Abstract
The epidermal growth factor (EGF) transduces its actions via the EGF receptor (EGFR), which can traffic from the plasma membrane to either the cytoplasm or the nucleus. However, the mechanism by which EGFR reaches the nucleus is unclear. To investigate these questions, liver cells were analyzed by immunoblot of cell fractions, confocal immunofluorescence and real time confocal imaging. Cell fractionation studies showed that EGFR was detectable in the nucleus after EGF stimulation with a peak in nuclear receptor after 10 min. Movement of EGFR to the nucleus was confirmed by confocal immunofluorescence and labeled EGF moved with the receptor to the nucleus. Small interference RNA (siRNA) was used to knockdown clathrin in order to assess the first endocytic steps of EGFR nuclear translocation in liver cells. A mutant dynamin (dynamin K44A) was also used to determine the pathways for this traffic. Movement of labeled EGF or EGFR to the nucleus depended upon dynamin and clathrin. This identifies the pathway that mediates the first steps for EGFR nuclear translocation in liver cells.
Keywords: Epidermal growth factor receptor, Nuclear translocation, Liver, Clathrin
1. Introduction
EGFR is a receptor tyrosine kinase (RTK) that is activated by binding to its ligands [1,2]. The EGFR ligand complex forms homo or hetero dimers resulting in EGFR autophosphorylation and activation [1]. The activated EGFR is rapidly internalized into early endosomes and subsequently recycled to the plasma membrane or sorted to late endosomes–lysosomes for degradation [3,4]. Internalization into endosomal vesicles and degradation in lysosomes is thought to be the mechanism to terminate receptor activation [5].
Recent evidence has identified additional intracellular destinations for the internalized receptor as well, including the nucleus [6,7]. Over the past decade, evidence has accumulated showing that either full-length or fragmented EGFR family members can be shuttled from the plasma membrane to the nucleus [8–12]. Increased nuclear localization of the EGFR is associated with poor prognosis of tumors and treatment resistance [13].
The mechanisms proposed for nuclear transport of membrane proteins include the activity of transmembrane domain-binding chaperones, endosome-mediated nuclear translocation, and retrotranslocation by endoplasmic reticulum (ER)-associated trafficking machinery [7,14–16]. Although certain intracellular trafficking destinations for activated EGFRs, such as the lysosome or recycling to the cell surface, are relatively well understood [17], little is known regarding the mechanism by which this or other cell surface receptors reach the nucleus.
Regardless of some authors assume that EGFR is internalized by clathrin dependent pathway, there is no available data directly showing the roles of this pathway on EGFR nuclear translocation. Hence, the aim of this work was to elucidate the first endocytic step that mediates the EGFR nuclear translocation in liver cells.
2. Materials and methods
2.1. Cells and cell culture
SKHep-1 liver cell line was obtained from ATCC (Manassas, VA). Cells were cultured at 37 °C in 5% CO2 in Dulbecco’s modified Eagle’s medium (Invitrogen, CA) containing 10% fetal bovine serum, 1 mM sodium pyruvate, 50 units/ml penicillin, and 50 g/ml streptomycin (Invitrogen, CA). Hepatocytes were isolated from the livers of male Sprague–Dawley rats (190–200 g; Charles River Laboratories, Wilmington, MA) by collagenase perfusion as described previously [18,19]. Primary hepatocytes were cultured at 37 °C in 5% CO2/95% O2 in Williams’ medium E containing 10% fetal bovine serum, 50 units/mL penicillin, and 50 g/mL streptomycin (Invitrogen, CA) and plated on collagen-coated coverslips (50 g/mL) (BD Biosciences, CA). Hepatocytes were used 4–6 h after isolation. Viability of the hepatocytes was greater than 85% and was measured by trypan blue exclusion.
