Abstract
We studied the role of classical phagocytic NADPH oxidase (Nox) in the pathogenesis of kidney allograft tubulointerstitial fibrosis. Immunofluorescence studies showed that Nox-2 and p22phox (electron transfer subunits of Nox) colocalized in the tubulointerstitium of human kidney allografts. Tubular Nox-2 also colocalized with α -SMA in areas of injury, suggestive of epithelial-to-mesenchymal transition (EMT). Interstitial macrophages (CD68+) and myofibroblasts (α -SMA+) expressed Nox-2 while graft infiltrating T cells (CD3+) and mature fibroblasts (S100A4+) were Nox-2−. These results were confirmed in the Fisher-to-Lewis rat kidney transplant model. Areas of tubulitis were associated with Nox-2 and α -SMA, suggestive of EMT. Immunoblot analyses showed that Nox-2 upregulation was associated with oxidative stress (nitrotyrosine) and fibrogenesis (α -SMA and phospho-Smad2) at 3 weeks and 6 months. Allografts treated with Nox inhibitors (DPI or apocynin) for 1 week showed reduced fibronectin and phospho-Smad2 and increased E-cadherin levels. Cyclosporine A, TGF-β1 and angiotensin II increased Nox-2 mRNA levels 2- to 7-fold in vitro (NRK52E cells). Treatment with specific Nox inhibitors (DPI or apocynin) prevented the downregulation of E-cadherin and upregulation of fibronectin transcripts. In aggregate, these studies suggest that Nox-2 is involved in the pathogenesis of allograft tubulointerstitial fibrosis via activation transcription factor Smad2, EMT and myofibroblasts.
Keywords: EMT, macrophage, myofibroblast, Nox, oxidative stress, smad
Introduction
Tubulointerstitial fibrosis is an important cause of late allograft loss and is associated with significant patient morbidity and mortality (1–5). Importantly, calcineurin inhibitors (i.e. cyclosporine A and tacrolimus), the backbone of immunosuppression, are also key mediators of long-term allograft injury and tubulointerstitial fibrosis (1,3,6). A major challenge to the future of kidney transplantation is to dissect out the identifiable causes of chronic tubulointerstitial fibrosis and to develop cause-specific treatment strategies (3).
Oxidative stress (OS) may be involved in the pathogenesis of chronic allograft tubulointerstitial fibrosis (7). It is a term that signifies damage to DNA, proteins, lipids, carbohydrates, cells and tissues caused by reactive oxygen species (ROS). These ROS include superoxide anion , hydrogen peroxide (H2O2), hydroxyl radical (OH°−) and peroxynitrite (ONOO°−). The balance between ROS production and antioxidant defenses defines the degree of OS in a given tissue. OS is associated with epithelial to mesenchymal transition (EMT) and chronic tubulointerstitial fibrosis in the kidney allograft (7,8). However, association does not imply causation and the few studies that looked at the effect of antioxidants in human and experimental kidney allografts had inconsistent results (9–11). Some showed improved outcomes (10) while others showed no benefit from antioxidant therapy (9,11). One explanation could be the type of intervention. These studies utilized classical ROS scavengers including vitamin E and superoxide dismutase (SOD) mimetics. Because OS is a multifaceted and redundant system, it is likely that the effects of ROS scavengers are far downstream in complex biological organisms. An alternative strategy would therefore be to intervene earlier in the OS pathway and inhibit the generation of ROS. More specifically, one could target molecules involved in the generation of superoxide anion.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) enzymes are important sources of ROS generation in the kidney (12,13). The phagocytic Nox has several subunits including Nox-2 (originally named gp91phox, the electron-transfer unit), p22phox, p47phox, p67phox, p40phox and the GTPase Rac and is primarily involved in immune responses such as the oxidative burst (14). Nonphagocytic Nox generates ROS that act as second messengers for several transcription factors including NF-κB, AP-1 and p38MAPK and ERK1/2 (12,14). Typically, Nox-2 activation requires the assembly of membrane-bound p22phox that stabilizes the proteins and docks cytosolic subunits (14). Nox enzymes play an important role in tissue remodeling and fibrosis as evidenced by their involvement in EMT (15,16), atherosclerosis (17) and cardiac and liver fibrosis (18,19). Furthermore, evidence suggests that Nox is involved in the pathogenesis of diabetic, hypertensive and glomerular kidney disease (20–24). However, little is known regarding the contribution of Nox to the pathogenesis of chronic allograft tubulointerstitial fibrosis. We hypothesized that Nox-2 is increased in kidney allografts undergoing tubulointerstitial fibrosis where it could be activated by angiotensin II, TGF-β1 and cyclosporine A (CsA); and that it may contribute to matrix accumulation by activating redox-sensitive pathways and profibrotic transcription factors.
Methods
Patients
Adult kidney transplant recipients undergoing diagnostic biopsies between October 2006 and March 2007 were invited to participate in a study to examine the role of OS in kidney allograft tubulointerstitial fibrosis. Pathologists blinded to the study read the biopsies after H&E, PAS, PAMM, Trichrome and immunohistochemical studies. Patients were included if histopathological findings were consistent with interstitial fibrosis and tubular atrophy not otherwise specified (IFTANOS) (25,26). Chronic injury was assessed and graded according to the modified Banff 1997 classification scheme and the proportion of cortical area affected: grade 0 < 6% of the cortical area affected; grade 1: 6–25%; grade 2: 26–50%; and grade 3 > 50% affected (25,26). Interstitial, tubular, vascular and glomerular chronic injury scores were reported as ct, ci, cv and cg, respectively. Control human kidney sections were prepared from tumor-free areas following resection surgery for malignancy. Data were presented as median and range of chronic injury scores. Data on demographics, kidney allograft function, immunosuppression and histopathological findings were collected. Leftover tissue was then examined for OS and fibrosis biomarkers using standard immunostaining protocols. The Human Subjects Committee and the Institutional Review Board at the University of Wisconsin Madison School of Medicine and Public Health approved this study.
Animals
Adult (9–11-week-old) male Fisher 344 and Lewis rats were purchased from Harlan Sprague–Dawley (Indianapolis, IN). Animals were housed in the animal care facility at the William Middleton VA Hospital in Madison, WI and the procedures were performed in accordance with the Animal Care Policies at the VA Hospital and the UW. Kidney transplants were performed as previously described (27). Briefly, the left kidney of the Fisher or Lewis donor rat was isolated, perfused (with 10 mL cold University of Wisconsin preservation solution), excised and transplanted orthotopically into weight-matched Lewis recipients following the excision of the native kidney. Total cold ischemic time was less than 30 min. End-to-end anastomosis of donor and recipient renal artery, vein and ureter was performed with 10–0 prolene sutures. The contralateral native kidney was excised 10 days later and the graft was checked macroscopically to exclude hydronephrosis. Allograft recipients were treated with low-dose cyclosporine A (CsA, 1.5 mg/kg/day) for 10 days posttransplant to prevent graft loss due to acute rejection. Studies were performed at 1 week, 3 weeks and 6 months post transplant. In addition, some rats were treated with specific chemical inhibitors of Nox: apocynin (4-hydroxy-3-methoxyacetophenone) and diphenyleneiodonium (DPI) for 1 week. The doses of apocynin (16 mg/kg/day in the drinking water) and DPI (1.5 mg/kg/day intraperitoneal injections) were based on studies where they reduced ROS accumulation and kidney injury (21,28,29). Animals were euthanized by exanguination under general anesthesia. There were six rats in each experimental group. All recipients were 3 months old at the time of transplant and weighed from 250 to 300 g.
