Abstract
Purpose
The effects of radiation +/− hypogravity on immunologic function were investigated using the Partial Weight Suspension (PWS) model (Wagner et al. 2010).
Materials and methods
Mice were exposed to 0.5, 1, or 2 Gray (Gy) dose of gamma radiation and then placed in the PWS system for 4, 24, 48 hours, or 4 days. Spleens were excised and white blood cells were prepared for flow cytometry analyses.
Results
The combination of PWS + radiation (1 and 2 Gy doses only) resulted in decreased cell viability at the 24 h (~16% decrease), 48 h (~20% decrease), and 4 day (~20% decrease) time points, compared to the PWS (no radiation) and no treatment (non-suspended, non-irradiated) groups. The T lymphocyte (thymus-derived) population increased by ~10% (24 h, 48 h, and 4 day time points), while the B lymphocyte (bursal or bone marrow-derived) population decreased by ~10% (at all time points examined), when mice were exposed to PWS + radiation (2 Gy dose only), compared to the PWS or no treatment groups. T cell activation was observed in the PWS group and the 0.5 Gy +/− PWS groups at the 4 and 24 h time points, compared to the no treatment group. However, T cell activation was significantly suppressed (~85%) at the acute time points in the 2 Gy +/− PWS groups, comparable to the no treatment group.
Conclusions
Ionizing radiation in the absence and presence of simulated hypogravity results in acute lymphocyte dysfunction and compromised immune response.
Keywords: Space flight, lymphocytes, immunology
Introduction
Space radiation exposure in a reduced gravity environment is expected to induce physiological changes that hinder human health during long-duration space flight or interplanetary travel. Variable lymphocyte responses after space flight or exposure to simulated microgravity have been reported (Cogoli et al. 1984, Nash et al. 1991, Sonnenfeld and Shearer 2002, Wei et al. 2003). Additionally, ionizing radiation is known to induce changes in hematological parameters such as peripheral leukocyte viability, lymphocyte counts and function (Wambi et al. 2008, 2009) as well as splenic leukocyte counts and function (Shearer et al. 2005).
Lymphocytes are critical regulators of innate and adaptive immunity. The T (thymus-derived) lymphocyte subtype, also called T cells, express the surface antigen CD3 (CD standing for cluster of differentiation), and is primarily involved in fighting infection, while the B (bursal or bone marrow-derived) lymphocyte subtype (or B cells, expressing surface antigen CD19) is primarily involved with antibody production. Lymphopenia has been reported in both astronauts and rodents upon return from space missions (Lange et al. 1985, Taylor et al. 1986, Kajioka et al. 1999, Pecaut et al. 2003). Isolated lymphocytes have been shown to be dysfunctional, with a decreased response to mitogen activation, decreased cytokine production, and alterations in gene expression in response to radiation doses which could occur during space-flight (Gridley et al. 2009). Hughes-Fulford’s group (Boonyaratanakornkit et al. 2005) investigated gravity-dependent genes and pathways in vitro in a random positioning machine that simulated microgravity exposure, and reported reduced expression of genes involved in the pathway of T cell activation.
Consistent with the literature on the effects of ionizing radiation on lymphocyte counts, reports on lymphocyte counts after space flight or simulated microgravity experiments have indicated a similar decrease in lymphocyte counts. For example, upon landing, rat splenic and peripheral blood lymphocyte numbers were decreased after a nine day space flight mission, along with decreased CD4+, CD8+, and B cells (Allebban et al. 1994). CD4 is expressed by T helper cells and CD8 is expressed by cytotoxic T cells. Using a hindlimb unloading system for mice, Wei et al. (2003) demonstrated that all CD4+, CD8+, and B cell splenocytes continually decrease at the 2-day, 4-day, and 10-day time points observed. Alternatively, significant increases in total leukocyte percentages, including CD4+ lymphocytes and B lymphocytes, were observed in the peripheral blood of astronauts after 4–16 days in space flight, while the CD 8+ lymphocyte number were not altered (Mills et al. 2001). While there are conflicting reports on lymphocyte subtype and subset changes in response to space flight, more consistent reports on lymphocyte activation and function are available. These effects have been associated with potentially important clinical effects, including depressed mitogen-induced T cell activation (Cogoli et al. 1984, Nash and Mastro 1992, Shearer et al. 2005), reduced antibody response to bacteriophage immunization (Shearer et al. 2009), reduced delayed-type hypersensitivity to antigens (Taylor 1993), and decreased expression of genes involved in the T cell activation pathway (Gridley et al. 2009, Hughes-Fulford et al. 2005).
