Summary
Although multiple kinetic components of synaptic vesicle endocytosis have been identified, it has remained unclear whether neurons can differentially modulate these components. Using membrane capacitance measurements from isolated goldfish bipolar cell terminals, we found that the kinetics of endocytosis in retinal slices (single exponential decay; τ > 10 s) were significantly slower than those in acutely dissociated terminals (double exponential decay; τfast ≈ 1–2 s; τslow > 10 s). Surprisingly, GABAA and/or GABAC receptor antagonists restored the fast component of endocytosis to terminals in retinal slices. Blocking GABAergic feedback from reciprocal synapses or removing external Cl− ions also allowed for fast endocytosis. Elevating internal Cl− via the patch pipette invariably slowed endocytosis, even in terminals dialyzed with increased Ca2+ buffer. These results suggest a new role for GABA and Cl− ions in blocking the trigger for fast endocytosis at this ribbon-type synapse.
Introduction
Endocytosis plays a vital role in the ongoing cycle of supply and demand for synaptic vesicles at a nerve terminal. When vesicles fuse with the plasma membrane to liberate their transmitter into the synaptic cleft, endocytosis quickly retrieves the vesicular membrane into the cytosol where it can be recycled to form new vesicles of uniform size (Zhang et al., 1999). Endocytosis therefore serves the essential function of maintaining chemical transmission during prolonged nerve activity (Brodin et al., 1997). This is most clearly evident in the Drosophila mutant shibire, where a temperature-sensitive defect in the endocytosis protein dynamin quickly halts transmission as synaptic vesicles are depleted (Delgado et al., 2000). Similarly, changes in the kinetics of endocytosis that alter vesicle replenishment may also have repercussions for sustained synaptic signaling. These kinetic changes would be particularly influential in nerve terminals that experience frequent or prolonged depolarizations and continuous exocytosis, such as the ribbon synapses of the retina.
Synaptic vesicle endocytosis at ribbon-type synapses has been reported to depend on intracellular free calcium ([Ca2+]i) (von Gersdorff and Matthews, 1994b; Rouze and Schwartz, 1998; Beutner et al., 2001), ATP-Mg2+ (Heidelberger, 2001), hydrostatic pressure, and osmolarity (Heidelberger et al., 2002). Although a mechanistic description of endocytosis at ribbon synapses remains to be elucidated, molecular evidence from other synapses indicates that synaptic vesicle endocytosis is driven by a complex cascade of protein-protein and protein-lipid interactions (DeCamilli et al., 2001; Morgan et al., 2002). At the goldfish retinal bipolar cell terminal, capacitance measurements have lent support to the view of endocytosis as a multistep process by revealing at least two distinct kinetic components (von Gersdorff and Matthews, 1994a; Neves et al., 2001). These kinetic components have stereotypical time constants, but the relationship between rate-identified steps and their underlying physiological mechanisms is not known. By comparison, membrane retrieval at conventional active zone nerve terminals has at least three distinct components (Gandhi and Stevens, 2003), and these can be Ca2+ sensitive (Klingauf et al., 1998; Sankaranarayanan and Ryan, 2001; Teng and Wilkinson, 2003) or insensitive (Ramaswami et al., 1994; Sun et al., 2002; Wu and Betz, 1996). Given the large number of molecules and functional complexity involved in synaptic vesicle endocytosis, it is not surprising that this process is sensitive to several regulatory and homeostatic factors. Such sensitivity, however, also underscores the importance of experimental conditions when performing and evaluating measurements of synaptic vesicle endocytosis.
To date, endocytosis at retinal bipolar cell terminals has only been measured in acutely dissociated preparations. Although acutely dissociated presynaptic terminals are easily accessible for patch-clamp recordings and have a unique advantage for controlled biophysical experiments, the dissociation process may disrupt cytoskeletal elements, the plasma membrane morphology, or other structures involved in synaptic vesicle endocytosis. Time-resolved membrane capacitance measurements can monitor real-time net changes in cell surface area that reflect exocytosis and endocytosis in single live cells (Neher and Marty, 1982). Here, we use capacitance measurements to study endocytosis in situ by recording directly from bipolar cell terminals in the retinal slice. Together with whole-cell and perforated patch recordings in dissociated terminals, we find that both GABA-mediated Cl− influx and elevated presynaptic [Cl−]i inhibit the fast component of synaptic vesicle endocytosis. These data suggest a novel “second messenger”-type role for Cl− ions that is distinct from their well-established function in changing the membrane potential.
Results
Isolated Terminals in Slices: GABAergic Inputs
In goldfish retinal slices, isolated Mb-type bipolar cell terminals retain their normal synaptic architecture and morphology (Figure 1A; compare with Sherry and Yazulla, 1993). The primary inputs to the bipolar cell terminal come from GABAergic amacrine cell boutons (Witkovsky and Dowling, 1969). Amacrine cells in the goldfish retina make both reciprocal and conventional synapses directly onto bipolar cell terminals, and there are about 300 GABAergic boutons per bipolar cell terminal that cover nearly its entire surface and provide around 98% of its total input (Marc and Liu, 2000). This synaptic input from amacrine cells to the bipolar cell terminal has been described previously in several species (Dong and Werblin, 1998; Protti and Llano, 1998; Hartveit, 1999; Singer and Diamond, 2003). In our preparation, reciprocal GABAergic input can be elicited by depolarizing the bipolar cell presynaptic terminal. This depolarization results in glutamate release from the bipolar cell and a fast GABAergic feedback that produces large chloride currents capable of completely masking the bipolar terminals’ slowly inactivating L-type Ca2+ current (Heidelberger and Matthews, 1992; Figures 1B and 2A). The calculated reversal potential for chloride under recording conditions with 15 mM internal chloride and 130 mM external chloride is about −55 mV. Therefore, the synaptic chloride currents are outward upon depolarization to 0 mV (indicating inward Cl− flux), and the calcium-activated chloride tail current (plus any conventional synaptic chloride input) is inward upon repolarization of the membrane to −60 mV (indicating outward Cl− flux).
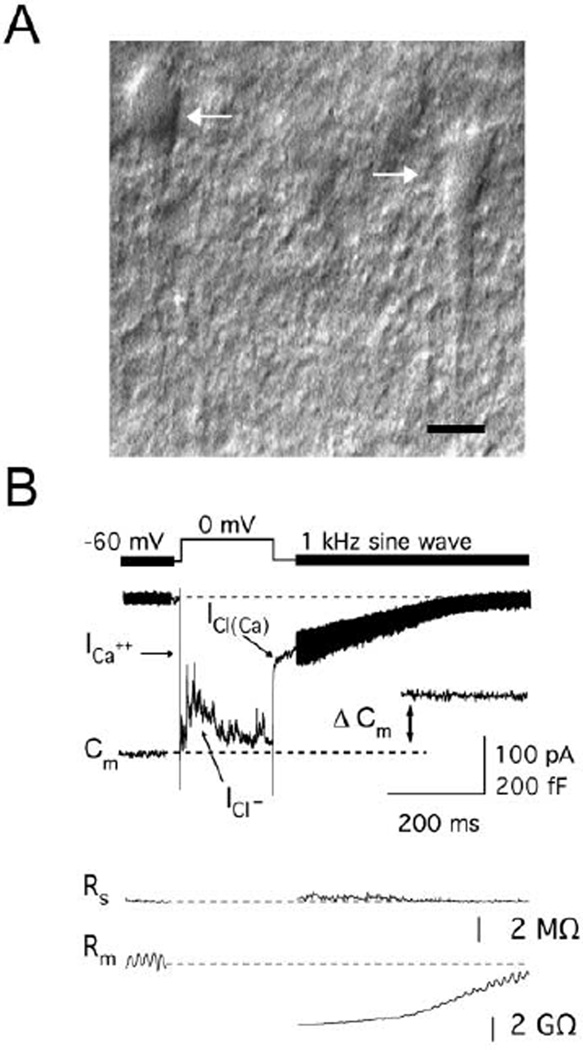
Figure 1. Typical Voltage-Clamp Recording and DIC Image from Bipolar Cell Terminals in a Retinal Slice.
(A) DIC image of Mb1 bipolar cell terminals (arrows) from a goldfish retinal slice (scale bar, 5 µm). These terminals reside in the inner plexiform layer close to the ganglion cells and send their axons to somata in the inner nuclear layer (axons run downwards from terminals).
(B) Voltage-clamp record of a calcium current (ICa) with superimposed outward GABAergic currents (ICl−, inward Cl− flux) at an isolated bipolar cell terminal. Membrane capacitance (Cm) was measured using a 1 kHz sine wave and reflects exocytosis (ΔCm) following calcium influx. The Ca2+-activated chloride tail current [ICl(Ca)] follows Ca2+ influx and is inward (reflecting Cl− efflux) at a holding potential of −60 mV. Series resistance (Rs) remains constant following a depolarization, and the change in membrane resistance (Rm) reflects the transient conductance associated with ICl(Ca). Note that Cm measurements do not correlate with Rs or Rm.