2.2. Immunoblotting
SKHep-1 cell immunoblots were performed as described previously [11,19–21]. Briefly, cells were washed twice with ice-cold phosphate-buffered saline, harvested by scraping, and lysed in a lysis buffer (20 mM HEPES, pH 7.0, 10 mM KCL, 2 mM MgCl2, 0.5% Nonidet P-40). After incubation on ice for 10 min, the cells were homogenized by vortex. The homogenate was centrifuged at 1500g for 5 min to sediment the nuclei. The supernatant was then centrifuged at a maximum speed of 16,100g for 20 min, and the resulting supernatant formed the non nuclear fraction. The nuclear pellet was washed three times with lysis buffer to remove any contamination from cytoplasmic membranes, and the purity of the nuclei was confirmed by light microscopy. To extract nuclear proteins, the isolated nuclei were resuspended in NETN buffer (150 mM NaCl, 1 mM EDTA, 20 mM Tris–HCl, pH 8.0, 0.5% Nonidet P-40), and the mixture was sonicated briefly to aid nuclear lysis. Nuclear lysates were collected after centrifugation at 16,100g for 20 min at 4 °C. Protease and phosphatase inhibitors (Sigma, MO) were added to all buffers. The purity of nuclear and non-nuclear fractions was confirmed using Lamin B1 (Abcam, MA) as a nuclear marker and α-tubulin (Sigma, MO) as a non nuclear marker [19–21]. The membrane was blocked with 5% skim milk in TBST (TBS plus 0.1% Tween 20) for 60 min and then incubated with primary antibody. Commercially available antibodies for EGFR (Santa Cruz, CA), anti-phospho-EGFR (Tyr1173) (Millipore, MA) and anticlathrin heavy chain 2 (BD, CA) were used. Incubations were carried out overnight. After three washes with TBST, the membranes were incubated with peroxidase-conjugated secondary antibody (1:5000) (Sigma, MO) for 1 h at room temperature. Blots were visualized by enhanced chemiluminescence and quantitatively analyzed using ImageJ software.
2.3. Confocal fluorescence microscopy
Both confocal immunofluorescence and time lapse imaging were performed. Confocal immunofluorescence was performed as described previously [19–21]. Cells were double-labeled with a polyclonal antibody against EGFR (Santa Cruz, CA) and a monoclonal antibody against the nuclear membrane marker Lamin-B1, and then incubated with secondary antibodies conjugated to Alexa 488 and 555 (Invitrogen, CA), respectively. Intracellular trafficking of EGF was examined by time lapse confocal imaging of EGF labeled with Alexa 488 or 555 (Invitrogen, CA). All confocal images were collected with a Zeiss LSM 510 confocal microscope using a 63×, 1.4 NA objective lens with excitation at 488 nm and observation at 505–550 nm to detect Alexa 488, and excitation at 543 nm and observation at 560–610 nm to detect Alexa 555.
2.4. Cell transfection
SKHep-1 cells were transfected using Lipofectamine 2000 according to the manufacturer’s instructions (Invitrogen, CA) with 50 nM siRNA for Clathrin Heavy Chain (CHC2) (siRNA ID AM16106) (Ambion, TX). Constructs encoding GFP-dynamin and GFP-dynamin K44A were kindly provided by Pietro De Camilli (Yale University). Transfected cells were incubated at 37 °C in an atmosphere of 5% CO2, 95% O2 for 48 h prior to use.
2.5. Statistical Analysis
One-way ANOVA followed by Bonferroni’s post hoc comparisons tests were performed in all statistical analyses. Data are represented as mean ± standard error.