Ground studies investigating weightlessness are commonly performed in rodents utilizing hindlimb or tail suspension, providing full unloading of the animals’ hindquarters. Recently, the Partial Weight Suspension (PWS) system has been established for simulating partial weightbearing such as that expected on the moon (Wagner et al. 2010). The expected gravity on the moon is 1/6 that on Earth. Therefore, the PWS system enables the subject to apply 16% of its body weight in a quadrupedal-loading fashion, as opposed to the traditional tail suspension model in which there is full unloading of the subject’ s hindlimbs and 50% of the body weight is applied to the forelimbs (Morey-Holton and Globus 2002). It is now clear that the impact of both microgravity and radiation on the immune system could significantly affect astronaut health, especially when considered in the context of the space flight environment, which includes other stressors (e.g., psychological stress and sleep disruption). In the studies reported here, we sought to investigate the effects of partial weightbearing, with and without radiation exposure, on lymphocyte subtypes and subsets. We hypothesized that moderate doses of radiation in combination with partial weightbearing would compromise the subtype populations and the integrity of splenic lymphocytes.
Materials and methods
Animals
Female ICR (Imprinting Control Region) mice (Taconic Farms, Inc., Hudson, NY, USA) 6–8 weeks of age were group-housed in standard laboratory vivarium caging with ad libitum access to both food and water for at least 24 h. Animals were randomly assigned into treatment groups. On the first day of acclimation to the custom built PWS cages, previously described by Wagner et al. (2010), all mice were anesthetized and placed in a forelimb ‘jacket’ to enable later suspension. The jackets were made of moleskin, providing flexibility, and secured by velcro. A separate group of mice were not anesthetized because they were not subjected to the forelimb jacket. This group of mice was compared separately to the jacket-bearing mice. All mice were singly-housed and acclimated to the cages and the jacket for 3 days. All groups started with five animals each, but due to self-inflicted tail injuries or animals freeing themselves from the jackets, group sizes stabilized such that the sample size was 3–5 animals/group. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, Philadelphia, PA, USA.
Radiation and PWS
A total of 12 experiments were performed (three radiation doses × 4 time points). Non-anesthetized, animals (in jackets) were exposed to whole body gamma irradiation (J.L. Sheperd Mark I, Model 30, Cesium-137 irradiator, Nordion, Ottowa, ON, Canada) at a dose rate of 0.45 Gy/min or were sham-irradiated on day 0. All animals were immediately placed in their respective cages, either fully weightbearing or suspended at 16% weightbearing. Jacketed controls were pair-fed according to the previous day’s average consumption of the PWS mice. The designated experimental end-points were 4 hours (4 h), 24 h, 48 h, and 4 days (4 d) after dosing. A separate group of jacketed controls were harvested at each time point.
At the completion of each study, animals were euthanized by carbon dioxide asphyxiation. Cardiac blood was collected for complete blood count (CBC) analyses by Antech Diagnostics (Lake Success, NY, USA); these data will be reported separately (Wilson, J. et al. unpublished data).