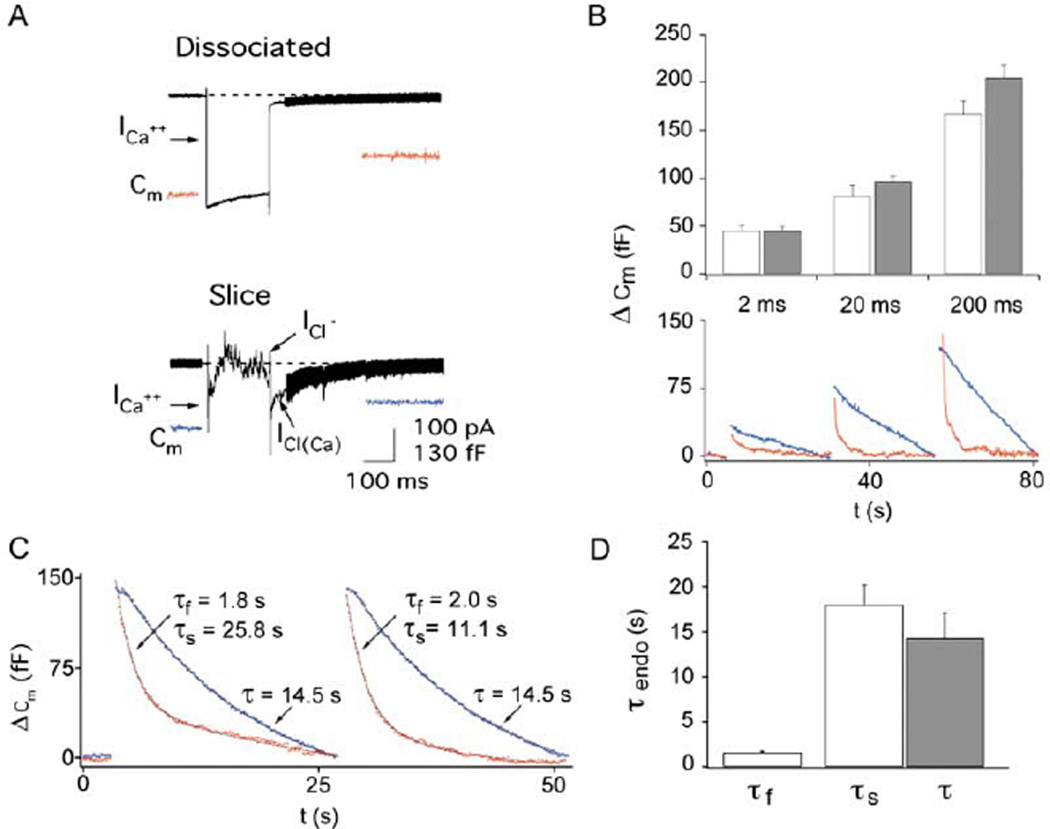
Figure 2. Exo-Endocytosis in Slice versus Acutely Dissociated Synaptic Terminals.
(A) Calcium currents in acutely dissociated terminals and isolated terminals in slice. Depolarizing the terminal in slice induces a ICa current and reciprocal GABAergic chloride feedback currents followed by a large inward Ca2+-activated chloride current [ICa(Cl)].
(B) Exocytosis (ΔCm) is the same in acutely dissociated terminals (open bars) and isolated terminals in the slice (gray bars) for depolarizations (−60 to 0 mV) of 2 ms (dissociated, n = 9; slice, n = 6), 20 ms (dissociated, n = 10; slice, n = 8), and 200 ms (dissociated, n = 23; slice, n = 35). Cm traces are from a typical cell in dissociation (red) and slice (blue) with consecutive 2, 20, and 200 ms depolarizations from −60 to 0 mV. Note that the percentage of fast endocytosis increases with pulse duration.
(C) Endocytosis is slower when recorded in slice (blue) as compared to dissociated terminals (red). Two successive 200 ms depolarizations each elicit endocytosis with both fast and slow kinetic components in acutely dissociated terminals, but only a single, slow kinetic component exists in terminals from the slice preparation.
(D) The average kinetics of endocytosis in the acute dissociation (open bars) were faster than those of the slice preparation (gray bars). The fast component of endocytosis in the acutely dissociated terminals is significantly different (p < 0.001) from the slow component of endocytosis in the same dissociated terminals (τslow = 18.0 ± 2.2 s; n = 23) as well as the single component of endocytosis from the slice preparation (τ = 14.3 ± 2.8 s; n = 26). Note that τslow in the dissociated terminals is not different from the τ of endocytosis in the slice (p = 0.32).
Cm Recordings: Slices versus Dissociated Terminals
We first sought to determine whether isolated bipolar cell terminals in slice exhibited similar capacitance responses to acutely dissociated bipolar cell terminals. Thus, we measured exocytosis and endocytosis from terminals in both preparations following voltage-clamp depolarizations from −60 to 0 mV for 2, 20, and 200 ms (Figures 2A and 2B). Exocytosis (Cm jump) was not significantly different between terminals in slice and acute dissociation (Cm jumps, slice: 2 ms = 46 ± 4 fF, n = 6; 20 ms = 96 ± 6, n = 10; 200 ms = 205 ± 14 fF, n = 35; Cm jumps, dissociated: 2 ms = 45 ± 5 fF, n = 9; 20 ms = 81 ± 12 fF, n = 8; 200 ms = 167 ± 14 fF, n = 23). Although the differences are not significant, averaged Cm jumps were slightly larger in the slice preparation. This is not surprising, however, as the average resting membrane capacitance and peak calcium current were also larger in terminals from the slice preparation (slice: resting Cm = 5.2 ± 0.3 pF, peak ICa2+ = 296 ± 20 pA; dissociated: resting Cm = 3.3 ± 0.2 pF, peak ICa2+ = 270 ± 22 pA), and larger terminals tend to have larger Cm jumps (von Gersdorff et al., 1996).
In acutely dissociated terminals, the kinetics of endocytosis following 2, 20, and 200 ms depolarizing pulses from −60 to 0 mV were usually best described with double exponentials that had both a fast and slow component (Figure 2; see Experimental Procedures for a description of exponential fitting). After 200 ms depolarizations, endocytosis exhibited a large fast component and a smaller slow component (τfast = 1.6 ± 0.1 s; 67% of ΔCm; τslow = 18.0 ± 2.2 s; n = 23; Figures 2C and 2D). Two kinetic components with similar time constants were also observed after the shorter 2 and 20 ms depolarizations (2 ms: τfast = 1.2 ± 0.5 s; 43% of ΔCm; τslow = 13.7 ± 5.5 s; n = 4; 20 ms: τfast = 1.4 ± 0.7 s; 46% of ΔCm; τslow = 20.4 ± 15.5 s; n = 4). The time constants for fast and slow endocytosis, therefore, did not depend on ΔCm for pulses of 200 ms or shorter. The amount of membrane retrieved by the fast component of endocytosis was, however, reduced following these shorter depolarizations. This change in the proportion of membrane retrieved by fast endocytosis may relate to the smaller calcium influxes associated with shorter depolarizations (Neves et al., 2001) or to the coupled decrease in vesicle fusion (smaller Cm jumps) associated with less calcium influx.
Although most control endocytosis data were best fit with double exponentials, we note that endocytosis is sometimes well described by single exponential kinetics (e.g., see Figure 3C). In these cases, our criteria for fitting double exponentials (see Experimental Procedures) were not met, and single exponentials were fit and categorized as 100% τfast for fits with time constants less than 5 s. Single exponential fits with time constants greater than 5 s were considered 100% τslow. As in previous experiments (Guatimosim et al., 2002), single exponential time constants typically ranged from 1 to 3 s in dissociated terminals (see below). The kinetics of endocytosis measured here thus agree closely with other published time constants for endocytosis in acutely dissociated bipolar cell terminals (von Gersdorff and Matthews, 1994a; Neves et al., 2001).
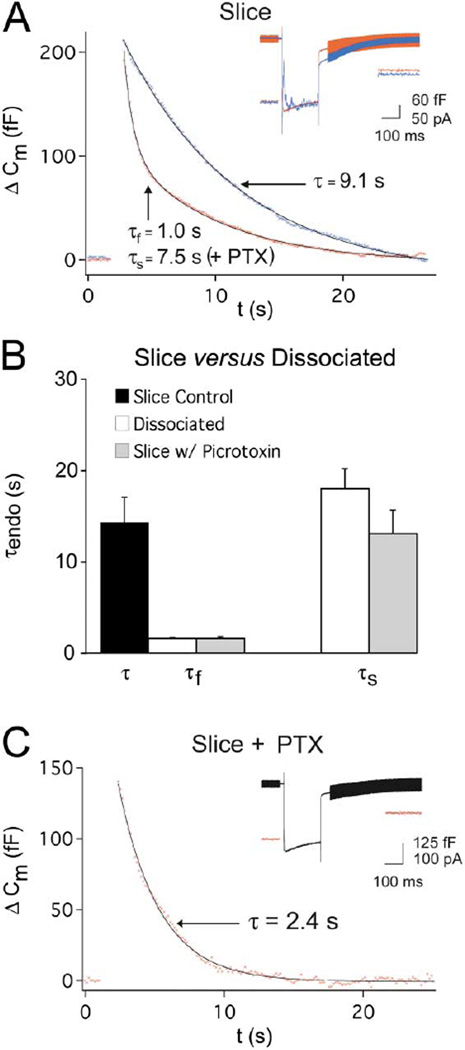
Figure 3. Picrotoxin Alters the Kinetics of Endocytosis in Isolated Presynaptic Terminals from the Retinal Slice.
(A) (Inset) Ca2+ currents and Cm. Picrotoxin (PTX; 50 µM; red trace) blocks GABAergic chloride currents in the slice preparation (blue trace, no picrotoxin). Note that GABAergic feedback is large in the blue trace as indicated by the initial amplitude of ICa (initial amplitude of ICa is much smaller in the red trace). Endocytosis (follows Ca2+ currents shown at inset, same two cells) has two kinetic components in the presence of PTX (red) and only one component in control (blue).
(B) In the presence of PTX (gray bars), the average kinetics of fast and slow endocytosis are not different from the fast and slow endocytosis in the dissociated terminals (open bars) (τfast, p = 0.92; τslow, p = 0.18). The slow components of endocytosis in both dissociation and PTX are not different from the single component of endocytosis (black bar) in slice without PTX (p = 0.45).
(C) An example recording from a slice in PTX where the decay in Cm was well fit by a single fast exponential according to our fitting criteria (see Experimental Procedures). The inset shows the corresponding Ca2+ currents and Cm jump. Note the lack of synaptic currents in PTX.
