Abstract
Immunotherapy, in recent times, has found its application in a variety of immunologically mediated diseases. Oral immunotherapy may not only increase patient compliance but may, in particular, also induce both systemic as well as mucosal immune responses, due to mucosal application of active agents. To improve the bioavailability and to trigger strong immunological responses, recent research projects focused on the encapsulation of drugs and antigens into polymer particles. These particles protect the loaded antigen from the harsh conditions in the GI tract. Furthermore, modification of the surface of particles by the use of lectins, such as Aleuria aurantia lectin, wheatgerm agglutinin or Ulex europaeus-I, enhances the binding to epithelial cells, in particular to membranous cells, of the mucosa-associated lymphoid tissue. Membranous cell-specific targeting leads to an improved transepithelial transport of the particle carriers. Thus, enhanced uptake and presentation of the encapsulated antigen by antigen-presenting cells favor strong systemic, but also local, mucosal immune responses.
Immunotherapy is a widely accepted and applied therapeutic approach for a range of immunologically mediated diseases including autoimmunity, cancer and allergy. It aims to restore deficient functions of the immune system by the administration of biological substances, which either enhance or suppress immune responses. Although immunotherapy is often applied parenterally, the oral administration route is preferred due to higher compliance of patients and lower complication rates [1]. However, the harsh conditions of the gastrointestinal (GI) environment constitute a substantial challenge for designing immunotherapeutic drugs, which often suffer from low bioavailability when administered via the oral route. Therefore, there have been extensive research efforts to improve and optimize drugs intended for oral application. During recent years, one of these novel approaches has been the encapsulation of therapeutic agents into polymer particles within the nano- or micro-meter range in size. These micro particles (MPs) or nanoparticles (NPs) display several features, which make them suitable for oral application and increase bioavailability of encapsulated substances.
Besides describing the characteristics and advantages of polymer particles for oral immunotherapy, we will focus on recent studies investigating lectins as bioactive substances for functionalizing the surface of particles to improve intestinal uptake in this review.
Preparation techniques of particle carriers for oral immunotherapy
Polymer particles used for oral immunotherapy are often made from polylactide-co-glycolide (PLGA) or polylactide (PLA) not only due to their special carrier properties, but also because of their adjuvant effects [2]. Both PLGA and PLA are characterized by high biocompatibility and biodegradability, as their degradation products are eliminated via the Krebs cycle, which is why their application in human patients has been approved by the US FDA [3–5].
Numerous studies have focused on the develop ment and improvement of the preparation processes for vaccine-loaded particles. It has become apparent that two techniques, the double-emulsion technique and spray-drying technique, are typically being applied today.
The water-in-oil-in-water (w/o/w) or double-emulsion technique allows for entrapment of hydrophilic substances such as vaccines. A small volume of an aqueous vaccine solution is emulsified in a PLGA- or PLA-rich organic solution, for example, dichlormethane or ethyl acetate, to form the primary water-in-oil emulsion. Dispersion of this primary emulsion in an excess of a second, aqueous surfactant solution yields the w/o/w emulsion followed by formation and hardening of the particles due to evaporation or extraction of the organic solvent. The surfactants in use are mostly poly(vinyl alcohol), but also poloxamer 188 (Pluronic® F-68) and poly(ethylene-alt-maleic acid) [6–8]. Although this w/o/w method is a relatively simple and economic technique [9], the sometimes low encapsulation efficiency or payload as well as the huge initial burst release, because of the limited speed of removal of organic solvent, are disadvantageous [10]. Therefore, different modifications were investigated in order to overcome these obstacles. The solid-in-oil-in-water method differs from the double-emulsion technique in as much as the first aqueous phase is replaced by an aqueous, very fine suspension of the antigen. Since the solid state is most favorable for protein stability and, thus, bioactivity, this technique might be useful for entrapment of unstable antigens [11]. Another variation of w/o/w method is the water-in-oil-in-oil technique, also termed as co acervation method. In this, a nonsolvent is added to the primary emulsion yielding coacervate droplets, which are finally hardened by another organic solvent [12].
The spray-drying technique is a second, widely used method to prepare PLGA or PLA particles. A fine suspension or emulsion containing the vaccine and the polymer is sprayed into the air for atomization and the organic solvent is evaporated at an elevated temperature to yield solid particles. This technique allows easy tuning of particle characteristics and scale-up, but high product loss, especially in small batches, might be crucial [13].
Recently, several new methods to manufacture PLGA or PLA particles have been reported, such as the ultrasonic atomization method [14,15], the electrospray method [16], the microfluidic method [17,18], the pore-closing and thermoreversible-gel method [19,20], and the microfabrication method [21]. Although these innovative techniques are being applied rarely for the preparation of vaccine-loaded particles as of now, they still offer new possibilities for manufacturing particles with improved characteristics.
Characteristics of particles for oral immunotherapy & their fate upon ingestion
The use of particle carriers as an oral-delivery system aims to protect the encapsulated substances and deliver them in an intact form to the immunocompetent cells of the GI tract. Thus, systemic or even mucosal immune responses, when being administered via the mucosal surface, can take place. Under normal conditions, upon ingestion, proteins or carbohydrates are exposed to changing pH levels and digestive enzymes of the oral cavity, stomach, pancreas and bile as well as brush-border hydrolases during GI transit resulting in degradation [22]. Additionally, after mucosal uptake hapten drugs encounter the hepatic first-pass metabolism leading to low bioavailability of active substances [5]. Encapsulation of antigens into particle carriers protects against these harsh GI degrading conditions [23], enabling an intestinal uptake of intact antigens and drugs, and the circumvention of the hepatic first-pass metabolism due to protection of the payload by the particle matrix. Furthermore, functionalization of particles by special targeting substances can direct particle binding to specific cells and enhance the particle uptake capacity via intestinal cells.
In the intestinal mucosa, two different cell types are responsible for the uptake of particulate structures: enterocytes and ‘membranous’ or ‘microfold’ cells (M cells). M cells are part of the follicle-associated epithelium overlying the organized gut-associated lymphoid tissue, which can either be located in isolated form or within organized follicular clusters, the so-called Peyer’s patches [24,25]. With the loss of brush-border enzymes, such as alkaline phosphatase, M cells show a reduced digestive function, which makes them even more interesting for drug targeting [26,27]. They are mainly responsible for the uptake of intact, particulate structures [28]. Although the proportion of M cells in follicle-associated epithelium of Peyer’s patches can be high in rabbits and mini-pigs, the occurrence in rodents (10%) and humans (<5%) is much lower [29]. These cells are responsible for antigen-specific intestinal immune responses by transporting foreign material from the gut lumen to the mucosa-associated lymphatic tissue in an intact form, making them an optimal entry for antigens in oral immunotherapy. The capacity of transcytosis through the intestinal epithelial layer depends on the size, with a particle size of below 1 μm for optimal M-cell absorption [30], but also on the charge and hydrophilicity of the particle carrier [31–33]. Once bound to the apical side of M cells, macromolecules are endocytosed via clathrin-coated vesicles, thereby reaching the lysosomal compartment, from which they are exocytosed to the basolateral site to be taken up by antigen presenting cells (APCs) [34,35].
The particles’ size is pivotal for stability, distribution, release of the encapsulated antigens and induction of immune responses [36,37]. Furthermore, the particles’ size determines not only the uptake, but also the retention time at M-cell sites. NPs are rapidly internalized mainly by a process that involves receptor-mediated endocytosis, and are then disseminated systemically [5,38]. In contrast, MPs greater than 5 μm remain trapped in the Peyers patches for up to 35 days [39]. The size of particles able to cross the intestinal barrier has been narrowed down to ranges between 700 nm and 10 μm [40]. In recent in vitro studies using the colon carcinoma cell line Caco-2 as an intestinal epithelial model, particles of different sizes from nano- to micro-meter range have been investigated for their interaction with intestinal cells and their properties for cellular uptake. NPs of 100-nm size showed a high interaction rate of more than 6000 PLGA and 200,000 polystyrene (PS) particles per Caco-2 cell. Particles with a size of 1 or 2 μm interacted in very low numbers (12 and below) with one Caco-2 cell [41]. Furthermore, while small NPs were detected intracellularly, also within the nuclei, particles bigger than 300 nm were attached to the apical membrane of Caco-2 cells. Thus, a very small difference in particle size is decisive for either sole cell-binding or even internalization [41]. Upon shuttling of NPs through intestinal epithelial cells encapsulated drugs circumvent the P-glycoprotein efflux from enterocytes and bypass liver metabolism, which enhance their oral bioavailability [42]. Additionally, slight differences of NP size have an impact on the availability of encapsulated drugs in the circulation. Particles with a size of 150 nm show a prolonged circulation time and sustained blood levels compared with particles with a size lower than 100 nm or above 200 nm, which may be taken up by the reticulo-endothelial system cells. In comparison, NPs between 100 and 200 nm can escape reticulo-endothelial system uptake [5,43]. A further property of PLGA and PLA particles is their negative surface charge [44–45]. As epithelial cells and mucus of the GI tract are negatively charged, the uptake of PLGA and PLA particles without surface modification may be poor. However, positively charging PLGA particles, for example using positively charged stabilizers, increases the epithelial penetration and systemic uptake of encapsulated, slightly soluble drugs. These favorable characteristics are not found in particulate carriers prepared with anionic stabilizers [5,46]. Therefore, the characteristics of particles, such as size and surface charge, influence the interaction, uptake and distribution by intestinal epithelial cells. The impact of particle hydrophilicity on intestinal uptake has been described very controversially in literature. Coating of PS particles with poloxamer, which increases hydrophilicity, was demonstrated to decrease the GI uptake [47]. A similar effect was shown for NPs made of polymers with different hydrophilic properties [48]. However, surface modification with poloxamer or particle preparation with different polymers influences the surface charge of the particles, which could contribute to reduced intestinal uptake [49]. A recent study by Gaumet et al. reported that modification of surface hydrophilicity by coating PLGA particles with chitosan, which did not affect surface charge or particle size, enhanced the interaction of particles with intestinal Caco-2 cells [49].