3. Results
3.1. EGF/EGFR complex translocates to the nucleus
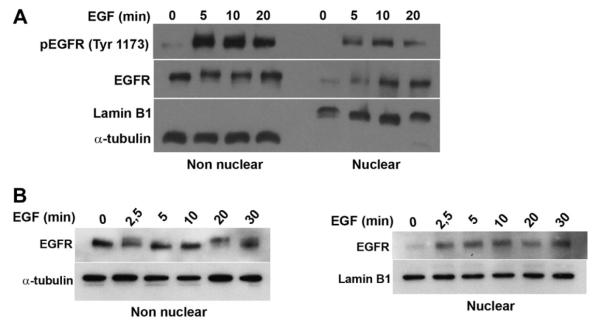
Studies were performed in the SKHep-1 human liver cell line and primary hepatocytes because of the well known effects of EGF in liver [22]. Immunoblot analysis of extracts from SKHep-1 cells was used to observe total and phosphorylated EGFR translocation to the nucleus. Cell fractionation showed that EGFR appears in the nucleus and reaches a peak there within 10 min (Fig. 1A). Phosphorylated receptor can be detected in the nucleus within 5 min of stimulation, which is the earliest time point examined (Fig. 1A). The EGFR also translocates to the nucleus in hepatocytes (Fig. 1B). SKHep-1 cells were used for all next experiments to circumvent technical difficulties associated with transient transfection of primary hepatocytes.
Fig. 1.
EGF receptor rapidly translocates to the nucleus in liver cells. (A) Immunoblots of total and phosphorylated EGF receptor (pEGFR) in non nuclear and nuclear fractions of SKHep-1 cells stimulated with EGF (100 ng/mL) are shown. α-tubulin and Lamin B1 were used as purity controls for the non nuclear and nuclear fractions, respectively. Total as well as phosphorylated EGFR are detected in the nucleus within 5 min of stimulation. (B) Immunoblots of total EGF receptor in non nuclear and nuclear fractions of hepatocytes stimulated with EGF (100 ng/mL). Blots are representative of what was observed in three separate experiments.
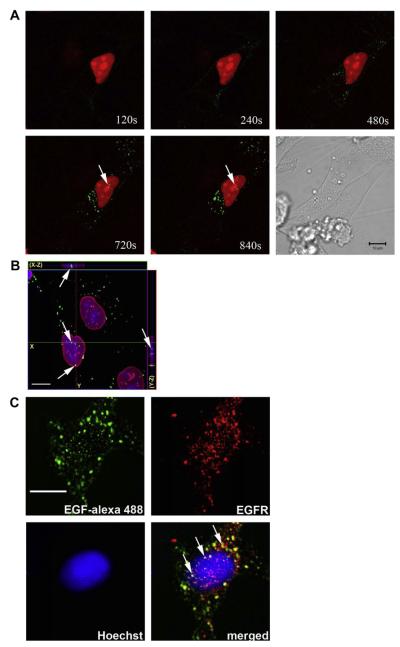
Confocal microscopy was performed to confirm the nuclear localization of the EGF/EGFR complex in SKHep-1 cells. First, time lapse confocal imaging was used to track the movement of labeled EGF-Alexa 488. SKHep-1 cells were transfected with monomeric Red Fluorescent Protein (mRFP) tagged with a nuclear localization signal (mRFP-NLS) to identify the nuclear region, and then stimulated with EGF-Alexa 488 (100 ng/mL). EGF was detected within the nucleus 10–12 min after stimulation, as observed by the colocalization of EGF with mRFP-NLS (Fig. 2A). Three-dimensional reconstruction of serial confocal immunofluorescence images confirmed that the EGF-Alexa 488 was localized to the nuclear interior (Fig. 2B). Confocal immunofluorescence was performed to visualize the subcellular distribution of the EGF/EGFR complex (Fig. 2C). The images show that ligand/receptor complex can be found within both the cytoplasm and nucleus (Fig. 2C). Together, these findings demonstrate that both total and activated EGFR rapidly translocate to the nucleus upon stimulation of cells with EGF, and suggest that EGF can move with its receptor to the nucleus.
Fig. 2.
