Abstract
Background. We documented the introduction of 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) into South Africa and describe its clinical presentation, epidemiology, and transmissibility.
Methods. We conducted a prospective descriptive study of the first 100 laboratory-confirmed cases of A(H1N1)pdm09 infections identified through active case finding and surveillance. Infected patients and the attending clinicians were interviewed, and close contacts were followed up to investigate household transmission.
Findings. The first case was confirmed on 14 June 2009, and by 15 July 2009, 100 cases were diagnosed. Forty-two percent of patients reported international travel within 7 days prior to onset of illness. Patients ranged in age from 4 to 70 years (median age, 21.5 years). Seventeen percent of household contacts developed influenza-like illness, and 10% of household contacts had laboratory-confirmed A(H1N1)pdm09 infection. We found a mean serial interval (± SD) of 2.3 ± 1.3 days (range, 1–5 days) between successive laboratory-confirmed cases in the transmission chain.
Conclusions. A(H1N1)pdm09 established itself rapidly in South Africa. Transmissibility of the virus was comparable to observations from outside of Africa and to seasonal influenza virus strains.
The 2009 pandemic influenza A virus subtype H1N1 (A[H1N1]pdm09) spread rapidly worldwide following its discovery in Mexico and the United States [1]. The introduction of a novel pathogen requires the timely understanding of its virulence, transmission potential, and epidemiologic characteristics to inform public health policies and interventions.
At the time of introduction of A(H1N1)pdm09 into Africa, the vast majority of A(H1N1)pdm09 infections reported elsewhere were among adolescents and adults, and disease had been generally described as mild to moderate in severity [2, 3]. Transmissibility was considered to be substantially higher than for seasonal influenza, with a secondary attack rate (SAR) of 22%–33%, compared with 5%–15% for seasonal influenza [3–5]. Revised estimates of the SAR of A(H1N1)pdm09 show that transmissibility is comparable to that of seasonal influenza strains concurrently circulating (8% and 9%, respectively) [6]. The serial interval (SI) of seasonal influenza is generally accepted to range from 2 to 4 days [7–9]. Retrospective estimates of the mean SI from the 1918 influenza pandemic include 1–3 days within closed study settings [10, 11] but range more widely (2–8 days) in community settings [10]. In a systematic review, Boëlle et al summarized 13 previous studies of the mean SI of A(H1N1)pdm09 [12], reporting an overall SI estimate of 3 days (95% confidence interval, 2.4–3.6), which decreased to 2.6 days when accounting for tertiary transmission. These findings are, however, largely based on the observations from more-developed countries and do not incorporate data from Africa.
In the early stages after A(H1N1)pdm09 introduction in South Africa, we conducted detailed investigations into laboratory-confirmed cases, with the objective of rapidly documenting the clinical presentation of illness, epidemiology, and transmissibility of the virus at the household level, all of which may differ from characteristics in more-developed countries, owing to South Africa's status as a developing country with relatively higher rates of underlying illness. Because of logistical limitations, and in accordance with World Health Organization (WHO) recommendations to limit case finding and laboratory testing, these studies were restricted to investigation of the first 100 laboratory-confirmed infections. We report the findings of this study to, first, document the introduction of A(H1N1)pdm09 into South Africa and describe its characteristics when first introduced in the country and, second, to provide comments on the suitability of these rapid assessments in informing the public health response during the early stages of a pandemic.