Cell culture experiments
Normal rat kidney proximal epithelial cells (NRK52E)were obtained from the American Type Culture Collection (ATCC, Rockwell, MD) and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were seeded at 2.5 × 105 cells per well into six-well culture plates in DMEM (high glucose) containing 5% heat inactivated FBS, 44 mM NaHCO3, 5000 IU Penicillin and 5000 µg/mL Streptomycin (Cellgro, VA). At 80% confluency, media was changed to serum- free DMEM supplemented with 0.1% BSA for 12 h to arrest growth and synchronize cell activity. Nox induction studies were performed using angiotensin II (A9525, Sigma, St. Louis, MO), cyclosporine A (Bedford Labs, Bedford, OH) and TGF-β1 (Sigma) at indicated concentrations. Specific chemical inhibitors of Nox: apocynin (100 µM, 178385, Calbiochem, San Diego, CA) and DPI (150 nM, 300260, Calbiochem) were added 60 min prior to TGF-β1 treatment.
Antibodies
Primary antibodies against α-SMA (Clone 1A4, A2547, Sigma), GAPDH (ab8245, 6C5, Abcam, Cambridge, MA), E-cadherin (Clone 36, 610181, BD Pharmigen, San Diego, CA), Nox-2 (611414, BD Pharmigen), p22phox (sc20781, Santa Cruz Biotechnology, Santa Cruz, CA), S100A4 (A5114, Dako, Carpinteria, CA), CD3 (CP215A, Biocare Medical, Concord, CA), CD68 (MCA341R, Serotec, Raleigh, NC), phospho-Smad2 (05–953, Upstate, Billerica, MA), Smad2 (51–1300, Zymed), Nitrotyrosine (AB5411, Chemicon, Temecula, CA) TGF-β1 (AHG0051, Invitrogen, Carlsbad, CA), β-actin (A0760–40 US Biologicals, Swampscott, MA) were used for immunoblot, immunofluorescence and immunohistochemical analyses.
RNA extraction, purification and real-time PCR analyses
Total RNA extraction was performed as previously described (27,30). Briefly, RNA was extracted from cell lysates using the Trizol protocol (GibcoBRL, Life Technologies, Rockville, MD). Following centrifugation, the clear supernatant containing RNA was transferred into a fresh tube and chloroform (200 µL/tube) was added for phase separation. Isopropanol (500 µL/tube) was then added and following another centrifugation the supernatant was removed and the RNA pellet washed with 1 mL 75% EtOH in DEPC water. The sample was centrifuged and supernatant removed allowing the pellet to dry at room air. Pellets were then resuspended in DEPC water and RNA Easy kit (Qiagen cat 74104) was used for purification. RNA samples were eluted with sterile DEPC-treated water and spectrophotometric readings were done at 260/280 nm to determine the final concentration. We utilized rat fibronectin (Unigene ID: Rn.1604, Exon boundary 23–24), E-cadherin (Unigene ID:Rn.1303, Exon Boundary 3–4) and S26 internal control (Refseq: XM 001066146.1 Exon Boundary 1–1) primers. cDNA synthesis was performed using the First Strand cDNA synthesis kit (Roche cat 1483188) as described previously (27,30). Briefly, RNA (2.5 µg), 10× reaction Buffer (4.0 µL), 25 mM MgCl (8.0 µL), dNTPs (4.0 µL), RNase Inhibitor (2.0 µL), AMV RT polymerase (1.6 µL), Oligo dT (4.0 µL) were mixed in a 500 µL Eppendorf tube. Samples were mixed and incubated at 25°C for 10 min, 42°C for 60 min, 99°C for 5 min followed by a 4°C hold in a thermocycler. PCR reactions were then performed using the primers described above and the GeneAmp 5700 Sequence detection system (Applied Biosystems). Mean Ct, standard deviation (SD) and delta Ct (compared to S26 internal control) for fibronectin and E-cadherin were evaluated and presented. Experiments were performed in triplicates and average fold changes (±SD) were presented as bar graphs.
Immunoblotting
Western blotting was performed on protein lysates obtained from whole kidney tissue or cell lysates as described earlier (27,30). After separation by SDS-Polyacrylamide Gel Electrophoresis (10–20% gradient PAGE, Biorad, Hercules, CA) proteins were transferred electrophoretically (100 V, 30 min) to nitrocellulose membranes (Bio-Rad) that were then blocked with a solution containing 5% carnation nonfat milk, 50 mM Tris, HCl, pH 7.4, NaCl 150 mM, Tween 20 0.05% (TBS-Tween) overnight at 4°C. Membranes were incubated the next day with antibodies against Nox-2 (1:250), α-SMA (1:2000), nitrotyrosine (1:750), phospho-Smad2 (1:5000), Smad2 (2 µg/mL), GAPDH (1:5000), β-actin (1:7500). All primary antibodies were diluted in the solution containing 5% carnation nonfat milk 50 mM Tris, HCl, pH 7.4, NaCl 150 mM, Tween 20 0.05% (TBS-Tween). Binding of primary antibodies was followed by incubation for 1 h at room temperature with a secondary HRP-conjugated IgG in 1% nonfat milk. Signals were visualized by enhanced chemiluminescence signals captured on x-ray films. Data were normalized to GAPDH or β-actin internal control. Densitometry was done using the NIH Image J software, downloaded from http://rsb.info.nih.gov/ij. Results were displayed as representative assays of three sets of independent experiments.
Immunohistochemical studies
A portion of the kidney tissue was excised promptly after euthanasia. It was immediately placed in 10% neutral-buffered formalin. Tissue was fixed for 2 h in formalin and processed for paraffin embedding following standard protocols and then sectioned (4 mm) for antibody staining. Double staining experiments for α-SMA and Nox-2 were performed after sections were deparaffinized and hydrated. Heat-induced antigen retrieval was performed using a 10 mM Citrate solution (pH = 6.0) at 25 psi for 2 min in a decloaking chamber. Nonspecific staining was blocked using Sniper (Biocare Medical) for 9 min. Slides were incubated overnight with Nox-2 (1:25), then washed and incubatedwith3%hydrogen peroxide for 30 min. MACH 2HRP polymer detection system (Biocare Medical) and DAB substrate were used to tag and stain the first primary antibody brown. Slides were then incubated with the second primary antibody (α-SMA, 1:50 000) at room temp for 1 h. MACH 2HRP polymer detection system and VIP substrate (Vector Laboratories, Burlingame, CA) were used to tag and stain the second primary antibody purple. Tissue sections were washed in distilled water, counter-stained with hematoxylin, dehydrated through an ethanol series and mounted with coverslips.