In contrast with acutely dissociated terminals, endocytosis was consistently slower when recorded from terminals in the slice preparation. Following short 2 and 20 ms depolarizations, no fast component of endocytosis was evident in the slice (2 ms, n = 6; 20 ms, n = 10; Figure 2B). Following 200 ms depolarizations, the fast component of endocytosis was absent in 26 of 35 terminals. Because the 200 ms depolarizations provide large Cm jumps and yield the largest fraction of fast endocytosis in dissociated terminals, we chose this paradigm as the basis for further comparing exo-endocytosis. After 200 ms depolarizations, endocytosis in most slice terminals was best fit with single exponentials with an average τ = 14.3 ± 2.8 s (n = 26; Figure 2C). Nine terminals in slice exhibited both fast and slow endocytic components (τfast = 1.9 ± 0.4 s; 50% of ΔCm; τslow = 26.0 ± 11.4 s). The slow, single exponential kinetics of endocytosis found in the majority of terminals in the slice corresponded closely with the second, slower phase of endocytosis recorded in the acutely dissociated terminals (Figure 2D). To reinforce the difference between the kinetics of endocytosis in dissociation and the majority of cells in slice, we also forced single exponential fits to all dissociated cell data. When only single exponentials were used, the more rapid kinetics of endocytosis in dissociated terminals were still clear even though these fits were often less accurate (τdissoc = 2.4 ± 0.1 s; n = 23).
A major difference between presynaptic terminals in slice and in acute dissociation is the GABAergic currents that result from conventional and reciprocal synapses with amacrine cells (Lukasiewicz and Shields, 1998; McGillem et al., 2000). In acutely dissociated goldfish bipolar cell terminals, exogenous GABA elicits large chloride currents (Tachibana and Kaneko, 1987; Matthews et al., 1994). In the goldfish retinal slice, we find that these chloride currents can be strongly evoked via reciprocal synapses by depolarizing the bipolar cell terminal. Furthermore, the currents are completely blocked with the GABAA/GABAC receptor antagonist picrotoxin (50 µM; Figure 3A). Surprisingly, when ionotropic chloride input was blocked with picrotoxin (50 µM), endocytosis had a fast component in all terminals and displayed kinetics similar to those measured in the acutely dissociated terminals (τfast = 1.6 ± 0.2 s; 42% of ΔCm; τslow = 13.1 ± 2.6 s; n = 11; Figure 3).
To further examine a putative role of Cl− influx on endocytosis in the slice preparation, we tested the effects of the GABAA and GABAC receptor antagonists bicuculline and TPMPA. Bath application of bicuculline-methiodide (BMI; 20 µM) selectively blocked the fast, transient GABAergic reciprocal feedback currents normally observed during depolarization. This significantly reduced the total chloride current and resulted in a fast component of endocytosis (τfast = 1.9 ± 0.3 s; 39% of ΔCm; τslow = 22.0 ± 4.1 s; n = 9; Figure 4A; compare with Figure 2A currents). We also found that a large component of the reciprocal GABAergic feedback was mediated by GABAC receptors and that TPMPA (100 µM) significantly reduced the total GABAergic input by eliminating a slower, more sustained GABAC current. In slices treated with TPMPA, endocytosis also had a fast component (τfast = 1.7 ± 0.6 s; 52% of ΔCm; τslow = 17.8 ± 3.3 s; n = 5; Figure 4B). Although blocking either GABA receptor subtype was sufficient to allow fast endocytosis, it is important to note that the amount of GABAergic feedback attributable to each receptor type is quite variable (data not shown). The leftover feedback following inhibition of either GABAA or GABAC receptors was unusually small in these terminals, and this may have allowed for fast endocytosis. It is therefore possible that selectively blocking one receptor subtype would not be sufficient to allow fast endocytosis in terminals with a larger leftover GABAA or GABAC component.
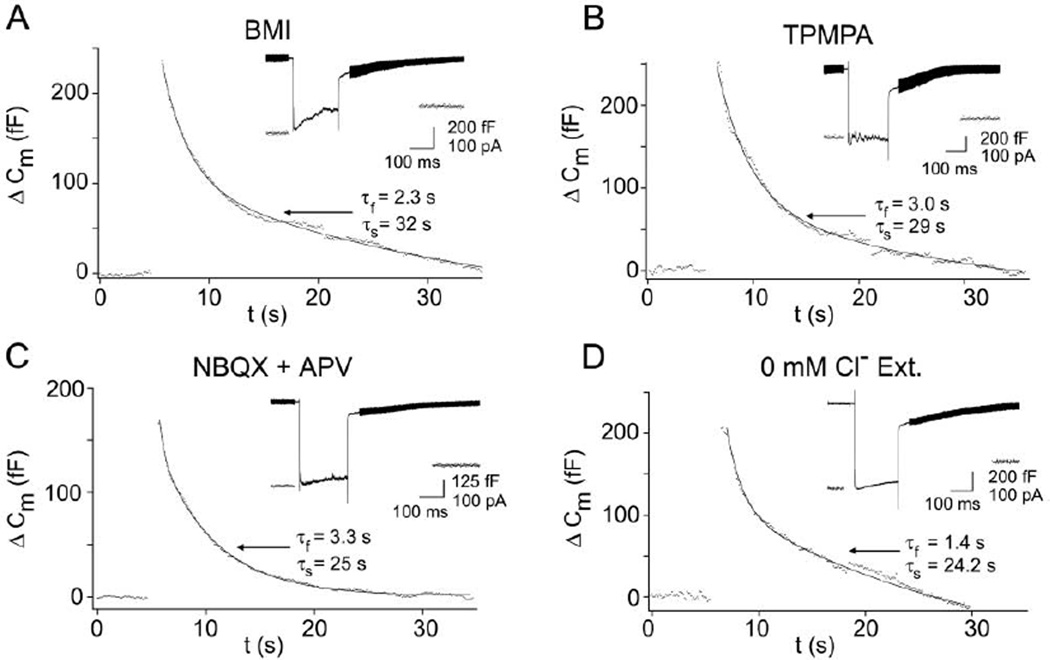
Figure 4. Inhibiting Cl− Influx in the Slice Preparation Allows Normal Endocytosis with Both Fast and Slow Components.
(A) Bath-applied bicuculline-methiodide (BMI; 20 µM) dramatically reduced the total Cl− current by eliminating the fast, transient reciprocal GABAergic Cl− feedback currents (inset; compare with Figures 1B and 2A) and produced endocytosis (Cm decay) with a major fast component.
(B) Fast endocytosis was also observed after treating slices with TPMPA (100 µM) to block GABAC receptors and reduce the total Cl− current by eliminating the slower rising, sustained component of the feedback.
(C) NBQX (25 µM) and APV (50 µM), which block AMPA and NMDA receptors on amacrine cells, also dramatically inhibited Cl− feedback by blocking the reciprocal synapse and allowed fast endocytosis.
(D) Removal of Cl− from the external bath (substituted with 125 mM methanesulfonate) resulted in a Ca2+ current without superimposed Cl− feedback and endocytosis with a large fast component. ICl(Ca) was also larger due to the increased driving force for Cl− extrusion at −60 mV after depolarization.
In addition to blocking GABA receptors, it is also possible to “short-circuit” the reciprocal synapse using glutamate receptor antagonists. When the AMPA receptor antagonist NBQX (25 µM) and NMDA receptor antagonist APV (50 µM) were used to block amacrine cell glutamate receptors, reciprocal feedback to the bipolar terminal was completely blocked (though minor spontaneous GABAergic input remained; Figure 4C). Under these conditions, endocytosis was again fast (τfast = 2.0 ± 0.5 s; 54% of ΔCm; τslow = 20.1 ± 3.4 s; n = 9; Figure 4C). Finally, to prevent chloride influx without application of any drugs, Cl− was removed from the external solution and replaced by methanesulfonate (125 mM). When this nearly chloride-free solution was used, no GABAergic Cl− currents appeared during the standard 200 ms depolarization, and endocytosis was fast (τfast = 1.7 ± 0.5 s; 44% of ΔCm; τslow = 12.2 ± 4.0 s; n = 7; Figure 4D). Taken together, these recordings suggest that Cl− influx through ionotropic GABA receptors can inhibit fast endocytosis.
Elevated [Cl−]i Inhibits Endocytosis
Over the course of our whole-cell mode recordings, endocytosis typically slowed with successive depolarizations until it completely “washed out” (see below). For this reason, we used the fastest endocytosis from each recording (which usually occurs within the first 2 min of whole-cell recording) as a measure of each terminal’s capacity for endocytosis. Though nystatin-perforated patch can slow this washout, we were unable to reliably record with nystatin from isolated terminals in slices. Exocytosis in the bipolar cell requires around 25 s between 200 ms depolarizing pulses to achieve a reproducible Cm jump with compensatory endocytosis (von Gersdorff et al., 1998; Neves and Lagnado, 1999). With this time frame as a limiting factor, and the complication of time-dependent washout, we were unable to consistently accelerate endocytosis by washing picrotoxin onto slices for direct comparison of membrane retrieval kinetics before and after inhibiting Cl− influx. We therefore turned to the acutely dissociated terminals in order to test the hypothesis that elevated chloride inhibits endocytosis in a more restrictive and controlled preparation that avoids complex synaptic interactions.
