Abstract
This single-center, retrospective analysis evaluated long-term bosentan treatment in adult patients (n = 7) with both Down and Eisenmenger syndromes (DS-ES). Laboratory tests, 6-minute walk distance (6MWD), functional class, and Doppler echocardiography were assessed at baseline and during 2 years’ follow-up. Improvements or maintenance of 6MWD were observed (68 m improvement from baseline at month 12) after bosentan initiation. 6MWD was maintained up to year 2. Overall, 6 patients experienced a significant improvement in functional class during 2 years’ therapy (P = 0.01). There were no significant changes in parameters measured by Doppler echocardiography. None of the patients required either hospitalization or additional pulmonary arterial hypertension (PAH) therapy because of PAH progression. Bosentan treatment was generally well tolerated; no liver function abnormalities or serious adverse drug reactions were noted. In this DS-ES cohort, bosentan seemed to be well tolerated and clinically effective.
Keywords: pulmonary arterial hypertension, Eisenmenger syndrome, Down syndrome, congenital heart disease, bosentan, endothelin receptor antagonist
Introduction
Pulmonary arterial hypertension (PAH) is a rare, progressive disease characterized by remodeling of the pulmonary vasculature leading to a progressive increase in pulmonary vascular resistance (PVR) and mean pulmonary arterial pressure.1 PAH is commonly associated with congenital heart disease (CHD) and can develop earlier or later in life depending on size and location of the underlying defect. Recent data from a US registry reported that PAH-CHD was the second most prevalent etiology within the associated PAH patient subgroup.2
Patients with PAH-CHD are at risk of developing right-to-left cardiac shunting, that is, Eisenmenger syndrome (ES). ES patients must make certain lifestyle adjustments due to limited functional capacity. Exercise capacity and quality of life (QOL) are also diminished.3 Moreover, there are a number of associated life-threatening complications and life expectancy is poorer than in the general population.4
Congenital heart defects are highly prevalent (40%–58%) in patients with Down syndrome (DS).5,6 Patients with DS and CHD are considered to be at higher risk for PAH3,7 and ES3 (Defined within the Pulmonary Hypertension World Health Organisation (WHO) clinical classification system (Dana Point 2008) as Group 1), probably due to different pathogenetic factors.7–12
According to European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines,13 ES represents the most advanced form of PAH-CHD and is characterized by a reversed pulmonary-to-systemic (right-to-left) shunt and central cyanosis. Several studies in adult patients with ES have reported a persistent beneficial effect of bosentan (an oral dual endothelin receptor antagonist) on exercise capacity and clinical status without any worsening of pulmonary-to-systemic shunt.14–17 In an open-label study, 24 patients with DS-ES were treated with bosentan with a median follow-up period of 11.5 months.18 The authors concluded that medical treatment was well tolerated in DS-ES patients and that results in patients with DS did not deviate from those observed in ES alone.18 The same authors in a retrospective study reported on the effect of almost 2 years of bosentan treatment on exercise capacity and QOL in 58 adult patients with PAH-CHD with and without DS.19 The results showed that in DS, patient’s 6-minute walk distance (6MWD) and QOL were stable during treatment. The aim of our retrospective study was to evaluate the effect of long-term treatment with bosentan in adult patients with DS-ES.
Methods
This was a retrospective analysis of data collected prospectively from all patients with DS-ES treated and undergoing active follow-up at our center (Department of Internal and Vascular Medicine IRCCS, Policlinico, San Donato Milano, Italy) between September 2008 and December 2010. Permission to prospectively collect patient data was obtained from the parents or the patients’ legal guardians. The authors of this manuscript have certified that they comply with the ethical standards of the responsible committee and with the Helsinki Declaration.20
Eisenmenger syndrome was diagnosed prior to, or following, referral to our center, based on clinical and echocardiographic features and confirmed by right heart catheterization. It was required that patients included in this analysis had stable PAH for at least 1 month prior to the study, that is, had no sign of acute heart failure. Patients with concomitant causes of pulmonary hypertension, such as lung, liver, or connective tissue disease, were excluded from this analysis.
