1. Structure
Lutein (C40H56O2) is one of the known 600 naturally occurring carotenoids in the plant kingdom but is not manufactured by animal kingdom. Based on its molecular contents, it belongs to class of the xanthophyll family (contains oxygen), one of the two major carotenoid families. The yellow color of lutein is directly linked to its structure and as a result gives yellow color to egg yolk, animal fat and human eye retinal macula. Lutein contains 40 carbon atoms hence known as tetraterpenoids. The biochemical structure of lutein is in the form of an alternate conjugated double bonds and single bonds along the polyene chain terminated by oxygen containing rings on either side (Fig. 1). The chemical reactivity of lutein is attributed to the presence of a conjugated polyene chain which is highly reactive and electron-rich system. The most striking characteristic is π-electrons which efficiently delocalized over the entire length of the polyene chain. Upon oxidation the resultant lutein degradation products have the polyene chain with a varied length and end group such as aldehyde, ketone, etc. which might render these products as highly reactive compounds (Kalariya et al. 2011). Such products have been identified in the human as well as the monkey retina.
Fig. 1.
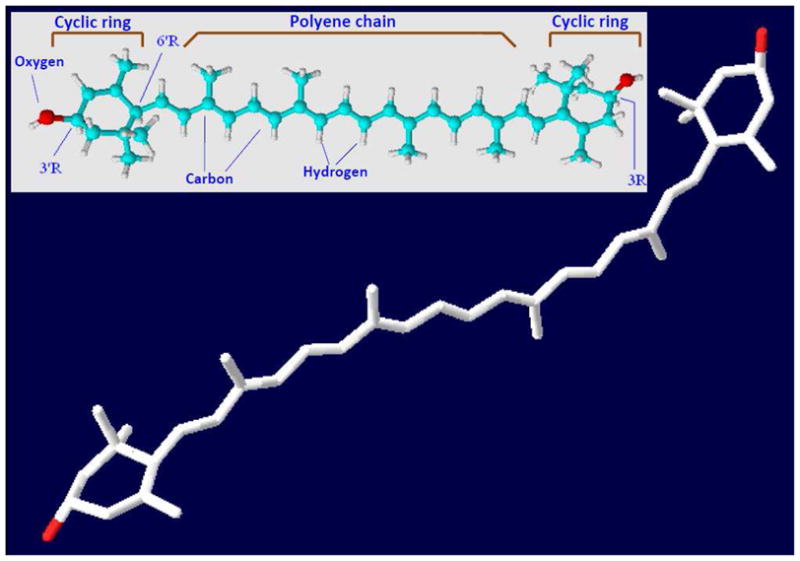
Structure of lutein. 3-D structure (PDF file of a light harvesting protein with bound lutein downloaded from Protein data bank (http://www.pdb.org/pdb/home/home.do). Using Swiss-Pro/PDB viewer the light harvesting protein was turned off to expose lutein. 3-D structure of lutein can also be viewed on http://www.worldofmolecules.com/3D/lutein_3d.htm. Fundamental chemical structure (Inset) reproduced from http://www.chm.bris.ac.uk/motm/carotenoids/carotenoids.htm with permission and modified as warranted.
2. Function
The presence of lutein in specific eye tissues makes it unique among all carotenoids exists in the human body. Lutein is highly concentrated in the human eye retinal macula. The selective uptake of lutein in macular region has been attributed to the existence of specific carotenoid binding proteins which help to concentrate carotenoids in the macula. So far, the identified carotenoid binding proteins in human and/or primate retinal macular region are pi isoform of glutathione S-transferase (GSTP1) (as a zeaxanthin-binding protein), a member of the steroidogenic acute regulatory domain (StARD) family (as a lutein-binding protein), and tubulin as a less specific but higher capacity site for carotenoid binding. The characterization and identification of macular carotenoid binding proteins has recently been reviewed (Li et al. 2010). Besides retina, the lens and ciliary bodies are other ocular tissues possess lutein. Lutein known to have two functions in the human eye, it acts as a potent antioxidant and filters high energy blue light which has potential damaging effects. Recent studies suggest that lutein may also help in suppressing various ocular diseases such as age-related macular degeneration (AMD), ocular inflammation, cataract, and photophobia. Lutein has also been demonstrated to be a potential therapeutic agent in a variety of non-ocular diseases but it is known mostly for its importance in eye health.