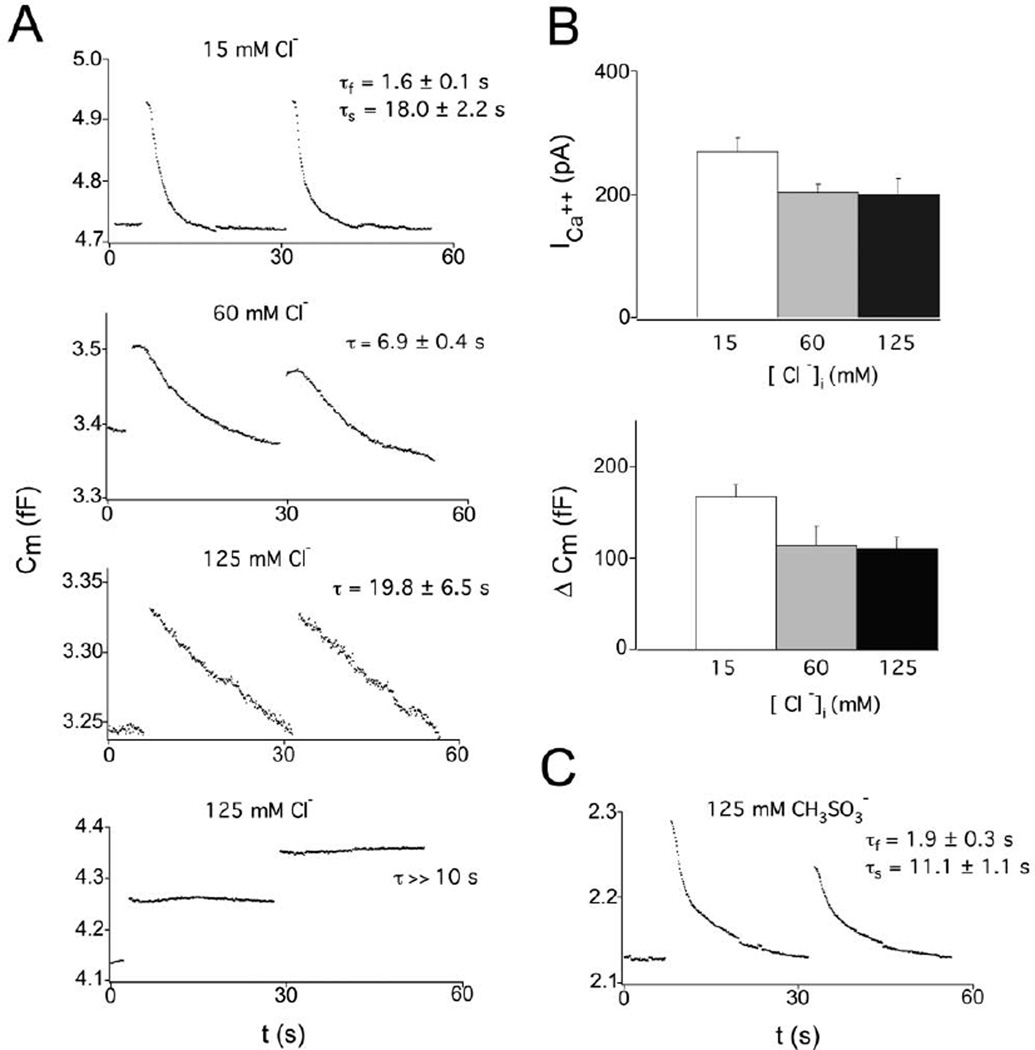
In acutely dissociated terminals, elevated internal chloride slowed the kinetics of endocytosis in a dose-dependent manner (Figure 5A). We tested the following three concentrations of internal chloride: 15 mM, 60 mM, and 125 mM. As reported in Figure 2, endocytosis in 15 mM internal chloride had a fast kinetic component with a time constant of about 2 s and a slow component with a time constant of more than 10 s (τfast = 1.6 ± 0.1 s; τslow = 18.0 ± 2.2 s; n = 23). At 60 mM internal chloride, endocytosis began after a brief delay and had only one slow kinetic component (τendo = 6.9 ± 0.4 s; n = 14). At 125 mM internal chloride, the fast component of endocytosis was also completely blocked (τendo = 19.8 ± 6.5 s; n = 6), and we frequently observed step-like capacitance changes with no endocytosis (τ >> 10 s; n = 7; Figure 5A). Internal chloride has been measured at 22 mM in the terminals of ON-type bipolar cells of the rat retina (Billups and Attwell, 2002), and GABA-mediated chloride influx has been shown to raise internal chloride to 90 mM above resting levels in the somata of cultured hippocampal cells (Kuner and Augustine, 2000). More recently, resting somatic chloride concentrations in mouse bipolar cells have been shown to vary between 5 and 40 mM with two-photon imaging (J. Dübel et al., 2004, ARVO, abstract). Because the bipolar cell terminal is completely enveloped by GABAergic boutons, the levels of chloride tested here may represent a reasonable approximation of the physiological range. Also, we suggest that global chloride elevations may not be necessary under physiological conditions, because GABA receptors are ideally situated to transiently raise internal Cl− concentrations directly beneath the membrane where endocytosis must occur (see Discussion and Figure 9).
Figure 5. Elevated Internal Cl− Slows the Kinetics of Endocytosis in Acutely Dissociated Terminals.
(A) Endocytosis (Cm decay) was measured under internal Cl− concentrations of 15 (n = 23), 60 (n = 14), and 125 (n = 13) mM. Inhibition of endocytosis in elevated chloride was observed following the first depolarization after 10 s of whole-cell mode break-in. In 125 mM Cl−, six cells exhibited extremely slow endocytosis, and seven terminals had no endocytosis.
(B) The average calcium current (ICa++) was approximately 70 pA smaller in the terminals with 60 and 125 mM internal chloride, and exocytosis was accordingly reduced.
(C) A pipette solution with 110 mM methanesulfonate and 15 mM Cl− did not alter the kinetics of endocytosis as compared with gluconate (n = 7).
Figure 9. The Bipolar Cell to Amacrine Cell Reciprocal Synapse.
(A) TEM of the goldfish Mb-type bipolar cell terminal ribbon synapse. The bipolar cell terminal (top) has glutamate-filled vesicles in the cytoplasm and a synaptic ribbon anchored to the plasma membrane. The ribbon is surrounded by a halo of synaptic vesicles. A reciprocal GABAergic synapse (arrow) from an amacrine cell is located near the ribbon. Note the cluster of vesicles (arrow) and narrow synaptic cleft.
(B) Schematic representation of the synaptic ribbon and reciprocal synapse with the relative locations of important receptors and channels. The proximity of GABA channels to the ribbon and putative sites of endocytosis implies that a local, submembrane increase in [Cl−]i (yellow region) can influence endocytosis. The duration and spread of these high-[Cl−]i “microdomains” will depend on the location and density of Ca2+-activated Cl− channels [Cl−(Ca)] and ECl. We have placed the Cl−(Ca) channels further from Ca2+ channels than the docked vesicles because ICl(Ca) can be blocked by 5 mM EGTA (despite its high Ca2+ affinity), whereas 27% of exocytosis (ΔCm jump) is resistant to this concentration of EGTA.
In dissociated terminals with low internal chloride, we observed larger Ca2+ currents and Cm jumps on average (Figure 5B). The averaged I–V relationship showed a 4.5 mV hyperpolarized shift in the peak ICa2+ under 125 mM internal chloride (n = 4) as compared with 15 mM chloride (n = 10), although there was no change in the kinetics of ICa2+ activation (data not shown). This I–V shift is not large enough to completely explain the difference in ICa2+ amplitudes. However, under 5 mM internal EGTA, we found that Ca2+ currents and Cm jumps were the same under high and low internal chloride (see Figure 5). The Ca2+ currents under 5 mM EGTA were also the same as those recorded in 0.5 mM EGTA with 60 and 125 mM chloride. Thus, only the low-chloride, low-EGTA Ca2+ currents were somewhat different. Although the reason for this difference is unclear, we note that the amplitude of Ca2+ currents varies between terminals and that the currents measured here fall well within the range of expected variability for these acutely dissociated terminals. Elevated internal chloride did not change the length of recordings, the degree of washout, or the reproducibility of multiple rounds of exocytosis (data not shown), suggesting that there was no change in the overall health of terminals with elevated internal chloride. Interestingly, low external Cl− has been shown to reduce L-type Ca2+ currents in photoreceptors (Thoreson et al., 2000).
To determine whether the effect of internal Cl− could be reproduced with other internal anions, we also tested internal solutions with 125 mM methanesulfonate (CH3SO3−) and nitrate (NO3−). Methanesulfonate did not alter the kinetics of endocytosis as compared with our standard counteranion gluconate (τfast = 1.9 ± 0.3 s; τslow = 11.1 ± 1.1 s; n = 7; Figure 5C). Internal nitrate, however, produced an unidentified standing inward current and exceptionally large tail currents due to its high permeability through chloride channels. The sustained inward nitrate currents and large tail currents made capacitance measurements unreliable (data not shown), so endocytosis could not be measured. It thus appears that high concentrations of Cl− anions selectively block endocytosis, whereas the much larger methanesulfonate or gluconate (C6H11O7−) anions do not have this ability.
Cl− Inhibition of Endocytosis and Ca2+ Buffering
Because membrane capacitance measures only net changes in membrane surface area, we wanted to ensure that the apparent slowing of Cm decay (or endocytosis) was not simply due to continued exocytosis masking normal endocytosis (Rieke and Schwartz, 1996). Exocytosis elicited by a 200 ms depolarizing pulse from −60 to 0 mV has been shown to exhaust the readily releasable pool of vesicles, saturating the capacitance response and resulting in a postexocytic capacitance measurement that reflects the much slower process of endocytosis (von Gersdorff and Matthews, 1994a). Asynchronous release was also shown to last at most 300 ms after a strong depolarizing pulse of 200 ms (von Gersdorff et al., 1998) and was also not detected for 200 ms pulses with FM1-43 fluorescence (Neves and Lagnado, 1999). It is possible, however, that raising internal chloride somehow disrupts the terminal’s calcium buffering capacity so that asynchronous exocytosis contaminates the postdepolarization Cm measurement. To test this idea, we measured endocytosis with 5 mM internal EGTA in high- and low-chloride solutions to more tightly buffer internal calcium without completely blocking release (Mennerick and Matthews, 1996; Sakaba et al., 1997). EGTA (5 mM) reduced exocytosis by ~73% in both 15 and 125 mM chloride, but high chloride still slowed the kinetics of endocytosis (Figure 6). Endocytosis was fast in all cells with 5 mM EGTA and low internal chloride, but we observed both single and double exponential kinetics for endocytosis. For this reason, and to directly compare the rate of endocytosis under different conditions, all individual jumps were scaled to the approximate mean jump (50 fF) and averaged for exponential fitting of endocytosis in high and low chloride (Figure 6B). In 15 mM internal chloride with 5 mM EGTA, the averaged endocytosis trace had a single exponential time constant of 2.7 s (n = 8). Alternatively, averaging the fast component of endocytosis from terminals with double exponential kinetics with the single component of endocytosis from the remaining terminals yields a measurement of τfast = 1.5 ± 0.3 s (n = 8). This rate of fast endocytosis in 5 mM EGTA is not different from the rate measured in dissociated terminals with 0.5 mM EGTA (τfast = 1.9 ± 0.2 s; p = 0.14). In contrast, 125 mM internal chloride and 5 mM EGTA produced an averaged endocytosis trace with a single time constant of 10.1 s (n = 6).