All patients received bosentan (Tracleer®, Actelion Pharmaceuticals Ltd., Allschwil, Switzerland) treatment, which was started at an oral dose of 62.5 mg twice a day and increased to the target dose of 125 mg twice a day after 4 weeks. Patients started on bosentan therapy following a diagnosis of ES and subsequent assessment by our center confirming that they were suitable candidates for this treatment, that is, they were in New York Heart Association (NYHA) functional class (FC) above II. Bosentan is the first-line treatment of choice for ES-DS patients at our center.
Submaximal exercise capacity was assessed using 6MWD according to the guidelines of the American Thoracic Society (ATS), and transcutaneous arterial oxygen saturation was measured by continuous pulse oximetry monitoring (ATS guidelines) before and after 6MWD testing.21 Functional capacity was assessed using the NYHA FC system, by the same representative parent or guardian on behalf of the patient at each assessment point. Right ventricular function was measured using tricuspid annular plane systolic excursion (TAPSE), and systolic pulmonary arterial pressure (PAPs) was estimated using peak velocity of the tricuspid regurgitation jet with continuous wave Doppler. These parameters were evaluated at baseline and every 6 months during bosentan therapy. Parameters such as mortality, hospitalization, additional PAH therapy, and cardiac surgery that collectively signify clinical worsening were also recorded and assessed. Laboratory tests (including hemoglobin, liver function, and uric acid) were evaluated every 2 weeks during the first 2 months and every 2 months thereafter. All patients were subjected to a baseline electrocardiogram and a chest X-ray.
Statistical analysis
Statistical analysis was performed applying the Shapiro-Wilk W test. Measured variables (6MWD, oxygen saturation, NYHA FC, PAPs, and TAPSE) were statistically analyzed at baseline, 12 months, and at the end of the study period (24 months). Clinical and instrumental parameter changes from baseline were controlled and a P value < 0.05 was considered as a level of statistical significance.
Results
Seven adult patients with DS-ES, five male (72%) and two female (28%) with a mean age 31.7 years (range 20–50 years), were included in this analysis. The majority of patients (5/7) had ventricular septal defect (VSD) (Table 1). Patients had not received corrective cardiac surgery. As these patients were referred to our center in adulthood, the reasons why they were not operated on in infancy were unknown. No patient received anticoagulant therapy or sildenafil therapy; concomitant medications for each patient are provided on Table 2.
Table 1.
Individual patient characteristics at baseline.
| Patient | Gender | Age, years | Weight, kg | Body mass index, kg/m2 | CHD | NYHA FC | 6MWD, m |
|---|---|---|---|---|---|---|---|
| 1 | Male | 34 | 47 | 17 | AVC | IV | 220 |
| 2 | Male | 24 | 108 | 42 | VSD | III | 310 |
| 3 | Female | 20 | 55 | 20 | VSD | IV | 180 |
| 4 | Male | 21 | 64 | 22 | VSD | III | 360 |
| 5 | Male | 41 | 74 | 27 | VSD | III | 200 |
| 6 | Male | 50 | 65 | 24 | VSD | III | 300 |
| 7 | Female | 32 | 60 | 24 | AVC | III | 200 |
Abbreviations: AVC, atrioventricular canal; VSD, ventricular septal defect.
Table 2.
Individual baseline medications.