MATERIALS AND METHODS
We conducted a prospective investigation of the first 100 laboratory-confirmed A(H1N1)pdm09 cases identified in South Africa through active case finding and surveillance activities. Case finding and surveillance methods during the study period are described in detail elsewhere [13]. From 28 April 2009 onward, healthcare workers throughout South Africa were requested to collected nasal and throat swab specimens from all individuals who presented to any healthcare facility and met the suspected case definition, including individuals with recent onset of influenza-like illness (ILI) and either a history of travel to an area reporting a confirmed community-wide outbreak or close contact with a suspected or confirmed case ≤7 days prior to the onset of symptoms. We defined ILI as self-reported fever plus 1 or more of the following: sore throat, rhinorrhea, cough, myalgia, or diarrhea. Specimens from all patients with suspected cases were transported for testing to the National Influenza Centre at the National Institute for Communicable Disease (NICD), a division of the National Health Laboratory Service (NHLS). The National Influenza Centre was solely responsible for laboratory investigations in South Africa during this period. Testing was performed using the real-time polymerase chain reaction (PCR) protocol developed by the WHO Collaborating Centre for Influenza, Centers for Disease Control and Prevention (CDC; Atlanta, GA), for the detection and characterization of A(H1N1)pdm09 [14].
Immediately following laboratory confirmation, we interviewed by telephone both the patient and the attending healthcare physician. We used a standardized questionnaire to capture demographic and clinical information, and we requested patients to identify all close contacts presenting with ILI. We defined a close contact as any individual sleeping within the same dwelling as an index patient for the period of ≤7 days before to <14 days after onset of their illness. We conducted a second interview of the index patients ≥14 days after symptom onset to identify contacts who may have developed symptoms after the initial interview. We also interviewed symptomatic contacts by using the same protocol, instructed them to visit their healthcare professional for a full clinical assessment, and collected specimens for laboratory testing. In the event that a patient with a confirmed case could not be reached for interview or refused to participate, we included the next consecutive patient with a confirmed case identified by active case finding or surveillance in this study.
Data from completed questionnaires were captured, cleaned, and analyzed using EpiInfo v3.5.1 (CDC). To assess the presence of malnutrition among patients, we calculated the body mass index (BMI; defined as the weight in kilograms divided by the square of the height in meters). Cases with a BMI of <18.5 were classified as underweight, and those with a BMI of ≥30 were classified as obese.
We estimated the transmissibility of A(H1N1)pdm09 through the estimation of the SI (ie, the mean time between onset of illness in 2 successive patients in the chain of transmission) and the SAR at the household (ie, residential living unit) level. We defined the household SAR as the total number of secondary cases among contacts of index cases per the total number of susceptible close contacts. Individuals with epidemiologically linked cases who had a date of symptom onset <1 day from that of the index patient were classified as coprimary; 1–8 days, as secondary; and 9–14 days, as tertiary. We calculated the SI and SAR for laboratory-confirmed cases. We additionally considered suspected cases that did not have an opportunity for laboratory testing in these calculations. Secondary cases without a date of symptom onset or with a laboratory finding other than A(H1N1)pdm09 infection (ie, negative test results or detection of seasonal influenza virus infection) were excluded from analyses. Given previous estimates of a mean SI of 3 days between successive cases in the transmission chain [12], we further explored the robustness of our SI and SAR estimates by excluding patients with an onset of illness ≥6 days after onset of illness in their index patient, which may constitute tertiary transmission.
Ethics clearance for essential communicable disease surveillance was granted to the NICD-NHLS by the Human Medical Research Ethics Committee of the University of the Witwatersrand, Johannesburg (protocol number M060449, reference R14/49 Schoub). This includes outbreak investigations related to notifiable medical conditions under surveillance.
RESULTS
The first laboratory-confirmed case of A(H1N1)pdm09 infection was identified in an international traveler from the United States, who landing in South Africa on 14 June 2009 with onset of illness 1 day prior to arrival. Thereafter, we observed 7 additional travel-associated cases, followed by the first identified locally acquired infection on 24 June 2009. By 15 July 2009, a total of 108 cases were confirmed at the NICD-NHLS from among 762 samples tested (detection rate, 14%). Eight cases were lost to follow-up during this period and are excluded from this analysis.
Patients with the first 100 confirmed cases ranged in age from 4 to 70 years (median age, 21.5 years), and 60% were male (Table 1). The largest proportion of cases (54%) was detected in Gauteng Province. Fifty-eight percent of cases acquired infection within South Africa, indicating that sustained local transmission had occurred during this period. Of 42 patients with imported cases, the largest proportion traveled from European (15 [36%]) and Asian (8 [19%]) countries. Although clusters of 2 or 3 cases were found within households, one large cluster, associated with a university squash tournament held from 30 June 2009 to 2 July 2009 in Gauteng Province, accounted for 66% of locally acquired infections (38 of 58) described here.
