Abstract
Background. Following the emergence of 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) in the United States and Mexico in April 2009, A(H1N1)pdm09 spread rapidly all over the world. There is a dearth of information about the epidemiology of A(H1N1)pdm09 in Africa, including Morocco. We describe the epidemiologic characteristics of the A(H1N1)pdm09 epidemic in Morocco during 2009–2010, including transmissibility and risk factors associated with fatal disease.
Methods. We implemented influenza surveillance for patients presenting with influenza-like illness (ILI) at 136 private and public clinics for patients with severe acute respiratory illness (SARI) at 16 regional public hospitals from June 2009 through February 2010. Respiratory samples and structured questionnaires were collected from all enrolled patients, and samples were tested by real-time reverse-transcription polymerase chain reaction for influenza viruses. We estimated the risk factors associated with fatal disease as well as the basic reproduction number (R0) and the serial interval of the pandemic virus.
Results. From June 2009 through February 2010, we obtained 3937 specimens, of which 1452 tested positive for influenza virus. Of these, 1398 (96%) were A(H1N1)pdm09. Forty percent of specimens from ILI cases (1056 of 2646) and 27% from SARI cases (342 of 1291) were positive for A(H1N1)pdm09. Sixty-four deaths occurred among laboratory-confirmed A(H1N1)pdm09 SARI cases. Among these cases, those who had hypertension (age-adjusted odd ratio [aOR], 28.2; 95% confidence interval [CI], 2.0–398.7), had neurological disorders (aOR, 7.5; 95% CI, 1.5–36.4), or were obese (aOR, 7.1; 95% CI, 1.6–31.1), as well as women of gestational age who were pregnant (aOR, 2.5; 95% CI, 1.1–5.6), were at increased risk of death. Across the country, elevated numbers of locally acquired infections were detected 4 months after the detection of the first laboratory-confirmed case and coincided with the expected influenza season (October–January) in Morocco. We obtained an R0 estimate of 1.44 (95% CI, 1.32–1.56) and a mean serial interval (±SD) of 2.3 ± 1.4 days (95% CI, 1.6–3.0).
Conclusion. Widespread but delayed community transmission of A(H1N1)pdm09 occurred in Morocco in 2009, and A(H1N1)pdm09 became the dominant influenza virus subtype during the 2009–2010 influenza season. The transmissibility characteristics were similar to those observed in other countries.
Following the emergence of 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) in the United States and Mexico in April 2009, A(H1N1)pdm09 spread rapidly all over the world [1]. On 11 June 2009, the World Health Organization (WHO) raised the pandemic alert to level 6 after human-to-human transmission and global spread of A(H1N1)pdm09 was confirmed [2]. Studies have since identified differences between seasonal influenza virus and A(H1N1)pdm09: factors associated with A(H1N1)pdm09 infection include a younger age distribution [3]; certain comorbidities, such as obesity [4]; and symptoms not frequently associated with seasonal influenza virus infection, such as diarrhea and vomiting [5]. There is a dearth of information about the epidemiology of A(H1N1)pdm09 in Africa, including Morocco. We describe the epidemiologic characteristics of the epidemic of A(H1N1)pdm09 infection in Morocco during 2009–2010, including transmissibility and risk factors associated with fatal disease.
METHODS
Study Design and Settings
Morocco is a middle-income country located in North Africa and has an estimated population of about 32 million people [6]. Routine influenza sentinel surveillance in Morocco is based on the collection of epidemiologic and virologic data from patients presenting with influenza-like illness (ILI), acute respiratory illness (ARI), and severe acute respiratory illness (SARI) at selected private and public health clinics and public hospitals. A questionnaire and nasopharyngeal and oropharyngeal swab specimens are collected from all enrolled SARI and ILI cases; samples are tested for influenza virus types and subtypes at regional reference laboratories or at the National Influenza Center (NIC; the National Institute of Hygiene) [7, 8].