Figure 6. High Cl− Inhibits Endocytosis in Dissociated Terminals under 5 mM Internal EGTA.
(A) At 0.5 mM EGTA, Ca2+-activated chloride tail currents [ICl(Ca); arrows] are present under both high- and low-Cl− internal solutions. In contrast, ICl(Ca) is completely blocked with both high- and low-chloride internals in 5 mM EGTA.
(B) Membrane capacitance was averaged for six terminals with high chloride (blue) and eight terminals with low chloride (red) under 5 mM EGTA. In high chloride, the averaged endocytosis was best fit with a single, slow exponential. Averaged endocytosis was significantly faster under low chloride and was best fit with a single exponential.
(C and D) The amplitude of ICa++ was the same for high and low Cl− under 5 mM EGTA, and exocytosis was reduced by ~73% as compared to 0.5 mM EGTA.
We also elevated chloride and exogenous calcium buffer in terminals from the slice preparation. With 0.5 mM EGTA and 125 mM Cl− in the patch pipette, endocytosis was slow with a single time constant (τ = 12.7 ± 1.4 s) in four terminals and completely absent in six other terminals. In an additional ten slice terminals with high internal chloride (125 mM) and picrotoxin (50 µM) in the external bath, endocytosis was also slow (τ = 12.2 ± 1.2 s; n = 8; no endocytosis in two terminals; Figure 7A). These data indicate that picrotoxin cannot somehow accelerate endocytosis by itself when Cl− is elevated via the patch pipette. When the exogenous internal calcium buffer was elevated in the slice to 5 mM EGTA (with 15 mM internal Cl− and 50 µM external picrotoxin), endocytosis remained as fast as it was under control conditions (τfast = 1.5 ± 0.2 s; 34% of ΔCm; τslow = 20.7 ± 4.9 s; n = 8; Figure 7B). In this experiment, all eight terminals exhibited double exponential endocytosis kinetics. When the exogenous calcium buffer was elevated to 5 mM EGTA with 125 mM internal Cl−, however, endocytosis was slow in nine of nine terminals (τendo = 22.6 ± 5.8 s; n = 7; no endocytosis in two remaining terminals). This difference in endocytosis between high and low internal Cl− solutions with 5 mM EGTA was not affected by variability in Ca2+ current amplitudes. Larger Ca2+ currents have been reported to increase the relative amount of fast endocytosis (but not its absolute time constant) under elevated internal EGTA (Neves et al., 2001). As shown in Figures 7A and 7B, Ca2+ current amplitudes did not affect the inhibition of endocytosis by internal Cl−. In these examples, Ca2+ current amplitudes (insets) were larger in high internal Cl−, and endocytosis still remained slower. Notably, exocytosis was larger in the slice preparation under 5 mM EGTA than in dissociated terminals with the same amount of calcium buffer (5 mM EGTA; Cm jumps, slice: 200 ms = 96 ± 7 fF, n = 18; Cm jumps, dissociated: 200 ms = 46 ± 4 fF, n = 15). Moreover, though the resting capacitance and calcium currents were larger on average in the slice preparation (as reported above), the difference in exocytosis between preparations under 5 mM EGTA was much greater than the difference under 0.5 mM EGTA. Thus, terminals in the slice have twice the amount of EGTA-resistant exocytosis, suggesting the possibility of tighter coupling between Ca2+ channels and docked vesicles in slice terminals.
Figure 7. High Cl− Inhibits Endocytosis in Terminals from the Slice Treated with 50 µM External PTX under 5 mM Internal EGTA.
(A) At 0.5 mM internal EGTA, Ca2+-activated chloride tail currents [ICl(Ca)] are present under both high- (blue) and low (red)-chloride internal solutions in the PTX-treated slice (arrow on inset). As in the dissociated terminals, endocytosis has both a fast and slow component with 15 mM internal Cl− (red Cm trace). However, at 125 mM internal Cl− endocytosis has no fast component (blue Cm trace) and is well fit with slower, single exponentials.
(B) At 5 mM internal EGTA, endocytosis remains fast in PTX-treated slices under low internal Cl− (red Cm trace), and ICl(Ca) is nearly completely abolished (arrow on inset). Note that high EGTA does not slow endocytosis per se. At 125 mM internal Cl− and 5 mM EGTA, endocytosis in PTX-treated slices again has no fast component (blue Cm trace) and can be fit with a slow, single exponential.
In summary, the overall kinetics of endocytosis were consistently about an order of magnitude slower under high internal chloride in both the dissociated and slice preparations, whether 0.5 or 5.0 mM EGTA was used. We were confident that 5 mM EGTA tightly buffered calcium in both high and low Cl− because the Ca2+-activated chloride tail current was completely absent in both cases (Figure 6A; Okada et al., 1995). In addition, calcium-activated chloride tail currents [ICl(Ca)] had the same decay kinetics with 0.5 mM internal EGTA under both high and low internal chloride, suggesting that internal calcium buffering was not disrupted by high chloride (15 mM Cl−: τCl(Ca) = 0.54 ± 0.07 s, n = 13; 125 mM Cl−: τCl(Ca) = 0.48 ± 0.08 s, n = 13). These time constants are very similar to those reported by Okada et al. (1995) and Burrone et al. (2002).
Endocytosis with Endogenous Ca Buffering
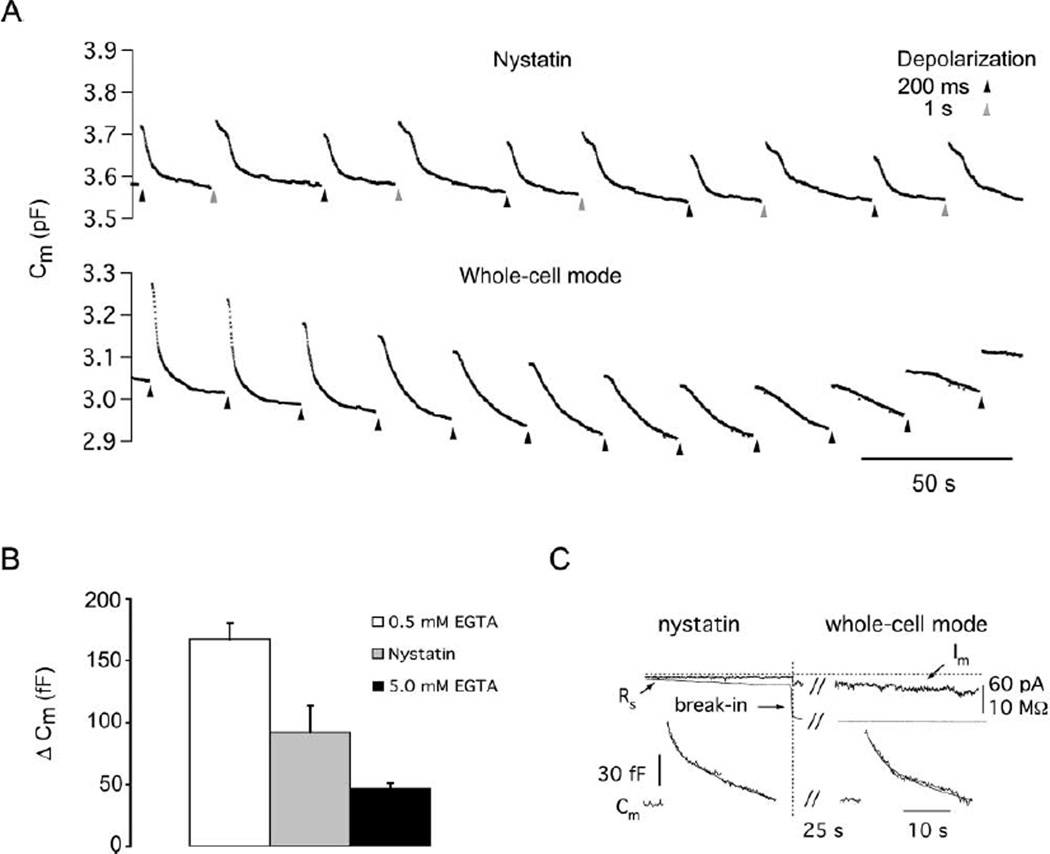
In acutely dissociated bipolar cell terminals, endogenous calcium buffer has been reported to lie near the equivalent of 0.4 mM BAPTA or 2 mM EGTA (Burrone et al., 2002). Given previous reports that calcium can inhibit (von Gersdorff and Matthews, 1994b; Rouze and Schwartz, 1998) or alter the amount of slow endocytosis (Neves et al., 2001), we wanted to ensure that our control endocytosis under low Cl− and 0.5 mM EGTA was comparable with endocytosis under endogenous calcium buffers. We therefore measured endocytosis under nystatin-perforated patch with low internal Cl− in acutely dissociated terminals (Figure 8A). The kinetics of endocytosis under nystatin-perforated patch (τfast = 1.7 ± 0.6 s; 51% of ΔCm; τslow = 19.7 ± 4.5 s; n = 10) were similar to those recorded in the whole-cell mode with low internal Cl− and 0.5 EGTA. However, washout of endocytosis was less pronounced under nystatin-perforated patch, and the gradual drift in baseline capacitance was reduced (see Supplemental Figure S1 at http://www.neuron.org/cgi/content/full/44/3/469/DC1/). These aspects of endocytosis with perforated patch recordings have also been observed in other preparations (Parsons et al., 1994; Smith and Neher, 1997). Measurements of exocytosis following 200 ms depolarizations under nystatin-perforated patch (92 ± 21 fF; n = 10) were intermediate between 0.5 mM EGTA (167 ± 14 fF; n = 23) and 5 mM EGTA (47 ± 5 fF; n = 9) in the dissociated terminals under whole-cell mode (Figure 8B). Thus, these data support the estimate of 2 mM EGTA as close to the endogenous calcium buffer at the bipolar cell terminal (Burrone et al., 2002). Additionally, as previously reported by Neves et al. (2001), we find that raising the exogenous calcium buffer does not alter either the fast or slow time constant of endocytosis (though the percentage of membrane retrieved by the fast component of endocytosis can be reduced under endogenous calcium buffer). These rate constants remained fixed under all calcium buffering conditions with low internal Cl−, including experiments where the nystatin-perforated patch was intentionally ruptured into whole-cell mode with 0.5 mM EGTA in the patch pipette (nine terminals; Figure 8C). Under both low and high internal EGTA, however, we were consistently able to abolish fast endocytosis by elevating internal Cl−, leading us to conclude that the effect of Cl− on endocytosis is distinct from any role of internal free calcium.