Table 1.
Demographic Characteristics, Travel History, and Clinical Presentation Associated With the First 100 Laboratory-Confirmed Cases of 2009 Pandemic Influenza A Virus Subtype H1N1 Infection, South Africa, 2009
| Characteristic | Value |
|---|---|
| Male sex | 60 |
| Age, y | |
| Median (range) | 21.5 (4–70) |
| 0–9 | 7 |
| 10–19 | 31 |
| 20–29 | 36 |
| 30–39 | 11 |
| 40–49 | 5 |
| ≥50 | 10 |
| International travel ≤7 d before symptom onset | |
| Any | 42 |
| Europe | 15/42 |
| Asia | 8/42 |
| North America | 6/42 |
| South America | 5/42 |
| Australia | 5/42 |
| Africa (excluding South Africa) | 3/42 |
| Symptom | |
| Cough | 85 |
| Sore throat | 72 |
| Feeling feverish | 72 |
| Nasal congestion | 65 |
| Muscle pain | 64 |
| Headache | 62 |
| Fever (temperature, >38°C) | 55/93 |
| Sneezing | 44 |
| Shortness of breath | 30 |
| Nausea | 25 |
| Diarrhea | 17 |
| Vomiting | 13 |
| Conjunctivitis | 7 |
| Comorbid disease | |
| Any | 11 |
| Asthma | 7 |
| Cardiovascular diseasea | 4 |
| Underweightb | 7/71 |
| Obesityb | 3/71 |
| Hospitalization | 11 |
Data are for 100 patients, unless otherwise indicated.
a Includes hypertension.
bAssessed using body mass index (BMI) calculated as the weight in kilograms divided by the square of the height in meters. Underweight was defined as a BMI of <18.5, and obese was defined as a BMI of ≥30.
From the households of the patients with primary cases, we identified a cumulative total of 158 susceptible contacts—a ratio ( ± SD) of 2.2 ± 1.8 susceptible contacts per primary case (range, 0–8). Among these contacts, we identified 1 patient with a laboratory-confirmed coprimary case, 16 with confirmed secondary cases, 11 with secondary cases who developed ILI but did not have an opportunity for laboratory testing (ie, suspected cases), and 1 with a confirmed tertiary case. We therefore estimated a household SAR of 10%, when including laboratory-confirmed secondary cases only, and a SAR of 17%, when additionally including suspected secondary cases. The median delay from onset of illness to collection of laboratory specimens from symptomatic contacts was 2 days (range, 0–9 days).
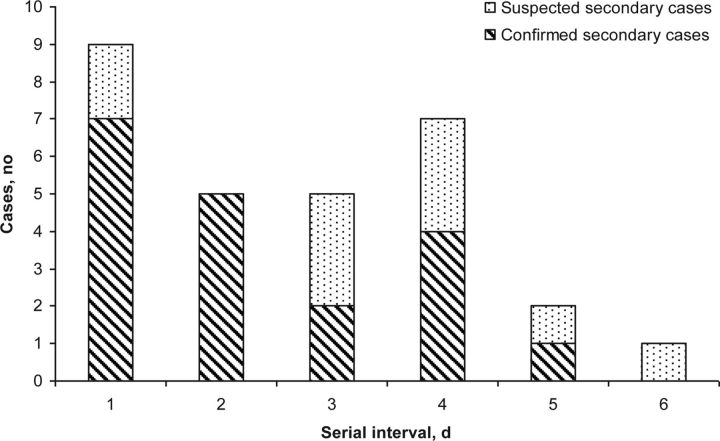
We observed 29 instances of transmission from a primary case to a secondary case in which the dates of symptom onset were known for both cases, including 27 instances of household transmission (16 laboratory confirmed and 11 suspected) and 2 laboratory-confirmed instances of transmission in a hotel or university-residence setting. We found a mean SI ( ± SD) of 2.3 ± 1.3 (range, 1–5 days) when including laboratory-confirmed cases only. This slightly increased to 2.7 ± 1.5 days (range, 1–6 days) when suspected cases were included (Figure 1).