Immunofluorescence studies
Formalin fixed paraffin embedded sections were cut into 4–5 µm sections. Slides were deparaffinized and rehydrated from xylene through a graded ethanol series to dH2O. Antigen retrieval was performed at 25 psi for 2 min. Nonspecific background staining was blocked using Sniper (Biocare Medical). Double-staining studies were performed with Nox-2 (1:15) and p22phox (1:50), α-SMA (1:50 000), S100A4 (1:25), CD3 (1:300), CD68 (1:100) antibodies. Cover slips were secured using the ProLong Gold Antifade reagent with DAPI.
All slides were viewed on a Nikon Eclipse E600 microscope with an Olympus DP70 camera. Images were analyzed using the DP70 imaging software.
Statistical analysis
Students’ t-test and the nonparametric Mann–Whitney rank sum test (Sigma Stat Software, Jandel Scientific) were utilized when appropriate to compare differences in gene and protein expression between groups. p-Values ≤ 0.05 were considered significant.
Results
Baseline characteristics of patients undergoing biopsies
There were 12 patients in the study (Table 1). All were Caucasian, 9 were female and half had diabetes as the cause of kidney failure. There were 5, 4 and 3 deceased-donor, living-donor and kidney-pancreas transplant recipients, respectively. At the time of biopsy, all patients were receiving prednisone while 11 were on mycophenolic acid and 7 on calcineurin inhibitors. There were no patients on sirolimus and there were no obvious differences between the immunosuppressive regimens. Three patients were on active vitamin D for metabolic bone disease. Otherwise no antioxidant supplements were noted in patients’ medical records. Most patients had moderate fibrosis (grade 2), moderate interstitial fibrosis (ci = 2) and tubular atrophy (ct = 2). Median serum creatinine and eGFR levels were 2.3 mg/dL and 25.5 mL/min/1.73 m2, respectively corresponding to moderate reduction of kidney allograft function and stage 3 of chronic kidney disease.
Table 1.
Baseline characteristics of kidney transplant recipients with tubulointerstitial fibrosis
| Number of patients | 12 |
| Female | 9 |
| Caucasian | 12 |
| Age (years)* | 52 (28–70) |
| Transplant to biopsy interval (years)* | 9.5 (2–27) |
| Cause of ESRD (DM/GD/ADPKD/HTN) | 6/3/2/1 |
| Transplant type (DD/LD/KP) | 5/4/03 |
| Immunosuppression (Pred/MPA/CNI) | 12/11/07 |
| Banff grade* | 2 (1–3) |
| ci score* | 2 (1–3) |
| ct score* | 2 (1–3) |
| cv score* | 1.5 (0–3) |
| cg score* | 1.5 (0–3) |
| Serum creatinine (mg/dL)* | 2.3 (1.4–6.2) |
| eGFR (mL/min/1.73 m2)* | 25.5 (10–45) |
Patients were included if histopathological findings were consistent with interstitial fibrosis and tubular atrophy not otherwise specified. Chronic injury was assessed and graded according to the modified Banff 1997 classification scheme and the proportion of cortical area affected: grade 0 < 6% of the cortical area affected; grade 1: 6–25%; grade 2: 26–50%; and grade 3 >50%. Interstitial, tubular, vascular and glomerular chronic injury scores were reported as ct, ci, cv and cg, respectively.
Data presented as median (range).
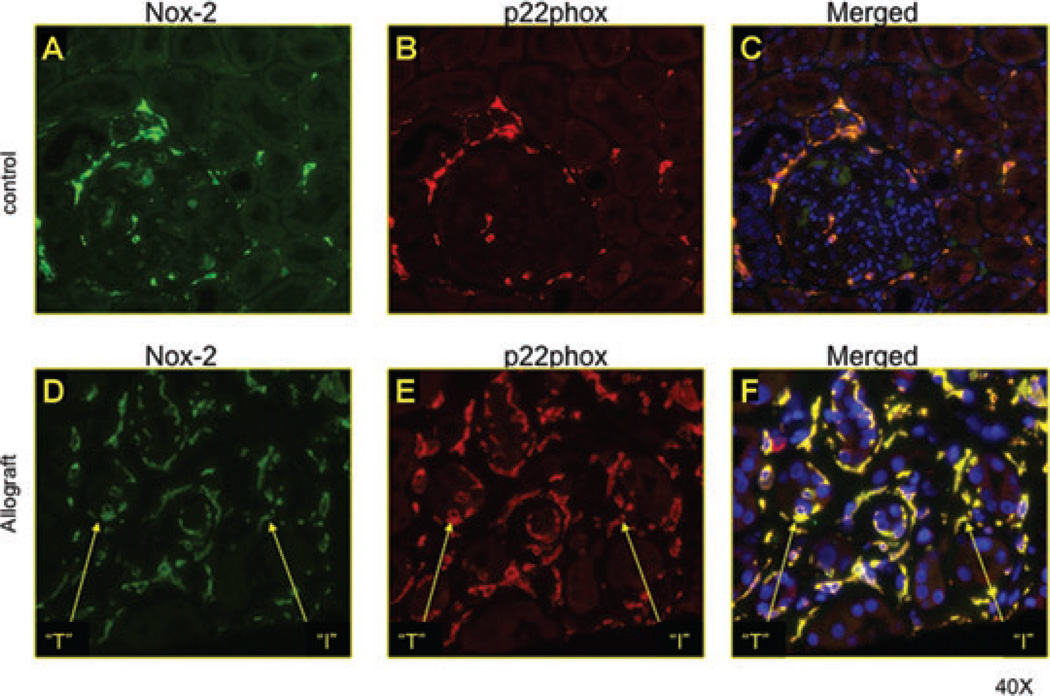
Nox-2 and p22phox were increased in areas of tubulointerstitial injury in human allografts
There is no information on the expression pattern of Nox-2 and p22phox, electron-transferring subunits of the classical phagocytic Nox, in kidney allografts. To address this question, we performed double-staining immunofluorescence studies in kidney allografts with IFTANOS compared to control native kidneys (Figure 1). In the control kidney, few interstitial cells stained for Nox-2 and p22phox. The molecules costained, suggesting a functional role for Nox (14). Kidney allografts showed significantly greater interstitial staining for both molecules. In addition, tubular epithelial cells upregulated Nox in areas of injury, suggestive of increased ROS generation by renal tubules.
