Abstract
Objective
To estimate the magnitude of the association between overweight, moderate, and extreme childhood obesity and the risk of idiopathic intracranial hypertension (IIH).
Study design
Risk estimates were obtained from the Kaiser Permanente Southern California Children’s Health Study (n = 913 178). Weight classes were assigned by body mass index specific for age and sex. A combination of electronic database searches followed by complete medical records review was used to identify all children diagnosed with IIH between 2006 and 2009.
Results
We identified 78 children with IIH, the majority of whom were girls (n = 66, 84.5%), age 11-19 (n = 66, 84.5%), non-Hispanic Whites (n = 37, 47.4%), and overweight or obese (n = 57, 73.1%). The adjusted ORs and 95% CIs of IIH with increasing weight class were 1.00, 3.56 (1.72-7.39), 6.45 (3.10-13.44), and 16.14 (8.18-31.85) for underweight/normal weight (reference category), overweight, moderately obese and extremely obese 11-19 year olds, respectively (P for trend < .001). Other independent IIH risk factors included White non-Hispanic race/ethnicity for all age groups and female sex, but only in older children. Overweight/obese children also had more IIH symptoms at onset than normal weight children.
Conclusions
We found that childhood obesity is strongly associated with an increased risk of pediatric IIH in adolescents. Our findings suggest that the childhood obesity epidemic is likely to lead to increased morbidity from IIH particularly among extremely obese, White non-Hispanic teenage girls. Our findings also suggest careful screening of these at risk individuals may lead to earlier detection and opportunity for treatment of IIH.
Idiopathic intracranial hypertension (IIH) is a disorder that typically presents with headache and blurred vision and is diagnosed by the presence of papilledema and elevated intracranial pressure in the absence of infectious, vascular, or structural causes. It most often affects obese or overweight adult females and can lead to blindness in up to 10% of patients, particularly if it is not recognized or treated promptly.1
Once thought to be rare, IIH is becoming more common with an estimated incidence of 15-19 cases per 100 000 among overweight or obese women ages 20-44.2 This increasing incidence has been attributed to the growing obesity epidemic among adults.1
Over the last 30 years the prevalence of pediatric obesity has tripled. Yet, whether obesity or other putative IIH risk factors like the use of tetracyclines or retinoids for acne are more important in pediatric IIH is unclear. The few studies that have examined the relationship between pediatric IIH and obesity3-8 have yielded conflicting results. Some studies suggested that obesity is only a risk factor for IIH in postpubertal age children.3 All had significant methodological limitations including descriptive case series design with obesity information on cases only, small sample sizes (15-50), and referral center bias.3-8 None of these studies reported risk estimates, which are necessary when counseling obese patients to prevent IIH. The purpose of this study was to identify risk factors for pediatric IIH and estimate the magnitude of the association between overweight, moderate, and extreme childhood obesity and the risk of pediatric IIH in a population-based, multiethnic cohort of children.
Methods
The institutional review board at Kaiser Permanente Southern California (KPSC) approved this study. Informed consent was waived as this was a database and chart review study only without direct patient contact.
For this cross-sectional study, we used data on children enrolled in the KPSC Children’s Health Study, which is described in detail elsewhere.9 KPSC is a large prepaid health maintenance organization with over 3.2 million members including over 900 000 members 18 years and younger. It provides comprehensive health care coverage to ~20% of the population in the geographic area it serves. The cost of specialist consultations, hospitalizations, magnetic resonance imaging scans, other diagnostic tests, and medications are fully covered. The KPSC pediatric membership is representative of the general pediatric population in Southern California with respect to ethnicity, age, sex, and socioeconomic status with the exception of an under-representation of the lowest and highest ends of the socioeconomic spectrum.9 After exclusion of 265 241 members who did not have any medical encounters in 2007-2009, 1 030 730 patients were eligible for participation in the cohort study. Out of these patients, 920 034 patients (89.2% of eligible patients) had at least 1 valid weight and height in the 3-year study period. After exclusion of pregnant patients (n = 6856), 913 178 patients were included in the final analytical cohort. This cohort is described in detail elsewhere.10
To identify potentially incident cases, we searched electronic databases for any mention of International Classification of Diseases, 9th Edition diagnostic code for IIH (348.2) between January 1, 2006-December 31, 2009 including all in-patient and outpatient encounters since enrollment into the health plan (n = 167). Diagnoses were confirmed and additional clinical details were extracted through full medical records abstraction including all inpatient and outpatient records, computed tomography and magnetic resonance imaging scans, and diagnostic test results by two neurologists (A.G. and S.B.) according to criteria for pediatric IIH proposed by Rangwala and Lui.11 Briefly, IIH was defined as presence of papilledema and elevated opening cerebrospinal fluid pressure (>180 mm H20 if less than age 8 and >250 mm H20 if age 8 or older); normal cerebrospinal fluid composition, no evidence of hydrocephalus, vascular or structural central nervous system lesions; and normal mental status.11 Because Tanner stage information on our background population was not complete, age <11 years was used as a surrogate for prepuberty.