Therefore it is obvious that a ‘fine-tuning’ of particle properties and design by the choice of size and hydrophilicity is substantial for optimizing the uptake and the resulting immunological response. While the intestinal epithelial transport of particles in the nanometer range might be optimal, one has to keep in mind that nanospheres are taken up into the cells with distinct intracellular distribution, which might lead to side effects not yet known.
Immune response triggered by particles
In addition, the type and intensity of the elicited immune response are also influenced by th character istics of the particle carrier. PLGA is not only a highly biocompatible and bio degradable material, it further affects the immune response via its adjuvant properties [50], which were hypothesized to be due to the particulate structure of the allergen carrier and due to the sustained allergen release from the particles [51]. Various studies demonstrated that the capacity of PLGA particles to act as vaccine adjuvant was high and comparable to the conventional aluminum-adsorbed vaccine formulations [52–54]. Subcutaneously applied NPs encapsulating the birch pollen allergen Bet v1 induced comparable IgG1, IgG2a and IgG2b titers in BALB/c mice as subcutaneous treatment with allergen combined with aluminum [55]. In animal studies, intraperitoneal immunization with PLGA MPs loaded with pollen extract even induced a Th1-dominant immune response including IgG2a antibodies and IFN-γ production by spleen cells [56,57]. In contrast, the widely used vaccine component aluminum hydroxide triggers a Th2-driven immune response. Therefore, the use of PLGA particles for immunomodulating purposes may be beneficial, especially in situations where the immune system is biased towards a Th2 environment, such as in allergic diseases.
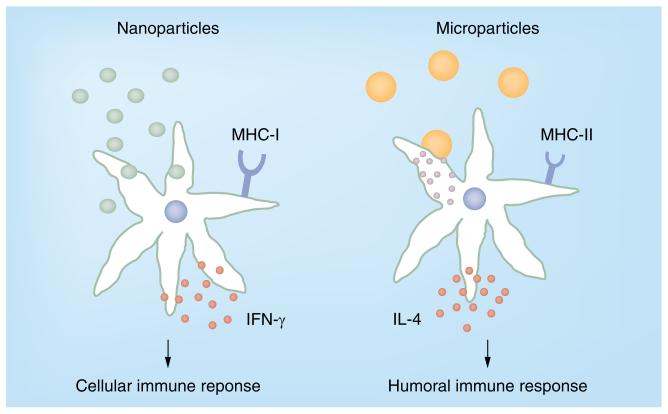
Also with regards to immune response, the particle size determines the outcome. Only MPs between 1 and 10 μm in size promote humoral responses, whereas NPs below 1 μm mainly induce cellular responses (Figure 1) [58]. Particle structures smaller than 5 μm are taken up by APCs, such as dendritic cells (DCs) and macrophages [59–61], and are transported directly to the lymph node [58], where the encapsulated antigen can be presented to T cells.
Figure 1.
Particle-induced immune response.
NPs loaded with FITC–bovine serum albumin were shown to be phagocytosed by APCs, leading to a two–threefold increase of cell size, and were then continuously endocytosed and exocytosed from the cells. Thus, only low concentrations of intra cellularly released antigen could be observed over time [2]. Interestingly, immunizations with NPs containing hepatitis B surface antigen (HBsAg) led to low production of IgG, secretion of IFN-γ and upregulation of MHC-I on APCs, which indicated the induction of a cellular immune response. The uptake of NPs may result in the production and release of pro-inflammatory cytokines, such as IL-1, IL-5, IL-8, IL-10, IL-18 and TNF-α [62,63]. Semete et al., however, observed no increase in cytokine production [64]. Fluorescent MPs (2–8 μm) were found to attach to the cell surface of APCs in a nonspecific manner [2]. Additionally, the encapsulated antigen is slowly and continuously released into the cell leading to enhanced antibody responses, increased production of IL-4 and upregulation of MHC-II molecules, which in concert triggers a predominant humoral response. The high concentration of released soluble antigen leads to direct binding of the antigen to MHC-II molecules and subsequent presentation [2]. Besides the different impact on the release pattern of the entrapped antigen by hydrophobic particles in comparison to more hydrophilic carriers, the hydrophobicity of polymer microspheres and a rough surface further increase the attachment to the surface of APCs [65], and enhance the antibody response [66]. Interestingly, the capacity of phagocytosis by macrophages can be increased by coating microspheres with targeting substances, such as wheatgerm agglutinin (WGA), arginine–glycine-aspartic acid-containing peptide or mannose-polyethylene glycol(PEG)3-NH2 [67].
The uptake of NPs and the intensity of antigen presentation by APCs can be modulated by the use of substances bound to the particle surface, specifically targeting APCs, such as DC-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) antibodies. With this DC-specific targeting approach, antigen-specific cellular responses can be stimulated at 10–100-fold lower particle concentrations than with nontargeted NPs [68]. By the incorporation of fluorescent labeled peptides and the inclusion of iron oxide, encapsulated antigens can be microscopically tracked without the loss of vaccine function. In this study, peptides loaded into NPs, which specifically targeted DC-SIGN on DCs, were found in the lysosomal compartment of DCs within 24 h and were presented to T cells despite the presence of fluorescent labels [68]. Interestingly, the surface modification with a DC-SIGN-specific antibody did not enhance uptake and presentation of MP encapsulated antigens. Recently, NPs’ surface modification with an antibody against the DC lectin DEC-205 receptor induced the production of IL-10 by DCs and T cells in vitro. When these modified particles were intraperitoneally injected into ovalbumin (OVA) primed mice, a similar IL-10 pattern was observed, which was dependent on the density of surface modification [69]. Previous studies have shown that mannosylated particles can specifically deliver antigens and drugs to DCs and result in enhanced presentation to T cells [70]. When a dimannosyl ligand was used for liposomal vesicles targeting, which contained the Toll-like receptor (TLR)-2 ligand Pam2CAG for strong immune stimulation and further peptide sequences of ERbB2 and Th-helper epitopes, tumors expressing ErbB2 were eradicated more efficiently in an artificial mouse tumor model [71]. This combination of mannose receptor and TLR targeting beneficially affects the binding to APCs and the stimulation of cellular immune responses. Mannan or PEG coating was shown to protect against protein opsonization, enabling specific receptor-ligand binding. However, mannose coupling to microspheres initiated the phagocytosis by C-type lectin receptor, it could not stimulate DC maturation and activation toward T-cell responses [72].
After the induction of the first immune responses, the longevity of the protective effects after vaccination is determined by the induction of a memory response. The continuous release of inoculated antigens from PLGA particles after the first burst is advantageous to restimulate memory cells and responsible for a long protection time even after one vaccination [52], underlining the beneficial effects of PLGA as a carrier substance for novel vaccination approaches.
Particles for oral immunotherapy
Oral immunomodulation with polymeric particulate carriers has been investigated for a wide range of therapeutic applications, such as immuno suppressive drugs [42], viral and bacterial antigens [58,65], hormones [5,73], anticancer drugs [74], and allergens [75]. The majority of these studies focused on the investigation of PLGA particle carriers loaded with drugs or antigen but without further functionalization of the particle surface for cell targeting in the GI tract.
Recently, it was observed that the encapsulation of antigens into PLGA particles did not only protect from GI digestion, but also initiated specific immune responses by the means of IgG and IFN-γ production at very low antigen amounts [76]. In this study, encapsulation of the food allergen OVA, which was found to be degraded under physiological conditions during the GI transit [77], remained stable when entrapped within PLGA NPs. Oral application of OVA-loaded PLGA nanospheres, which were further coated with PEG to reduce particle aggregation and to enhance particulate blood circulation [78], initiated a high OVA-specific IgG response at very low allergen amounts (5 μg). However, PEGylation of glycan-modified liposomes interfered with the targeting potential to DCs, which has to be taken into consideration for designing novel formulations [79]. Moreover, very low IL-4 and significantly higher IFN-γ production of spleen cells was observed in animals immunized with particles compared with mice being fed unprotected OVA, which was even more pronounced when the β1 integrin on M cells was specifically targeted by an RGD-containing peptide [76].