Figure 1. Nox-2 and p22phox increased in tubular epithelial cells in human allografts.
We performed double-staining immunofluorescence studies in kidney allografts with IFTANOS compared to control native kidneys. In the control kidney, few interstitial cells stained for Nox-2 and p22phox. The molecules costained, suggesting a functional role for Nox. Kidney allografts showed significantly greater interstitial staining for both molecules. In addition, tubular epithelial cells upregulated Nox in areas of injury, suggestive of increased ROS generation by renal tubules.
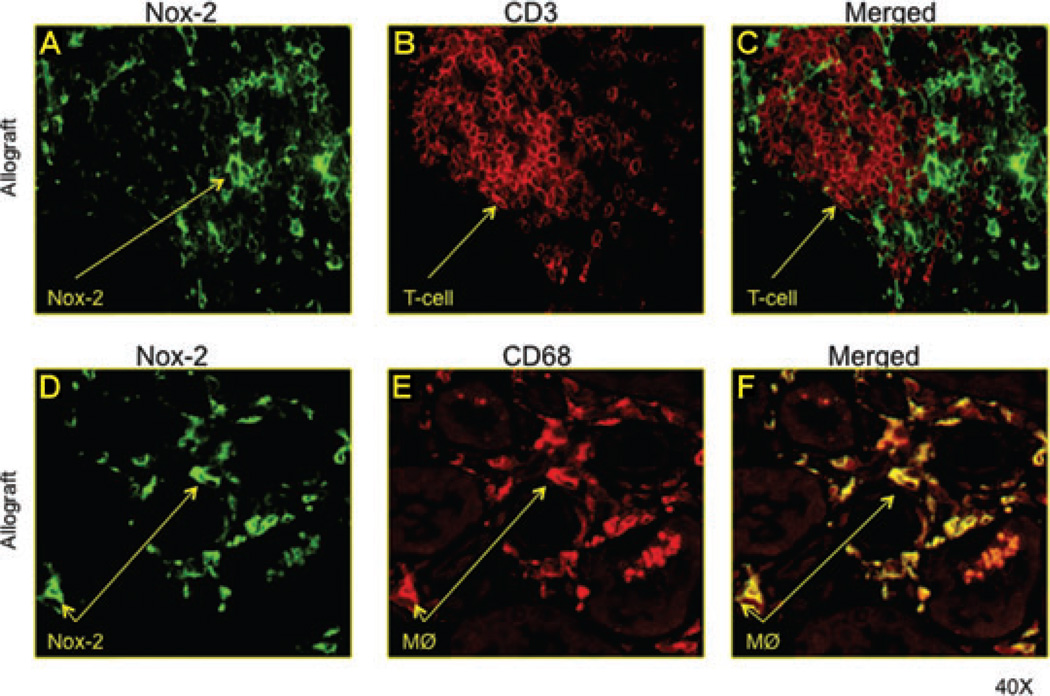
Interstitial macrophages but not T cells expressed Nox-2
To determine which graft infiltrating cells were a source of interstitial Nox-2; we performed double-staining immunofluorescence studies for CD68 (macrophages), CD3 (T cells) and Nox-2 (Figure 2). Merged image analyses showed that Nox-2 and CD68 colocalized (Figure 2E). There was no costaining between CD3 and Nox-2 (Figure 2C), suggesting that graft infiltrating macrophages and not T cells are a source of Nox-2.
Figure 2. Interstitial macrophages but not T cells expressed Nox-2.
To determine which graft infiltrating cells were a source of interstitial Nox-2; we performed double-staining immunofluorescence studies for CD68 (macrophages), CD3 (T cells) and Nox-2. Merged image analyses showed that Nox-2 and CD68 colocalized (E). There was no costaining between CD3 and Nox-2 (C), suggesting that graft infiltrating macrophages and not T cells are a source of Nox-2.
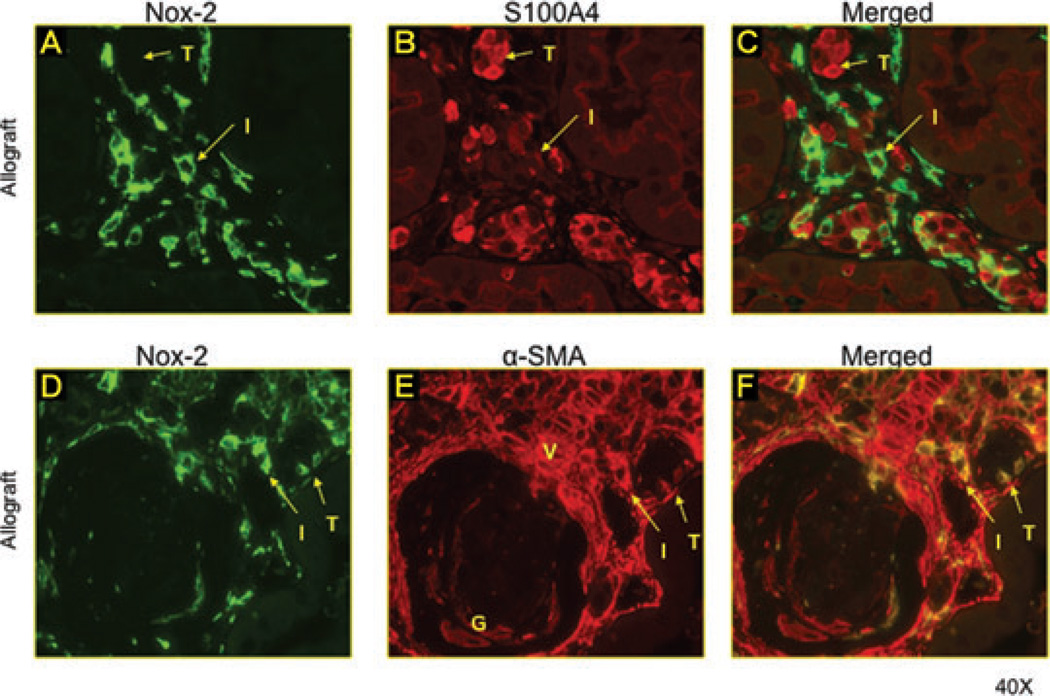
Myofibroblasts but not mature fibroblasts expressed Nox-2
Myofibroblasts are the active form of fibroblasts and a primary source of fibrogenesis in the kidney. To evaluate which cell type expressed Nox-2 within the allograft; double-staining immunofluorescence studies were performed using α-SMA (myofibroblasts) S100A4 (fibroblasts) and Nox-2 antibodies (Figure 3). Some tubular cells expressed α-SMA or S100A4 suggestive of EMT (Figure 3B, E). Tubular and interstitial cells expressing S100A4 did not costain for Nox-2 (Figure 3A–C). However, the large majority of α-SMA+ interstitial and tubular cells were also positive for Nox-2 (Figure 3D, E). These studies suggest that myofibroblasts but not mature fibroblasts are a source of Nox-2 in the allograft.