Spleens were excised at the time of euthanasia and prepared for flow cytometry. Briefly, a single cell suspension was prepared by teasing the spleen apart with the plunger of a syringe in a Petri dish containing staining buffer (eBiosciences, San Diego, CA, USA). The cell suspension was passed through a cell strainer to remove debris. Total spleen cells were collected by centrifugation and erythrocytes were lysed using Lysis buffer (eBiosciences). The Lysis buffer was neutralized with the addition of phosphate buffered saline and leukocytes were counted using a Z Series Coulter Counter (Beckman Coulter, Inc., Brea, CA, USA).
Flow cytometry
Cells were washed with staining buffer (eBiosciences), and incubated with purified anti-mouse CD16/32 to block non-specific Fc receptor binding. Three separate tubes were prepared for cell surface phenotyping. The first tube was a cocktail of anti-CD3 (phycoerythrin [PE]-conjugated), anti-CD4 (allophycocyanin [APC]-conjugated), anti-CD8 (flourescein [FITC]-conjugated), and anti-CD69 (peridinin chlorophyll 5.5 [PerCP-Cy5.5]-conjugated). The second tube was a cocktail of anti-CD3 (PE-conjugated) and anti-CD19 (FITC-conjugated). All antibodies were purchased from eBiosciences. The last tube contained propidium iodide (Sigma-Aldrich, St Louis, MO, USA) to discriminate viable vs. dead cells. Cells were stained in the dark at 4°C for 30 min. with subsequent washes of staining buffer. Cells were analyzed on a FACSCalibur (BD Biosciences, San Jose, CA, USA) with Cell-Quest Pro software (BD Biosciences) and further analyzed using FlowJo Analysis Software (Tree Star Inc., Ashland, OR, USA). A total of 10,000 events were collected.
Statistical analyses
All histograms represent the average percentage of stained cells +/− standard deviation. One-way analysis of variance (ANOVA) with Tukey’ s Multiple Comparison Test was used to determine whether there were statistically significant differences between treatment groups. Differences were considered significant at p < 0.05.
Results
Non-jacketed versus jacketed controls
Forelimb jacketed animals were compared to non-jacketed animals. For all endpoints studied here, lymphocyte viability, lymphocyte subtype and subset populations, and T cell activation (4 and 24 h timepoints only), the differences observed for the animals wearing the forelimb jackets versus the animals not wearing the jackets were not statistically significant (data not shown). Therefore, the radiation treatments +/− PWS groups were compared to the jacketed control group or the PWS control group. The non-jacketed animals were not included in the following data analyses.
Lymphocyte viability
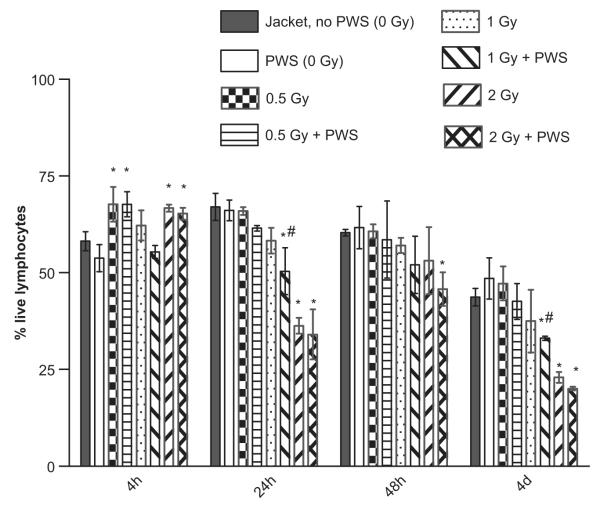
The total live lymphocyte population was detected by propidium iodide (PI) staining in the animals exposed to gamma irradiation (or sham-irradiation) and subsequently placed in the PWS system. The normal weightbearing animals (jacketed controls), were either gamma or sham-irradiated, and subsequently placed in the PWS caging system with no suspension. PI intercalates in double-stranded nucleic acids. It is excluded by viable cells but can penetrate cell membranes of dying or dead cells. At the 4 h time point, lymphocyte viability was increased in animals exposed to 0.5 Gy +/− PWS and 2 Gy +/− PWS, when compared to PWS alone (Figure 1). At the 24 h and 4 day time points, lymphocyte counts decreased significantly in the 1 Gy +/− PWS and 2 Gy +/− PWS groups compared to the PWS (no radiation) group (Figure 1). Cell viability was significantly decreased in the 2 Gy + PWS group at the 48 h time point, compared to PWS alone, while at the 4 day time point, decreases in cell viability were observed in the 1 Gy + PWS and the 2 Gy +/− PWS groups (Figure 1).