EGF from the plasma membrane travels with EGFR to the nucleus. (A) Time lapse confocal images of SKHep-1 cells stimulated with EGF-Alexa 488 (100 ng/mL). Cells were transfected with mRFP tagged with a nuclear localization signal (mRFP-NLS) (Red) and after 48 h the cells were stimulated with EGF-Alexa 488 (Green). EGF-Alexa 488 labeling is initially along the plasma membrane and then moves into the cytoplasm and then the nucleus (arrows). Images are representative of what was observed in three separate experiments. (B) Confocal immunofluorescence images 10 min after stimulation with EGF-Alexa 488. EGF-Alexa 488 is in green, Lamin B1 is in red and the nucleus is stained with TO-PRO-3 (blue). Serial optical sections were collected for three-dimensional reconstruction; x-z sections are shown at the top, and y-z sections are shown at the right of each image. Note that EGF-Alexa 488 is in the interior of the nuclear region (arrows). The images confirm that the EGF moves to the nucleus. Images are representative of what was observed in 11 separate cells. (C) Confocal immunofluorescence images 10 min after stimulation with EGF-Alexa 488 (100 ng/mL). The left top panel shows subcellular distribution of EGF-Alexa 488 (Green), the top right EGFR (Red), the bottom left nuclear staining with Hoechst (Blue), and the bottom right panel shows the merged image. The merged image demonstrates that EGFR co-localizes with EGF within both the cytoplasm and nucleus (arrows). Images are representative of what was observed in 20 separate cells. Scale Bar = 10 μm.
3.2. Internalization of EGF is dynamin-dependent
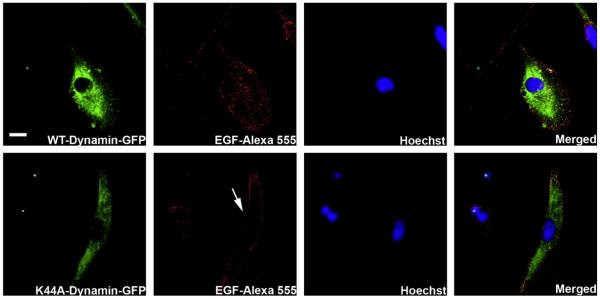
To investigate the dependence of dynamin on EGFR nuclear translocation we over-expressed dynamin or a dominant negative form of dynamin (K44A), each conjugated to a Green Fluorescent Protein (GFP). The dominant negative form of dynamin lacks the GTPase activity needed to activate the function of this protein [23]. SKHep-1 cells were transfected with either construct and were stimulated 48 h later with EGF-Alexa 555 (100 ng/ml) for 10 min (Fig. 3). Cells that over-expressed the wild type form of dynamin showed EGF distributed throughout the cell (Top panel, Fig. 3). In contrast, EGF accumulated along the region of the plasma membrane in cells over-expressing the dominant negative form of dynamin (K44A-GFP) (Bottom panel, Fig. 3) and consequently blocks the nuclear trafficking of EGF. These results suggest that internalization of EGF is dynamin-dependent, as has been suggested by others [24,25].
Fig. 3.
Internalization of EGFR is dynamin-dependent. Confocal immunofluorescence images are shown 10 min after stimulation with EGF-Alexa 555 (red). SKHep-1 cells were transfected with a WT Dynamin (top panels) or a dominant negative form of Dynamin (K44A) (bottom panels), both tagged with a green fluorescent protein (GFP) (green). Nuclear staining with Hoechst is shown in blue. Cells were stimulated with EGF-alexa 555 (100 ng/mL) 48 h after transfection. Note that EGF-Alexa 555 is internalized in cells transfected with WT dynamin but remains concentrated along the plasma membrane region in cells transfected with Dynamin K44A (arrow). Images are representative of what was observed in 15 separate cells under each experimental condition. Scale Bar = 10 μm.