Charts of cases were also abstracted for any mention of other potential risk factors for IIH including oral contraceptive use, thyroid replacement, other hormone use, high-dose over the counter vitamins, otitis, sinusitis, polycystic ovary syndrome, and metabolic syndrome.
We confirmed the diagnosis of IIH in 78 children. The diagnosis of IIH was suspected but not confirmed in another 33 children because of insufficient documentation (eg, opening pressure noted as “high” but not recorded or no documentation of fundoscopic exam), lumbar puncture was not done (eg, obese child with bilateral papilledema and new onset headache with unsuccessful lumbar puncture attempts), or because papilledema was absent (n = 5). Another 56 children had diagnostic codes for IIH entered in error (rule out diagnosis, later diagnosed with migraine, infection, or structural lesion), resulting in a positive predictive value (PPV) of 66.1% for confirmed and suspected cases, and a PPV of 46.4% for confirmed cases only.
Body weight and height were extracted from electronic health records when available from the same day. Body mass index (BMI) was calculated as weight (kilograms) divided by the square of the height (meters). For patients enrolled into the study in years 2007, 2008, and 2009, the median BMI-for-age of all encounters in the year of study enrollment for a patient was used for analysis. Based on a validation study including 15 000 patients with 45 980 medical encounters, the estimated error rate in body weight and height data was <0.4%.12
Definitions for overweight and obesity in children and adolescents are based on the sex-specific BMI-for-age growth charts developed by the Centers for Disease Control and Prevention and World Health Organization definitions for overweight and obesity in adults.13-15 Children were categorized as underweight (BMI-for-age <5th percentile), normal weight (BMI-for-age ≥5th and <85th percentile), overweight (BMI-for-age ≥85th percentile or a BMI ≥25 kg/m2), moderately obese (BMI-for-age ≥95th percentile or a BMI ≥30 kg/m2), and extremely obese (BMI-for-age ≥1.2 × 95th percentile or a BMI ≥35 kg/m2).13
Race and ethnicity information were obtained from health plan administrative records and birth certificates. We categorized race/ethnicity as non-Hispanic White, Hispanic White, Black (regardless of ethnicity), Asian or Pacific Islander, other, or multiple race/ethnicity, and unknown due to missing information (52.5%). A validation study compared health plan administrative records and birth certificate records of 325 810 children.16 The PPV for Hispanic ethnicity was 95.6%. PPV for White, Black, Asian/Pacific Islander, American Indian/Alaskan Native, multiple, and other was 89.3%, 86.6%, 73.8%, 18.2%, 51.8% and 1.2%, respectively.
For unknown race and ethnicity information, administrative records were supplemented by an imputation algorithm based on surname lists and address information derived from the US Census Bureau.17-19 Hispanic ethnicity and Asian race were assigned based on surnames. For Blacks and Non-Hispanic Whites, the child’s home address was used to link racial/ethnic information from the US Census Bureau. Race/ethnicity was hierarchically assigned using probability cut-offs of >50% for Asian surname, >50% for Hispanic surname, >75% for Black race from geocoding if probability for Asian surname was <50% (Hispanic Blacks are assigned to Black race for this study), and White race >45% from geocoding if no other assignment could be made before. The specificity and PPVs were >98% for all major racial/ethnic groups.9
We used Medi-Cal status as an indicator for low socioeconomic status. Medi-Cal is the California state-subsidized program providing health care coverage for more than 6 million low-income children and families as well as elderly, blind, or disabled individuals.