Figure 1.
Acute exposure to the PWS system +/− gamma radiation alters lymphocyte viability. Radiation + PWS exposure at the 4 hour time point results in a brief increase in lymphocyte viability when compared to the PWS group and the Jacketed control group, particularly at the 0.5 Gy and 2 Gy doses +/− PWS (*). At the 24 hour time point, the 1 Gy and 2 Gy doses of radiation +/− PWS result in a significant decrease in lymphocyte viability, when compared to the PWS and Jacket, no PWS groups (*). The differences between the results for the 1 Gy + PWS group compared to those from the 1 Gy dose group are statistically significant (#). At the 48 hour time point, only the 2 Gy + PWS group results in a statistically significant decrease in viability when compared to the PWS and Jacket, no PWS groups. At the 4 day time point, the 2 Gy +/− PWS groups, as well as the 1 Gy + PWS group result in significant decreases in cell viability when compared to the PWS and Jacket, no PWS groups (*). Also at this time point, the differences in the results for the 1 Gy + PWS group compared to the results from the 1 Gy dose group are statistically significant (#). Error bars indicate standard deviation for n = 3–5.
Lymphocyte subtypes
The T lymphocyte population was measured by CD3 surface expression. The T lymphocyte population was increased in a statistically significant manner by 24 h after exposure to combined radiation and PWS (2 Gy + PWS group only) compared to PWS alone and compared to the 2 Gy radiation group alone (Figure 2). Surface expression was up-regulated at the 48 h and 4 day time points in response to the 2 Gy + PWS treatment. The statistically significant increase in expression of CD3 on lymphocytes was observed after 2 Gy radiation exposure alone at the 4 day time point, when compared to PWS alone (Figure 2), suggesting that at later time points, lymphocyte sensitivity to radiation plus AND minus PWS is increased. No changes in CD3 expression (T lymphocyte population) were observed in the groups receiving 0.5 or 1 Gy +/− PWS.
Figure 2.
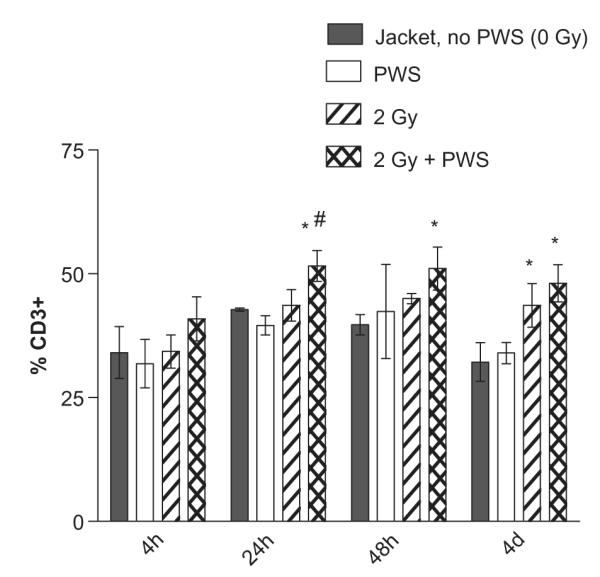
Acute exposure to PWS in combination with gamma radiation up-regulates CD3 expression at the 2 Gy dose. No statistically significant changes in CD3 expression are observed at the 0.5 Gy and 1 Gy dose of radiation +/− PWS. 2 Gy gamma radiation + PWS exposure induces CD3 expression up to 4 days of the combination treatment. Only at the 24 hour time point, is the 2 Gy + PWS significantly increased, compared to radiation alone (2 Gy, denoted by #). At the 48 hour and 4 day time points, there is a statistically significant increase in CD3 expression with 2 Gy + PWS, compared to Jacket, no PWS (*). Additionally, only at the 4 day time point there is a statistically significant increase with the 2 Gy dose of radiation when compared to the jacketed controls with no PWS (*). Error bars indicate the standard deviation for n = 3–5.