In order to design PLGA particles as oral therapeutics, the size, lipophilic properties and surface charge have to be taken into consideration. However, the development of these formulations becomes even more complicated when hydrophilic drugs, such as insulin, are subject to encapsulation. This problem can be solved by the use of specific ampholytic surfactants, such as phospholipids. In a recent study, PLGA NPs loaded with insulin for oral application in diabetic rats revealed slower absorption and sustained elimination, while serum insulin concentration steadily increased over 6 h. Thus, the insulin absorption was markedly enhanced by the use of an insulin–soybean phosphatidyl choline complex loaded into PLGA NPs compared with subcutaneous insulin injection [73].
Comparing the oral route with others, a recent study focused on the encapsulation of viruses, such as rotavirus in MPs. PLA and PLGA microspheres containing rotavirus were evaluated for in vivo immunogenicity in mice using intra nasal and oral administration as different routes of immunization. A single particle administration elicited an increase of systemic rotavirus-specific IgA and IgG antibody titers. Orally applied PLA microspheres induced a more persistent and longer-lasting serum IgA antibody response, which could be explained by the higher hydro phobicity of PLA and the slower polymer degradation. PLGA microspheres showed an improved antibody response when being applied via the intranasal route. However, it is questionable whether antibody titers in serum are significant for protection against rotaviruses as it is well known that mucosal IgA protection is most efficient [80]. Therefore, the authors hypothesized that oral immunization with these microspheres might induce a strong intestinal antibody response, although mucosal IgA levels have not been analyzed in this study [58]. This was also the subject of investigation in a murine study analyzing the systemic and mucosal immune response after oral immunization with Helicobacter pylori lysate-loaded PLGA NPs, in order to evaluate their potential as a novel antimicrobial treatment strategy against H. pylori infections. Kim et al. demonstrated that after two oral administrations of H. pylori-PLGA nanospheres, H. plyori-specific serum IgG1 and IgG2b levels and gut lavaged IgA were significantly elevated [81].
Another field of application is anticancer therapy with particulate carriers incorporating anticancer drugs [82–83]. It was reported that the bioavailability of chemotherapeutic drugs, such as tamoxifen or doxorubicin, encapsulated in particles significantly increased [83–84]. Furthermore, in a 7,12-dimethylbenz[a]anthracene-induced breast cancer rat model, oral administration of doxorubicin loaded PLGA NPs revealed a higher antitumor efficacy as a result of enhanced bioavailability, tumor permeation and retention effect [74]. In addition, incorporation of doxorubicin into PLGA nanospheres significantly reduced the doxorubicin-induced cardiotoxicity [74].
Targeting via surface bound lectin improves intestinal uptake of particles
While the above mentioned oral application approaches of PLGA particles seem to work appropriately in these experimental models, a further strategy to improve the quality of PLGA carriers is the functionalization of particle surface with bioactive substances. Following this approach, mucosal cells are specifically targeted, thereby improving the cellular uptake as well as transepithelial transport and inducing not only a systemic, but also a strong mucosal immune response.
Enterocytes represent the majority of epithelial cells in the intestinal tract. Thus, enterocyte-specific targeting, for example by WGA, substantially increases the transepithelial transport, which may especially be of interest for drug development where no mucosal immune response induction is needed. Enterocyte-specific targeting induces a rather systemic IgG-mediated immune response [22]. As a second group of intestinal epithelial cells that are of interest, M cells can be specifically addressed by particle functionalization. The idea of M-cell-specific uptake and the induction of subsequent immune responses derives from nature. A number of enteropathogenic microorganisms, such as Vibrio cholerae [85,86], Salmonella typhimurium [87,88], Shigella flexneri [89–91], Yersinia pseudotuberculosis and Yersinia enterocolotica [92–94], and reoviruses [95], but also nonenteropathogenic pathogens, such as influenza virus A [96], or human immunodeficiency virus-1 (HIV-1) [97], are primarily taken up by M cells, as reviewed by Corr et al. [98]. Although underlying mechanisms of the M-cell uptake are in many cases not fully understood to date, it is suspected that pathogens use specific binding approaches to surface structures on M cells to invade the human body via transmucosal transport [99]. Possible interaction partners might be M-cell expressed TLR4, platelet activating factor receptor and α5β1 integrin [100], carbohydrates and Claudin 4. The latter functions as a receptor for Clostridium perfringens enterotoxin (CPE) and has been recent focus for targeting approaches [101]. CPE30, the C-terminal 30 amino acid part of CPE, was coated to PLGA NPs, which increased the uptake by upper airways when administered intranasally or by intestinal M cells after ingestion [101]. However, in depth investigation of pathogen M-cell-binding partners has been quite difficult due to the lack of suitable in vitro and in vivo models. Due to the low number of M cells in the intestinal tract and due to the inter-species variability in surface receptors [99], results obtained in experimental animal models can only be extrapolated into the human situation when taking into account certain limitation. Furthermore, it has to be kept in mind that the distribution of surface structures on human M cells of different intestinal regions may also differ [24], which may indicate a certain local functionality. Therefore, the most convenient approach for M-cell studies might represent the human M-cell-like co-culture model, where M cells are generated by co-culturing Caco-2 cells, a colon carcinoma cell line with intestinal phenotype, together with Raji-B cells, a cell line derived from Burkitt lymphoma [102]. As this model is based on the use of carcinoma cell lines, it should be noted that epithelial glycosylation patterns in the gut are altered in colon cancer, questioning the value of this experimental model [24]. However, due to the lack of information on M-cell-specific carbohydrate residues in humans, the M-cell-like co-culture model might represent the only valuable experimental model currently available. Using this model, it has become obvious that bacterial and viral transcytosis via M cells might, in some cases, be initiated by a receptor-mediated process [99].
Numerous studies rely on the idea of specifically targeting M cells to increase the mucosal transport. Several bioactive substances, especially lectins, have been the focus of extensive research to date. Lectins are proteins that selectively bind to carbohydrate residues of epithelial cells’ glycocalyx and may serve as molecules on particle surface for specifically targeting M cells [103,104].
Lectins, derived from Ulex europaeus (UEA-I), Lycopersicon esculentum (tomato lectin), Bandeiraea simplicifolia I, Canavalia ensiformis, Triticum vulgare (WGA) and Aleuria aurantia lectin (AAL) have been propagated for intestinal cell targeting, however, they differ in their specificity for the intestinal cell type, as reviewed by Clark [105].
WGA for targeting enterocytes
WGA represents a dietary nontoxic lectin, which is contained in wheat flour [22]. WGA specifically binds to N-acetyl-d-glucosamine and sialic acid moieties of both, epithelial cells and M cells [106,107]. The usability of WGA-coated, allergen-loaded PLGA microspheres for oral immunotherapy was assessed in animal studies. After two gavages of WGA-coated microspheres, mice revealed a higher allergen-specfic IgG production compared with feedings of uncoated particles [108]. However, when the efficacy of WGA-coated allergen-loaded microspheres was evaluated in birch pollen-sensitized mice, oral treatment did not induce an allergen-specific isotype switch towards IgG2a or the production of the Th1 cytokine IFN-γ in comparison to gavages of plain microspheres [74]. Additionally, WGA attached to carbopol has recently been used for surface modification of liposomes, which significantly increased the receptor mediated binding to Caco-2 cells and further enhanced the pharmaco logical efficacy of calcitonin, when loaded into the nanocarriers [109].
As targeting of orally applied particle carriers to epithelial cells in situations of Th2 modulation does not seem sufficient to trigger immune responses, a M-cell-specific targeting approach may increase the efficiency, because transcytosed particles reach the lymphatic tissue underlying the M cells and induce a strong mucosal, as well as systemic immune response.
Concanavalin A for interaction with intestinal epithelial cells & immune cells
Concanavalin A is a lectin from Canavalia ensiformis (Jack bean), which specifically recognizes glycoproteins and glycolipids containing α-d-mannosyl and α-d-glucosyl residues. Concanavalin A can not only interact with the intestinal epithelium but also with immune cells, such as macrophages, resulting in their activation and subsequent upregulation of TLRs as well as the production of NO and proinflammatory cytokines [110–112].
As the lectins listed below bind specifically to α-l-fucose, their use as targeting moieties should result in M-cell-specific adherence.
Ulex europaeus I
The lectin UEA-I has recently been used for the functionalization of HBsAg incorporating NPs [113]. These NPs were shown to specifically bind via α-l-fucose to mouse M cells ex vivo. After three oral immunizations with UEA-I coated, HBsAg loaded NPs and a booster immunization after 3 weeks, mice developed HBsAg-specific serum antibody titers comparable to levels measured after intramuscular injection of HBsAg adsorbed to aluminum hydroxide. Furthermore, gavages of these UEA-I coated NPs-induced mucosal secretion of sIgA and the production of IL-2 and IFN-γ in spleens, indicating a Th1-dominant immune response [113].
Although UEA-I may be a promising targeting substance, its origin is a highly toxic plant, which makes its beneficial application in humans questionable.