Figure 3. Myofibroblasts but not mature fibroblasts expressed Nox-2.
Double-staining immunofluorescence studies were performed using α-SMA (myofibroblasts) S100A4 (fibroblasts) and Nox-2 antibodies. Some tubular cells expressed α-SMA or S100A4 suggestive of EMT (B and E). Tubular and interstitial cells expressing S100A4 did not costain for Nox-2 (A–C). However, the large majority of α-SMA+ interstitial (I) and tubular (T) cells were also positive for Nox-2 (D–E). These studies suggest that myofibroblasts but not mature fibroblasts are a source of Nox-2 in the allograft.
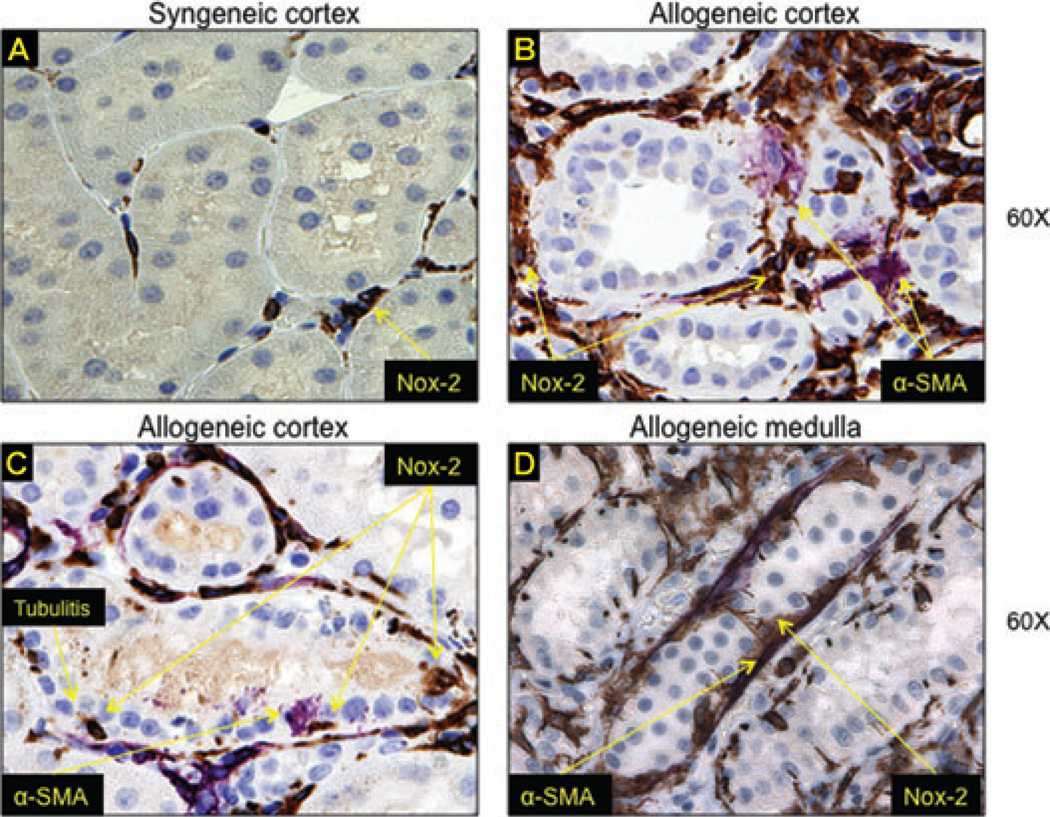
Nox-2 was associated with EMT and tubulitis in rat kidney allografts
To determine the reproducibility of our findings, to quantify the amount of Nox proteins in the allograft and to prepare for in vivo inhibition studies, we first examined Nox-2 expression in the classical Fisher-to-Lewis rat transplant model. Akin to human studies, allogeneic kidneys had significant upregulation of Nox-2 in interstitial and tubular cells (Figure 4C, D) compared to syngeneic grafts (Figure 4A). There was also evidence of tubulitis (Figure 4C) where invading mononuclear cells showed intense Nox-2 immunoreactivity. Interestingly, tubular epithelial cells upregulated α-SMA in areas of tubulitis suggestive of EMT (Figure 4C). These findings were confirmed in the medulla where injured tubules coexpressed α-SMA and Nox-2 (Figure 4D).
Figure 4. Nox-2 was associated with EMT and tubulitis in rat kidney allografts.
We examined Nox-2 expression in the classical Fisher-to-Lewis rat transplant model. Allogeneic kidneys had significant upregulation of Nox-2 in interstitial and tubular cells (C–D) compared to syngeneic grafts (A). There was also evidence of tubulitis (C) where invading mononuclear cells showed intense Nox-2 immunoreactivity. Tubular epithelial cells upregulated α-SMA in areas of tubulitis suggestive of EMT (C). These findings were confirmed in the medulla where injured tubules coexpressed α-SMA and Nox-2 (D).
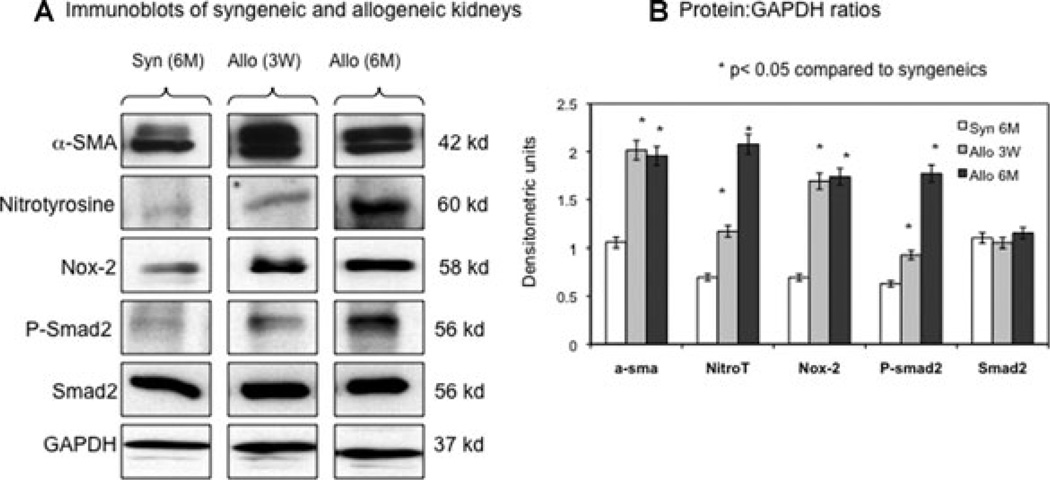
Nox-2 was associated with fibrogenesis in rat kidney allografts
Correlations between animal and human studies led us to further investigate Nox-2, OS (nitrotyrosine protein adducts) and proteins involved in fibrogenesis (α-SMA and Smad2 profibrotic signaling pathway) by immunoblot analyses. Syngeneic transplants at 6 months were compared to 3-week and 6-month posttransplant kidney allografts (Figure 5A, B). Allografts showed a significant increase in Nox-2 associated with greater α-SMA, nitrotyrosine and phosphorylated Smad2 levels whereas total Smad2 did not change significantly.