Figure 8. The Kinetics of Endocytosis under Whole-Cell Mode and Nystatin-Perforated Patch Mode.
(A) Cm traces with no baseline subtraction. Under nystatin-perforated patch, the drift in baseline Cm is less severe, and the kinetics of endocytosis persist for longer than under whole-cell dialysis. In nystatin mode, depolarizing pulses of 200 ms and 1 s were applied in alternation, while in whole cell only 200 ms pulses were given every 25 s. Note that endocytosis washes out more quickly than exocytosis in the whole-cell mode.
(B) Exocytosis (ΔCm jump) under nystatin-perforated patch is intermediate between 0.5 mM EGTA and 5 mM EGTA whole-cell recordings.
(C) Upon break-in from nystatin-perforated patch to whole-cell mode, the endocytosis remains the same for a short period before washout (n = 9). The vertical dashed line indicates the time of break-in to whole-cell mode. The horizontal dashed line is the 0 pA current level. Im is the leak current at a holding potential of −60 mV. Rs started at 25 MΩ and dropped from 23 to 11 MΩ immediately at break-in to whole-cell mode. For the endocytosis responses, nystatin: τfast = 1.5 s; 20% of ΔCm; τslow = 34 s; whole-cell mode: τfast = 3.0 s; 31% of ΔCm; τslow = 28 s.
Discussion
We have observed a 5- to 10-fold slower rate of endocytosis (Cm decay) in terminals from retinal slices as compared to acutely dissociated terminals. We propose that this striking difference is due to the GABAergic synapses that cover the bipolar cell terminals’ surface in the slice and produce an elevated [Cl−]i that selectively inhibits fast endocytosis. In contrast, we find that exocytosis (Cm jump, under 0.5 mM internal EGTA) is not significantly different between bipolar cell terminals in slice and acute dissociation. This suggests that acute dissociation does not disrupt the cytoskeleton to a degree that alters exocytosis. Synaptic ribbons, the preferred sites of exocytosis, are thus also likely to remain largely intact in freshly dissociated Mb bipolar cells (Zenisek et al., 2003).
GABAergic Synapses and the Site of Endocytosis
We have shown that manipulations that prevent Cl− influx though presynaptic ionotropic GABA receptors also allow a fast component of endocytosis. This raises the interesting possibility that the most physiologically relevant factor governing the kinetics endocytosis in the slice is not global changes in Cl− concentration, but rather local increases in Cl− near the plasma membrane that result from Cl− influx through GABA receptors. This implies that the molecular trigger for fast endocytosis is sensitive to elevations of [Cl−]i near the plasma membrane. At this synapse, the site of reciprocal GABA feedback is very close to the synaptic ribbons where exocytosis occurs (Figure 9; see also Marc and Liu, 2000) and where endocytosis is also believed to occur (Roos and Kelly, 1999; Lenzi et al., 2002). Thus, GABA receptors may be ideally located near the site of endocytosis, where they could produce transient microdomains of high Cl−. And, although Cl− would not remain elevated for long, it may only need to be high when fast endocytosis, is initiated immediately after exocytosis. We have never observed an endocytosis event with a late fast component, so it is likely that fast endocytosis is triggered immediately after exocytosis or not at all. Thus, a transient and local Cl− elevation may be sufficient to block the triggering of fast endocytosis.
Ca2+- and Cl−-Dependent Endocytosis: Distinct Phenomena
Fast and slow forms of endocytosis have been reported in the frog neuromuscular junction (Miller and Heuser, 1984; Richards et al., 2000), where high-frequency activity slows the rate of endocytosis (Wu and Betz, 1996). Similarly, fast and slow forms of endocytosis have also been reported in Drosophila photoreceptors (Koenig and Ikeda, 1996). Recent capacitance measurements at mammalian nerve terminals have also revealed a fast component of endocytosis (τendo = 0.2–2.0 s) following short depolarizations (Hsu and Jackson, 1996; Sun et al., 2002). At goldfish bipolar cell terminals, short depolarizing pulses of less than 250 ms produce fast endocytosis (von Gersdorff and Matthews, 1994b). We confirm these latter findings by showing that pulses of 2, 20, and 200 ms result in similar rates of Cm decay (Figure 2B). Pulses of 200 ms generate about 100 times more Ca2+ influx than 2 ms pulses, but the endocytosis time constants are nevertheless the same for both pulses. These data suggest that the time constants of fast and slow endocytosis following depolarizations of ≤ 200 ms are independent of [Ca2+]i (see also Neves et al., 2001). However, we note that the percentage of fast endocytosis can increase with pulse duration (see Figure 2B). Thus, we suggest that the percentage of membrane retrieved by fast endocytosis may depend on the amount of preceding exocytosis.
In this study, we have shown that high internal Cl− blocks the fast component of endocytosis, even in the presence of 5 mM EGTA. Elevating internal Cl− does not therefore slow endocytosis by disrupting internal calcium buffering or somehow raising [Ca2+]i beyond normal levels, an action which could produce artificially slow Cm decay due to asynchronous release. Elevated [Ca2+]i caused by reduced Ca2+ buffering can also slow endocytosis independent of asynchronous release (von Gersdorff and Matthews, 1994b; Cousin and Robinson, 2000), but the results of our 5 mM EGTA experiments again argue against this possibility.
Since pipette pressure and osmolarity were carefully controlled in all experiments, the effect of elevated [Cl−]i on endocytosis cannot be attributed to either of these critical experimental conditions (see Experimental Procedures). As further evidence, we note that the fast component of endocytosis in bipolar cell terminals has been shown to be independent of pipette pressure and osmolarity changes (Heidelberger et al., 2002). Neurotoxic effects of high [Cl−]i are also unlikely to explain our results, because they occur only after a long exposure period (Chen et al., 1998). A similar contradiction to the idea that elevated Cl− is acutely toxic comes from rat melanotrophs, where high [Cl−]i has been shown to enhance Ca-dependent exocytosis via G protein modulation (Rupnik and Zorec, 1995). We therefore conclude that the [Cl−]i-mediated inhibition of fast endocytosis is not an epiphenomenon of these previously suggested mechanisms and that it constitutes an independent regulatory mechanism for endocytosis in ribbon synapses.
Factors Influencing the Time Course of [Cl−]i Decay
Although we suggest that only a transient elevation in [Cl−]i near the membrane during exocytosis may be required to block the triggering of fast endocytosis, the time course of internal Cl− elevation may influence the recovery of fast synaptic vesicle endocytosis. For this reason, we note that there are several mechanisms for Cl− extrusion from the bipolar cell terminals. Aside from normal clearance by pumps and ion cotransporters, such as the K+-Cl− (KCC2) cotransporter (Billups and Attwell, 2002; Vu et al., 2000), the calcium-activated Cl− tail current may provide a fast way for the bipolar cell terminal to remove internal Cl−. Interestingly, the normal rate of endocytosis is not affected by using high internal K+ in the patch pipette (von Gersdorff and Matthews, 1994b). Though it is not known how fast the internal Cl− transient might decay in our synaptic terminals, the time course of global Cl− changes and decay in other neurons can be very slow (i.e., several seconds; Kuner and Augustine, 2000; Marandi et al., 2002).
Fast Endocytosis: Clathrin Independent and Cl Sensitive
Little is known about the molecular mechanism of endocytosis at ribbon synapses. Evidence from electron microscopy suggests that vesicular membrane can be retrieved as large endosomes that are only later recycled into synaptic vesicles (Figure 9; Paillart et al., 2003). Paillart et al. report that clathrin-coated pits and vesicles are conspicuously absent from the bipolar cell terminal, though this evidence does not rule out the possibility of rapid clathrin-mediated endocytosis and uncoating that may escape capture due to the time required for EM fixation. Dynamin, a GTPase that is thought to be essential for endocytosis, has also been linked to clathrin-independent endocytosis (Artalejo et al., 1995; Daly et al., 2000). It is therefore surprising that GTPγS and GDPβS, which would be expected to inhibit dynamin, do not slow or prevent endocytosis in dissociated bipolar cell terminals (Heidelberger, 2001). Together, these results suggest that at least some fraction of endocytosis at the bipolar cell terminal may be clathrin and dynamin independent. Other studies, however, have found enriched quantities of the endocytosis proteins dynamin, amphiphysin, and clathrin in the ribbon-type synaptic terminals of photoreceptors (von Kriegstein et al., 1999; Yao et al., 2002). Early EM studies of Gray and Pease (1971) also clearly indicate that clathrin coats are found in the vicinity of photoreceptor synaptic ribbons. If clathrin-mediated endocytosis does occur at the bipolar cell terminal, it may be more likely to account for the slow form of endocytosis (De Camilli et al., 2001). At hippocampal boutons, where fast and slow forms of endocytosis have also been described, fluorescent imaging of clathrin has shown that there is a long-lasting delay before the onset of clathrin coat formation (Mueller et al., 2004). This finding suggests that any fast endocytosis would be clathrin independent. Transient vesicle fusion without complete collapse, termed kiss-and-run, has been implicated as a clathrin-independent form of fast endocytosis at Drosophila synapses (Verstreken et al., 2002). Because single vesicle imaging has shown no evidence for kiss-and-run endocytosis at the goldfish bipolar terminal (Zenisek et al., 2002), there is an emerging sense that this synapse contains an as yet unidentified form of fast, clathrin-independent endocytosis.