Lotus tetragonolobus
The Lotus tetragonolobus agglutinin (LTA) from Asparagus pea represents another lectin candidate for functionalization of particles. Similar to UEA-I, LTA targets M cells specifically via α-l-fucose. Encapsulation of HBsAg in LTA-coated PLGA NPs can effectively be delivered to murine M cells. Upon oral immunization these particle carriers elicited a significantly higher sIgA response compared with uncoated nanospheres. Furthermore, mice immunized orally with these LTA-coated NPs developed a significantly higher Th1 response by means of IgG2a/IgG1 production ratio compared with intra muscular aluminum hydroxide-based HBsAg injections. This was underlined by higher levels of the Th1 cytokines IFN-γ and IL-2, suggesting the induction of cell-mediated immunity [114].
Aleuria aurantia lectin
AAL from the edible, nontoxic orange peel mushroom Aleuria aurantia also possesses α-l-fucose specificity and, thus, was shown to bind to murine Peyer’s patches [115]. Additionally, a common in vitro M cell-like co-culture model of human origin was used to confirm AAL binding to human M cells. AAL coating of fluospheres resulted in an increased transcytosis of MPs by M cells compared with plain particles [115]. In the same study, AAL-coated PLGA microspheres induced a high production of the Th1 cytokines IL-2 and IFN-γ compared with only small quantities of IL-5 and IL-10 in peripheral blood mononuclear cells of allergic individuals. These data supported the suitability of AAL-coated microspheres for allergy therapy.
In subsequent studies, AAL-coated PLGA MPs were loaded with an allergen in order to evaluate their efficacy as oral allergy immunotherapy in vivo. Naive BALB/c mice were orally immunized with AAL-coated, birch-pollen-loaded MPs six-times. This oral immunization regimen induced a three-times higher IgG2a antibody response compared with noncoated MPs [116]. The immunotherapeutic efficacy of these AAL functionalized MPs was further evaluated by oral treatment of birch pollen sensitized mice. Animals fed with AAL-functionalized, allergen-loaded microspheres for 15-times revealed an increase of birch pollen-specific IgG2a, whereas IgE and IgG1 were not affected, indicating the induction of a prominent Th1-biased immune response. In addition, spleen cells of animals being treated with AAL MPs via the oral route secreted significantly more T-regulatory cytokine IL-10 and Th1 cytokine IFN-γ [75]. These studies corroborate the potential of AAL functionalized MPs as a novel strategy for oral immunotherapy against Th2-biased diseases, such as allergy.
Although lectin targeting prolongs the retention time in the intestine and may, therefore improve the mucosal uptake and the subsequent immune response, one has to keep in mind that lectins are often toxic and susceptible to proteolytic degradation in the GI tract, which renders some of them inappropriate for in vivo application [105,117]. Several studies have focused on novel approaches to overcome the limitations of the toxicity of lectins, such as UEA-I. The production of recombinant lectins with modified properties but with the same binding characteristics has been described for the recombinant mistletoe lectin [118]. Additionally, synthesized peptides, which mimic function and characteristics of lectins, such as size and stability, have been designed. These are promising novel interaction partners and can reduce costs. A tetragalloyl d-lysine amide construct, as the main compound of two UEA1 mimetics, leads to successful delivery of coated PS particles to mouse M cells and might specifically bind via α-l-fucose [117]. Based on this study, Misumi et al. have recently synthesized a tetragalloyl-d-lysine dendrimer (TGDK) and evaluated its use for M-cell binding in nonhuman primates and in vitro M cell-like culture model [119]. After gavage of TGDK to rhesus macaques, the dendrimer was efficiently delivered to rhesus Peyer’s patches’ M cells in vivo. Additionally, oral immunization with TGDK-conjugated multi antigens containing bovine serum albumin and a rhesus CCR5-derived cyclopeptide, which has recently been discussed as an attractive target for mucosal HIV-1 vaccines, elicited mucosal rcDDR5-specific IgA with neutralizing activity. However, dose-finding studies need to be performed in addition to address the functional aspects of these induced autoantibodies [119].
Although these novel approaches of synthesizing lectin mimetics may be promising for future application, the question remains whether synthetic targets are superior to lectins derived from nature, which also feature characteristics suitable for oral application in humans. The targeting substances WGA [120] and AAL are nontoxic and highly resistant against gastric digestion [Diesner SC, Schultz C, Wang X-Y et al., Unpublished Data] and enhance the retention time and transepithelial transport of MPs at the GI mucosa, finally resulting in improved efficacy, as reviewed by Des Rieux et al. [23].
Preparation of WGA & AAL microspheres
For preparation of WGA- and AAL-grafted MPs, the uncapped type of PLGA-containing terminal carboxylate groups or PLA is used as a matrix for anchorage of the lectins at the particle surface. For coupling, the carbodiimide method is applied, which represents the most popular zero-length cross-linked coupling technique [121]. Here, the carboxylic groups of PLGA or PLA react with N-substituted carbodiimides to yield highly reactive, but highly instable O-acylisourea intermediates. The latter can react with nucleophiles such as primary amino groups of the lectins to form stable amide bonds yielding isourea as a by-product. To achieve optimum coupling rates, the pH is maintained between 4.5 and 7.5. Usually, the water-soluble 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide is applied, which is dissolved rapidly and used immediately to prevent extensive loss of activity. Furthermore, in presence of N-hydroxysuccinimide (NHS) or N-hydroxysulfosuccinimide the stability of the active intermediate is extended to a few hours by preventing rapid hydrolysis. Moreover, upon use of NHS, the reaction can be performed in two steps. In the first step, the carboxylic groups are activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/NHS. After removing the excess coupling reagents by washing, the amine component is added and allowed to react with the active ester. The advantage of this being that the coupling of proteins, which contain both carboxylate and amine groups, may be improved by avoiding the cross-linking of protein molecules with each other. Altogether, the addition of NHS may improve the efficiency of the reaction [122].
Current highlights & pitfalls of PLGA-based immunotherapy
Irrespective of the route of application, PLGA particles have been further developed for allergy vaccination encapsulating immunomodulatory motives. Beside the above-mentioned studies focusing on lectin-coated microspheres for allergy treatment, recent data indicated the counterbalance of Th2 responses by the use of PLGA particles co-encapsulated with the immuno stimulatory TLR9 ligand oligodeoxy-nucleotide (CpG), protamine and phospholipase A2 from bee venom [123]. Whether or not this approach may be effective in human allergic patients remains to be elucidated as especially the receptor of CpG, TLR9, is diversely expressed in mice, while in humans its expression is restricted to B-cells and plasmayctoid DCs [124], probably leading to different immunological responses.
Further novel approaches of immunomodulation in various diseases, such as infections, autoimmunity and cancer, represent the adoptive immunotherapy [125]. Antigen-specific cytotoxic T cells expand by ex vivo stimulation with activated APCs and are subsequently reinfused into patients. The generation and the use of APCs, however, carry the risk for infections and therefore attempts are made to replace them by novel approaches, such as artificial APC (aAPC) systems. In 2008, the concept of aAPCs using PLGA particles encapsulating IL-2 for T-cell stimulation presenting adaptor elements for recognition ligands as well as co-stimulatory ligands in high density was first introduced [126]. With this aAPC approach, peptide-specific cytotoxic T cells were even more efficiently activated than when using autologous cellular APCs [125]. By the coupling of different peptides and cytokines, aAPC systems based on PLGA particles might represent a promising novel approach for a number of different diseases in the future, inducing a very specific, beneficial T-cell response. Additionally, PLGA particles encapsulating tumor antigen were recently introduced for intracellular loading of DCs, which should then become efficient activators of a cytotoxic anti-tumor T-cell response [127,128].
In contrast with these recently published highlights in the development of novel immunotherapeutical approaches, one should also critically reflect on current research regarding PLGA-based immunotherapies. Due to numerous studies within recent decades, our knowledge on optimizing properties of PLGA particle carriers, such as size, functionalization or preparation methods, has extensively broadened. However, clinical trials in human patients proving the usability of PLGA particles for oral immunotherapy application are still missing, making conclusions on their efficacy and applicability impossible. Due to substantial differences of M-cell distribution and surface receptor expression between mice and men [99], data generated in animal studies might not reflect the situation in humans. The only valuable model currently available, the M cell-like co-culture model, is based on the formation of M cells from the colon cancer cell line, which is known to differently express carbohydrate residues [24]. As data on the expression of specific receptors on human M cells is lacking, the development of M-cell-specific targeting strategies, such as the coating of particles with lectins or lectin mimetics [117,119], might be more difficult than expected. It was even hypothesized that the role of M cells for luminal antigen uptake is overrated as these cells account for only a minority in the intestinal epithelium and might exert diverse functions in different intestinal regions [24], making the field of M-cell targeting even more complex.
Therefore, as long as suitable and physiological M-cell models, as well as human clinical studies are missing, conclusions on the applicability of lectin-functionalized PLGA particles for oral immunotherapy in humans have to be drawn with great caution.