Figure 5. Nox-2 was associated with fibrogenesis in rat kidney allografts.
Immunoblot (A) and densitometry analyses (B) of kidney lysates from syngeneic transplants at 6 months were compared to kidney allografts 3 weeks and 6 months posttransplant. Data represented in (B) were normalized to GAPDH internal control and represent averages of three independent experiments. Allografts showed a significant increase in Nox-2 associated with greater α-SMA, nitrotyrosine and phosphorylated Smad2 levels whereas total Smad2 did not change significantly.
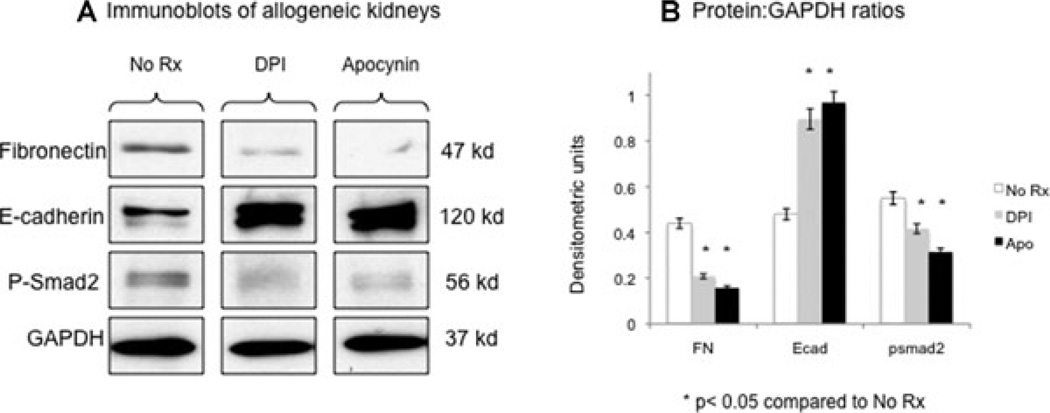
Nox inhibition was associated with decreased fibrogenesis in rat allografts
Allograft recipients were treated with specific inhibitors of Nox (DPI and apocynin) for 1 week (Figure 6). There was a significant downregulation of fibronectin and phospho-Smad2 associated with greater E-cadherin levels, proof of principle that the relationship between Nox and fibrogenesis in the allograft is causal rather than associative at least in the early posttransplant period.
Figure 6. Nox inhibition was associated with decreased fibrogenesis in rat allografts.
Allograft recipients were treated with specific inhibitors of Nox (DPI and apocynin) for 1 week (Figure 6). Western blot analyses (A) showed that there was a significant downregulation of fibronectin and phospho-Smad2 associated with greater E-cadherin levels. Data represented in (B) were normalized to GAPDH internal control and represent averages of three independent experiments.
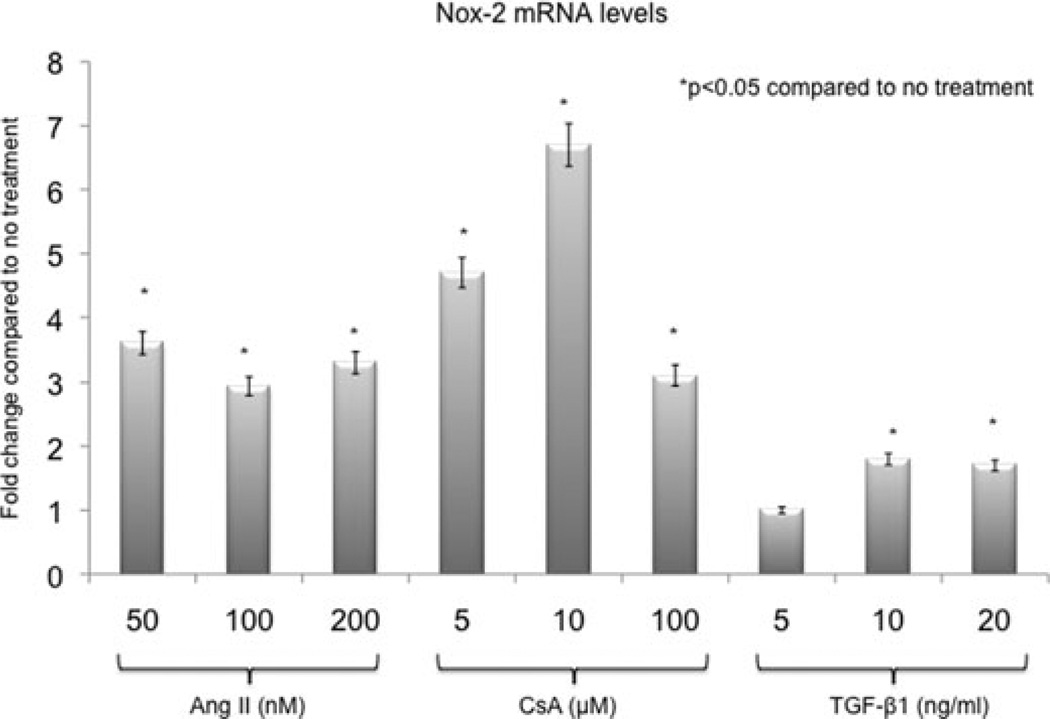
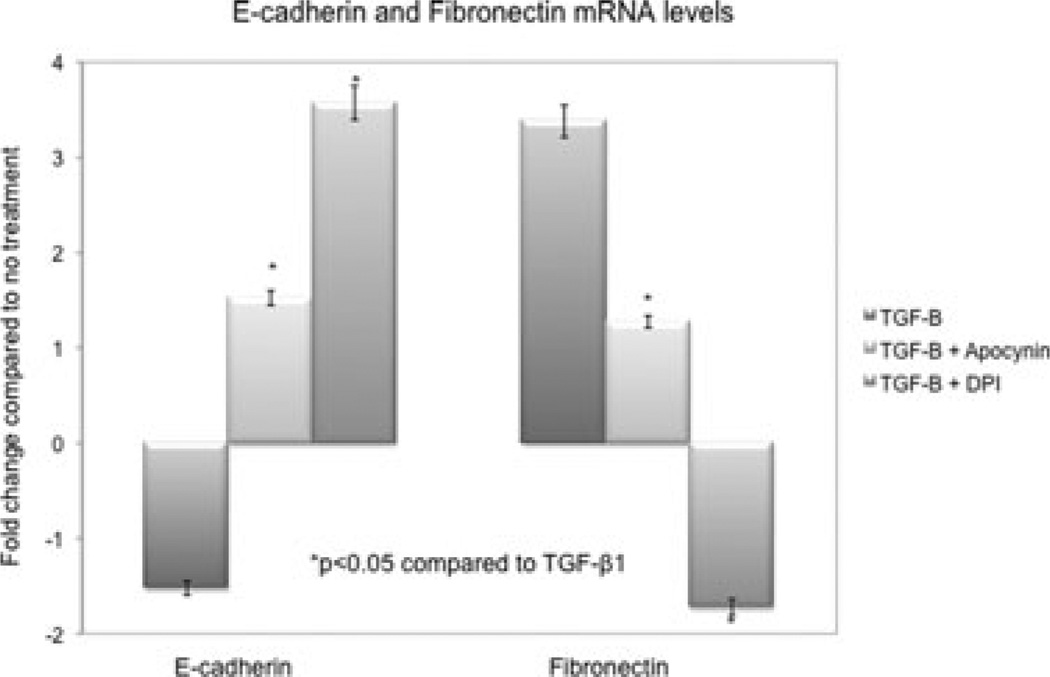
In vitro inhibition of Nox was associated with the inhibition of EMT
NRK52E were treated with increasing doses of angiotensin II, CsA and TGF-β1 to determine tubular Nox-2 response to key profibrotic molecules (Figure 7). Nox-2 mRNA levels measured by real-time PCR increased significantly (2- to 7-fold) after 4 h of treatment in all three groups. Pretreatment with DPI and apocynin inhibited TGF-β1 (20 ng/mL)-induced EMT by preventing the decrease in E-cadherin and the increase in fibronectin mRNA (Figure 8).