3.3. Translocation of EGFR to the nucleus depends upon clathrin
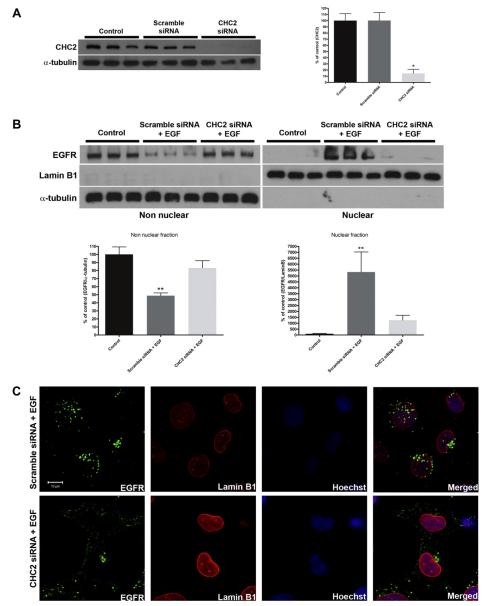
A previously Ambion validated clathrin (CHC2) siRNA reduced clathrin expression in SKHep-1 cells by 85.4 ± 6.5% (p < 0.01) (Fig. 4A). Scrambled siRNA had no effect on clathrin expression (p > 0.05) (Fig. 4A). SKHep-1 cells were transfected using these siRNAs and then stimulated for 10 min with EGF 48 h later. Immunoblots of nuclear and non-nuclear cell fractions demonstrated that treatment of cells with clathrin siRNA block the translocation of EGFR to the nucleus (p > 0.05) (Fig. 4B). Confocal immunofluorescence was performed to directly visualize the subcellular localization of EGFR in these cells (Fig. 4C). After 10 min of EGF stimulation EGFR was localized in the nucleus in Scramble siRNA-treated cells, but was excluded from the nucleus in the CHC2 siRNA-treated cells (Fig. 4C). Together, these results show that clathrin is necessary for EGFR nuclear translocation.
Fig. 4.
Knockdown of Clathrin blocks translocation of EGFR to the nucleus. (A) SKHep-1 cells were transfected with clathrin heavy chain 2 (CHC2) siRNA (50 nM). Immunoblots was performed to quantify the knockdown of CHC2 (left panel) and densitometric analysis confirms that it reduced protein expression by 85.4 ± 6.5% (n = 3, *p < 0.05) (right panel). Scramble siRNA was used and it didn’t reduce CHC2 expression level. (B) SKHep-1 cells were transfected with CHC2 or Scramble siRNAs and stimulated 48 h later with EGF (100 ng/mL) for 10 min. The amount of EGFR in the treated group with CHC2 siRNA and EGF didn’t alter in the non nuclear and in the nuclear fractions compared with control cells (p > 0.05). α-tubulin and Lamin B1 were used as purity controls for the non nuclear and nuclear fraction, respectively. Blot is representative of what was observed in nine separate experiments. (C) Confocal immunofluorescence images 10 min after stimulation with EGF (100 ng/mL). SKHep-1 cells were trasfected with Scramble or CHC2 siRNAs 48 h before the stimulation with EGF. EGFR and Lamin B1 were labeled with Alexa-488 (green) and Alexa-555 (red), respectively. Nuclear staining with Hoechst is shown in blue. Note that in CHC2 siRNA-treated cells the EGFR are concentrated outside the nucleus. Images are representative of what was observed in 20 cells under each experimental condition. **p < 0.001. Scale Bar = 10 μm.
4. Discussion
EGF acts through the EGFR, and evidence from other RTKs suggests that translocation to the nucleus may be a common feature for this class of receptors. A number of RTKs have been found in the nucleus, including the hepatocyte growth factor receptor (c-met), fibroblast growth factor receptor (FGFR), insulin receptor, vascular endothelial growth factor receptor (VEGFR), and the four members of the EGFR family [8,19,21,26–30]. Total and phosphorylated EGF receptor can be found in the nucleus within 5 min of stimulation with EGF, and reaches peak levels within 10 min. Here we demonstrated that EGF and its receptor translocates to the nucleus in liver cells. EGF nuclear transport was monitored by real time imaging suggesting that the ligand travels with its receptor to the nucleus. It had been demonstrated that clathrin-mediated endocytosis is required for EGFR internalization and that it plays roles in attenuation of some EGFR responses and also controlling specific signaling pathways [31,32]. However, whether EGFRs destined for the nucleus also use this endocytic pathway has not been examined directly in liver cells.