Future perspective
As reviewed in this article, functionalized particles loaded with active agents to be delivered to the gut-associated lymphoid tissue, might represent a successful novel treatment strategy, especially in situations when the immune system is biased towards Th2, such as allergic diseases. Without any doubt, the murine system mirrors only partially the situation in human patients [129]. Therefore, clinical studies to prove the therapeutic efficacy of the proposed novel oral immunotherapy formulations are of paramount importance. However, additional strategies may still be improved in the future. In ongoing current research efforts to approach the aim of successful oral immunotherapy, two functionalization strategies of particle carriers emerged, being of special interest when screening the scientific literature:
Active targeting of apical receptors on M cells as highlighted by Brayden et al. [130]. Since α-l-fucose is part of the sparsely mucus-coated glycocalyx of M cells, binding of active substances can be mediated by lectin-coated formulations. For oral administration, however, these certain lectins have to resist the harsh conditions during GI transit. Additional properties required are nontoxicity as well as non-immunogenicity of the carrier material, which can be achieved by synthetic modification of the carriers or by the careful choice of naturally derived targeting substances, such as WGA and AAL. Furthermore, cellular interaction is mediated by only small units of most complex structured lectin molecules. Thus, undesired effects might be avoided by designing ‘neolectins’, which represent exclusively the binding domain, by using phage display techniques for example. Nevertheless, one should keep in mind that cell-binding is only the first stage of a complex journey through the epithelial layer to the subjacent immune cells until an efficient immune response is elicited.
The transepithelial uptake as the second step can be enhanced by physical properties of the particulate structure. It is generally accepted that particles below 1 μm are optimally taken up by Peyer’s patches and can thus, lead to sufficient antigen processing. Since the volume and, therefore, the loading capacity of MPs by far exceeds that of NPs, the usage of particles in the micrometer range seems to be most suitable in the case of encapsulating large proteins for allergen-specific immunotherapy. Thus, the combination of both targeting strategies using a biodegradable polymeric matrix with adjuvant properties might represent the deciding prerequisite for successful oral immunotherapy in the future but have to be investigated in-depth for their suitability in humans.
Executive summary.
Preparation techniques of particle carriers for oral immunotherapy
▪ The double-emulsion technique sometimes yields low payload and huge initial burst release of particles, which might be overcome by the use of solid antigens or coacervation.
▪ The spray-drying technique allows fine tuning of particle characteristics and easy scaling-up. However, high product loss occurs upon processing of small batches.
Characteristics & gastrointestinal fate of particles for oral immunotherapy
▪ The particle matrix protects encapsulated antigens from harsh conditions in the GI tract and helps them escape the hepatic first-pass metabolism.
▪ Positive surface charge facilitates adhesion of particles to the negatively charged cell membrane and hydrophilicity seems to enhance the interaction with cells. Particles <1μm are taken up by M-cells as opposed to epithelial cells, whereas particles >300nm remain bound to the cell membrane and smaller ones accumulate intracellularly.
Immune response triggered by particles
▪ Polylactide-co-glycolide possesses adjuvant properties comparable to aluminum-adsorbed formulations. In this regard, microparticles trigger a humoral response, whereas nanoparticles elicit a cellular response.
▪ The sustained release of antigens restimulates memory cells.
Lectin-modified particles for oral immunotherapy
▪ Lectins can improve uptake and transcellular transport of particles inducing not only a systemic but also a strong mucosal response.
▪ Wheat-germ agglutinin-grafted microparticles interacting with both M-cells and absorptive enterocytes, induce higher IgG-levels than plain ones.
▪ Particles modified with α-l-fucose binding lectins such as Ulex europaeus-I, Lotus tetragonolobus agglutinin and Aleuria aurantia lectin induce a Th1-dominant response.
Future challenges
▪ (Bio)synthesis of nontoxic lectin-mimetics exhibiting gastrointestinal stability, low-molecular weight and high specificity of carbohydrate binding as targeting ligands for site-specific delivery of particles will promote progress in the field.
▪ Development of preclinical models with glycosylation patterns similar to that in humans and clinical trials in humans will be milestones for further advances in oral immunotherapy.
Acknowledgements
The authors gratefully acknowledge VE Assmann for proofreading the manuscript.
This work was supported by the Austrian science fund project P21884.
Key Terms
- Oral immunotherapy
Oral application of drugs and antigens for immunomodulation
- Lectins
(Glyco)proteins selectively binding to carbohydrate residues of epithelial cells’ glycocalyx
- PLGA particles
Delivery vehicles composed of polylactic-co-glycolic acid within the micro- or nano-meter range
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Varma MV, Khandavilli S, Ashokra JY, et al. Biopharmaceutic classification system: a scientific framework for pharmacokinetic optimization in drug research. Curr. Drug Metab. 2004;5(5):375–388. doi: 10.2174/1389200043335423. [DOI] [PubMed] [Google Scholar]
- 2.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28(35):5344–5357. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Scholl I, Kopp T, Bohle B, Jensen-Jarolim E. Biodegradable PLGA particles for improved systemic and mucosal treatment of Type I allergy. Immunol. Allergy Clin. North Am. 2006;26(2):349–364. doi: 10.1016/j.iac.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Bala I, Hariharan S, Kumar MN. PLGA nanoparticles in drug delivery: the state of the art. Crit. Rev. Ther. Drug Carrier Syst. 2004;21(5):387–422. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.20. [DOI] [PubMed] [Google Scholar]
- 5.Hariharan S, BhardwaJ V, Bala I, Sitterberg J, Bakowsky U, Ravi Kumar MN. Design of estradiol loaded PLGA nanoparticulate formulations: a potential oral delivery system for hormone therapy. Pharm. Res. 2006;23(1):184–195. doi: 10.1007/s11095-005-8418-y. [DOI] [PubMed] [Google Scholar]
- 6.Crotts G, Park TG. Preparation of porous and nonporous biodegradable polymeric hollow microspheres. J. Control. Release. 1995;35(2–3):91–105. [Google Scholar]
- 7.Herrmann J, Bodmeier R. Biodegradable, somatostatin acetate containing microspheres prepared by various aqueous and nonaqueous solvent evaporation methods. Eur. J. Pharm. Biopharm. 1998;45(1):75–82. doi: 10.1016/S0939-6411(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 8.Tamber H, Johansen P, Merkle HP, Gander B. Formulation aspects of biodegradable polymeric microspheres for antigen delivery. Adv. Drug Deliver. Rev. 2005;57(3):357–376. doi: 10.1016/j.addr.2004.09.002. [DOI] [PubMed] [Google Scholar]; ▪ ▪ Describes the different preparation techniques of polymeric formulations for antigen delivery and possible improvements thereof.
- 9.Ruan G, Feng SS, Li QT. Effects of material hydrophobicity on physical properties of polymeric microspheres formed by double emulsion process. J. Control. Release. 2002;84(3):151–160. doi: 10.1016/s0168-3659(02)00292-4. [DOI] [PubMed] [Google Scholar]
- 10.Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release. 2010;146(2):241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]; ▪ Describes the current status of protein microencapsulation using biodegradable materials, as well as the comparison of the characteristics of microparticles (MPs) prepared by different techniques.
- 11.Castellanos IJ, Crespo R, Griebenow K. Poly(ethylene glycol) as stabilizer and emulsifying agent: a novel stabilization approach preventing aggregation and inactivation of proteins upon encapsulation in bioerodible polyester microspheres. J. Control. Release. 2003;88(1):135–145. doi: 10.1016/s0168-3659(02)00488-1. [DOI] [PubMed] [Google Scholar]
- 12.Thomasin C, Ho NT, Merkle HP, Gander B. Drug microencapsulation by PLA/PLGA coacervation in the light of thermodynamics. 1. Overview and theoretical considerations. J. Pharm. Sci. 1998;87(3):259–268. doi: 10.1021/js970047r. [DOI] [PubMed] [Google Scholar]
- 13.Ratzinger G, Fillafer C, Kerleta V, Wirth M, Gabor F. The role of surface functionalization in the design of PLGA micro- and nanoparticles. Crit. Rev. Ther. Drug. 2010;27(1):1–83. [Google Scholar]; ▪ ▪ Describes current techniques of preparation, characterization and especially surface modification of polylactide-co-glycolide (PLGA) particles.