SARI Surveillance
Routine SARI surveillance is conducted throughout the year at the pediatric, pulmonary, and internal medicine wards of 1 regional public hospital in each of the 16 regions of the country. A SARI case is defined as a hospitalized patient ≥5 years of age with fever (temperature, >38°C), cough, and shortness of breath or difficulty breathing with a duration of illness of <7 days. In patients aged 2 months through <5 years, SARI is defined as a child hospitalized for cough or difficulty breathing, with or without wheezing and stridor in a calm child, or chest indrawing. In patients aged 1 week through <2 months, SARI is defined as hospitalization for a respiratory illness associated with one of the following signs: convulsions, tachypnea (≥60 breaths per minute in a calm infant), chest indrawing, nasal flaring, grunting, lethargy or unconsciousness, fever (temperature of >38°C or warm to the touch), or hypothermia (temperature of <36°C or cold to the touch). All SARI cases were eligible for enrollment if they provided verbal informed consent or, for patients <15 years of age, if consent was obtained from the patient's caregiver.
ILI and ARI Surveillance
Routine ILI and ARI surveillance is implemented in 495 public and private outpatient clinics across the country in 2 separate networks: the public health center network and the private practitioners' network. The public health center network includes 375 public clinics that collect aggregated data on total ILI and ARI consultations and total outpatient visits throughout the year. Of these, 16 (4.2%; 1 health center in each region) collect nasopharyngeal and oropharyngeal specimens from a maximum of 5 ILI cases per day for virologic testing. The private practitioner network comprises 120 physicians (including general practitioners, pediatricians, and lung specialists) located in 9 large cities across the country (Tanger, Fes, Meknes, Oujda, Casablanca, Rabat, Marrakech, Agadir, and Laayoun). The physicians enroll a maximum of 5 ILI cases per day and provide the NIC with aggregated data on the total number of ILI and ARI cases during October–April.
An ILI case is defined as an outpatient with fever (temperature, ≥38°C) and cough or sore throat in the absence of a specific diagnosis and with symptom onset <5 days prior to presentation. An ARI case is defined as an outpatient with sudden onset of ≥1 respiratory sign (cough, difficulty breathing, rhinitis, or coryza) and ≥1 general symptom (fever, headache, fatigue, or myalgia) <5 days before presentation. ILI cases were eligible for enrollment if they provided verbal informed consent or, for patients <15 years of age, if consent was obtained from the patient's caregiver.
Enhanced Surveillance During the A(H1N1)pdm09 Epidemic
The national SARI surveillance system was maintained throughout the A(H1N1)pdm09 epidemic in the country. Furthermore, the private sentinel network (120 clinics), which usually operates only during the influenza season (October–April in Morocco), was activated outside the influenza season, and oropharyngeal and nasopharyngeal specimens were requested to be collected from all consenting ILI patients presenting at the private (120) and public (16) clinics that routinely implement ILI virologic surveillance. In addition, daily reporting of laboratory-confirmed cases and deaths was set up.
For each ILI or SARI case enrolled, a structured questionnaire was used to collect demographic, epidemiologic, and clinical information, and the data were entered into a Web-based database (http://grippe.sante.gov.ma/INH). In addition, the Department of Epidemiology and Disease Control investigated all laboratory-confirmed A(H1N1)pdm09 SARI cases and deaths in order to collect information on the patients' medical history.
Sample Collection and Laboratory Procedures During the A(H1N1)pdm09 Epidemic
During the study period, oropharyngeal and nasopharyngeal swab specimens were collected from ILI and SARI cases enrolled at the sentinel surveillance sites. Oropharyngeal and nasopharyngeal swab specimens collected from the same patient were placed in 1 cryovial, stored at 4°C at the health facilities, and transported within 48 hours to the NIC or the regional reference laboratories. For transportation, the specimens were packaged using a standard triple packaging system and were transported in cool boxes.
The NIC and 4 regional laboratories conducted all diagnostic testing using the real-time polymerase chain reaction protocol developed by the US Centers for Disease Control and Prevention (CDC) for the detection of A(H1N1)pdm09 [9].
Data Analysis
We analyzed the epidemiologic characteristics of ILI, SARI, and deaths among laboratory-confirmed A(H1N1)pdm09 cases from June 2009 through February 2010. We assessed the increased risk for mortality due to underlying medical conditions among laboratory-confirmed A(H1N1)pdm09 SARI cases, using age-adjusted logistic regression.
In addition, we estimated the basic reproduction number (R0) during the growth phase of the epidemic; the growth phase was defined as the period from the occurrence of an increasing number of cases over 5 consecutive days to the epidemic peak [10]. The R0 is a key transmissibility parameter and is defined as the average number of secondary cases caused by a primary case in an entirely susceptible population [10].