Figure 1.
Estimated serial interval (ie, time between onset of illness in 2 successive patients in the chain of transmission) from investigations into the first 100 laboratory-confirmed cases of 2009 pandemic influenza A virus subtype H1N1 infection, South Africa, 2009.
One instance of suspected transmission was observed in which the SI was ≥6 days. Although no other symptomatic cases were observed in this household, there remains a possibility that infection may have arisen in this patient because of tertiary transmission. When we adjusted for this, we observed limited change in our estimate that incorporates suspected and confirmed cases (SAR, 26 of 158 [16%]; mean SI [ ± SD], 2.6 ± 1.4 days; range, 1–5 days).
The most frequent symptoms reported among the first 100 confirmed cases included cough (85%) and sore throat (72%); a smaller proportion experienced gastrointestinal symptoms (nausea, in 25%; diarrhea, in 17%; and vomiting, in 13%) (Table 1). Despite inclusion of fever in the definition of suspected cases, specimen collection often included individuals without documented or self-reported fever at the time of presentation. We found that 72% of the patients with the first 100 cases reported feeling feverish and, of 93 for whom temperatures were recorded, 59% (n = 55) were pyrexial (temperature, ≥38°C). One patient with a laboratory-confirmed case who was tested in the early stages of the outbreak because of a history of being a contact of a confirmed case was asymptomatic. Of 11 hospitalized case-patients, 6 were admitted as an isolation precaution, 2 were admitted for observation because of an underlying condition (asthma in one and heart disease in the other), and 3 were admitted because of the development of complications (2 received a diagnosis of pneumonia, and 1 received a diagnosis of myocarditis). The overall prevalence of comorbid disease among the patients with the first 100 cases was 11%. Underlying medical conditions included asthma (in 7%) and hypertension (in 4%); no patients were pregnant or reported to be infected with human immunodeficiency virus (HIV). A BMI was recorded for 71 cases, of which 10% (7) were found to be underweight (BMI, <18.5), and 4% (3) were obese (BMI, ≥30). Seventy-two cases received treatment with oseltamivir after a clinical diagnosis of influenza virus infection was made. No chronic sequelae or fatalities were reported during or subsequent to the follow-up period among the patients with the first 100 A(H1N1)pdm09 cases.
DISCUSSION
While international travel played an important role in the global spread of this novel pathogen, sustained local transmission occurred rapidly in South Africa, and the first 100 cases were confirmed within a period of 1 month following virus introduction. We estimated the household SAR of A(H1N1)pdm09 to be 17% and the SI to be 2.3 days. These transmissibility characteristics are consistent with findings from most studies of A(H1N1)pdm09 outside Africa and are broadly similar to those of seasonal influenza viruses [3–9, 12].
The highest rate of infection was among young adults (median age, 21.5 years) within the initial case group, which is in contrast to elevated infection rates observed in younger subjects (median age, 15.5 years) when one examines all A(H1N1)pdm09 cases detected in South Africa throughout 2009 [13]. In addition, males were slightly more affected than females. These differences are likely due to the higher rates of international travel among older age groups and the large proportion of the initial infections associated with a university sporting event predominated by young adult males. We additionally found that infection with A(H1N1)pdm09 was a self-limiting, mild-to-moderate disease characterized by cough, sore throat, and fever; these findings are similar to those observed elsewhere [2–4, 15–17].
We note that the introduction of A(H1N1)pdm09 in South Africa, in particular, and in Africa, in general, was relatively delayed in comparison to the rapid spread of this novel virus to other continents. Although the occurrence of infections in our setting prior to these documented cases cannot be conclusively excluded, we believe this report accurately reflects the introduction of this novel virus into South Africa, given extensive case-finding activities, surveillance, and laboratory investigations conducted from late April 2009 onward.