Statistical Analyses
Differences in the distribution of basic demographics across groups defined by weight class were assessed with the χ2 test. T test was used to compare normally distributed variables across groups. For IIH cases, age was assigned as age at first mention of IIH diagnosis. For non-IIH cases, age was assigned based on the age on July 1 of the year of study enrollment.
Multiple logistic regression models were generated to estimate ORs and their 95% CIs for IIH vs weight class (under-weight/normal weight [reference], overweight, moderate obesity, extreme obesity) and adjusted for sex, age group (2-10, 11-14, or 15-19 years) and race/ethnicity. All analyses were conducted using SPSS release 18.0 (SPSS Inc, Chicago, Illinois).
Results
Children and adolescents in KPSC who were overweight, moderately, or extremely obese were more likely to be 11 years or older (P < .001), male (P < .001), and Hispanic or Black, than those of normal weight (P < .001; Table I).
Table I. Demographic characteristics of the study population according to weight class*.
| Under weight (n = 27 447) |
Normal weight (n = 551 676) |
Over-weight (n = 159 064) |
Moderately obese (n = 118 789) |
Extremely obese (n = 56 202) |
|
|---|---|---|---|---|---|
| Male (%) | 51.2 | 48.7 | 49.1 | 55.3 | 57.1 |
| Age group (%) | |||||
| 2-10 y | 62.0 | 51.8 | 42.5 | 47.6 | 36.5 |
| 11-14 y | 14.1 | 20.5 | 24.4 | 26.1 | 27.4 |
| 15-19 y | 23.8 | 27.7 | 33.0 | 23.3 | 36.1 |
| Race/ethnicity (%) | |||||
| Non-Hispanic White | 25.2 | 23.6 | 19.4 | 14.9 | 12.8 |
| Hispanic | 40.6 | 47.1 | 54.4 | 60.9 | 62.1 |
| Black | 6.8 | 7.4 | 7.6 | 7.1 | 9.5 |
| Asian or Pacific Islander | 12.6 | 7.8 | 5.5 | 4.6 | 3.4 |
| Others | 14.7 | 14.1 | 13.1 | 12.5 | 12.2 |
| Medi-Cal (%) | 11.5 | 12.8 | 13.5 | 15.1 | 14.5 |
Definition of weight class: underweight was defined BMI-for-age ≤5th percentile, overweight as BMI-for-age ≥85th percentile or a BMI ≥25 kg/m2, moderate obesity as ≥95th percentile or a BMI ≥30 kg/m2, and extreme obesity ≥1.2 × 95th percentile or a BMI ≥35 kg/m2.
The 78 children with clinically definite IIH, were also more likely to be 11 years or older at diagnosis (n = 66, 84.5%; Table II). However, in contrast to the general study population, IIH cases were more likely to be female (n = 66, 84.5%) and White, non-Hispanic (n = 37, 47.5%). Fifty-seven (73.1%) children and adolescents with IIH were overweight or obese. Increasing weight class was strongly associated with IIH with adjusted ORs and 95% CIs 1.00, 3.23 (1.70-6.13), 4.29 (2.78-10.08), and 15.37 (8.43-28.01) for underweight/normal weight (reference category), overweight, moderately obese, and extremely obese children, respectively (P for trend < .001).
Table II. Demographic characteristics and weight class of children with IIH according to age group.