We based the R0 estimates for Morocco on dates of symptom onset, using daily counts of laboratory-confirmed cases obtained from the Web-based influenza database. When the date of symptom onset was missing, we imputed this value from the date of specimen collection (which was available for all confirmed cases). We modeled the lag time from the date of symptom onset to the date of specimen collection from cases with complete data via a Poisson regression model, using predictors statistically significant at a P value of < .05 (ie, public or private institution, date of sample collection, and age), and we imputed the estimated date of symptom onset by using a random sampling process from a Poisson distribution. The imputation process was repeated 1000 times for each missing value. We used 2 methods to estimate the R0 value in Morocco.
In method 1, we first estimated the exponential growth rate (which represents the average increase in the proportion of cases per increase in unit time [days]) of the epidemic, using Poisson regression. We then derived R0 by assuming that the serial interval (SI; defined as the time between successive cases in a chain of transmission) values followed a gamma distribution [11, 12], using the formula
in which 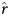 is the estimated exponential growth rate, µ is the mean serial interval, and k is the coefficient of variation (SD/mean) of the serial interval.
is the estimated exponential growth rate, µ is the mean serial interval, and k is the coefficient of variation (SD/mean) of the serial interval.
Since no SI estimates were available from Morocco or from other African countries, an estimated value of the SI (mean [±SD], 2.7 ± 1.1; n = 32) observed in the Netherlands [13] was used. Because, for this method, we used the mean SI observed in another country, we performed a sensitivity analysis to assess the variation of R0 on the basis of different mean SI values (ie, 2 and 4 days). In addition, we performed a sensitivity analysis to assess the variation of the exponential growth rates estimates (and related R0 values) over different periods of the growth phase of the epidemic.
In method 2, we used a likelihood-based method for the simultaneous estimation of R0 and SI [10, 13–15]. This method is well suited for estimating R0 and SI in real time with observed aggregated daily counts of new cases, denoted by N = {N0, N1, …, NT}, where T is the last day of observation and N0 is the initial number of seed cases that begin the outbreak. Ni values are assumed to be composed of a mixture of cases that were generated by the previous k days, where k is the maximal value of the serial interval. Xji is the number of cases that appear on day i that were infected by individuals with onset of symptoms on day j. The method assumes that the number of infectees generated by infectors with symptoms on day j follows a Poisson distribution with parameter R0Nj. Additionally, Xj = {Xj,j+1, Xj,j+2, … ,Xj,j+k+1}, the vector of cases infected by the Nj individuals, follows a multinomial distribution with parameters p, k, and Xj. Here p is a vector of probabilities that denotes the serial interval distribution. The following likelihood was used for the estimation:
in which 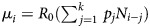 .
.
For the R0 calculations using the maximum likelihood method, we used 6 days as the maximal value of the SI (k). In addition, we implemented a sensitivity analysis to assess the variation of the R0 and SI estimates vis-à-vis different values of k and over different periods of the growth phase of the epidemic. All analyses were performed using R, version 2.12.0.
RESULTS
Detection and Spread of A(H1N1)pdm09 in Morocco
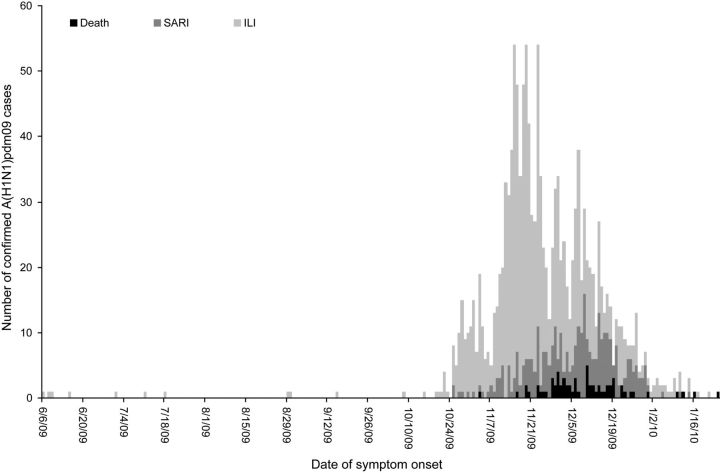
From June 2009 through February 2010, 1398 laboratory-confirmed A(H1N1)pdm09 cases were detected in Morocco. The first laboratory-confirmed A(H1N1)pdm09 case was identified on 10 June 2009 in a 19-year-old woman travelling from Canada to Morocco. From June through October 2009, cases were either imported or were part of local clusters linked to imported cases. Low-level transmission was observed until late October (Figure 1), when an increased number of locally acquired infections was detected.