The B lymphocyte population was measured by CD19 surface expression in all groups of mice exposed to PWS alone, or in combination with radiation. CD19 expression was decreased in a statistically significant manner by 4 h after the combined treatment of the 2 Gy dose of radiation exposure + PWS, compared to PWS group and the 2 Gy group (without suspension, Figure 3). This was observed at the 24 h time point. At the 48 h time point, the 2 Gy + PWS was decreased in a statistically significant manner compared to PWS, but not compared to the 2 Gy group (without suspension), suggesting that lymphocyte populations are more sensitive at the later time points when mice are exposed to radiation alone and in combination with PWS.
Figure 3.
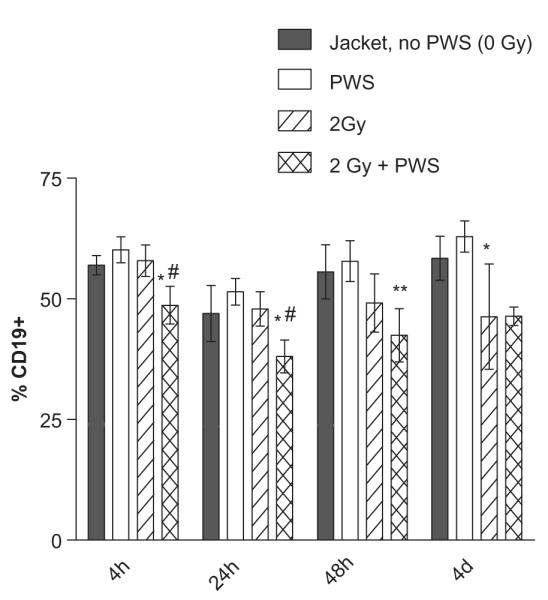
Gamma radiation + PWS exposure decreases CD19 expression at all time points of the combination treatment. The 2 Gy + PWS treatment results in a statistically significant decrease in CD19 surface expression all time points, compared to the PWS group (*). Only at the 4 and 24 hour time points is the 2 Gy + PWS group significantly different from the 2 Gy group (#). Additionally, the 2 Gy dose of radiation alone resulted in a statistically significant reduction of CD19 expression, when compared to PWS alone at the 4 day time point. Error bars indicate the standard deviation for n = 3–5.
T cell activation
To determine whether the combined effect of radiation + PWS treatment affected T cell activation, the Very Early Activation (VEA) marker, also known as surface marker CD69, was monitored in the T lymphocyte subtype population. Activation of T cells rapidly up-regulates the surface expression of CD69 and the maximum percentage of CD69 + cells was observed within 3 days of lymphocyte activation (Simms and Ellis 1996). In our system, CD69 expression was not detectable at the 4 day time point. T cells became activated when the animals were exposed to the PWS system alone for 4 and 24 h, suggesting that stress occurred in these animals during the early time points, compared to the non-suspended, jacketed controls (*, Figure 4). CD69 expression was upregulated in the 0.5 Gy +/− PWS and the 1.0 Gy +/− PWS groups at the 4 h time point, compared to the jacketed controls (*, Figure 4). At the 24 h time point, the upregulation of CD69 expression was not observed in the 1 Gy +/− PWS or the 2 Gy +/− PWS groups, compared to the jacketed controls. These data indicate a compromised immune response at the early time points in response to the selected doses of radiation with and without PWS exposure.
Figure 4.