| 2-10 y, n = 12 |
11-14 y, n = 25 |
15-19 y, n = 41 |
Total, n = 78 |
|||||
|---|---|---|---|---|---|---|---|---|
| n (%) | P value* | n (%) | P value* | n (%) | P value* | n (%) | P value* | |
| Female sex | 7(58) | .57 | 20 (80) | <.001 | 39 (95) | <.001 | 66 (84.6) | <.001 |
| Race/ethnicity | ||||||||
| Non-Hispanic White | 7(58) | .002 | 13(52) | <.001 | 17(41) | <.001 | 37 (47.4) | <.001 |
| Hispanic White | 1 (8) | 6(24) | 11 (27) | 18(23.1) | ||||
| Black | 2(17) | 2 (8) | 8 (20) | 12(15.4) | ||||
| Asian/Pacific Islander | 1 (8) | 0 | 0 | 1 (1.2) | ||||
| Other | 1 (8) | 4(16) | 5(12) | 10(12.8) | ||||
| Weight class | ||||||||
| Normal weight | 7 (58) | .87 | 2 (8) | <.001 | 12(29) | <.001 | 21 (26.9) | <.001 |
| Overweight | 2(17) | 6 (24) | 9 (22) | 17(21.8) | ||||
| Moderately obese | 2(17) | 8 (32) | 7(17) | 17(21.8) | ||||
| Extremely obese | 1 (8) | 9(36) | 13(32) | 23 (29.5) | ||||
χ2 test.
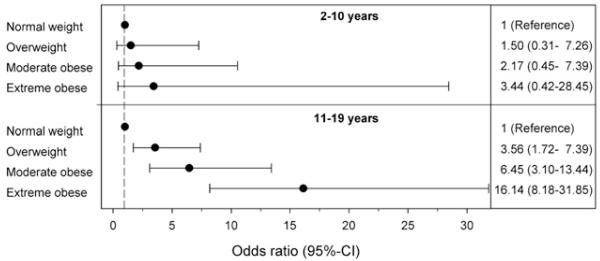
The association between female sex, obesity, and IIH was most pronounced in children 11 years or older (Tables II and III and Figure). IIH was uncommon in children ages 2-10 years (n = 12) and the magnitude of association between female sex, increasing weight class, and IIH was significantly weaker and did not reach statistical significance (P = .17; Figure). The female preponderance was more prominent in patients 15-19 years of age and 11-14 years of age than in patients 2-10 years of age (95.1%, 80.0%, and 58.3% female, respectively; P = .006). However, the magnitude of association between obesity, increasing weight class, and IIH was most striking in children ages 11-14 years of age (Table II and Figure).
Table III. Other IIH risk factors by age group.
| Adjusted OR (95% CI) |
||
|---|---|---|
| 2-10 y | 11-19 y | |
| Female sex | 1.56(0.49-4.89) | 8.33 (3.80-18.27) |
| Race/ethnicity | ||
| Non-Hispanic White n (%) | 1.00 (Ref) | 1.00 (Ref) |
| Hispanic White n (%) | 0.04 (0.05-0.35) | 0.20(0.11-0.37) |
| Black n (%) | 0.67(0.14-3.27) | 0.65(0.32-1.35) |
| Asian/Pacific Islander n (%) | 0.35 (0.04-2.82) | 0.41 (0.20-0.88) |
| Other/unknown n (%) | 0.19(0.23-1.53) | - |
ORs are adjusted for sex, race, and weight class.
Figure.
Association between weight class and IIH by age group. Depicted are the adjusted OR and 95% CI of IIH with increasing weight class compared with normal/underweight children (reference category) stratified by age group (2-10 years or 11-19 years). Increasing weight class was associated with increasingly higher OR for IIH among the older age group (P for trend < .001). A trend toward increased risk of IIH with increasing weight class was also present in the younger age group although the magnitude of this effect is much less pronounced and did not reach statistical significance (P = .17). ORs are adjusted for sex and race/ethnicity.
The clinical characteristics and clinical symptoms of IIH by weight class are presented in Table IV. Normal weight children with IIH were more likely to have been exposed to tetracyclines (43%) or have another IIH risk factor, than overweight or obese children (7%, P = .001; Table IV). Normal weight children were less likely to complain of headache (P = .004) or blurred vision, have a sixth nerve palsy, and had slightly lower opening pressures then overweight or obese children with IIH, although these later findings did not reach statistical significance (Table IV). Girls were more likely to be affected than boys regardless of weight class although the female preponderance was less striking among normal weight children with IIH than overweight or obese cases (71.4% and 89.5%, respectively, P = .05).