Figure 1.
Number of 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) cases associated with nonfatal influenza-like illness (ILI), nonfatal severe acute respiratory illness (SARI), and death, by date of illness onset, Morocco, 2009–2010.
The period from November 2009 through January 2010 was characterized by an elevated number of locally acquired infections, indicating sustained community transmission. The A(H1N1)pdm09 epidemic in Morocco peaked in late November 2009 and was over by the end of January 2010. By the end of the epidemic, all 16 regions in Morocco had been affected.
Epidemiologic Characteristics of A(H1N1)pdm09 in Morocco
From June 2009 through February 2010, 3937 specimens were collected from ILI (2646; 67%) and SARI (1291; 33%) case patients at surveillance sites; 872 (22%) were from the 16 public health centers, 1774 (45%) were from the private sentinel surveillance network, and 1291 (32%) were from the hospital network. Of these specimens, 1452 (37%) tested positive for influenza virus. Among the positive samples, 1398 (96%) were A(H1N1)pdm09, 17 (1.1%) were seasonal influenza A virus subtype (A[H1N1]), 4 (0.2%) were influenza A virus subtype H3N2 (A[H3N2]), and 33 (2.3%) were influenza B virus. Among the A(H1N1)pdm09-positive cases, there were 716 (51%) males and 682 (49%) females.
The percentage positive for A(H1N1)pdm09 was 36% (1398 of 3937) overall, 40% (1056 of 2646) in ILI cases, and 27% (342 of 1291) in SARI cases (Table 1). The highest percentages of positive cases among ILI (490 of 911; 54%) and SARI (73 of 220; 33%) cases were observed in the 5–14-year age group.
Table 1.
Proportion of Specimens Positive for 2009 Pandemic Influenza A Virus Subtype H1N1 Among Influenza-Like Illness (ILI) and Severe Acute Respiratory Illness (SARI) Cases, by Age, Morocco, 2009–2010
| Age | ILI Cases, Proportion (%) | SARI Cases, Proportion (%) |
|---|---|---|
| <5 y | 104/332 (31.1) | 100/518 (19.3) |
| 5–14 y | 490/911 (53.8) | 73/220 (33.2) |
| 15–24 y | 226/560 (40.4) | 37/138 (26.8) |
| 25–59 y | 226/732 (30.9) | 118/358 (32.9) |
| >60 y | 10/111 (9.0) | 14/57 (24.6) |
| Total | 1056/2646 (39.9) | 342/1291 (26.5) |
Sixty-four deaths occurred among SARI cases with laboratory-confirmed A(H1N1)pdm09. Deaths related to A(H1N1)pdm09 were reported in all age groups; 4 (6%) were in children aged <5 years, 3 (5%) were in children aged 5–14 years, 15 (23%) were in individuals aged 15–24 years, 40 (63%) were in adults aged 25–59 years, and 2 (3%) were in adults aged ≥60 years. Forty-nine of the 64 fatal cases (77%) had 1 or more underlying medical conditions and/or smoked cigarettes. Among the SARI cases with laboratory-confirmed A(H1N1)pdm09, those who had hypertension (age-adjusted odds ratio [aOR], 28.2; 95% confidence interval [CI], 2.0–398.7), had neurological disorders (aOR, 7.5; 95% CI, 1.5–36.4), or were obese (aOR, 7.1; 95% CI, 1.6–31.1), as well as women of gestational age who were pregnant (aOR, 2.5; 95% CI, 1.1–5.6), were at increased risk of death (Table 2).
Table 2.