Given the lack of an identified mechanism for fast endocytosis at the bipolar cell terminal, it is difficult to pinpoint the action of chloride. One possibility to explain the effect of chloride ions on endocytosis relates to intracellular chloride channels (ClCs). ClCs are present in the membrane of synaptic vesicles, and their associated chloride gradient is important for vesicular acidification and transmitter filling. Vesicular pH may in turn regulate the conformation or expression of receptors in the vesicular membrane that recruit signaling molecules (Faundez and Hartzell, 2004). In the mouse, ClC-5 mutations have defects of endocytosis in renal epithelial cells, and ClC-3 knockouts have impaired synaptic vesicle acidification that leads to degeneration of the hippocampus and retina (Jentsch et al., 2002).
Another possible action of chloride ions could be to change internal surface charges. Surface charges regulate the electrostatic interactions of G proteins and several signaling molecules (e.g., PKC) with the phospholipid bilayer (Murray and Honig, 2002). Because signaling molecules such as PKC and calcineurin can modulate synaptic vesicle endocytosis (Liu et al., 1994; Marks and McMahon, 1998; Chan and Smith, 2003), and several other molecules with complex interactions have been implicated in endocytosis (Morgan et al., 2002), this mechanism should also be tested by future experiments.
Finally, we point out that chloride ions have recently been suggested to play an important role beyond their well-known function of changing membrane potential in other systems. For example, the outer hair cell transmembrane protein Prestin is a molecular motor that uses cytoplasmic chloride ions as extrinsic voltage sensors (Dallos and Fakler, 2002). Several chloride-dependent kinases and enzymes have also been described in the literature (Lanius et al., 1993; Lytle and Forbush, 1996; D’Amico et al., 2000). Together with our study, these results increasingly suggest that chloride may play a role akin to a “second messenger,” triggering and modulating cellular processes close to the plasma membrane. Interestingly, a recent report has shown that NO can modulate the rate of endocytosis in nerve terminals using a PIP2-mediated pathway (Micheva et al., 2003). Because the amount of endocytosis can change the output of a synapse by altering the availability of vesicles for release, modulating the kinetics of endocytosis by retrograde and ionic messengers (like Ca2+ and Cl−) may be a general property of synapses.
Experimental Procedures
Bipolar Cell Terminal Preparation
Bipolar cell terminals from the goldfish retina were acutely dissociated according to Heidelberger and Matthews (1992). All recordings were performed within 2–3 hr of plating. Retinal slice preparation and optics follow the methods of Palmer et al. (2003). Bipolar cell terminals with severed axons were identified in the inner plexiform layer based on the following: (1) resting membrane time constant (single exponential for isolated terminal; see Supplemental Figure S1 at http://www.neuron.org/cgi/content/full/44/3/469/DC1/); (2) the presence of an L-type calcium current, GABAergic input from amacrine cells, and Cm jump; and (3) Mb-shaped terminal morphology (Figure 1).
Electrophysiology and Capacitance Measurements
Standard external recording solutions contained the following: 120 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.0 mM MgCl2, 10 mM HEPES, and 12 mM glucose (pH 7.2; osmolarity 260–265). Patch pipettes were pulled from leaded capillary glass with a PP-830 Narishige vertical puller and coated with dental wax to reduce pipette capacitance. Internal pipette solutions, depending on experiment, contained one of the following solutions. Standard (low chloride): 110 mM Cs-Gluconate, 15 mM TEA-Cl, 25 mM HEPES, 3 mM Mg-ATP, 0.5 mM Na-GTP, and 0.5 mM EGTA; 60 mM chloride: same as standard solution except that 65 mM Cs-Gluconate, 50 mM CsCl, and 10 mM TEA-Cl were used instead of 110 mM Cs-Gluconate; high chloride (125 mM): same as standard solution except that 115 mM CsCl and 10 mM TEA-Cl were used; cesium methanesulfonate: same as standard solution except that 110 mM Cs-methanesulfonate was used instead of Cs-gluconate; cesium nitrate: same as standard solution except that 110 mM Cs-nitrate was used instead of Cs-gluconate. All internals were set to pH 7.2 with CsOH and an osmolarity of 260 (checked with a vapor pressure osmometer; Wescor, Vapro 5520). EGTA (5 mM) experiments were conducted in Cs-gluconate or Cs-Cl internals. Perforated patch recordings were made with nystatin (250 µg/ml, 0.4% DMSO). All recordings were performed with a digital manometer (WPI) attached to the pipette holder tubing for recording and controlling pipette pressure. Pipette pressure was kept slightly negative (−0.01 psi) to achieve the most reproducible endocytosis (Heidelberger et al., 2002). Picrotoxin (50 µM), BMI, TPMPA, and NBQX/APV were bath applied under constant perfusion. All chemicals were purchased from Sigma.
Isolated bipolar cell terminals were voltage clamped in the whole-cell or perforated patch mode using a HEKA EPC-9 double patch-clamp amplifier in conjunction with Pulse software running the X-chart extension (Pulse v. 8.53). The Sine + DC technique was used for real-time measurements of membrane capacitance. Briefly, a 30 mV peak-to-peak 1 kHz sine wave was superimposed on the cells’ holding potential (−60 mV) and used by online analysis software to calculate time-resolved membrane capacitance. Offline data analysis was performed using “IgorPro” software (Wavemetrics, Lake Oswego, OR). The decay in membrane capacitance due to endocytosis was best fit with either single or double exponentials using f(t) = y0 + A1e(−t/τ) for single exponentials or f(t) = y0 + A1e[−t/τ(fast)] + A2e[−t/τ(slow)] for double exponentials, where y0 is the horizontal asymptote defined by the baseline capacitance; A1 and A2 are the amplitudes of each exponential component; and τfast and τslow are the time constants for the first and second exponential components, respectively. We accepted double exponential fits if the time constants for fast (τfast) and slow (τslow) endocytosis differed by more than 2-fold and the amplitude of τfast was at least 10% of ΔCm. Because τfast ranged between 0.5 and 3 s, it could be easily distinguished from τslow (≥10 s). In most recordings, we observed a slow, exponential decay in the baseline capacitance that was subtracted from the raw X-chart traces (Supplemental Figure S1 at http://www.neuron.org/cgi/content/full/44/3/469/DC1/). Depolarizing pulses of 2, 20, and 200 ms were separated by 25 s to allow sufficient recovery time from synaptic depression. Series resistance (RS) in the whole-cell mode averaged about 14 MΩ (see the Supplemental Data for detailed recording parameters). Statistics were calculated using Prism (v. 3; GraphPad Software) with Student’s t tests for paired data sets and one-way ANOVAs for comparison of three or more data sets.
Supplementary Material
Acknowledgments
We thank Steven Yazulla (SUNY at Stony Brook, NY) for generously donating the goldfish bipolar cell electron micrograph. We thank Craig Jahr, Gary Matthews, Mary J. Palmer, and Jozsef Vigh for discussions. This work was supported by a grant from the NEI (H.v.G.) and an NRSA predoctoral fellowship (C.H.).