Table IV. Clinical characteristics at initial presentation according to weight class.
| Normal weight, n = 21 |
Overweight, n = 17 |
Moderately obese, n = 17 |
Extremely obese, n = 23 |
Total, n = 78 |
||
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | P value* | |
| Female sex | 15(71.4) | 15 (88.2) | 15(88.2) | 21 (91.3) | 66 (84.6) | .05 |
| Clinical symptoms at presentation | ||||||
| Headache | 15(71) | 17(100) | 16(94) | 21 (91) | 69 (88) | .004 |
| Blurred vision | 9 (43) | 10(59) | 13(76) | 14(61) | 46 (59) | .08 |
| Diplobia | 5 (24) | 9 (53) | 4 (24) | 6 (26) | 24(31) | .58 |
| Sixth nerve palsy | 1 (5) | 6(13) | 4 (24) | 4(17) | 15(19) | .06 |
| Opening pressure (median, range) | 315 (226-550) | 390 (280-500) | 440 (250-600) | 365 (250-570) | 370 (226-600) | .09 |
| Presence of other putative factors† | ||||||
| Use of tetracyclines‡ | 9 (43) | 3(18) | 1 (6) | 1 (4) | 14(18) | .001 |
| Other | 1§ (5) | 0 | 0 | 0 | 1 (1) |
χ2 normal weight vs overweight and obese.
The only other putative risk factors found were use of tetracyclines and sickle cell anemia.
Tetracycline, doxycycline, and minocycline were used.
Sickle cell anemia with normal magnetic resonance venogram.
Discussion
Whether obesity and female sex are risk factors for pediatric IIH is unclear. Few studies have examined pediatric IIH risk factors and most are descriptive case series with BMI or weight information available only for cases.3-8 Some studies have found pediatric IIH cases to be predominantly over-weight or obese adolescent females3,8 similar to adults with IIH, and others have reported no increase in BMI4,5,7 or female preponderance among cases.5,7 Methodological limitations including small sizes (ranging from 15-50 cases with available weight data)3-8 lack of standardized definitions of pediatric obesity (which vary by age and sex),4-8 and referral center bias3-8 may explain the discrepancies between studies.
Alternatively, obese children with IIH may have been less common in older pediatric IIH studies because of a birth cohort effect. In our contemporary cohort, extreme obesity peaked at age 10 in boys, and girls had a bimodal peak at age 12 and 18 years.9 Even if obesity is a true risk factor for pediatric IIH, cohorts that were accrued before the pediatric obesity epidemic5,7 would be more likely to have a higher proportion of normal weight vs obese children with IIH presenting to their ophthalmology or neurology referral centers than cohorts accrued during the obesity epidemic. Because prior studies could not calculate risk estimates and included predominantly normal weight children in their base population, these case-only descriptions may have missed an association with childhood obesity.
The finding from our study that exposure to tetracyclines was a more likely explanation for IIH in normal weight vs overweight or obese children supports the possibility of a birth cohort effect. As might be expected from our finding, these older studies of mostly normal weight children with IIH reported a higher prevalence of other IIH risk factors5-7 than more recent studies where obesity is a prominent risk factor. We chose not to exclude cases with tetracycline exposure from our analyses as has been recommended by some authors1 because the association with this risk factor has been described in small case series and removal would only inflate the association with obesity.
Consistent with two previous studies,3,8 we found that female sex and obesity first emerge as strong IIH risk factors in postpubertal age children. Our analysis, showing an increasing risk of IIH with increasing weight class, is the strongest evidence to-date for an existence of an association between childhood obesity and IIH. Extremely obese adolescents were 16 times more likely than normal weight children to have IIH whereas moderately obese or overweight children were only 3.5-6 times more likely to have IIH, respectively. However, similar to previous studies3,8 we were not able to demonstrate a clear association between obesity or female sex and IIH in prepubertal age children. Our findings suggest that prepubertal IIH, although associated with White non-Hispanic race/ethnicity, otherwise differs considerably from postpubertal onset with a much lower incidence, only slight female predilection and a much weaker or no association with obesity. Like those previous studies,3,8 we had very few children with IIH under the age of 11 and, therefore, cannot exclude the possibility that female sex and obesity increase IIH risk in this age group albeit much less dramatically than in adolescents.