Prevalence of Underlying Medical Conditions and Risk Factors for Mortality Among Patients With or Without Fatal Severe Acute Respiratory Illness (SARI) and Laboratory-Confirmed 2009 Pandemic Influenza A Virus Subtype H1N1 Infection, Morocco, 2009–2010
| Underlying Condition | Died, No. (%) (n = 64) | Survived, No. (%) (n = 278) | Age-Adjusted OR (95% CI) | P |
|---|---|---|---|---|
| Obesitya | 7 (10.9) | 3 (1.0) | 7.1 (1.6–31.1) | .009 |
| Cardiopathy | 6 (9.4) | 18 (6.5) | 1.2 (.4–3.4) | .706 |
| Hypertension | 5 (7.8) | 1 (0.4) | 28.2 (2.0–398.7) | .013 |
| Pregnancyb | 17 (40.5) | 18 (11.1) | 2.5 (1.1–5.6) | .034 |
| Diabetes | 11 (17.2) | 18 (6.5) | 2.0 (.8–4.8) | .110 |
| Asthma | 6 (9.4) | 34 (12.2) | 0.7 (.3–1.8) | .523 |
| Neurologic disorder | 4 (6.3) | 7 (2.5) | 7.5 (1.5–36.4) | .013 |
| Chronic respiratory disease | 3 (4.7) | 7 (2.5) | 2.2 (.5–10.5) | .321 |
| Renal failure | 4 (6.3) | 0 | … | |
| Othersc | 4 (6.3) | 21 (7.5) | 0.9 (.3–2.9) | .864 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aObesity was determined by subjective judgment.
bFor women of gestational age only (42 deaths and 162 nonfatal SARI cases).
cAllergy, anemia, immune deficiency, medullar aplasia, rheumatism, cirrhosis, and trisomy 21.
Transmissibility of A(H1N1)pdm09 in Morocco
Of 1398 laboratory-confirmed A(H1N1)pdm09 cases, 237 (17%) did not have information on the date of symptom onset. For these cases, the date of symptom onset was estimated using multiple imputation techniques. From the available surveillance data, the growth phase of the A(H1N1)pdm09 epidemic in Morocco started on 21 October 2009 and extended for 29 days, to 18 November 2009. The exponential growth rate was estimated at 0.13 (95% CI, .11–.15) (ie, an average increase of 13% in the number of cases for each increase in unit time [days]) for the period from 24 October 2009 through 15 November 2009 (Supplementary Table 1). For this period, using the SI estimates from the Netherlands study (mean [±SD], 2.7 ± 1.1) [14], the estimated R0 was 1.40 (95% CI, 1.34–1.48) (method 1). Sensitivity analysis that was implemented over different periods of the growth phase of the epidemic and used different mean SI estimates revealed that the R0 values were in the range of 1.24 (95% CI, 1.19–1.28) for the period from 21 October 2009 through 12 November 2009, with a mean SI of 2 days, and 1.94 (95% CI, 1.73–2.17) for the period from 27 October 2009 through 18 November 2009, with a mean SI of 4 days.
We used the likelihood-based method for the simultaneous estimation of R0 and the SI (method 2) and obtained an R0 estimate of 1.44 (95% CI, 1.32–1.56) and a mean SI (±SD) of 2.3 ± 1.4 days (95% CI, 1.6–3.0) for the period 24 October 2009 through 15 November 2009 and a maximal value (length) of the SI of 6 days (Supplementary Table 2). In the sensitivity analysis, R0 estimates ranged from 1.36 (95% CI, 1.27–1.45) for the period from 21 October 2009 through 12 November 2009, with a maximal SI of 4 days, to 1.58 (95% CI, 1.47–1.69) for the period from 27 October 2009 through 18 November 2009, with a maximal SI of 8 days. For the same periods and maximal values of the SI, the estimated mean SI (±SD) ranged from 1.7 ± 1.2 days (95% CI, 1.1–2.3) to 3.0 ± 1.7 days (95% CI, 1.9–4.1).