- 14.Felder CHB, Blanco-Prieto MJ, Heizmann J, Merkle HP, Gander B. Ultrasonic atomization and subsequent polymer desolvation for peptide and protein microencapsulation into biodegradable polyesters. J. Microencapsul. 2003;20(5):553–567. doi: 10.1080/0265204031000148059. [DOI] [PubMed] [Google Scholar]
- 15.Freitas S, Rudolf B, Merkle HP, Gander B. Flow-through ultrasonic emulsification combined with static micromixing for aseptic production of microspheres by solvent extraction. Eur. J. Pharm. Biopharm. 2005;61(3):181–187. doi: 10.1016/j.ejpb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Berkland C, Pack DW, Kim KK. Controlling surface nanostructure using flow-limited field-injection electrostatic spraying (FFESS) of poly(d,l-lactide-co-glycolide) Biomaterials. 2004;25(25):5649–5658. doi: 10.1016/j.biomaterials.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Hung LH, Teh SY, Jester J, Lee AP. PLGA micro/nanosphere synthesis by droplet microfluidic solvent evaporation and extraction approaches. Lab. Chip. 2010;10(14):1820–1825. doi: 10.1039/c002866e. [DOI] [PubMed] [Google Scholar]
- 18.Rhee M, Valencia PM, Rodriguez MJ, Langer R, Farokhzad OC, Karnik R. Synthesis of size-tunable polymeric nanoparticles enabled by 3D hydrodynamic flow focusing in single-layer microchannels. Adv. Mater. 2011;23(12):H79–H83. doi: 10.1002/adma.201004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HK, Chung HJ, Park TG. Biodegradable polymeric microspheres with ‘open/closed’ pores for sustained release of human growth hormone. J. Control. Release. 2006;112(2):167–174. doi: 10.1016/j.jconrel.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Leo E, Ruozi B, Tosi G, Vandelli MA. PLA-microparticles formulated by means a thermoreversible gel able to modify protein encapsulation and release without being co-encapsulated. Int. J. Pharm. 2006;323(1–2):131–138. doi: 10.1016/j.ijpharm.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Rolland JP, Maynor BW, Euliss LE, Exner AE, Denison GM, Desimone JM. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J. Am. Chem. Soc. 2005;127(28):10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 22.Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv. Drug Deliv. Rev. 2004;56(4):459–480. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]; ▪ ▪ Detailed description of the intracellular fate of lectins with its implications to drug delivery. Also discussed issues such as toxicity, influence of nutrients and immunogenicity.
- 23.Des Rieux A, Fievez V, Garinot M, Schneider YJ, Preat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J. Control. Release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Giannasca PJ, Giannasca KT, Leichtner AM, Neutra MR. Human intestinal M cells display the sialyl Lewis A antigen. Infect. Immun. 1999;67(2):946–953. doi: 10.1128/iai.67.2.946-953.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jepson MA, Clark MA, Hirst BH. M cell targeting by lectins: a strategy for mucosal vaccination and drug delivery. Adv. Drug Deliv. Rev. 2004;56(4):511–525. doi: 10.1016/j.addr.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Kerneis S, Bogdanova A, Colucci-Guyon E, Kraehenbuhl JP, Pringault E. Cytosolic distribution of villin in M cells from mouse Peyer’s patches correlates with the absence of a brush border. Gastroenterology. 1996;110(2):515–521. doi: 10.1053/gast.1996.v110.pm8566599. [DOI] [PubMed] [Google Scholar]
- 27.Tyrer P, Ruth Foxwell A, Kyd J, Harvey M, Sizer P, Cripps A. Validation and quantitation of an in vitro M-cell model. Biochem. Biophys. Res. Commun. 2002;299(3):377–383. doi: 10.1016/s0006-291x(02)02631-1. [DOI] [PubMed] [Google Scholar]
- 28.Davis IC, Owen RL. The immunopathology of M cells. Springer Semin. Immunopathol. 1997;18(4):421–448. doi: 10.1007/BF00824051. [DOI] [PubMed] [Google Scholar]
- 29.Buda A, Sands C, Jepson MA. Use of fluorescence imaging to investigate the structure and function of intestinal M cells. Adv. Drug Deliv. Rev. 2005;57(1):123–134. doi: 10.1016/j.addr.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Brayden DJ, Baird AW. Microparticle vaccine approaches to stimulate mucosal immunisation. Microbes Infect. 2001;3(10):867–876. doi: 10.1016/s1286-4579(01)01445-9. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowitz E, Jacob JS, Jong YS, et al. Biologically erodable microspheres as potential oral drug delivery systems. Nature. 1997;386(6623):410–414. doi: 10.1038/386410a0. [DOI] [PubMed] [Google Scholar]
- 32.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997;14(11):1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 33.Florence AT. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov. Today Technol. 2005;2(1):75–81. doi: 10.1016/j.ddtec.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 34.Neutra MR, Phillips TL, Mayer EL, Fishkind DJ. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer’s patch. Cell Tissue Res. 1987;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- 35.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol. Rev. 2003;83(3):871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 36.Lamprecht A, Schafer U, Lehr CM. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18(6):788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- 37.Shakweh M, Besnard M, Nicolas V, Fattal E. Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by peyer’s patches in mice. Eur. J. Pharm. Biopharm. 2005;61(1–2):1–13. doi: 10.1016/j.ejpb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Sahana DK, Mittal G, BhardwaJ V, Kumar MN. PLGA nanoparticles for oral delivery of hydrophobic drugs: influence of organic solvent on nanoparticle formation and release behavior in vitro and in vivo using estradiol as a model drug. J. Pharm. Sci. 2008;97(4):1530–1542. doi: 10.1002/jps.21158. [DOI] [PubMed] [Google Scholar]
- 39.Eldridge JH, Meulbroek JA, Staas JK, Tice TR, Gilley RM. Vaccine-containing biodegradable microspheres specifically enter the gut-associated lymphoid tissue following oral administration and induce a disseminated mucosal immune response. Adv. Exp. Med. Biol. 1989;251:191–202. doi: 10.1007/978-1-4757-2046-4_18. [DOI] [PubMed] [Google Scholar]
- 40.Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J. Pharm. Pharmacol. 1990;42(12):821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 41.Gaumet M, Gurny R, Delie F. Localization and quantification of biodegradable particles in an intestinal cell model: the influence of particle size. Eur. J. Pharm. Sci. 2009;36(4–5):465–473. doi: 10.1016/j.ejps.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Italia JL, Bhatt DK, BhardwaJ V, Tikoo K, Kumar MN. PLGA nanoparticles for oral delivery of cyclosporine: nephrotoxicity and pharmacokinetic studies in comparison to Sandimmune Neoral. J. Control. Release. 2007;119(2):197–206. doi: 10.1016/j.jconrel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Yu J. Poly(lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus. Fish Shellfish Immunol. 2011;30(1):109–117. doi: 10.1016/j.fsi.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Duncanson WJ, Figa MA, Hallock K, Zalipsky S, Hamilton JA, Wong JY. Targeted binding of PLA microparticles with lipid-PEG-tethered ligands. Biomaterials. 2007;28(33):4991–4999. doi: 10.1016/j.biomaterials.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas C, Gupta V, Ahsan F. Influence of surface charge of PLGA particles of recombinant hepatitis B surface antigen in enhancing systemic and mucosal immune responses. Int. J. Pharm. 2009;379(1):41–50. doi: 10.1016/j.ijpharm.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 46.El-Shabouri MH. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002;249(1–2):101–108. doi: 10.1016/s0378-5173(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 47.Hillery AM, Florence AT. The effect of adsorbed poloxamer 188 and 407 surfactants on the intestinal uptake of 60-nm polystyrene particles after oral administration in the rat. Int. J. Pharm. 1996;132(1–2):123–130. [Google Scholar]
- 48.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–2722. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 49.Gaumet M, Gurny R, Delie F. Interaction of biodegradable nanoparticles with intestinal cells: the effect of surface hydrophilicity. Int. J. Pharm. 2010;390(1):45–52. doi: 10.1016/j.ijpharm.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 50.O’Hagan DT, Singh M. Microparticles as vaccine adjuvants and delivery systems. Expert Rev. Vaccines. 2003;2(2):269–283. doi: 10.1586/14760584.2.2.269. [DOI] [PubMed] [Google Scholar]
- 51.Schöll I, Boltz-Nitulescu G, Jensen-Jarolim E. Review of novel particulate antigen delivery systems with special focus on treatment of type I allergy. J. Control. Release. 2005;104(1):1–27. doi: 10.1016/j.jconrel.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 52.Gupta RK, Chang AC, Siber GR. Biodegradable polymer microspheres as vaccine adjuvants and delivery systems. Dev. Biol. Stand. 1998;92:63–78. [PubMed] [Google Scholar]
- 53.Peyre M, Sesardic D, Merkle HP, Gander B, Johansen P. An experimental divalent vaccine based on biodegradable microspheres induces protective immunity against tetanus and diphtheria. J. Pharm. Sci. 2003;92(5):957–966. doi: 10.1002/jps.10361. [DOI] [PubMed] [Google Scholar]
- 54.Johansen P, Moon L, Tamber H, Merkle HP, Gander B, Sesardic D. Immunogenicity of single-dose diphtheria vaccines based on PLA/PLGA microspheres in guinea pigs. Vaccine. 1999;18(3–4):209–215. doi: 10.1016/s0264-410x(99)00191-7. [DOI] [PubMed] [Google Scholar]
- 55.Schöll I, Weissenböck A, Förster-Waldl E, et al. Allergen-loaded biodegradable poly(d,l-lactic-co-glucolic)acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin. Exp. Allergy. 2004;34(2):315–321. doi: 10.1111/j.1365-2222.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- 56.Igartua M, Hernandez RM, Gutierro I, Gascon AR, Pedraz JL. Preliminary assessment of the immune response to Olea europaea pollen extracts encapsulated into PLGA microspheres. Pharm. Dev. Technol. 2001;6(4):621–627. doi: 10.1081/pdt-120000299. [DOI] [PubMed] [Google Scholar]
- 57.Batanero E, Barral P, Villalba M, Rodriguez R. Encapsulation of Ole e 1 in biodegradable microparticles induces Th1 response in mice: a potential vaccine for allergy. J. Control. Release. 2003;92(3):395–398. doi: 10.1016/s0168-3659(03)00337-7. [DOI] [PubMed] [Google Scholar]
- 58.Nayak B, Ray AR, Panda AK, Ray P. Improved immunogenicity of biodegradable polymer particles entrapped rotavirus vaccine. J. Biomater. Appl. 2011;25(5):469–496. doi: 10.1177/0885328209353642. [DOI] [PubMed] [Google Scholar]
- 59.Torche AM, Le Corre P, Albina E, Jestin A, Le Verge R. PLGA microspheres phagocytosis by pig alveolar macrophages: influence of poly(vinyl alcohol) concentration, nature of loaded-protein and copolymer nature. J. Drug Target. 2000;7(5):343–354. doi: 10.3109/10611869909085517. [DOI] [PubMed] [Google Scholar]