| Patient | Medication |
|---|---|
| 1 | Digoxin + diuretics + allopurinol + iron |
| 2 | Diuretics + aspirin + iron |
| 3 | Iron |
| 4 | Diuretics + allopurinol |
| 5 | Diuretics + iron |
| 6 | Digoxin + diuretics + allopurinol |
| 7 | Iron |
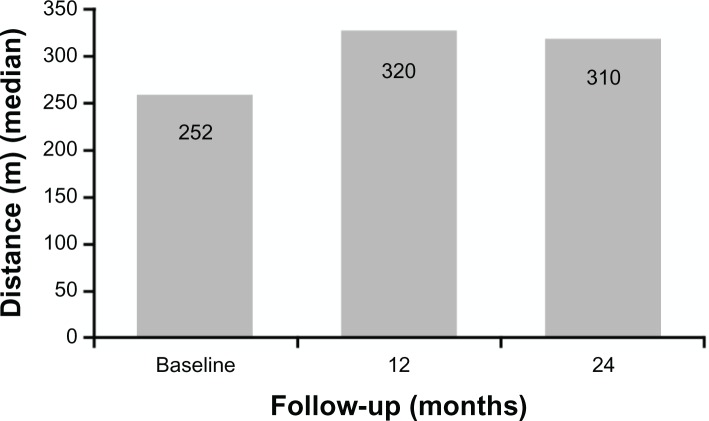
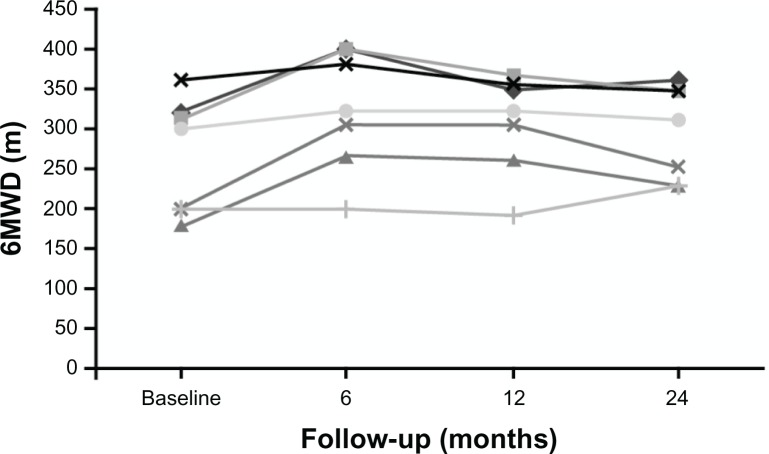
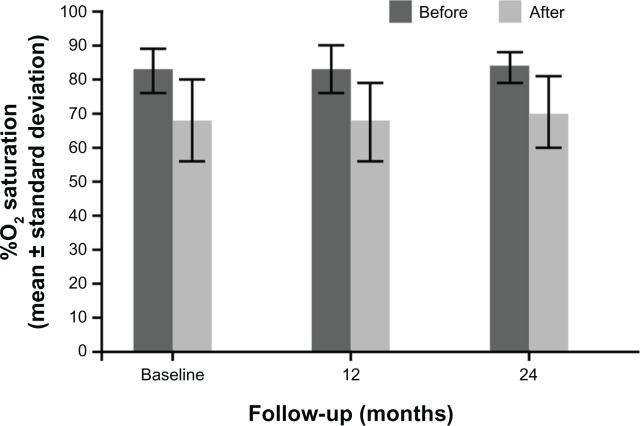
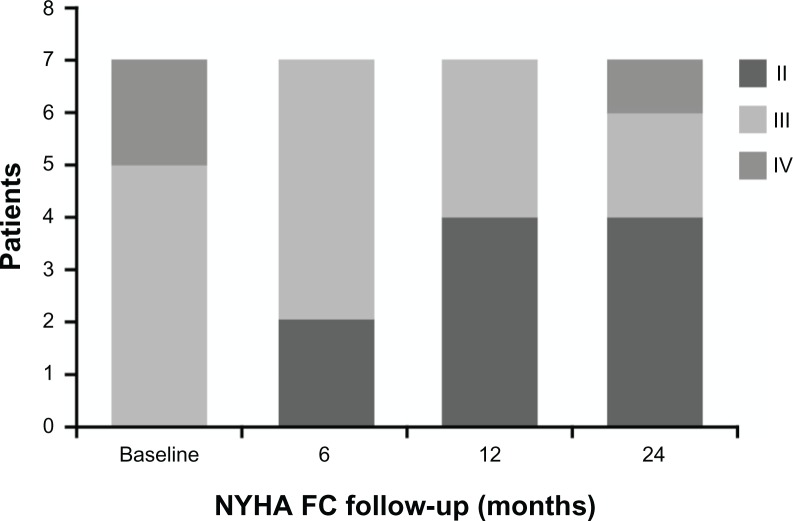
In the first 6 and 12 months of bosentan therapy, patients showed an increase in 6MWD compared with baseline values (Figs. 1 and 2). Median 6MWD increased from 252 m at baseline to 320 m at 12 months (P = 0.10) and after 24 months 6MWD was maintained compared with the 12 month value (median 310 m, P = 0.20). Mean ± standard deviation 6MWD was 261 ± 64 m at baseline, 306 ± 62 m at 12 months, and 298 ± 60 m at 24 months. There was no significant variation of oxygen saturation before and after 6MWD testing over the 2-year course of follow-up (Fig. 3). Overall, patients experienced a statistically significant improvement in NYHA FC (Fig. 4; P = 0.01). After 24 months of therapy, two patients improved from their baseline NYHA FC from IV to III, and four patients, from NYHA FC III to II. One 50 year-old male patient with perimembranous VSD and aortic valve insufficiency who initially remained in NYHC FC III worsened into NYHA FC IV after 24 months’ treatment. There was no significant change in Doppler echocardiography data (TAPSE, PAPs, and absence of pericardial effusion) observed. No patients required hospitalization for PAH or additional PAH therapy because of progression of disease in the 2-year observation period, and no patients required cardiac surgery.