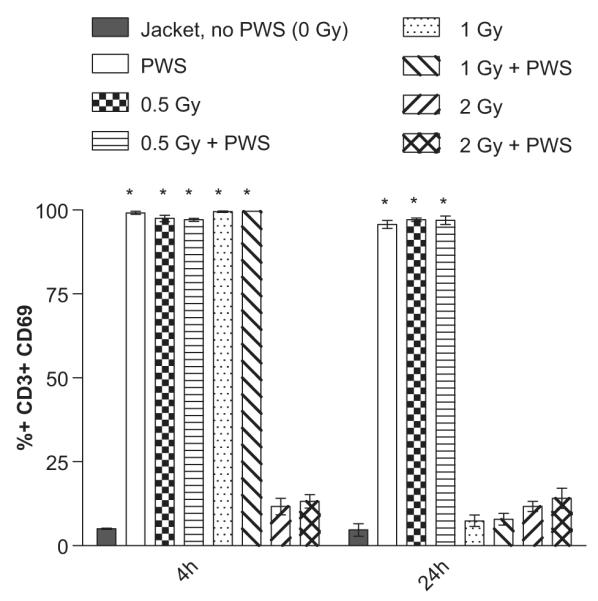
Acute T cell activation is suppressed in animals exposed to the combination of 2 Gy radiation +/− PWS (4 hour time point) and both 1 and 2 Gy +/− PWS (24 hour time point). CD69 + surface expression is increased in a statistically significant manner in the 0.5 Gy +/− PWS groups (at both time points) and the 1.0 Gy +/− PWS groups (only at the 4 hour time point), compared to the jacketed, no PWS group (*). The suppression in T cell activation indicates that the moderate dose of radiation alone, and in combination with PWS exposure, compromised T lymphocyte activation. Error bars indicate standard deviation for n = 3–5.
Discussion
Using the Partial Weight Suspension (PWS) system in the absence and presence of ionizing radiation, we have characterized the effects of partial weightbearing in combination with radiation exposure on splenic lymphocytes. We have found that the splenic lymphocyte population and T lymphocyte activation are compromised at the moderate doses of 1 and 2 Gy gamma radiation in the absence and presence of PWS.
Here we show that total splenic lymphocyte viability is decreased following acute exposure to ionizing radiation in combination with PWS in what appears to be a dose-dependent manner (Figure 1). From the mice exposed to the 2 Gy dose of radiation and PWS, it is apparent that viability remains suppressed over the time points observed in this study, out to 4 days (Figure 2). We also observed a shift in lymphocyte subtype and subset after the combination treatment of radiation and PWS in only the 2 Gy +/− PWS groups (Figures 2 and 3). We did not, however, observe any significant changes in the T lymphocyte subset populations (data not shown). This may be due to the lymphoid organ studied, as others report inconsistent fluctuations in CD4:CD8 ratios and percentages. For example, Meehan et al. (1992) reported no changes in the number of CD4+ or CD8+ blood cells in astronauts when comparing the pre-flight percentages to the post-landing percentages (3 days total flight), while Gridley et al. (2002) reported a decrease in blood and splenic CD4+ and CD8+ cells at 4 days post space-relevantradiation exposure and Wei et al. (2003) report decreased CD4+ and CD8+ cells in the spleen and thymus of rodents subjected to hindlimb unloading for 2 days.
The detection of the early activation marker CD69 using fluorescence-activated cell sorting has been postulated as a simpler, faster, and safer assessment of T-cell function than the traditional [3H]-thymidine (tritium labeled) incorporation or in vitro mitogen-activated proliferation (Maino et al. 1995). We report that all T cells express CD69 within the first 24 hours of mice being exposed to the PWS system (Figure 4). This acute activation of T cells suggests an initial period of increased stress, presumably from the suspension. The higher doses of radiation exposure (1 and 2 Gy) with and without PWS exposure do not result in an increase in T cell activation, as was observed in the 0.5 Gy dose of radiation with and without PWS groups and in the PWS group, indicating that the higher doses of radiation may hinder T cell activation.