Figure 7. Angiotensin II, CsA and TGF-α1 induced Nox-2 mRNA expression in proximal tubular epithelial cells in vitro.
Normal rat proximal tubular epithelial cells (NRK52E) were treated with increasing doses of angiotensin II, CsA and TGF-β1 for 4 h to determine tubular Nox-2 response to key profibrotic molecules. Nox-2 mRNA levels measured by real-time PCR increased significantly (2– to 7-fold) after treatment in all three groups. Gene expression was normalized to S26 internal control. Data presented as average fold difference compared to no treatment. All experiments were repeated three times.
Figure 8. Nox inhibition prevented EMT transcripts in vitro.
Pretreatment with DPI (150 nM) and apocynin (500 µM) inhibited TGF-β1 (20 ng/mL)-induced EMT after 4 h by preventing the decrease in E-cadherin and the increase in fibronectin mRNA levels. Gene expression was normalized to S26 internal control. Data presented as average fold difference compared to no treatment. All experiments were repeated three times.
Discussion
Our study shows that Nox-2 is involved in the pathogenesis of kidney allograft tubulointerstitial fibrosis. Nox-2 and p22phox, the electron transferring subunits of Nox were increased in the tubulointerstitium of human kidney allografts with fibrosis. Graft infiltrating macrophages but not T cells expressed large amounts of Nox-2. Similarly, activated myofibroblasts but not mature fibroblasts demonstrated greater Nox-2 levels. Renal tubular epithelial cells coexpressed α-SMA and Nox-2 in areas of injury suggestive of a role for Nox-2 in EMT. These studies were confirmed in the rat model of kidney allograft fibrosis demonstrating tubulitis-associated Nox-2 upregulation and EMT. Furthermore, Nox-2 upregulation was associated with fibrogenesis and activation of profibrotic Smad2 signaling pathway. Short-term in vivo inhibition of Nox-2 with DPI and apocynin reduced fibrogenesis in kidney allografts. In vitro studies showed that angiotensin II, CsA and TGF-β1 induced Nox-2 mRNA in rat proximal tubular epithelial cells and that pretreatment with DPI and apocynin prevented EMT. In aggregate, these human, animal and in vitro studies demonstrate that Nox-2 plays a role in kidney allograft fibrogenesis and that the modulation of its activity may have beneficial effects of tubulointerstitial injury.
Only a few studies have looked at the effect of antioxidants on kidney allograft outcomes (9–11,31). Vitamin E supplementation alone did not prevent chronic allograft injury in an experimental model (9). Yet in the same model, L-arginine decreased proteinuria and glomerulosclerosis (31). Similarly, one clinical trial showed that the i.v. injection of human recombinant SOD immediately prior and after allograft reperfusion resulted in no difference between kidney function up to 48 h after transplant (11). However, another clinical trial using intraoperative i.v. injections of recombinant human SOD demonstrated that the incidence of acute and chronic rejection decreased significantly (10). It seems therefore that the type, dose and timing of intervention therapies with antioxidants for the prevention of chronic allograft IF need be determined.
An appropriate strategy to evaluate the role of antioxidants would be to inhibit early events such as the generation of ROS by inhibiting the activity of key pro-oxidant molecules such as Nox. There are no interventional studies aimed to inhibit Nox activity in human, or experimental kidney transplantation. However, congruent with our findings, the inhibition of Nox-4 by anti-sense oligonucleotides prevented cortical and glomerular deposition of fibronectin in diabetic rats (20). Apocynin prevented mesangial matrix expansion, upregulation of Nox-2 and urinary excretion of hydrogen peroxide (21); and treatment with p22phox siRNA improved angiotensin II-induced hypertension in rats (22). Together, these studies suggest that Nox modulates native and transplant kidney tubulointerstitial fibrosis and that chemical and/or genetic manipulations of Nox activity may reduce the injury burden.
We described three cell types expressing Nox-2 in kidney allografts: macrophages, myofibroblasts and tubular epithelial cells. T cells and mature fibroblasts did not express Nox-2 suggesting that these cells are not a key source of ROS generation in the allograft. While it was not surprising to find high levels of Nox-2 in graft infiltrating macrophages, it was interesting to demonstrate its association with fibrogenesis (activated myofibroblasts, phosphorylation of Smad2) and EMT (tubular epithelial cells). Calcineurin inhibitors, angiotensin II and TGF-β1 are key profibrotic molecules involved in the pathogenesis of EMT and matrix accumulation in native and transplant kidney disease. Our in vitro studies demonstrated that all three molecules increased Nox-2 mRNA in tubular epithelial cells and that Nox inhibition by DPI and apocynin prevented EMT. In a relevant study, kidney allografts treated with high-dose tacrolimus (0.25 mg/kg) for 90 days had increased TGF-β1 (37-fold), Nox-1 (18-fold), p22phox (31-fold) and Rac-1 mRNA (20-fold) compared to untreated syngeneic transplants (32), suggesting that some of the chronic nephrotoxic effects of calcineurin inhibitors result from the upregulation of Nox.