These results are subject to a number of limitations. First, the transmissibility of A(H1N1)pdm09 documented here may be underestimated because of interventions taken to mitigate the spread of infection, including increased practice of personal infection-control precautions, owing to high community awareness and intense international media coverage, as well as possible (but limited) use of antiviral prophylaxis by contacts in the very early stages of the pandemic (this was not measured). Second, we were unable to obtain specimens for laboratory investigations from 11 of 27 individuals (41%) with secondary cases; therefore, the measured SAR of 17% may be overestimated. In secondary cases not tested, ILI may have been caused by seasonal influenza virus strains or other pathogens; however, we note that the introduction of A(H1N1)pdm09 occurred at the end of the seasonal influenza season in South Africa [18].
Third, our transmissibility parameters may be underestimated in that only secondary cases presenting with ILI were considered in our study. Studies elsewhere observed a proportion of individuals with A(H1N1)pdm09 infections who presented without fever or remained asymptomatic but who may have contributed to transmission of the virus [6, 19]. Furthermore, we based our measurement on the assumption that epidemiologically linked cases with a SI of <1 day were coprimary, those with a SI of 1–8 days were secondary, and those with a SI of 9–14 days were tertiary; however, with most estimates of the SI at <3 days, one might assume that cases presenting after a SI of ≥6 days may be tertiary cases. We identified one instance of transmission that would fit these criteria; however, given that this was observed during the period of the first 100 cases, it is believed that the risks of acquiring infection outside of the household would be minimal. Furthermore, no other secondary cases were identified in this household; therefore, in this instance, secondary transmission was more likely. We nonetheless excluded the case in a repeated analysis and observed limited change in our transmissibility estimates.
Finally, the clinical findings for this study are not generalizable to the entire South African population, because A(H1N1)pdm09 first affected a more affluent population, with a lower prevalence of comorbidities (including HIV infection) than observed in the general population. Pandemic influenza virus infection in poorer communities with high rates of known risk factors (including underlying medical conditions) may result in more-severe disease. Furthermore, the clinical findings shown here are affected by the study design and criteria used for case enrollment. One would anticipate a different pattern of illness presentation in patients who attend healthcare facilities, as well as a more frequent finding of subclinical or asymptomatic illness if all contacts were laboratory tested.
These limitations bring into question the suitability of the WHO recommendation to conduct in-depth investigations limited to the first 100 cases for the purposes of documenting clinical epidemiological and transmissibility characteristics of a novel influenza virus, which may be used to inform public health policies and interventions. Our experience and findings following such a study has demonstrated that these methods were effective in estimating selected transmissibility characteristics but that other epidemiological characteristics (eg, case demographic characteristics) were substantially biased by the case groups who were first affected (ie, travelers and young adults associated with university gathering). Measures of the clinical presentation and severity of illness and the risk factors for progression to severe disease may not be adequately described using this approach and are biased by the case definition and enrollment methods applied. Furthermore, the usefulness of these findings (other than transmissibility estimates) would be short-lived and superseded by epidemiological description of a larger cohort that might be achieved under similar resource constraints. In the event of future influenza pandemics, in a developing setting, greater emphasis should be place on adapting and using preestablished surveillance programs to better inform these characteristics.
Nonetheless, this study characterizes the initial stages of A(H1N1)pdm09 spread in South Africa. We show the transmissibility of this virus in South Africa to be comparable to that observed in more-developed countries and in seasonal influenza epidemics. Despite these findings, public health officials should not forget the ease and rapidity in which A(H1N1)pdm09 established itself locally and caused widespread morbidity and mortality. Greater efforts are needed should we hope to contain and mitigate future, and possibly more severe, pandemics.