DISCUSSION
Beginning in May 2009, the Moroccan Ministry of Health implemented robust influenza surveillance to monitor the spread of A(H1N1)pdm09 and its impact on public health. Our surveillance showed that A(H1N1)pdm09 did not spread swiftly after 10 June 2009, when the first case was detected. Elevated numbers of locally acquired infections were not detected until the onset of the expected influenza season (October–April) in the country [7, 8]. This is in contrast to the majority of temperate European countries near Morocco, where significant increases in numbers of newly reported cases were observed beginning in July 2009 [16]. Factors contributing to the delayed community spread of A(H1N1)pdm09 in Morocco may be the implementation of strict containment measures in May 2009, which included social distancing, early isolation of cases, and antiviral prophylaxis for close contacts of case patients. Another factor that could have contributed to reduced transmission was the summer school closure, which started during 15–29 June 2009 and extended through the month of Ramadan (22 August–21 September 2009) to 27 September 2009. However, delayed community transmission of A(H1N1)pdm09 (toward the end of 2009 or the beginning of 2010) was observed in other countries in west and central Africa (eg, Ghana, Senegal, Ivory Coast, Nigeria, Niger, Cameroon, Mauritania, Guinea, Cape Verde, and Mali), where no strict containment measures were implemented [17]. All of these countries had functional influenza surveillance systems that documented community transmission 3–5 months after the detection of the first case of A(H1N1)pdm09 infection in the country.
The percentage of ILI and SARI cases positive for A(H1N1)pdm09 in Morocco was highest among children aged 5–14 years. Most of the A(H1N1)pdm09-associated deaths occurred in the 25–59-year age group, which contrasts with seasonal influenza, where the highest numbers of deaths typically occur among individuals aged >65 years [18, 19]. We observed underlying conditions in 77% of fatal cases and found that conditions such as obesity, pregnancy, hypertension, and neurologic disorder were significantly more common among fatal SARI cases with A(H1N1)pdm09 infection, compared with nonfatal SARI cases. These are well-known risk factors for severe disease due to seasonal influenza virus infection, and they have been observed to be associated with severe disease due to A(H1N1)pdm09 in other countries [4, 18–20].
From the available literature, R0 estimates for A(H1N1)pdm09 varied globally, from 1.1 to 1.8 [15, 16, 21, 22]; the values were higher in school outbreaks [21, 23]. R0 is a measure of transmissibility that encompasses the intrinsic transmissibility of the pathogen, as well as the characteristics of the host population. Different population structures in different countries may explain the observed differences. Other factors that may explain these differences are the use of varied estimation methods, the time of the estimation (eg, during the early phases of the pandemic, when active case finding was implemented and no country- and pathogen-specific SI estimates were available), and the use of suspected and/or confirmed A(H1N1)pdm09 cases for the estimation. In our analysis, the 2 estimation methods that we used yielded similar R0 values. The maximum likelihood method for the simultaneous estimation of R0 and SI may be more appropriate in our setting, where no independent estimates of the SI are available. However, the Morocco R0 estimates obtained with both methods are within the range of values observed globally. The SI estimate for Morocco is also within the range of estimates from other countries [22].
Our findings are subject to limitations. Cases of A(H1N1)pdm09 infection may have been missed by the surveillance system, particularly if symptoms were mild or if cases were asymptomatic. In this regard, the use of only confirmed cases of A(H1N1)pdm09 infection for the R0 analysis may have resulted in underestimating the true R0. Errors such as underreporting and misclassification of outcome and risk factors are possible and might have concealed the real burden of disease.
The results of this study highlight the importance of the clinical, epidemiologic, and virologic influenza surveillance network in Morocco that permitted timely identification of pandemic influenza cases, monitoring of the spread of the epidemic, and isolation and characterization of the new circulating strains. Similar surveillance networks are needed in other African countries where influenza surveillance is lacking or suboptimal.