References
- Artalejo C, Henley J, McNiven M, Palfrey H. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+GTP, and dynamin but not clathrin. Proc. Natl. Acad. Sci. USA. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J. Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin L, Low P, Gad H, Gustafsson J, Pieribone V, Shupliakov O. Sustained neurotransmitter release: new molecular clues. Eur. J. Neurosci. 1997;9:2503–2511. doi: 10.1111/j.1460-9568.1997.tb01679.x. [DOI] [PubMed] [Google Scholar]
- Burrone J, Neves G, Gomis A, Cooke A, Lagnado L. Endogenous calcium buffers regulate fast exocytosis in the synaptic terminal of retinal bipolar cells. Neuron. 2002;33:101–112. doi: 10.1016/s0896-6273(01)00565-7. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J. Physiol. 2003;553:707–717. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Olney JW, Lukasiewicz PD, Almli T, Romano C. Ca2+-independent excitotoxic neurodegeneration in isolated retina, an intact neural net: A role for Cl− and inhibitory transmitters. Mol. Pharmacol. 1998;53:564–572. doi: 10.1124/mol.53.3.564. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ca2+ inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. J. Neurosci. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico S, Gerday C, Feller G. Structural similarities and evolutionary relationships in chloride-dependent α-amylases. Gene. 2000;253:95–105. doi: 10.1016/s0378-1119(00)00229-8. [DOI] [PubMed] [Google Scholar]
- Dallos P, Fakler B. Prestin, a new type of motor protein. Nat. Rev. Mol. Cell Biol. 2002;3:104–111. doi: 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- Daly C, Sugimori M, Moreira JE, Ziff EB, Llinás R. Synaptophysin regulates clathrin-independent endocytosis of synaptic vesicles. Proc. Natl. Acad. Sci. USA. 2000;97:6120–6125. doi: 10.1073/pnas.97.11.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCamilli P, Slepnev VI, Shupliakov O, Brodin L. Synaptic vesicle endocytosis. In: Cowen M, Sudhof TC, Stevens CF, editors. Synapses. Baltimore: The Johns Hopkins University Press; 2001. pp. 217–274. [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/s0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dong C, Werblin F. Temporal contrast enhancement via GABAC feedback at bipolar terminals in tiger salamander retina. J. Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- Faundez V, Hartzell HC. Intracellular chloride channels: determinants of function in the endosomal pathway. Sci. STKE. 2004;2004:re8. doi: 10.1126/stke.2332004re8. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Gray EG, Pease HL. On understanding the organization of retinal receptor synapses. Brain Res. 1971;35:1–15. doi: 10.1016/0006-8993(71)90591-9. [DOI] [PubMed] [Google Scholar]
- Guatimosim C, Hull C, von Gersdorff H, Prado MA. Okadaic acid disrupts vesicle trafficking in a ribbon-type synapse. J. Neurochem. 2002;82:1047–1057. doi: 10.1046/j.1471-4159.2002.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Reciprocal synaptic interactions between rod bipolar cells and amacrine cells in the rat retina. J. Neurophysiol. 1999;81:2923–2936. doi: 10.1152/jn.1999.81.6.2923. [DOI] [PubMed] [Google Scholar]
- Heidelberger R. ATP is required at an early step in compensatory endocytosis in synaptic terminals. J. Neurosci. 2001;21:6467–6474. doi: 10.1523/JNEUROSCI.21-17-06467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J. Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Zhou Z-Y, Matthews G. Multiple components of membrane retrieval in synaptic terminals revealed by changes in hydrostatic pressure. J. Neurophysiol. 2002;88:2509–2517. doi: 10.1152/jn.00267.2002. [DOI] [PubMed] [Google Scholar]
- Hsu S-F, Jackson MB. Rapid exocytosis and endocytosis in nerve terminals of the rat posterior pituitary. J. Physiol. 1996;494:539–553. doi: 10.1113/jphysiol.1996.sp021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik A. Molecular structure and physiological function of chloride channels. Phys. Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Klingauf J, Kavalali E, Tsien R. Kinetics and regulation of fast endocytosis at hippocampal synapses. Nature. 1998;394:581–585. doi: 10.1038/29079. [DOI] [PubMed] [Google Scholar]
- Koenig J, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J. Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Augustine GJ. A genetically encoded ratiometric indicator for chloride: capturing chloride transients in cultured hippocampal cells. Neuron. 2000;27:447–459. doi: 10.1016/s0896-6273(00)00056-8. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Pasqualotto BA, Shaw CA. Gamma-aminobutyric acid A receptor regulation by a chloride-dependent kinase and a sodium-dependent phosphatase. Brain Res. Mol. Brain Res. 1993;20:192–198. doi: 10.1016/0169-328x(93)90041-m. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Crum J, Ellisman MH, Roberts WM. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron. 2002;36:649–659. doi: 10.1016/s0896-6273(02)01025-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Sim A, Robinson P. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–973. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz PD, Shields C. Differential combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J. Neurophysiol. 1998;79:3157–3167. doi: 10.1152/jn.1998.79.6.3157. [DOI] [PubMed] [Google Scholar]
- Lytle C, Forbush B., III Regulatory phosphorylation of the secretory Na-K-Cl cotransporter: modulation by cytoplasmic Cl. Am. J. Physiol. 1996;270:C437–C448. doi: 10.1152/ajpcell.1996.270.2.C437. [DOI] [PubMed] [Google Scholar]
- Marandi N, Konnerth A, Garashuk O. Two-photon chloride imaging in neurons of brain slices. Pflugers Arch. 2002;445:357–365. doi: 10.1007/s00424-002-0933-7. [DOI] [PubMed] [Google Scholar]
- Marc RE, Liu W-LS. Fundamental GABAergic amacrine cell circuits in the retina: nested feedback, concatenated inhibition, and axosomatic synapses. J. Comp. Neurol. 2000;425:560–582. doi: 10.1002/1096-9861(20001002)425:4<560::aid-cne7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr. Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J. Neurosci. 1994;14:1079–1090. doi: 10.1523/JNEUROSCI.14-03-01079.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillem G, Rotolo T, Dacheaux R. GABA responses of rod bipolar cells in rabbit retinal slices. Vis. Neurosci. 2000;17:381–389. doi: 10.1017/s0952523800173067. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996;17:1241–1249. doi: 10.1016/s0896-6273(00)80254-8. [DOI] [PubMed] [Google Scholar]
- Micheva K, Buchanan J, Holz R, Smith S. Retrograde regulation of synaptic vesicle endocytosis and recycling. Nat. Neurosci. 2003;6:925–932. doi: 10.1038/nn1114. [DOI] [PubMed] [Google Scholar]
- Miller T, Heuser J. Endocytosis of synaptic vesicle membrane at the frog neuromuscular junction. J. Cell Biol. 1984;98:685–698. doi: 10.1083/jcb.98.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Augustine GJ, Lafer EM. Synaptic vesicle endocytosis: the races, places, and molecular faces. Neuromolecular Med. 2002;2:101–114. doi: 10.1385/NMM:2:2:101. [DOI] [PubMed] [Google Scholar]
- Mueller VJ, Wienisch M, Nehring RB, Klingauf J. Monitoring clathrin-mediated endocytosis during synaptic activity. J. Neurosci. 2004;24:2004–2012. doi: 10.1523/JNEUROSCI.4080-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D, Honig B. Electrostatic control of the membrane targeting of C2 domains. Mol. Cell. 2002;9:145–154. doi: 10.1016/s1097-2765(01)00426-9. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Lagnado L. The kinetics of exocytosis and endocytosis in the synaptic terminal of goldfish retinal bipolar cells. J. Physiol. 1999;515:181–202. doi: 10.1111/j.1469-7793.1999.181ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects for the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc. Natl. Acad. Sci. USA. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Horiguchi H, Tachibana M. Ca2+-dependent Cl current at the presynaptic terminals of goldfish bipolar cells. Neurosci. Res. 1995;23:297–303. doi: 10.1016/0168-0102(95)00955-8. [DOI] [PubMed] [Google Scholar]
- Paillart C, Li J, Matthews G, Sterling P. Endocytosis and vesicle recycling at a ribbon synapse. J. Neurosci. 2003;23:4092–4099. doi: 10.1523/JNEUROSCI.23-10-04092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J. Neurosci. 2003;23:4831–4841. doi: 10.1523/JNEUROSCI.23-12-04831.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons T, Lenzi D, Almers W, Roberts W. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Protti DA, Llano I. Calcium currents and calcium signalling in rod bipolar cells of rat retinal slices. J. Neurosci. 1998;18:3715–3724. doi: 10.1523/JNEUROSCI.18-10-03715.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytotic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/s0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Rieke F, Schwartz E. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J. Physiol. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, Kelly RB. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr. Biol. 1999;9:1411–1414. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Rouze N, Schwartz E. Continuous and transient vesicle cycling at a ribbon synapse. J. Neurosci. 1998;18:8614–8624. doi: 10.1523/JNEUROSCI.18-21-08614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnik M, Zorec R. Intracellular Cl− modulates Ca2+-induced exocytosis from rat melanotrophs through GTP-binding proteins. Pflügers Arch. 1995;431:76–83. doi: 10.1007/BF00374379. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Tachibana M, Matsui K, Minami N. Two components of transmitter release in retinal bipolar cells: exocytosis and mobilization of synaptic vesicles. Neurosci. Res. 1997;27:357–370. doi: 10.1016/s0168-0102(97)01168-1. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNARES at hippocampal synapses. Nat. Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sherry D, Yazulla S. Goldfish bipolar cells and axon terminal patterns: a Golgi study. J. Comp. Neurol. 1993;329:188–200. doi: 10.1002/cne.903290204. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J. Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J. Cell Biol. 1997;139:885–894. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J-Y, Wu X, Wu L-G. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. Gamma-aminobutyric acid exerts a local inhibitory action on the axon terminal of bipolar cells: evidence for negative feedback from amacrine cells. Proc. Natl. Acad. Sci. USA. 1987;84:3501–3505. doi: 10.1073/pnas.84.10.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. ’Delayed’ endocytosis is regulated by extracellular Ca2+ in snake motor boutons. J. Physiol. 2003;551:103–114. doi: 10.1113/jphysiol.2003.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB, Nitzan R, Miller RF. Chloride efflux inhibits single calcium channel open probability in vertebrate photoreceptors: Chloride imaging and cell-attached patch-clamp recordings. Vis. Neurosci. 2000;17:197–206. doi: 10.1017/s0952523800172025. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Kjaeruff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994a;367:735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994b;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence that vesicles on the synaptic ribbon of retinal bipolar neurons can be rapidly released. Neuron. 1996;16:1221–1227. doi: 10.1016/s0896-6273(00)80148-8. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Sakaba T, Berglund K, Tachibana M. Submillisecond kinetics of glutamate release from a sensory synapse. Neuron. 1998;21:1177–1188. doi: 10.1016/s0896-6273(00)80634-0. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Schmitz F, Link E, Sudhof TC. Distribution of synaptic vesicle proteins in the mammalian retina identifies obligatory and facultative components of ribbon synapses. Eur. J. Neurosci. 1999;11:1335–1348. doi: 10.1046/j.1460-9568.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J. Neurosci. 2000;20:1414–1423. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Dowling J. Synaptic relationships in the plexiform layers of carp retina. Z. Zellforsch. Mikrosk. Anat. 1969;100:60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Coleman PD, Calkins DJ. High-resolution localization of clathrin assembly protein AP180 in the presynaptic terminals of mammalian neurons. J. Comp. Neurol. 2002;447:152–162. doi: 10.1002/cne.10217. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Steyer JA, Feldman ME, Almers W. A membrane marker leaves synaptic vesicles in milliseconds after exocytosis in retinal bipolar cells. Neuron. 2002;35:1085–1097. doi: 10.1016/s0896-6273(02)00896-6. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J. Neurosci. 2003;23:2538–2548. doi: 10.1523/JNEUROSCI.23-07-02538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ganetzky B, Bellen HJ, Murthy VN. Tailoring uniform coats for synaptic vesicles during endocytosis. Neuron. 1999;23:419–422. doi: 10.1016/s0896-6273(00)80794-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.