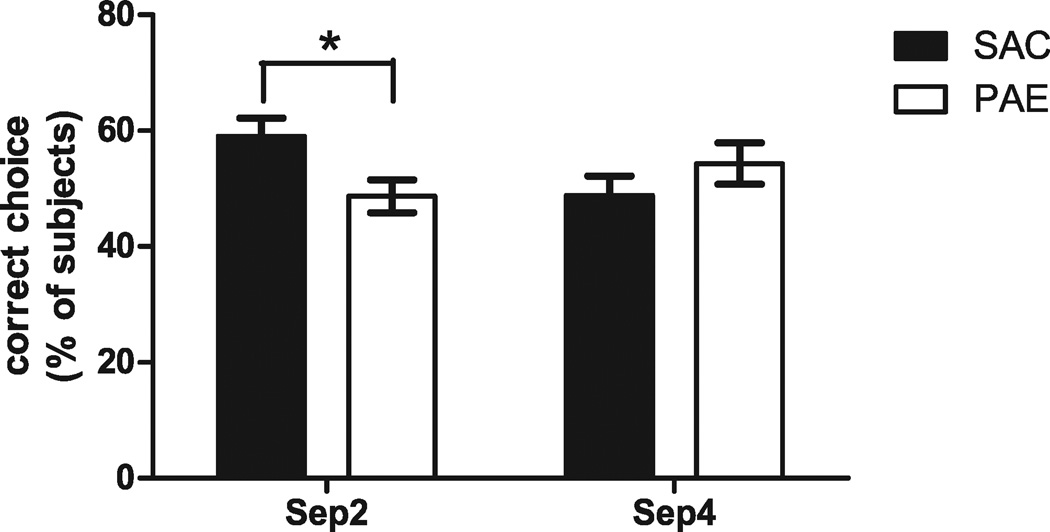
Because previous studies have demonstrated deficits in the dentate gyrus in PAE offspring (Samudio-Ruiz et al., 2009; Sutherland et al., 1997; Varaschin et al., 2010), we assessed dentate gyrus-dependent behavior in our PAE model. A recent study developed a DNMP task that is dentate gyrus-dependent (Clelland et al., 2009). Mice were tested for their ability to distinguish between 2 spatially separated locations, one of which was recently visited (the sample arm) and a second (the choice arm) which was previously unavailable, in an 8-way RAM. Two-way ANOVA indicated a significant interaction of prenatal treatment and arm separation, F(1, 20) = 5.924, p < 0.05. Post hoc Student’s t-test with Bonferroni correction indicated that PAE offspring made significantly fewer correct arm entries in the dentate gyrus-dependent form of the task, Sep 2 (SAC 59.00 ± 3.18%, PAE 48.67 ± 2.86%; t[10] = 2.415, p < 0.05; Fig. 7), but no difference in performance for Sep 4 (SAC 48.83 ± 3.34%, PAE 54.33 ± 3.58%; t[10] = 1.123, n.s.; Fig. 7), which does not require a functional dentate gyrus (Clelland et al., 2009).
Fig. 7.
Effect of prenatal ethanol exposure paradigm on a delayed nonmatching to place radial-arm maze (RAM) task. Adult male saccharin control (SAC) and prenatal alcohol exposure (PAE) mice were trained in a RAM paradigm as described in Materials and Methods. The number of correct arm entries for each trial per day across 3 days were determined and expressed as a percentage (n = 6). Two-way ANOVA indicated a significant interaction between prenatal treatment and separation, F(1, 20) = 5.924, p < 0.05, and a post hoc Student’s t-test indicated a significant difference in correct arm entries for Sep 2, t(10) = 2.415, *p < 0.05, but not for Sep 4, between SAC and PAE animals. Sep 2, separation 2; Sep 4, separation 4.
DISCUSSION
We utilized a PAE paradigm, in which dams had access to a saccharin-sweetened 10% ethanol solution for 4 hours a day prior to and throughout gestation, to characterize the effects of PAE on hippocampal-dependent learning and memory. The model produced consistent drinking levels, averaging 7.17 ± 0.17 g ethanol/kg body weight per day, with BECs reaching 68.54 ± 9.24 mg/dl after 2 hours of drinking and 88.32 ± 11.55 mg/dl after 4 hours. We found no significant differences in maternal food and water consumption, maternal weight gain, pup weight, or litter size between saccharin and ethanol groups, indicating that malnutrition is not a factor in our model. The limited access model yielded less variability in daily drinking than we previously observed (Caldwell et al., 2008) using a continuous access model: C57BL/6J dams given 22 h/d access consumed 9.55 ± 2.57 g ethanol/kg body weight per day. Although the ethanol intake of the dams in the present study was significantly correlated with the BECs reached after 2 and 4 hours of drinking, inspection of the data shows that there is sufficient variability to necessitate measurements of BECs as the model is employed in the future.