The higher risk of pediatric IIH in White non-Hispanic children is a novel finding and was consistent across age strata. To our knowledge, no previous studies in children or adults have examined the influence of race/ethnicity on IIH risk in the same study population although race/ethnicity may influence prognosis.20
That overweight/obese children had more IIH symptoms at onset than normal weight children is consistent with findings from a previous study that found that obese children (as defined by weight only) were less likely to have asymptomatic IIH than normal weight children.21 These findings would suggest that careful screening of overweight/obese adolescents for headache, blurred vision, and eye movement abnormalities may result in earlier detection and opportunity for IIH treatment.
IIH can cause permanent vision loss in up to 10% of children8 and severe headaches that may persist even after intracranial pressure is normalized.22 Acute treatment of IIH includes mild diuretics like acetazolamide, serial lumbar punctures, or if necessary, lumbo-peritoneal shunts or optic nerve sheath fenestration.23 During the acute phase, it is recommended that children are examined by an ophthalmologist at least monthly. Although the optimal duration of treatment is unknown, some experts recommend that treatment is continued for at least 6 months after visual status, and optic nerve appearance stabilize before tapering off medications.24 Chronic management focuses on symptomatic treatment of headaches and weight loss counseling to mitigate the 6%-22% risk of recurrence.23
The pathophysiologic mechanism(s) whereby obesity, particularly in postpubertal females, might lead to increased intracranial pressure is unknown. Based on our findings that the risk of IIH increases dramatically in postpubertal age girls particularly right around the time of menarche, it is tempting to speculate that menstrual cycle hypothalamic-pituitary-ovarian axis hormones play a role. However, it may be that these girls had rapid weight gain around the time of menarche and that this better explains the sudden increase in IIH risk. Future studies examining the duration and extent of obesity, anthropometric measurements,25 timing of puberty and comorbid polycystic ovary syndrome, and metabolic syndrome may reveal important insights into the pathophysiology of IIH.
Pediatric IIH is rare, particularly in prepubertal age children. In fact the primary limitation of this study is the small sample size particularly of very young IIH cases. Other limitations include the use of age cut-offs rather than Tanner stage to determine puberty and the relatively large number of suspected but not confirmed cases. Because we excluded these cases from our analysis—some of who were extremely obese with unsuccessful attempts at lumbar puncture that were successfully treated for clinician-diagnosed IIH—our estimates are conservative and may represent an underestimation of the true risk of IIH and childhood obesity particularly in prepubertal age children where our cohort is too small to draw conclusions.
This study confirms previous reports3,8 that prepubertal onset differs considerably from postpubertal onset IIH. These findings are novel and suggest that the risk of IIH is highest among overweight/obese White non-Hispanic teenage girls. Our findings also suggest that careful screening of these at risk individuals for headaches, blurred vision, and eye movement abnormalities may lead to earlier detection and, thus, opportunity for treatment to prevent vision loss.
Acknowledgments
Supported by the National Institute of Diabetes and Digestive and Kidney Disorders (R21DK085395, to C.K.) and Kaiser Permanente Direct Community Benefit Funds. The authors declare no conflicts of interest.