Our study is limited by the lack of long-term interventions inhibiting Nox activity in the allograft. However, we show that posttransplant tubulointerstitial fibrosis is a process that starts early in the rat allograft and we provide the proof of principle that Nox inhibition may prevent/delay injury. In conclusion, using human, animal and in vitro studies, we show that the classical phagocytic Nox subunits play an important role in nonphagocytic cells. Nox2 and p22phox were upregulated in graft infiltrating macrophages, but also in activated myofibroblasts and tubular epithelial cells in areas of injury including tubulitis. Nox-2 activation was associated with EMT and activation of profibrotic transcription factor Smad2. Last, chemical inhibition of Nox, in vitro and in vivo, resulted in the prevention of EMT and matrix accumulation. Nox inhibition strategies may have a beneficial effect on long-term allograft outcomes.
Acknowledgments
The parts of this work were supported by the NIH/NIDDK grant 5K08DK067981-05 and the ASN-AST John Merrill Grant to AD.
References
- 1.Halloran PF, Melk A, Barth C. Rethinking chronic allograft nephropathy: The concept of accelerated senescence. J Am Soc Nephrol. 1999;10:167–181. doi: 10.1681/ASN.V101167. [DOI] [PubMed] [Google Scholar]
- 2.Colvin RB. Chronic allograft nephropathy. N Engl J Med. 2003;349:2288–2290. doi: 10.1056/NEJMp038178. [DOI] [PubMed] [Google Scholar]
- 3.Cornell LD, Colvin RB. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2005;14:229–234. doi: 10.1097/01.mnh.0000165888.83125.07. [DOI] [PubMed] [Google Scholar]
- 4.Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. 2003;75:1291–1295. doi: 10.1097/01.TP.0000061602.03327.E2. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan B, Meier-Kriesche HU. Death after graft loss: An important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970–974. doi: 10.1034/j.1600-6143.2002.21015.x. [DOI] [PubMed] [Google Scholar]
- 6.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman J. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326–2333. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 7.Djamali A. Oxidative stress as a common pathway to chronic tubulointerstitial injury in kidney allografts. Am J Physiol Renal Physiol. 2007;293:F445–F455. doi: 10.1152/ajprenal.00037.2007. [DOI] [PubMed] [Google Scholar]
- 8.Bedi S, Vidyasagar A, Djamali A. Epithelial-to-mesenchymal transition and chronic allograft tubulointerstitial fibrosis. Transplant Rev. 2008;22:1–5. doi: 10.1016/j.trre.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottmann U, Oltersdorf J, Schaub M, et al. Oxidative stress in chronic renal allograft nephropathy in rats: Effects of long-term treatment with carvedilol, BM 91.0228, or alpha-tocopherol. J Cardiovasc Pharmacol. 2003;42:442–450. doi: 10.1097/00005344-200309000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Land W, Schneeberger H, Schleibner S, et al. The beneficial effect of human recombinant superoxide dismutase on acute and chronic rejection events in recipients of cadaveric renal transplants. Transplantation. 1994;57:211–217. doi: 10.1097/00007890-199401001-00010. [DOI] [PubMed] [Google Scholar]
- 11.Pollak R, Andrisevic JH, Maddux MS, Gruber SA, Paller MS. A randomized double-blind trial of the use of human recombinant superoxide dismutase in renal transplantation. Transplantation. 1993;55:57–60. doi: 10.1097/00007890-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 13.Zou AP, Li N, Cowley AW., Jr Production and actions of superoxide in the renal medulla. Hypertension. 2001;37(2 Part 2):547–553. doi: 10.1161/01.hyp.37.2.547. [DOI] [PubMed] [Google Scholar]
- 14.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 15.Rhyu DY, Yang Y, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 16.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 17.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 18.Rocic P, Lucchesi PA. NAD(P)H oxidases and TGF-beta-induced cardiac fibroblast differentiation: Nox-4 gets Smad. Circ Res. 2005;97:850–852. doi: 10.1161/01.RES.0000190403.87462.bf. [DOI] [PubMed] [Google Scholar]
- 19.De minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266–272. doi: 10.1016/j.abb.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorin Y, Block K, Hernandez J, et al. Nox4 NAD(P)H oxidase mediates hypertrophy and fibronectin expression in the diabetic kidney. J Biol Chem. 2005;280:39616–39626. doi: 10.1074/jbc.M502412200. [DOI] [PubMed] [Google Scholar]
- 21.Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 22.Modlinger P, Chabrashvili T, Gill PS, et al. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 23.Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: Reversal by lifelong antioxidant supplementation. Kidney Int. 2004;65:219–227. doi: 10.1111/j.1523-1755.2004.00372.x. [DOI] [PubMed] [Google Scholar]
- 24.Kondo S, Shimizu M, Urushihara M, et al. Addition of the antioxidant probucol to angiotensin II type I receptor antagonist arrests progressive mesangioproliferative glomerulonephritis in the rat. J Am Soc Nephrol. 2006;17:783–794. doi: 10.1681/ASN.2005050519. [DOI] [PubMed] [Google Scholar]
- 25.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 26.Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 meeting report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 27.Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant. 2005;5:500–509. doi: 10.1111/j.1600-6143.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 28.Cooper JM, Petty RK, Hayes DJ, Morgan-Hughes JA, Clark JB. Chronic administration of the oral hypoglycaemic agent diphenyleneiodonium to rats. An animal model of impaired oxidative phosphorylation (mitochondrial myopathy) Biochem Pharmacol. 1988;37:687–694. doi: 10.1016/0006-2952(88)90143-8. [DOI] [PubMed] [Google Scholar]
- 29.Abdelrahman M, Mazzon E, Bauer M, et al. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–114. doi: 10.1097/01.shk.0000151028.15377.f7. [DOI] [PubMed] [Google Scholar]
- 30.Djamali A, Reese S, Oberley T, Hullett D, Becker B. Heat shock protein 27 in chronic allograft nephropathy: A local stress response. Transplantation. 2005;79:1645–1657. doi: 10.1097/01.tp.0000164319.83159.a7. [DOI] [PubMed] [Google Scholar]
- 31.Albrecht EW, van Goor H, Smit-van Oosten A, Stegeman CA. Long-term dietary L-arginine supplementation attenuates proteinuria and focal glomerulosclerosis in experimental chronic renal transplant failure. Nitric Oxide. 2003;8:53–58. doi: 10.1016/s1089-8603(02)00132-5. [DOI] [PubMed] [Google Scholar]
- 32.Khanna AK, Pieper GM. NADPH oxidase subunits (NOX-1, p22phox, Rac-1) and tacrolimus-induced nephrotoxicity in a rat renal transplant model. Nephrol Dial Transplant. 2007;22:376–385. doi: 10.1093/ndt/gfl608. [DOI] [PubMed] [Google Scholar]