3. Disease Involvement
AMD is a leading cause of vision loss in North America and Europe. A number of epidemiological studies reported correlation between AMD and lower levels of lutein in the human macula. It has been reported that macular lutein declines with aging in humans. One of the possible factors for AMD development in these populations is low intake of green vegetables and fruits containing lutein. Therefore, increased dietary intake of lutein has been recommended as a preventive measure. In the last decade, lutein has been a major focus of ocular research and considered as a potential agent for prevention of AMD. Various studies have shown that its consumption and serum levels have inverse relationship to the risk for ocular diseases such as AMD (Delcourt et al. 2006). Moreover, increased macular pigment optical density (which could help to improve visual function in patients suffering with ocular pathologies such as AMD, cataract, uveitis, etc.) followed by ingestion of lutein containing foods or supplements has also been reported. However, there are also several epidemiological studies which did not find such an association between dietary or serum concentrations of lutein and AMD risk. It was also reported that increased intake of lutein, zeaxanthin and omega-3 fatty acids associated with progression of AMD (Robman et al. 2007). Thus the link between lutein intake and risk or prevention of AMD has not been entirely consistent. Besides AMD, several reports have studied the relationship between cataract causing opacities and serum levels of lutein. The Beaver Dam Eye Study prospectively examined nutrient intake in relationship to the incidence of nuclear cataracts. Lutein has been shown to prevent cataract development in diabetic rats. Moreover, lutein acts as a relatively broadband filter of blue light and hence photophobia or visual discomfort could be attenuated for much of the blue region of the visible spectrum.
4. Future Studies
Since there is no cure for dry AMD, preventive approaches are preferred. One such approach is a strong advocacy for lutein supplementation. However, due to variable epidemiological results on beneficial or harmful effects of lutein as supplementary carotenoids, it is imperative to address following aspects to understand the true potential of lutein as a therapeutic molecule for AMD. (1) There is a lack of data on determining therapeutic dosage of lutein based on age, gender, ethnicity, and degree of pathological conditions. (2) There is a wide individual variation in absorption of lutein through oral route. Hence other potential routes for fast and accurate delivery of lutein to retinal tissues needs to be explored. (3) Highly sensitive techniques to measure total intact lutein and its oxidized products in the macula are essential to determine their macular levels. (4) Most importantly, the structural and functional studies on oxidized lutein products with a possible role in ocular tissues are also required. A number of studies in the recent past have demonstrated that oxidized carotenoid products could cause cyto- and geno-toxicity. Lutein-derived products-induced toxicity in retinal pigment epithelial (RPE) cells as well as post-translational protein modification have been reported recently (Kalariya et al. 2011). These facts are consistent with the results of a previous study, which demonstrated that A2E (a component of human retinal lipofuscin with structural analogy to carotenoids) oxidized products can damage RPE cells as well as generate aldehydes, ketones, and epoxides which could be highly reactive. Recent studies have shown that carotenoid supplementation in humans and monkeys significantly increase levels of their metabolites in serum and ocular tissues. Various carotenoid metabolites such as (3R,3′S,6′R)-lutein (3′-epilutein), 3-hydroxy-β,ε-caroten-3′-one, 3-hydroxy-β-ionone, 3-hydroxy-14′-apocarotenal, ε-carotene-3,3′-diol, ε, ε-carotene-3,3′-dione, and 3′-hydroxy-ε, ε-caroten-3-one have been identified in human eye tissues. Besides, there are many other cleavage products believed to exist in ocular tissues but could not be identified either due to lack of sensitive technique or undetectable concentration with the existing methods. It is possible that antioxidant effect of lutein may be overwhelmed by oxidative stress in the retina of the AMD patients and therefore, preventive role could not be established by a number of epidemiological studies. Thus understanding the role of these diverse lutein-derived products in ocular tissues along with other aspects mentioned above could help us determine the true potential of lutein as a therapeutic agent for prevention of AMD.
Acknowledgments
Supported by Wilkins AMD Fund (FJGMvK), National Institutes of Health Grants EY015891 & GM071036 (KVR), and unrestricted grant from Research to Prevent Blindness. We would like to acknowledge Dr. James D. Johnson of Florida State University, Talahassee, FL for his help in capturing and preparing 3-D Lutein image for the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W. The POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: The POLA study. Invest Ophthalmol Vis Sci. 2006;47:2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- Kalariya NM, Ramana KV, Srivastava SK, van Kuijk FJGM. Posttranslational protein modification by carotenoid cleavage products. Biofctors. 2011;37:104–116. doi: 10.1002/biof.152. [DOI] [PubMed] [Google Scholar]
- Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010;11:1418–1425. doi: 10.1039/c0pp00126k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robman L, Vu H, Hodge A, Tikellis G, Dimitrov P, McCarty C, Guymer R. Dietary lutein, zeaxanthin, and fats and the progression of age-related macular degeneration. Can J Ophthalmol. 2007;42:720–726. doi: 10.3129/i07-116. [DOI] [PubMed] [Google Scholar]