Glossary
- BMI
Body mass index
- IIH
Idiopathic intracranial hypertension
- KPSC
Kaiser Permanente Southern California
- PPV
Positive predictive value
References
- 1.Ball AK, Clarke CE. Idiopathic intracranial hypertension. Lancet Neurol. 2006;5:433–42. doi: 10.1016/S1474-4422(06)70442-2. [DOI] [PubMed] [Google Scholar]
- 2.Durcan FJ, Corbett JJ, Wall M. The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol. 1988;45:875–7. doi: 10.1001/archneur.1988.00520320065016. [DOI] [PubMed] [Google Scholar]
- 3.Balcer LJ, Liu GT, Forman S, Pun K, Volpe NJ, Galetta SL, Maguire MG. Idiopathic intracranial hypertension: relation of age and obesity in children. Neurology. 1999;52:870–2. doi: 10.1212/wnl.52.4.870. [DOI] [PubMed] [Google Scholar]
- 4.Faz G, Butler IJ, Koenig MK. Incidence of papilledema and obesity in children diagnosed with idiopathic ‘benign’ intracranial hypertension: case series and review. J Child Neurol. 2010;25:1389–92. doi: 10.1177/0883073810364853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babikian P, Corbett J, Bell W. Idiopathic intracranial hypertension in children: the Iowa experience. J Child Neurol. 1994;9:144–9. doi: 10.1177/088307389400900208. [DOI] [PubMed] [Google Scholar]
- 6.Rose A, Matson DD. Benign intracranial hypertension in children. Pediatrics. 1967;39:227–37. [PubMed] [Google Scholar]
- 7.Scott IU, Siatkowski RM, Eneyni M, Brodsky MC, Lam BL. Idiopathic intracranial hypertension in children and adolescents. Am J Ophthalmol. 1997;124:253–5. doi: 10.1016/s0002-9394(14)70798-6. [DOI] [PubMed] [Google Scholar]
- 8.Kesler A, Fattal-Valevski A. Idiopathic intracranial hypertension in the pediatric population. J Child Neurol. 2002;17:745–8. doi: 10.1177/08830738020170101401. [DOI] [PubMed] [Google Scholar]
- 9.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. J Pediatr. 2010;157:26–31.e22. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koebnick C, Coleman KJ, Black MH, Smith N, Der-Sarkissian JK, Jacobsen SJ, et al. Cohort profile: the KPSC Children’s Health Study, a population-based study of 920,000 children and adolescents in southern California. Int J Epidemiol. 2011 Jan 20; doi: 10.1093/ije/dyq252. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52:597–617. doi: 10.1016/j.survophthal.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Smith N, Coleman KJ, Lawrence JM, Quinn VP, Getahun D, Reynolds K, et al. Body weight and height data in electronic medical records of children. Int J Pediatr Obes. 2010;5:237–42. doi: 10.3109/17477160903268308. [DOI] [PubMed] [Google Scholar]
- 13.Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–20. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;246:1–190. [PubMed] [Google Scholar]
- 15.World Health Organization . Obesity: preventing and managing the global epidemic. World Health Organization; Geneva: 2000. [PubMed] [Google Scholar]
- 16.Smith N, Iyer RL, Langer-Gould A, Getahun D, Strickland D, Jacobsen SJ, et al. Health plan administrative records versus birth certificate records: quality of race and ethnicity information in children. BMC Health Serv Res. 2010;10:316. doi: 10.1186/1472-6963-10-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiscella K, Fremont AM. Use of geocoding and surname analysis to estimate race and ethnicity. Health Serv Res. 2006;41:1482–500. doi: 10.1111/j.1475-6773.2006.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Census 2000 surnamelist [Accessed 07/11/2009];Bureau of Census. 2009 Available at: http://www.census.gov/genealogy/www/freqnames2k.html.
- 19.Word DL, Perkins RC. Building a Spanish surname list for the 1990s–a new approach to an old problem. US Bureau of the Census; Washington, DC: 1996. Technical Working Paper No. 13. [Google Scholar]
- 20.Bruce BB, Kedar S, Van Stavern GP, Monaghan D, Acierno MD, Braswell RA, et al. Idiopathic intracranial hypertension in men. Neurology. 2009;72:304–9. doi: 10.1212/01.wnl.0000333254.84120.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassan H, Berkner L, Stolovitch C, Kesler A. Asymptomatic idiopathic intracranial hypertension in children. Acta Neurol Scand. 2008;118:251–5. doi: 10.1111/j.1600-0404.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58:1551–3. doi: 10.1212/wnl.58.10.1551. [DOI] [PubMed] [Google Scholar]
- 23.Standridge SM. Idiopathic intracranial hypertension in children: a review and algorithm. Pediatr Neurol. 2010;43:377–90. doi: 10.1016/j.pediatrneurol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Friedman DI, Jacobson DM. Idiopathic intracranial hypertension. J Neuro-Ophthalmol. 2004;24:138–45. doi: 10.1097/00041327-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Kesler A, Kliper E, Shenkerman G, Stern N. Idiopathic intracranial hypertension is associated with lower body adiposity. Ophthalmology. 2010;117:169–74. doi: 10.1016/j.ophtha.2009.06.030. [DOI] [PubMed] [Google Scholar]