- 60.Prior S, Gander B, Blarer N, et al. In vitro phagocytosis and monocyte-macrophage activation with poly(lactide) and poly(lactide-co-glycolide) microspheres. Eur J. Pharm. Sci. 2002;15(2):197–207. doi: 10.1016/s0928-0987(01)00218-4. [DOI] [PubMed] [Google Scholar]
- 61.Luzardo-Alvarez A, Blarer N, Peter K, et al. Biodegradable microspheres alone do not stimulate murine macrophages in vitro, but prolong antigen presentation by macrophages in vitro and stimulate a solid immune response in mice. J. Control. Release. 2005;109(1–3):62–76. doi: 10.1016/j.jconrel.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 62.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J. Autoimmun. 2010;34(3):J234–J246. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Lee HM, Shin DM, Song HM, et al. Nanoparticles up-regulate tumor necrosis factor-alpha and CXCL8 via reactive oxygen species and mitogen-activated protein kinase activation. Toxicol. Appl. Pharmacol. 2009;238(2):160–169. doi: 10.1016/j.taap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Semete B, Booysen Li, Kalombo L, et al. In vivo uptake and acute immune response to orally administered chitosan and PEG coated PLGA nanoparticles. Toxicol. Appl. Pharmacol. 2010;249(2):158–165. doi: 10.1016/j.taap.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Nayak B, Panda AK, Ray P, Ray AR. Formulation, characterization and evaluation of rotavirus encapsulated PLA and PLGA particles for oral vaccination. J. Microencapsul. 2009;26(2):154–165. doi: 10.1080/02652040802211709. [DOI] [PubMed] [Google Scholar]
- 66.Raghuvanshi RR, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int. J. Pharm. 2002;245(1–2):109–121. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 67.Brandhonneur N, Chevanne F, Vie V, et al. Specific and nonspecific phagocytosis of ligand-grafted PLGA microspheres by macrophages. Eur J. Pharm. Sci. 2009;36(4–5):474–485. doi: 10.1016/j.ejps.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Cruz LJ, Tacken PJ, Fokkink R, et al. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control. Release. 2010;144(2):118–126. doi: 10.1016/j.jconrel.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 69.Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32(11):3094–3105. doi: 10.1016/j.biomaterials.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26(2):104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Thomann JS, Heurtault B, Weidner S, et al. Antitumor activity of liposomal ErbB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials. 2011;32(20):4574–4583. doi: 10.1016/j.biomaterials.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 72.Wattendorf U, Coullerez G, Voros J, Textor M, Merkle HP. Mannose-based molecular patterns on stealth microspheres for receptor-specific targeting of human antigen-presenting cells. Langmuir. 2008;24(20):11790–11802. doi: 10.1021/la801085d. [DOI] [PubMed] [Google Scholar]
- 73.Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro characterization and in vivo evaluation. J. Control. Release. 2006;114(2):242–250. doi: 10.1016/j.jconrel.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 74.Jain AK, Swarnakar NK, Das M, et al. Augmented anticancer efficacy of doxorubicin loaded polymeric nanoparticles after oral administration in breast cancer induced animal model. Mol. Pharm. 2011;8(4):1140–1151. doi: 10.1021/mp200011f. [DOI] [PubMed] [Google Scholar]
- 75.Roth-Walter F, Scholl I, Untersmayr E, et al. M cell targeting with Aleuria aurantia lectin as a novel approach for oral allergen immunotherapy. J. Allergy Clin. Immunol. 2004;114(6):1362–1368. doi: 10.1016/j.jaci.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 76.Garinot M, Fievez V, Pourcelle V, et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J. Control. Release. 2007;120(3):195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 77.Diesner SC, Knittelfelder R, Krishnamurthy D, et al. Dose-dependent food allergy induction against ovalbumin under acid-suppression: a murine food allergy model. Immunol. Lett. 2008;121(1):45–51. doi: 10.1016/j.imlet.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang BC, Dawson M, Lai Sk, et al. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc. Natl Acad. Sci. USA. 2009;106(46):19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joshi MD, Unger WW, Van Beelen AJ, et al. DC-SIGN mediated antigen-targeting using glycan-modified liposomes: formulation considerations. Int. J. Pharm. 2011;416(2):426–432. doi: 10.1016/j.ijpharm.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 80.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24(15):2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 81.Kim SY, Doh HJ, Jang MH, Ha YJ, Chung SI, Park HJ. Oral immunization with Helicobacter pylori-loaded poly(d, l-lactide-co-glycolide) nanoparticles. Helicobacter. 1999;4(1):33–39. doi: 10.1046/j.1523-5378.1999.09046.x. [DOI] [PubMed] [Google Scholar]
- 82.Astete CE, Sabliov CM. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006;17(3):247–289. doi: 10.1163/156856206775997322. [DOI] [PubMed] [Google Scholar]
- 83.Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MN. Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm. Res. 2009;26(3):492–501. doi: 10.1007/s11095-008-9763-4. [DOI] [PubMed] [Google Scholar]
- 84.Jain AK, Swarnakar NK, Godugu C, Singh RP, Jain S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials. 2011;32(2):503–515. doi: 10.1016/j.biomaterials.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 85.Owen RL, Pierce NF, Apple RT, Cray WW., Jr. M cell transport of Vibrio cholerae from the intestinal lumen into Peyer’s patches: a mechanism for antigen sampling and for microbial transepithelial migration. J. Infect. Dis. 1986;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- 86.Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277(5328):949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 87.Clark MA, Jepson MA, Simmons NL, Hirst BH. Preferential interaction of Salmonella typhimurium with mouse Peyer’s patch M cells. Res. Microbiol. 1994;145(7):543–552. doi: 10.1016/0923-2508(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 88.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 1994;180(1):15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siebers A, Finlay BB. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4(1):22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 90.Gebert A, Rothkotter HJ, Pabst R. M cells in Peyer’s patches of the intestine. Int. Rev. Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- 91.Jensen VB, Harty JT, Jones BD. Interactions of the invasive pathogens Salmonella typhimurium, Listeria monocytogenes and Shigella flexneri with M cells and murine Peyer’s patches. Infect. Immun. 1998;66(8):3758–3766. doi: 10.1128/iai.66.8.3758-3766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Clark MA, Hirst BH, Jepson MA. M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect. Immun. 1998;66(3):1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grassl GA, Bohn E, Muller Y, Buhler OT, Autenrieth IB. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 2003;293(1):41–54. doi: 10.1078/1438-4221-00243. [DOI] [PubMed] [Google Scholar]
- 94.Schulte R, Kerneis S, Klinke S, et al. Translocation of Yersinia entrocolitica across reconstituted intestinal epithelial monolayers is triggered by Yersinia invasin binding to beta1 integrins apically expressed on M-like cells. Cell Microbiol. 2000;2(2):173–185. doi: 10.1046/j.1462-5822.2000.00047.x. [DOI] [PubMed] [Google Scholar]
- 95.Helander A, Silvey KJ, Mantis NJ, et al. The viral sigma1 protein and glycoconjugates containing alpha2–3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 2003;77(14):7964–7977. doi: 10.1128/JVI.77.14.7964-7977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujimura Y, Takeda M, Ikai H, et al. The role of M cells of human nasopharyngeal lymphoid tissue in influenza virus sampling. Virchows Arch. 2004;444(1):36–42. doi: 10.1007/s00428-003-0898-8. [DOI] [PubMed] [Google Scholar]
- 97.Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP. Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc. Natl Acad. Sci. USA. 2002;99(14):9410–9414. doi: 10.1073/pnas.142586899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Corr SC, Gahan CC, Hill C. M-cells: origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008;52(1):2–12. doi: 10.1111/j.1574-695X.2007.00359.x. [DOI] [PubMed] [Google Scholar]
- 99.Kyd JM, Cripps AW. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26(49):6221–6224. doi: 10.1016/j.vaccine.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 100.Azizi A, Kumar A, Diaz-Mitoma F, Mestecky J. Enhancing oral vaccine potency by targeting intestinal M cells. PLoS Pathog. 2010;6(11):e1001147. doi: 10.1371/journal.ppat.1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajapaksa TE, Stover-Hamer M, Fernandez X, Eckelhoefer HA, Lo DD. Claudin 4-targeted protein incorporated into PLGA nanoparticles can mediate M cell targeted delivery. J. Control. Release. 2010;142(2):196–205. doi: 10.1016/j.jconrel.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gullberg E, Leonard M, Karlsson J, et al. Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem. Biophys. Res. Commun. 2000;279(3):808–813. doi: 10.1006/bbrc.2000.4038. [DOI] [PubMed] [Google Scholar]
- 103.Pohlmeyer I, Jorns J, Schumacher U, et al. Lectin histochemical investigations of the distal gut of chicks with special emphasis on the follicle-associated epithelium. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2005;52(3):138–146. doi: 10.1111/j.1439-0442.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 104.Hathaway LJ, Kraehenbuhl JP. The role of M cells in mucosal immunity. Cell Mol. Life Sci. 2000;57(2):323–332. doi: 10.1007/PL00000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clark MA, Hirst BH, Jepson MA. Lectin-mediated mucosal delivery of drugs and microparticles. Adv. Drug Deliv. Rev. 2000;43(2–3):207–223. doi: 10.1016/s0169-409x(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 106.Takata S, Ohtani O, Watanabe Y. Lectin binding patterns in rat nasal-associated lymphoid tissue (NALT) and the influence of various types of lectin on particle uptake in NALT. Arch. Histol. Cytol. 2000;63(4):305–312. doi: 10.1679/aohc.63.305. [DOI] [PubMed] [Google Scholar]
- 107.Wirth M, Hamilton G, Gabor F. Lectin-mediated drug targeting: quantification of binding and internalization of wheat germ agglutinin and Solanum tuberosum lectin using Caco-2 and HT-29 cells. J. Drug Target. 1998;6(2):95–104. doi: 10.3109/10611869808997885. [DOI] [PubMed] [Google Scholar]
- 108.Walter F, Scholl I, Untersmayr E, et al. Functionalisation of allergen-loaded microspheres with wheat germ agglutinin for targeting enterocytes. Biochem. Biophys. Res. Commun. 2004;315(2):281–287. doi: 10.1016/j.bbrc.2004.01.057. [DOI] [PubMed] [Google Scholar]; ▪ PLGA microspheres were coated with wheatgerm agglutinin and loaded with birch pollen-induced higher levels of allergen-specific IgG after oral administration.