Figure 1.
Changes in 6MWD at baseline, after 12 months (P = 0.1), and after 24 months (P = 0.2) of bosentan therapy in 7 patients with dual DS-ES.
Figure 2.
Individual change in 6MWD over 24 months of bosentan therapy in 7 patients with dual DS-ES.
Figure 3.
Arterial oxygen saturation before and after 6MWD testing in 7 patients with dual DS-ES.
Figure 4.
Changes in NYHA FC from baseline and after 6, 12, and 24 months of bosentan therapy (P = 0.01) in 7 patients with dual DS-ES.
Bosentan treatment was generally well tolerated and no serious adverse drug reactions were recorded. All seven patients with DS-ES tolerated induction of oral bosentan therapy and there was no indication of decreased arterial oxygen saturation over the treatment period studied. No liver function abnormalities (>3 × upper limit of normal) were noted and uric acid, hemoglobin, white blood cells, and platelet count were unchanged during bosentan treatment.
Discussion
Overall, the DS-ES patients treated with bosentan in our study showed initial improvement in exercise capacity, as shown by the median and mean increases from baseline at 12 months, which was subsequently maintained up to 2 years. Although the changes from baseline in 6MWD at 12 and 24 months were not statistically significant, they are clinically meaningful. Given the progressive nature of ES, the fact that three patients showed an increase in 6MWD and the other four remained stable over the course of 2 years (even in one patient who was overweight) is encouraging (see Fig. 2 for individual patient data). Furthermore, NYHA FC improved in six patients over the course of the study providing strong supportive evidence that most of these patients derived a benefit from bosentan treatment over the long term. In addition, patients in our study did not exhibit any of the clinical parameters associated with clinical worsening, that is, death, hospitalization due to PAH, or a requirement for additional PAH therapy or cardiac surgery, although one patient did worsen to NYHA FC IV after 24 months’ treatment. We have shown long-term benefits in exercise capacity (6MWD) and NYHA FC in DS-ES patients treated with bosentan combined with a favorable safety profile in accordance with previously published literature.15,18,19,22–25
In our center, bosentan is selected as the first-line treatment for patients with DS-ES, based on the recommendations given in guidelines for the treatment of ES patients13 and the fact that sildenafil may have sexual side effects in men. Sildenafil may be initiated in combination with bosentan if a patient’s 6MWD decreases by at least 10% compared with the previous value and there are other indicators consistent with deterioration and no ethical impediments. None of the patients received targeted PAH combination therapy during the study period.
The BREATHE-5 study was the first to demonstrate improvements in hemodynamics and exercise capacity after treatment with bosentan in patients with ES.16 The study’s open-label extension confirms the early results over a longer treatment period of 40 weeks.26 A subgroup analysis of the BREATHE-5 patient population showed that the location of septal defect was a key determinant of treatment response.27
In our study, during the follow-up period, DS-ES patients showed and maintained an increased 6MWD. Additionally, the patients’ FC improved significantly during follow-up. Our data add to and support the findings reported in the literature on the effects of targeted PAH therapies in this patient population. Duffels et al reported an improvement or stability in QOL and 6MWD with long-term bosentan administration in patients with DS-ES.18,19 A further study also showed an improved 6MWD in three DS-ES patients during long-term treatment with bosentan.28 More recently, several articles have been published regarding the efficacy of bosentan therapy in this subset of patients. Two long-term studies demonstrated a prolonged beneficial effect of bosentan treatment on exercise capacity and main hemodynamic parameters.22,23 Monfredi et al24 using the same method of follow-up, confirmed the efficacy and safety data of bosentan.