Previously, we reported a significant loss in total lymphocytes in peripheral blood in animals exposed to 1 Gy of X-ray irradiation (Wambi et al. 2008) or 1 Gy of proton irradiation (Wambi et al. 2009), at 4 and 24 h after total body irradiation. Furthermore, Gridley et al. (2002) report a significant decrease in splenic lymphocytes on day 4 after a 2 Gy dose of irradiation with iron ions in mice. They also report a significant change in the CD4:CD8 ratio in spleen and blood at this time point.
This is the first report of a ground-based partial weight-bearing system used in combination with radiation exposure to assess changes in splenic lymphocyte populations. These data indicate that moderate doses of gamma radiation in the presence of an acute reduced weightbearing environment can produce deleterious effects on lymphocyte population and function. These changes may contribute to a compromised immune system during space flight and should be explored in greater detail, particularly within other lymphoid organs to fully characterize lymphocyte distribution and dysfunction.
Acknowledgements
This research was supported by the National Space Biomedical Research Institute (NSBRI) Center of Acute Radiation Research (CARR) grant. The NSBRI is funded through the National Aeronautics and Space Administration (NASA) Class Code (NCC) 9-58.
Footnotes
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Allebban Z, Ichiki AT, Gibson LA, Jones JB, Congdon CC, Lange RD. Effects of spaceflight on the number of rat peripheral blood leukocytes and lymphocyte subsets. Journal of Leukocyte Biology. 1994;55(209):213. doi: 10.1002/jlb.55.2.209. [DOI] [PubMed] [Google Scholar]
- Boonyaratanakornkit JB, Cogoli A, Li CF, Schopper T, Pippia P, Galleri G, Meloni MA, Hughes-Fulford M. Key gravity-sensitive signaling pathways drive T cell activation. Federation of American Societies for Experimental Biology Journal. 2005;19:2020–2022. doi: 10.1096/fj.05-3778fje. [DOI] [PubMed] [Google Scholar]
- Cogoli A, Tschopp A, Fuchs-Bislin P. Cell sensitivity to gravity. Science. 1984;225:228–230. doi: 10.1126/science.6729481. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Pecaut MJ, Nelson GA. Total-body irradiation with high-LET particles: Acute and chronic effects on the immune system. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2002;282:R677–688. doi: 10.1152/ajpregu.00435.2001. [DOI] [PubMed] [Google Scholar]
- Gridley DS, Slater JM, Luo-Owen X, Rizvi A, Chapes SK, Stodieck LS, Ferguson VL, Pecaut MJ. Spaceflight effects on T lymphocyte distribution, function and gene expression. Journal of Applied Physiology. 2009;106:194–202. doi: 10.1152/japplphysiol.91126.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Fulford M, Sugano E, Schopper T, Li CF, Boonyaratanakornkit JB, Cogoli A. Early immune response and regulation of IL-2 receptor subunits. Cellular Signaling. 2005;17:1111–1124. doi: 10.1016/j.cellsig.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Kajioka EH, Gheorghe C, Andres ML, Abell GA, Folz-Holbeck, Slater JM, Nelson GA, Gridley DS. Effects of proton and gamma radiation on lymphocyte populations and acute response to antigen. In Vivo. 1999;13:525–533. [PubMed] [Google Scholar]
- Lange RD, Andrews RB, Gibson LA, Wright P, Dunn CD, Jones JB. Hematologic parameters of astrorats flown on SL-3. Physiologist. 1985;28:S195–196. [PubMed] [Google Scholar]
- Maino VC, Suni MA, Ruitenberg JJ. Rapid flow cytometric method for measuring lymphocyte subset activation. Cytometry. 1995;20:127–133. doi: 10.1002/cyto.990200205. [DOI] [PubMed] [Google Scholar]
- Meehan RT, Neale LS, Kraus ET, Stuart CA, Smith ML, Cintron NM, Sams CF. Alteration in human mononuclear leucocytes following space flight. Immunology. 1992;76:491–497. [PMC free article] [PubMed] [Google Scholar]