A confound in some PAE paradigms is that continued drinking during the postnatal period, or withdrawal symptoms during this period, may interfere with maternal care, leading to alterations in the offspring not directly due to PAE (Liu et al., 2000). However, we found no difference in pup weight, pup retrieval times, or time spent on the nest, indicating that tapering the nursing mothers off of the ethanol solution had minimal impact on maternal care.
A recent paper (Boehm et al., 2008) developed a similar model in C57BL/6Jmice, offering a 20% unsweetened ethanol solution for 2 hours a day, 3 hours into the dark cycle during pregnancy. Although there are some similarities, our model has key differences. We chose a 10% solution, rather than the 20% solution used by Boehm and colleagues (2008), to achieve moderate BECs in the dams and avoid malnutrition that may accompany higher doses of ethanol. By reducing the ethanol concentration to 10%, we found lower BECs than Boehm and colleagues (76 to 100 mg/dl after 4 hours drinking vs. approximately 130 to 170 mg/dl after 2 hours drinking). In addition, the Boehm and colleagues’ (2008) model appeared to produce malnutrition, as there was a decrease in pup weight at PD 21, which we did not find at a similar time point (PD 23). Although other studies have shown decreases in pup weight, the lack of an impact on pup weight in our model may be due both to lower ethanol consumption and similar caloric intake between our control and ethanol dams.
Boehm and colleagues (2008) did not examine the effect of PAE on hippocampal-dependent learning and memory. Previous studies have shown that prenatal ethanol-exposed offspring exhibit deficits in spatial tasks, similar to those seen in animals with hippocampal lesions (Berman and Hannigan, 2000). In our model, we observed deficits in PAE animals in contextual delay fear conditioning, a hippocampal-dependent task (McEchron et al., 1998; Quinn et al., 2002; Weitemier and Ryabinin, 2004). Prenatal ethanol-exposed offspring exhibited decreased freezing when returned to the training context, indicating a failure to learn the association between the context and the US shock. An important consideration in using an aversive conditioning paradigm to assess learning and memory deficits in PAE animals is the influence of pain perception on fear-conditioned learning, as studies have shown that animals prenatally exposed to alcohol exhibit hyperalgesia and increased analgesic response to morphine (Markel et al., 1986; Nelson et al., 1986). Young and Fanselow (1992) have described a negative-feedback model involving opioid-dependent analgesia that mediates, in part, the relationships between the perception of the intensity of a nociceptive US and the formation of the US–CS association. This mechanism is observed under conditions of low-to-moderate US intensity and becomes most apparent following more than 1 training session. We believe that the contribution of the feedback mechanism to conditioning in our animals is minimal under the conditions that we used (moderate-to-high shock intensity and a single training session). In addition, Lehner and colleagues (2010) have shown that reactivity to the US foot shock is not correlated with freezing in the training context. Thus, our finding of reduced freezing upon return to the training context is likely to be due to learning and/or memory deficits in PAE offspring, rather than altered pain perception or analgesic responses. The identification of a learning/memory deficit in adult offspring demonstrates that our model produces learning and memory deficits that persist into adulthood, similar to FASD.
In addition to deficits in delay fear conditioning, studies in rats have shown that PAE is associated with reduced trace-conditioned learning and memory (Hunt et al., 2009; Wagner and Hunt, 2006). In trace conditioning, an interval of time between the CS and the US creates a temporally noncontiguous relationship between the 2 stimuli. Learning the relationship between the CS and the US requires the hippocampus (McEchron et al., 1998; Quinn et al., 2002; Weitemier and Ryabinin, 2003, 2004). We found a deficit in freezing to the CS in PAE mice compared with SACs; this deficit appeared to be dependent on learning the CS–US association, because freezing to the CS tone in untrained animals was not different between the groups. This novel result demonstrates that the PAE mouse model exhibits a deficit in trace-conditioned learning and memory.