Prior to the era of targeted PAH therapies, survival of patients with ES was calculated to be 97% at 1 year, 89% at 2 years, and 77% at 3 years, which were higher than the comparable rates for patients with idiopathic PAH (77%, 69%, and 35%, at 1, 2 and 3 years, respectively).29 While these data may suggest that ES patients have a more stable disease course than idiopathic PAH patients, this does not mean that ES patients would not derive benefits from PAH therapy in terms of survival. Until recently, there has been little evidence regarding the survival benefits of targeted PAH therapy in patients with ES. A single center study has recently provided the first evidence that targeted PAH therapies can significantly reduce the risk of death in ES patients.27
Study strengths and limitations
The present study adds valuable evidence to the limited information reported in the literature regarding patients with DS-ES treated with the dual endothelin receptor antagonist, bosentan. This study demonstrated a significant and stable long-term clinical improvement with a favorable safety profile in accordance with previous reports. However, this study is subject to a number of limitations. These include the lack of a control group and the small sample size, but this is not surprising considering the low prevalence of this dual syndrome. Nevertheless, our observations are in line with other recent studies.18,19,22–25
Conclusions
In conclusion, in our experience and in accordance with latest literature, bosentan has been shown to be well tolerated and clinically effective in patients with DS-ES, and the clinical benefit is maintained over a long-term treatment period.
Acknowledgements and Funding
An English language editing service was provided by Rose Doyle PhD, Elements Communications Ltd., Westerham, UK and was funded by Actelion Pharmaceuticals Ltd., Allschwil, Switzerland.
Footnotes
This article is available from http://www.la-press.com.
Author Contributions
Planned and designed the study: GS, MCh, and MC. Treated patients and performed evaluations: MG, AM, DN, CL, and RD. Collected and statistically analyzed data: MG. Wrote the first draft of the manuscript: GS. All authors agree with manuscript results and conclusions, made critical revisions, and approved final version. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Houtchens J, Martin D, Klinger JR. Diagnosis and management of pulmonary arterial hypertension. Pulm Med. 2011;2011:845–64. doi: 10.1155/2011/845864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. 2012;21:8–18. doi: 10.1183/09059180.00008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaemmerer H, Mebus S, Schulze-Neick I, et al. The adult patient with Eisenmenger syndrome: a medical update after dana point part I: epidemiology, clinical aspects and diagnostic options. Curr Cardiol Rev. 2010;6:343–55. doi: 10.2174/157340310793566154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuuring MJ, Vis JC, Duffels MG, Bouma BJ, Mulder BJ. Adult patients with pulmonary arterial hypertension due to congenital heart disease: a review on advanced medical treatment with bosentan. Ther Clin Risk Manag. 2010;6:359–66. doi: 10.2147/tcrm.s8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cua CL, Blankenship A, North J, Hayes AL, Nelin LD. Increased incidence of idiopathic persistent pulmonary hypertension in Down syndrome neonates. Pediatr Cardiol. 2007;28:250–4. doi: 10.1007/s00246-006-0011-6. [DOI] [PubMed] [Google Scholar]
- 6.van Loon RLE, Roofthooft TR, Hillege HL, et al. Pediatric pulmonary hypertension in The Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. 2011;124:1755–64. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Yamaki S, Mimori S, et al. Pulmonary vascular disease in Down’s syndrome with complete atrioventricular septal defect. Am J Cardiol. 2000;86:434–7. doi: 10.1016/s0002-9149(00)00960-7. [DOI] [PubMed] [Google Scholar]
- 8.Byard RW. Forensic issue in Down syndrome fatalities. J Forensic Legal Med. 2007;14:475–81. doi: 10.1016/j.jflm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Chi TP, Krovetz JL. The pulmonary vascular bed in children with Down syndrome. J Pediatr. 1975;86:533–8. doi: 10.1016/s0022-3476(75)80142-9. [DOI] [PubMed] [Google Scholar]