The current work suggests that this process is dynamin-dependent, which is consistent with the previous observation that transient expression of the K44A dynamin dominant negative mutant in CHO cells dramatically reduces nuclear EGFR expression levels [23]. The current work provides evidence that dynamin and clathrin are necessary first steps for translocation of EGFR to the nucleus. It has been reported that treatment with colchicine and cytochalasin D inhibited the nuclear accumulation of EGFR [33]. In contrast, other report showed that ErbB3 nuclear translocation is clathrin-independent in prostate cancer cell lines [34]. This contradictory result may be due the different receptors or cells used for these experiments.
How does EGFR move to the nucleus after it has undergone endocytosis? Accumulating evidence suggests the existence of a novel pathway by which internalized cell surface RTKs within early endosomes are transported to the nucleus rather than back to the cell surface or to lysosomes [6,7]. A retrograde route from Golgi to ER is thought to be involved in EGFR trafficking to the nucleus. Nuclear transport of EGFR also is regulated by COPI-mediated vesicular trafficking from the Golgi to the ER [35]. In addition, the Sec61 translocon associates with EGFR, and is retrotranslocated from the ER to the cytoplasm [16,36]. HSP70 is essential in the retrotranslocation process and likely functions by interacting with the receptor transmembrane domain and thereby maintaining the receptor in a soluble state following extraction from the ER membrane [36]. EGFR as a cytoplasmic protein likely interacts with importin-β and then enters the nucleus through the nuclear pore complex [8,23]. Further work will be needed to understand the relative roles of nuclear and non-nuclear EGFR in cell signaling, and to identify how the proportions of EGFRs that are destined for the various possible intracellular locations are regulated.
Acknowledgments
This work was supported by NIH grants TW008709, DK57751, DK45710, DK61747, and DK34989, and by grants from FAPEMIG and CNPq.
References
- [1].Schlessinger J. Ligand-induced receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- [2].Carpenter G, Cohen S. Epidermal growth factor. J. Biol. Chem. 1990;265:7709–7712. [PubMed] [Google Scholar]
- [3].Lai WH, Cameron PH, Wada I, Doherty JJ, Kay DG, Posner BI, Bergeron JJ. Ligand-mediated internalization recycling, and downregulation of the epidermal growth factor receptor in vivo. J. Cell Biol. 1989;109:2741–2749. doi: 10.1083/jcb.109.6.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lai WH, Cameron PH, Doherty JJ, Posner BI, Bergeron JJ. Ligand-mediated autophosphorylation activity of the epidermal growth factor receptor during internalization. J. Cell Biol. 1989;109:2751–2760. doi: 10.1083/jcb.109.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- [6].Wang YN, Yamaguchi H, Hsu JM, Hung MC. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene. 2010;29:3997–4006. doi: 10.1038/onc.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carpenter G, Liao HJ. Trafficking of receptor tyrosine kinases to the nucleus. Exp. Cell Res. 2009;315:1556–1566. doi: 10.1016/j.yexcr.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- [9].Kim J, Jahng WJ, Di Vizio D, Lee JS, Jhaveri R, Rubin MA, Shisheva A, Freeman MR. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer Res. 2007;67:9229–9237. doi: 10.1158/0008-5472.CAN-07-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tao Y, Song X, Deng X, Xie D, Lee LM, Liu Y, Li W, Li L, Deng L, Wu Q, Gong J, Cao Y. Nuclear accumulation of epidermal growth factor receptor and acceleration of G1/S stage by Epstein-Barr-encoded oncoprotein latent membrane protein 1. Exp. Cell Res. 2005;303:240–251. doi: 10.1016/j.yexcr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- [11].Hsu SC, Hung MC. Characterization of a novel tripartite nuclear localization sequence in the EGFR family. J. Biol. Chem. 2007;282:10432–10440. doi: 10.1074/jbc.M610014200. [DOI] [PubMed] [Google Scholar]