Recently, a study showed that irradiation to the dentate gyrus, which ablated neurogenesis, impaired performance in the Sep 2, but not Sep 4, version of the DNMP RAM(Clelland et al., 2009). To our knowledge, no one has demonstrated a behavioral deficit in PAE offspring directly attributed to the dentate gyrus; we therefore decided to examine the effects of PAE on performance in this task. We found that PAE offspring were impaired in the Sep 2 version of the DNMP test, with no apparent deficits in the Sep 4 task. These results indicate that dentate gyrus function is impaired in PAE offspring in this model. It is believed that the dentate gyrus is important in spatial learning, especially more difficult pattern separations (Acsady and Kali, 2007; Hsu, 2007; Kesner, 2007; Leutgeb et al., 2007), and is also of interest because it is 1 of the 2major sites of neurogenesis in the adult brain (Deng et al., 2010; Parent, 2007). Studies examining the effects of PAE on the dentate gyrus have shown deficits in long-term potentiation (Christie et al., 2005; Sutherland et al., 1997; Varaschin et al., 2010), an electrophysiological model of the synaptic changes that underlie learning and memory, impaired survival of newly generated dentate granule neurons in response to an enriched environment in adult mice (Choi et al., 2005), and decreased NMDA receptor-dependent activation of extracellular signal-regulated kinase 1/2 (ERK1/2) in the adult mouse dentate gyrus (Samudio-Ruiz et al., 2009). ERK1/2 activation has been shown to be necessary in several learning tasks (Blum et al., 1999; Selcher et al., 1999), and alterations in adult neurogenesis in the dentate gyrus have been shown to affect performance in pattern separation tasks (Clelland et al., 2009; Sahay et al., 2011). Our findings provide a connection between dentate gyrus-specific biochemical and electrophysiological findings (Samudio-Ruiz et al., 2009; Sutherland et al., 1997; Varaschin et al., 2010) and dentate gyrus-specific behavioral deficits in PAE.
Although the paradigm that we detail appears to be a useful tool to study the effects of PAE, there is a caveat that needs to be acknowledged. Because the gestational period in mice is the equivalent of the first 2 trimesters in humans, our model, by primarily targeting these 2 trimesters combined with reduced exposure during the initial half of the third trimester equivalent postnatally through breast milk, does not fully mimic PAE occurring throughout pregnancy in humans. However, a recent study indicated that, from a cohort of about 95,000 women, most reported drinking in the first and second trimesters, with the levels of drinking decreasing greatly by the third trimester (Muhuri and Gfroerer, 2009). Therefore, the paradigm that we employed does model the most common pattern of PAE occurring in human populations.
In this study, we have described and characterized a mouse model of PAE. Dams exhibited moderate ethanol consumption during a 4-hour ethanol exposure period, achieving pharmacologically relevant BECs. This pattern of consumption produced learning deficits associated with hippocampal dysfunction in the offspring without any apparent influences from other confounds. This model will facilitate the study of PAE, particularly in examining the preclinical efficacy of potential treatments that could ameliorate the long-lasting alterations in hippocampal function that are associated with FASD.
ACKNOWLEDGMENTS
We thank Buz Tyler for computer and equipment support and Samantha Goggin for help scoring behavior. Sources of support: NIH-NIAAA award #1T32AA014127 (MLB), NIH-NIAAA award #1F31AA020434 (MLB), NIH-NIAAA award #1R03AA020101 (KKC), NIH-NIAAA award #1P20AA017068 (KKC), and Dedicated Health Research Funds from the University of New Mexico School of Medicine (AMA, KKC).
REFERENCES
- Acsady L, Kali S. Models, structure, function: the transformation of cortical signals in the dentate gyrus. Prog Brain Res. 2007;163:577–599. doi: 10.1016/S0079-6123(07)63031-3. [DOI] [PubMed] [Google Scholar]
- Allan AM, Chynoweth J, Tyler LA, Caldwell KK. A mouse model of prenatal ethanol exposure using a voluntary drinking paradigm. Alcohol Clin Exp Res. 2003;27:2009–2016. doi: 10.1097/01.ALC.0000100940.95053.72. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH. Effects of prenatal alcohol exposure on the hippocampus: spatial behavior, electrophysiology, and neuroanatomy. Hippocampus. 2000;10:94–110. doi: 10.1002/(SICI)1098-1063(2000)10:1<94::AID-HIPO11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Moore EM, Walsh CD, Gross CD, Cavelli AM, Gigante E, Linsenbardt DN. Using drinking in the dark to model prenatal binge-like exposure to ethanol in C57BL/6J mice. Dev Psychobiol. 2008;50:566–578. doi: 10.1002/dev.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell KK, Sheema S, Paz RD, Samudio-Ruiz SL, Laughlin MH, Spence NE, Roehlk MJ, Alcon SN, Allan AM. Fetal alcohol spectrum disorder-associated depression: evidence for reductions in the levels of brain-derived neurotrophic factor in a mouse model. Pharmacol Biochem Behav. 2008;90:614–624. doi: 10.1016/j.pbb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29:2053–2062. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21:1719–1726. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298:1063–1067. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH, Denning CE. Blood ethanol concentrations in rats drinking sucrose/ethanol solutions. Alcohol Clin Exp Res. 1999;23:1331–1335. [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl) 2005;178:471–480. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Weber MK, Denny C, O’Connor MJ. Prevention of fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:193–199. doi: 10.1002/ddrr.75. [DOI] [PubMed] [Google Scholar]
- Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: dose-response and timing effects. Alcohol. 2009;43:465–474. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo B, Harvey S, Novotny M. Promotion of the Whitten effect in female mice by synthetic analogs of male urinary constituents. Proc Natl Acad Sci USA. 1986;83:4576–4579. doi: 10.1073/pnas.83.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kesner RP. A behavioral analysis of dentate gyrus function. Prog Brain Res. 2007;163:567–576. doi: 10.1016/S0079-6123(07)63030-1. [DOI] [PubMed] [Google Scholar]
- Kleiber ML, Wright E, Singh SM. Maternal voluntary drinking in C57BL/6J mice: advancing a model for fetal alcohol spectrum disorders. Behav Brain Res. 2011;223:376–387. doi: 10.1016/j.bbr.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Lehner M, Wislowska-Stanek A, Maciejak P, Szyndler J, Sobolewska A, Krzascik P, Plaznik A. The relationship between pain sensitivity and conditioned fear response in rats. Acta Neurobiol Exp (Wars) 2010;70:56–66. doi: 10.55782/ane-2010-1774. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Markel E, Nyakas C, Tal E, Endroczi E. Changes in avoidance behaviour following ethanol treatment in rats of different ages. Acta Physiol Hung. 1986;68:175–181. [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Muhuri PK, Gfroerer JC. Substance use among women: associations with pregnancy, parenting, and race/ethnicity. Matern Child Health J. 2009;13:376–385. doi: 10.1007/s10995-008-0375-8. [DOI] [PubMed] [Google Scholar]
- Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PLoS ONE. 2010;5:e12418. doi: 10.1371/journal.pone.0012418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LR, Taylor AN, Lewis JW, Poland RE, Redei E, Branch BJ. Pituitary-adrenal responses to morphine and footshock stress are enhanced following prenatal alcohol exposure. Alcohol Clin Exp Res. 1986;10:397–402. doi: 10.1111/j.1530-0277.1986.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Koob GF. Operant self-administration of sweetened versus unsweetened ethanol: effects on blood alcohol levels. Alcohol Clin Exp Res. 1999;23:1151–1157. [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samudio-Ruiz SL, Allan AM, Valenzuela CF, Perrone-Bizzozero NI, Caldwell KK. Prenatal ethanol exposure persistently impairs NMDA receptor-dependent activation of extracellular signal-regulated kinase in the mouse dentate gyrus. J Neurochem. 2009;109:1311–1323. doi: 10.1111/j.1471-4159.2009.06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slone JL, Redei EE. Maternal alcohol and adrenalectomy: asynchrony of stress response and forced swim behavior. Neurotoxicol Teratol. 2002;24:173–178. doi: 10.1016/s0892-0362(01)00186-6. [DOI] [PubMed] [Google Scholar]
- Smolen A, Smolen TN, van de Kamp JL. Sensitivity of inbred and selectively bred mice to ethanol. Alcohol. 1987;4:57–62. doi: 10.1016/0741-8329(87)90061-9. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Bookstein FL, Sampson PD, Olson HC. The long-term neurocognitive consequences of prenatal alcohol exposure: a 14-year study. Psychol Sci. 1999;10:186–190. [Google Scholar]
- Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–190. doi: 10.1053/scnp.2000.6729. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus. 1997;7:232–238. doi: 10.1002/(SICI)1098-1063(1997)7:2<232::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Varaschin RK, Akers KG, Rosenberg MJ, Hamilton DA, Savage DD. Effects of the cognition-enhancing agent ABT-239 on fetal ethanol-induced deficits in dentate gyrus synaptic plasticity. J Pharmacol Exp Ther. 2010;334:191–198. doi: 10.1124/jpet.109.165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AF, Hunt PS. Impaired trace fear conditioning following neonatal ethanol: reversal by choline. Behav Neurosci. 2006;120:482–487. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GR, Wainwright PE. Prenatal ethanol and stress in mice: 2. Development and behavior of fostered offspring. Physiol Behav. 1989;45:541–549. doi: 10.1016/0031-9384(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Subregion-specific differences in hippocampal activity between delay and trace fear conditioning: an immunohistochemical analysis. Brain Res. 2004;995:55–65. doi: 10.1016/j.brainres.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Willford JA, Richardson GA, Leech SL, Day NL. Verbal and visuospatial learning and memory function in children with moderate prenatal alcohol exposure. Alcohol Clin Exp Res. 2004;28:497–507. doi: 10.1097/01.alc.0000117868.97486.2d. [DOI] [PubMed] [Google Scholar]
- Young SL, Fanselow MS. Associative regulation of Pavlovian fear conditioning: unconditional stimulus intensity, incentive shifts, and latent inhibition. J Exp Psychol Anim Behav Process. 1992;18:400–413. doi: 10.1037//0097-7403.18.4.400. [DOI] [PubMed] [Google Scholar]