Notes
Acknowledgments. We thank the many team members who have contributed to these investigations and the response to the pandemic in South Africa. These include Abraham Malaza, Amukelani Dlomu, David Mutonga, Lungelwa Quntana, Lungile Mbatha, Motshabi P. Modise, and Refilwe Mokgetle, of the South African Field Epidemiology and Laboratory Training Programme; Amelia Buys, Cardia Fourie, Jack Manamela, Nomathemba Gumede, Marthi Nieuwoudt, Sandrama Nadan, and Mariza Vos, of the Virology Division; Juno Thomas and Tsakane Nkuna, of the Outbreak Response Unit, NICD-NHLS; the South Africa Department of Health; and the participating NHLS and private diagnostic laboratories.
Financial support. This work was supported by the NICD-NHLS. Laboratory testing of the first 100 A(H1N1)pdm09 cases was funded, in part, by an unrestricted grant from the CDC (grant 5U51/IP000155).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84:185–9. [PubMed] [Google Scholar]
- 3.New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec. 2009;84:249–57. [PubMed] [Google Scholar]
- 4.Fraser C, Donnelly CA, Cauchemez S, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Sugimoto JD, Halloran ME, et al. The transmissibility and control of pandemic influenza A (H1N1) virus. Science. 2009;326:729–33. doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowling BJ, Chan KH, Fang VJ, et al. Comparative epidemiology of pandemic and seasonal influenza A in households. N Engl J Med. 2010;362:2175–84. doi: 10.1056/NEJMoa0911530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowling BJ, Fang VJ, Riley S, Malik Peiris JS, Leung GM. Estimation of the serial interval of influenza. Epidemiology. 2009;20:344–7. doi: 10.1097/EDE.0b013e31819d1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 9.Viboud C, Boelle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 10.White LF, Pagano M. Transmissibility of the influenza virus in the 1918 pandemic. PLoS One. 2008;3:e1498. doi: 10.1371/journal.pone.0001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sertsou G, Wilson N, Baker M, Nelson P, Roberts MG. Key transmission parameters of an institutional outbreak during the 1918 influenza pandemic estimated by mathematical modelling. Theor Biol Med Model. 2006;3:38. doi: 10.1186/1742-4682-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boelle PY, Ansart S, Cori A, Valleron AJ. Transmission parameters of the A/H1N1 (2009) influenza virus pandemic: a review. Influenza Other Respi Viruses. 2011;5:306–16. doi: 10.1111/j.1750-2659.2011.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Archer B, Cohen C, Naidoo D, et al. Interim report on pandemic H1N1 influenza virus infections in South Africa, April to October 2009: epidemiology and factors associated with fatal cases. Euro Surveill. 2009;14:11–5. doi: 10.2807/ese.14.42.19369-en. [DOI] [PubMed] [Google Scholar]
- 14.WHO. CDC protocol of realtime RTPCR for swine influenza A(H1N1) http://www.who.int/csr/resources/publications/swineflu/CDCrealtimeRTPCRprotocol_20090428.pdf. Accessed 23 December 2010. [Google Scholar]
- 15.Jain R, Goldman RD. Novel influenza A(H1N1): clinical presentation, diagnosis, and management. Pediatr Emerg Care. 2009;25:791–6. doi: 10.1097/PEC.0b013e3181c3c8f8. [DOI] [PubMed] [Google Scholar]
- 16.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–73. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessler J, Reich NG, Cummings DA, Nair HP, Jordan HT, Thompson N. Outbreak of 2009 pandemic influenza A (H1N1) at a New York City school. N Engl J Med. 2009;361:2628–36. doi: 10.1056/NEJMoa0906089. [DOI] [PubMed] [Google Scholar]
- 18.National Institute for Communicable Diseases. Respiratory virus surveillance, South Africa 2009. Commun Dis Surveill Bull. 2010;8:6–10. [Google Scholar]
- 19.Papenburg J, Baz M, Hamelin ME, et al. Household transmission of the 2009 pandemic A/H1N1 influenza virus: elevated laboratory-confirmed secondary attack rates and evidence of asymptomatic infections. Clin Infect Dis. 2010;51:1033–41. doi: 10.1086/656582. [DOI] [PubMed] [Google Scholar]