- [12].Marti U, Wells A. The nuclear accumulation of a variant epidermal growth factor receptor (EGFR) lacking the transmembrane domain requires coexpression of a full-length EGFR. Mol. Cell Biol. Res. Commun. 2000;3:8–14. doi: 10.1006/mcbr.2000.0177. [DOI] [PubMed] [Google Scholar]
- [13].Lo HW, Xia W, Wei Y, Ali-Seyed M, Huang SF, Huang MC. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 2005;65:338–348. [PubMed] [Google Scholar]
- [14].Wells A, Marti U. Signalling shortcuts: cell-surface receptors in the nucleus? Nat Rev. Mol. Cell Biol. 2002;3:697–702. doi: 10.1038/nrm905. [DOI] [PubMed] [Google Scholar]
- [15].Wang SC, Hung MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 2009;15:6484–6489. doi: 10.1158/1078-0432.CCR-08-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang YN, Yamaguchi H, Huo L, Du Y, Lee HJ, Lee HH, Wang H, Hsu JM, Hung MC. The translocon Sec61beta localized in the inner nuclear membrane transports membrane-embedded EGF receptor to the nucleus. J. Biol. Chem. 2010;285:38720–38729. doi: 10.1074/jbc.M110.158659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- [18].Boyer JL, Phillips JM, Graf J. Preparation and specific applications of isolated hepatocyte couplets. Methods Enzymol. 1990;192:501–516. doi: 10.1016/0076-6879(90)92090-z. [DOI] [PubMed] [Google Scholar]
- [19].Rodrigues MA, Gomes DA, Andrade VA, Leite MF, Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology. 2008;48:1621–1631. doi: 10.1002/hep.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, Cheng YC, Bennett AM, Nathanson MH. Nucleoplasmic calcium is required for cell proliferation. J. Biol. Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T, Bennett AM, Nathanson MH. c-Met must translocate to the nucleus to initiate calcium signals. J.Biol.Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- [23].Lo HW, Ali-Seyed M, Wu Y, Bartholomeusz G, Hsu SC, Hung MC. Nuclearcytoplasmic transport of EGFR involves receptor endocytosis, importin beta1 and CRM1. J. Cell Biochem. 2006;98:1570–1583. doi: 10.1002/jcb.20876. [DOI] [PubMed] [Google Scholar]
- [24].Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Henley JR, Krueger EW, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maher PA. Nuclear Translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J. Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang SC, Lien HC, Xia W, Chen IF, Lo HW, Wang Z, Ali-Seyed M, Lee DF, Bartholomeusz G, Ou-Yang F, Giri DK, Hung MC. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004;6:251–261. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- [29].Offterdinger M, Schofer C, Weipoltshammer K, Grunt TW. C-erbB-3: a nuclear protein in mammary epithelial cells. J. Cell Biol. 2002;157:929–939. doi: 10.1083/jcb.200109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ni CY, Murphy MP, Golde TE, Carpenter G. Gamma-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- [31].Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- [32].Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- [33].Cortes-Reynosa P, Robledo T, Salazar EP. Epidermal growth factor promotes epidermal growth factor receptor nuclear accumulation by a pathway dependent on cytoskeleton integrity in human breast cancer cells. Arch. Med. Res. 2009;40:331–338. doi: 10.1016/j.arcmed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- [34].Koumakpayi IH, Le Page C, Delvoye N, Saad F, Mes-Masson AM. Macropinocytosis inhibitors and Arf6 regulate ErbB3 nuclear localization in prostate cancer cells. Mol. Carcinog. 2011 doi: 10.1002/mc.20766. in press. [DOI] [PubMed] [Google Scholar]
- [35].Wang YN, Wang H, Yamaguchi H, Lee HJ, Lee HH, Hung MC. COPI-mediated retrograde trafficking from the Golgi to the ER regulates EGFR nuclear transport. Biochem. Biophys. Res. Commun. 2010:498–504. doi: 10.1016/j.bbrc.2010.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol. Biol. Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]