In conclusion, widespread but delayed community transmission of A(H1N1)pdm09 occurred in Morocco in 2009, and A(H1N1)pdm09 became the dominant influenza virus subtype during the 2009–2010 influenza season. The transmissibility characteristics were similar to those observed in other countries.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are deeply indebted to the Ministry of Health staff at the regional and national levels and the private practitioners who participate in the National Influenza Surveillance System, for their assistance in case identification and sample collection at sentinel sites. We are also grateful to Dr Adam L. Cohen, CDC–South Africa, for providing support on scientific and grammatical issues associated with the writing of this manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the Ministry of Health, Morocco, and through funding by the CDC (grant 5U51CI000469).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Chan M. World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html. Accessed 9 December 2009. [Google Scholar]
- 3.Kwan-Gett TS, Baer A, Duchin JS. Spring 2009 H1N1 influenza outbreak in King County, Washington. Disaster Med Public Health Prep. 2009;3(Suppl 2):S109–16. doi: 10.1097/DMP.0b013e3181c6b818. [DOI] [PubMed] [Google Scholar]
- 4.Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1) Clin Infect Dis. 2011;52:301–12. doi: 10.1093/cid/ciq152. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84:185–9. [PubMed] [Google Scholar]
- 6.Haut commisariat au plan. Maroc. http://ww.hcp.ma . Accessed 15 March 2012. [Google Scholar]
- 7.Barakat A, Benjouad A, Manuguerra JC, El Aouad R, Van der Werf S. Virological surveillance in Africa can contribute to early detection of new genetic and antigenic lineages of influenza viruses. J Infect Dev Ctries. 2011;5:270–7. doi: 10.3855/jidc.1065. [DOI] [PubMed] [Google Scholar]
- 8.Barakat A, Ihazmad H, Benkaroum S, et al. PLoS One. 2011;6:e24579. doi: 10.1371/journal.pone.0024579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC protocol of realtime RTPCR for influenza a (H1N1) Geneva: World Health Organization; 2009. http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf . Accessed 13 September 2012. [Google Scholar]
- 10.White LF, Wallinga J, Finelli L, et al. Estimation of the reproductive number and the serial interval in early phase of the 2009 influenza A/H1N1 pandemic in the USA. Influenza Other Respi Viruses. 2009;3:267–76. doi: 10.1111/j.1750-2659.2009.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts MG, Heesterbeek JA. Model-consistent estimation of the basic reproduction number from the incidence of an emerging infection. J Math Biol. 2007;55:803–16. doi: 10.1007/s00285-007-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallinga J, Lipsitch M. How generation intervals shape the relationship between growth rates and reproductive numbers. Proc Biol Sci. 2007;274:599–604. doi: 10.1098/rspb.2006.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahné S, Donker T, Meijer A, et al. Epidemiology and control of influenza A(H1N1)v in the Netherlands: the first115 cases. Euro Surveill. 2009;14 doi: 10.2807/ese.14.27.19267-en. pii = 19267 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19267 . Accessed 13 September 2012. [DOI] [PubMed] [Google Scholar]
- 14.White LF, Pagano M. A likelihood-based method for real-time estimation of the serial interval and reproductive number of an epidemic. Stat Med. 2008;27:2999–3016. doi: 10.1002/sim.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization (WHO) Transmission dynamics and impact of pandemic influenza A (H1N1) 2009 virus. Wkly Epidemiol Rec. 2009;84:481–4. [PubMed] [Google Scholar]
- 16.ECDC working group on influenza A(H1N1)v. Preminary analysis of influenza A(H1N1)v individual and aggregated case reports from EU and EFTA. Euo surveillance. 2009;14 doi: 10.2807/ese.14.23.19238-en. pii = 19238. [DOI] [PubMed] [Google Scholar]
- 17.206(Suppl 1):S101–7. doi: 10.1093/infdis/jis572. Talla Nzussouo N, Michalove J, Diop O, et al. Delayed 2009 Pandemic Influenza A Virus Subtype H1N1 Circulation in West Africa, May 2009–April 2010. J Infect Dis 2012. [DOI] [PubMed] [Google Scholar]
- 18.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl 1):S3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 19.Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine. 2005;23(Suppl 1):S1–9. doi: 10.1016/j.vaccine.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14 doi: 10.2807/ese.14.33.19309-en. pii = 19309. [DOI] [PubMed] [Google Scholar]
- 21.Nishiura H, Castillo-Chavez C, Safan M, Chowell G. Transmission potential of the new influenza A(H1N1) virus and its age-specificity in Japan. Euro Surveill. 2009;14 doi: 10.2807/ese.14.22.19227-en. pii: 19227 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19227 . Accessed 13 September 2012. [DOI] [PubMed] [Google Scholar]
- 22.Boëlle PY, Ansart S, Cori A, Valleron AJ. Transmission parameters of the A/H1N1 (2009) influenza virus pandemic: a review. Influenza Other Respi Viruses. 2011;5:306–16. doi: 10.1111/j.1750-2659.2011.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cauchemez S, Ferguson NM, Wachtel C, et al. Closure of schools during an influenza pandemic. Lancet Infect Dis. 2009;9:473–81. doi: 10.1016/S1473-3099(09)70176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.