- Mills PJ, Meck JV, Waters WW, D’Aunno D, Ziegler MG. Peripheral leukocyte subpopulations and catecholamine levels in astronauts as a function of mission duration. Psychosomatic Medicine. 2001;63:886–890. doi: 10.1097/00006842-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: Technical aspects. Journal of Applied Physiology. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Nash PV, Mastro AM. Variable lymphocyte responses in rats after space flight. Experimental Cell Research. 1992;202:125–131. doi: 10.1016/0014-4827(92)90411-z. [DOI] [PubMed] [Google Scholar]
- Nash PV, Bour BA, Mastro AM. Effect of hindlimb suspension simulation of microgravity on in vitro immunological responses. Experimental Cell Research. 1991;195:353–360. doi: 10.1016/0014-4827(91)90384-7. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Nelson GA, Peters LL, Kostenuik PJ, Bateman TA, Morony S, Stodieck LS, Lacey DL, Simske SJ, Gridley DS. Genetic models in applied physiology: Selected contribution: Effects of spaceflight on immunity in the C57BL/6 mouse. I. Immune population distributions. Journal of Applied Physiology. 2003;94:2085–2094. doi: 10.1152/japplphysiol.01052.2002. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Zhang S, Reuben JM, Lee BN, Butel JS. Effects of radiation and latent virus on immune responses in a space flight model. Journal of Allergy and Clinical Immunology. 2005;115:1297–1303. doi: 10.1016/j.jaci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Shearer WT, Ochs HD, Lee BN, Cohen EN, Reuben JM, Cheng I, Thompson B, Butel JS, Blancher A, Abbal M, Aviles H, et al. Immune responses in adult female volunteers during the bed-rest model of spaceflight: Antibodies and cytokines. Journal of Allergy and Clinical Immunology. 2009;123:900–905. doi: 10.1016/j.jaci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Simms PE, Ellis TM. Utility of flow cytometric detection of CD69 expression as a rapid method for determining poly- and oligoclonal lymphocyte activation. Clinical and Diagnostic Laboratory Immunology. 1996;3:301–304. doi: 10.1128/cdli.3.3.301-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenfeld G, Shearer WT. Immune function during space flight. Nutrition. 2002;18:899–903. doi: 10.1016/s0899-9007(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Taylor GR. Immune changes during short-duration missions. Journal of Leukocyte Biology. 1993;54:202–208. doi: 10.1002/jlb.54.3.202. [DOI] [PubMed] [Google Scholar]
- Taylor GR, Neale LS, Dardano JR. Immunological analyses of U.S. Space Shuttle crewmembers. Aviation, Space and Environmental Medicine. 1986;57:213–217. [PubMed] [Google Scholar]
- Wagner EB, Granzella NP, Saito H, Newman DJ, Young LR, Bouxsein ML. Partial weight suspension: A novel murine model for investigating adaptation to reduced musculoskeletal loading. Journal of Applied Physiology. 2010;109:350–357. doi: 10.1152/japplphysiol.00014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, Sayers CM, Baran M, Ware JH, Kennedy AR. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiation Research. 2008;169:384–396. doi: 10.1667/RR1204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambi CO, Sanzari JK, Sayers CM, Nuth M, Zhou Z, Davis J, Finnberg N, Lewis-Wambi JS, Ware JH, El-Deiry WS, Kennedy AR. Protective effects of dietary antioxidants on proton total-body irradiation-mediated hematopoietic cell and animal survival. Radiation Research. 2009;172:175–186. doi: 10.1667/RR1708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei LX, Zhou JN, Roberts AI, Shi YF. Lymphocyte reduction induced by hindlimb unloading: Distinct mechanisms in the spleen and thymus. Cell Research. 2003;13:465–471. doi: 10.1038/sj.cr.7290189. [DOI] [PubMed] [Google Scholar]