- 10.Hals J, Hagemo PS, Thaulow E, Sorland SJ. Pulmonary vascular resistance in complete atrioventricular septal defect. A comparison between children with and without Down’s syndrome. Acta Paediatrica. 1993;82:595–8. doi: 10.1111/j.1651-2227.1993.tb12763.x. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs IN, Gray RF, Todd NW. Upper airway obstruction in children with Down syndrome. Arch Otolaryngol Head Neck Surg. 1996;122:945–50. doi: 10.1001/archotol.1996.01890210025007. [DOI] [PubMed] [Google Scholar]
- 12.Yamaki S, Yasui H, Kado H, et al. Pulmonary vascular disease and operative indications in complete atrioventricular canal defect in early infancy. J Thorac Cardiovasc Surg. 1993;106:398–405. [PubMed] [Google Scholar]
- 13.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 14.D’Alto M, Vizza CD, Romeo E, et al. Long term effects of bosentan treatment in adult patients with pulmonary arterial hypertension related to congenital heart disease (Eisenmenger physiology): safety, tolerability, clinical and haemodynamic effect. Heart. 2007;93:621–5. doi: 10.1136/hrt.2006.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulos K, Inuzuka R, Goletto S, et al. Improved survival among patients with Eisenmenger syndrome receiving advanced therapy for pulmonary arterial hypertension. Circulation. 2010;121:20–5. doi: 10.1161/CIRCULATIONAHA.109.883876. [DOI] [PubMed] [Google Scholar]
- 16.Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114:48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 17.Raposo-Sonnenfeld I, Otero-Gonzales I, Blanco-Aparicio M, Ferrer-Barba A, Medrano-Lopez C. Treatment with sildenafil, bosentan or both in children and young people with idiopathic pulmonary hypertension and Eisenmenger’s syndrome. Rev Esp Cardiol. 2007;60:366–72. [PubMed] [Google Scholar]
- 18.Duffels MGJ, Vis JC, van Loon RLE, et al. Down patients with Eisenmenger syndrome: is bosentan treatment an option? Int J Cardiol. 2009;134:378–83. doi: 10.1016/j.ijcard.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Duffels MGJ, Vis JC, van Loon RLE, et al. Effect of bosentan on exercise capacity and quality of life in adults with pulmonary arterial hypertension associated with congenital heart disease with and without Down’s syndrome. Am J Cardiol. 2009;103:1309–15. doi: 10.1016/j.amjcard.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 20.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Postgrad Med. 2002;48:206–8. [PubMed] [Google Scholar]
- 21.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.D’Alto M, Romeo E, Argiento P, et al. Therapy for pulmonary arterial hypertension due to congenital heart disease and Down’s syndrome. [Published online ahead of print Jul 27, 2011] Int J Cardiol. doi: 10.1016/j.ijcard.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Vis JC, Duffels MG, Mulder P, et al. Prolonged beneficial effect of bosentan treatment and 4-year survival rates in adult patients with pulmonary arterial hypertension associated with congenital heart disease. [Published online ahead of print Jun 30, 2011] Int J Cardiol. doi: 10.1016/j.ijcard.2011.06.064. [DOI] [PubMed] [Google Scholar]
- 24.Monfredi O, Griffiths L, Clarke B, Mahadevan VS. Efficacy and safety of bosentan for pulmonary arterial hypertension in adults with congenital heart disease. Am J Cardiol. 2011;108:1483–8. doi: 10.1016/j.amjcard.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Kermeen FD, Franks C, O’Brien K, et al. Endothelin receptor antagonists are an effective long term treatment option in pulmonary arterial hypertension associated with congenital heart disease with or without trisomy 21. Heart Lung Circ. 2010;19:595–600. doi: 10.1016/j.hlc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Gatzoulis MA, Beghetti M, Galiè N, et al. BREATHE-5 Investigators Longer-term bosentan therapy improves functional capacity in Eisenmenger syndrome: results of the BREATHE-5 open-label extension study. Int J Cardiol. 2008;127:27–32. doi: 10.1016/j.ijcard.2007.04.078. [DOI] [PubMed] [Google Scholar]
- 27.Berger RM, Beghetti M, Galiè N, et al. Atrial septal defects versus ventricular septal defects in BREATHE-5, a placebo-controlled study of pulmonary arterial hypertension related to Eisenmenger’s syndrome: a subgroup analysis. Int J Cardiol. 2010;144:373–8. doi: 10.1016/j.ijcard.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 28.Quintana EM, Gonzales FR, Medina-Gil JM, Agredo-Munoz J, Nieto-Lago V. Clinical outcome in Down syndrome patients with congenital heart disease. Cir Cir. 2010;78:245–50. [PubMed] [Google Scholar]
- 29.Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or Eisenmenger syndrome. J Heart Lung Transplant. 1996;15(1, pt 1):100–5. [PubMed] [Google Scholar]