- 109.Makhlof A, Fujimoto S, Tozuka Y, Takeuchi H. In vitro and in vivo evaluation of WGA-carbopol modified liposomes as carriers for oral peptide delivery. Eur. J. Pharm. Biopharm. 2011;77(2):216–224. doi: 10.1016/j.ejpb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 110.Leon-Rodriguez L, Leiro-Vidal J, Blanco-Mendez J, Luzardo-Alvarez A. Incorporation of PVMMA to PLGA MS enhances lectin grafting and their in vitro activity in macrophages. Int. J. Pharm. 2010;402(1–2):165–174. doi: 10.1016/j.ijpharm.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Andrade JL, Arruda S, Barbosa T, et al. Lectin-induced nitric oxide production. Cell Immunol. 1999;194(1):98–102. doi: 10.1006/cimm.1999.1494. [DOI] [PubMed] [Google Scholar]
- 112.Sodhi A, Tarang S, Kesherwani V. Concanavalin A induced expression of Toll-like receptors in murine peritoneal macrophages in vitro. Int. Immunopharmacol. 2007;7(4):454–463. doi: 10.1016/j.intimp.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 113.Gupta PN, Khatri K, Goyal AK, Mishra N, Vyas SP. M-cell targeted biodegradable PLGA nanoparticles for oral immunization against hepatitis B. J. Drug Target. 2007;15(10):701–713. doi: 10.1080/10611860701637982. [DOI] [PubMed] [Google Scholar]
- 114.Mishra N, Tiwari S, Vaidya B, Agrawal GP, Vyas SP. Lectin anchored PLGA nanoparticles for oral mucosal immunization against hepatitis B. J. Drug Target. 2011;19(1):67–78. doi: 10.3109/10611861003733946. [DOI] [PubMed] [Google Scholar]; ▪ Lotus tetragonolobus agglutinin-coated PLGA nanoparticles, loaded with hepatitis B surface antigen elicited mucosal and systemic responses.
- 115.Roth-Walter F, Bohle B, Scholl I, et al. Targeting antigens to murine and human M-cells with Aleuria aurantia lectin-functionalized microparticles. Immunol. Lett. 2005;100(2):182–188. doi: 10.1016/j.imlet.2005.03.020. [DOI] [PubMed] [Google Scholar]; ▪ ▪ In in vitro M cell-like co-culture models, MPs with Aleuria aurantia lectin specifically target M cells and are taken up more efficiently.
- 116.Roth-Walter F, Scholl I, Untersmayr E, et al. Mucosal targeting of allergen-loaded microspheres by Aleuria aurantia lectin. Vaccine. 2005;23(21):2703–2710. doi: 10.1016/j.vaccine.2004.11.052. [DOI] [PubMed] [Google Scholar]; ▪ ▪ In in vitro M cell-like co-culture models, MPs with Aleuria aurantia lectin specifically target M cells and are taken up more efficiently.
- 117.Lambkin I, Pinilla C, Hamashin C, et al. Toward targeted oral vaccine delivery systems: selection of lectin mimetics from combinatorial libraries. Pharm. Res. 2003;20(8):1258–1266. doi: 10.1023/a:1025061317400. [DOI] [PubMed] [Google Scholar]
- 118.Eck J, Langer M, Mockel B, Witthohn K, Zinke H, Lentzen H. Characterization of recombinant and plant-derived mistletoe lectin and their B-chains. Eur. J. Biochem. 1999;265(2):788–797. doi: 10.1046/j.1432-1327.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- 119.Misumi S, Masuyama M, Takamune N, et al. Targeted delivery of immunogen to primate M cells with tetragalloyl lysine dendrimer. J. Immunol. 2009;182(10):6061–6070. doi: 10.4049/jimmunol.0802928. [DOI] [PubMed] [Google Scholar]
- 120.Gabor F, Stangl M, Wirth M. Lectin-mediated bioadhesion: binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. J. Control. Release. 1998;55(2–3):131–142. doi: 10.1016/s0168-3659(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 121.Ertl B, Heigl F, Wirth M, Gabor F. Lectin-mediated bioadhesion: preparation, stability and caco-2 binding of wheat germ agglutinin-functionalized poly(d,l-lactic-co-glycolic acid)-microspheres. J. Drug Target. 2000;8(3):173–184. doi: 10.3109/10611860008996863. [DOI] [PubMed] [Google Scholar]
- 122.Hermanson GT. Bioconjugate Techniques. Academic Press; San Diego, CA, USA: 1996. pp. 170–176. [Google Scholar]
- 123.Martinez Gomez JM, Fischer S, Csaba N, et al. A protective allergy vaccine based on CpG- and protamine-containing PLGA microparticles. Pharm. Res. 2007;24(10):1927–1935. doi: 10.1007/s11095-007-9318-0. [DOI] [PubMed] [Google Scholar]
- 124.Duthie MS, Windish HP, Fox CB, Reed SG. Use of defined TLR ligands as adjuvants within human vaccines. Immunol. Rev. 2011;239(1):178–196. doi: 10.1111/j.1600-065X.2010.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Han H, Peng JR, Chen PC, et al. A novel system of artificial antigen-presenting cells efficiently stimulates flu peptide-specific cytotoxic T cells in vitro. Biochem. Biophys. Res. Commun. 2011;411(3):530–535. doi: 10.1016/j.bbrc.2011.06.164. [DOI] [PubMed] [Google Scholar]
- 126.Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol. Ther. 2008;16(4):765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]; ▪ ▪ Polylactide-co-glycolide particles were used as artificial antigen-presenting cells for the activation of peptide-specific T cells.
- 127.Hanlon DJ, Aldo PB, Devine L, et al. Enhanced stimulation of anti-ovarian cancer CD8(+) T cells by dendritic cells loaded with nanoparticle encapsulated tumor antigen. Am. J. Reprod. Immunol. 2011;65(6):597–609. doi: 10.1111/j.1600-0897.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma W, Smith T, Bogin V, et al. Enhanced presentation of MHC class Ia, Ib and class II-restricted peptides encapsulated in biodegradable nanoparticles: a promising strategy for tumor immunotherapy. J. Transl. Med. 2011;9:34. doi: 10.1186/1479-5876-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J. Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 130.Brayden DJ, Baird AW. Apical membrane receptors on intestinal M cells: potential targets for vaccine delivery. Adv. Drug Deliv. Rev. 2004;56(6):721–726. doi: 10.1016/j.addr.2003.10.036. [DOI] [PubMed] [Google Scholar]