Abstract
Background
Overactive bladder (OAB) is a common condition affecting the elderly. The mainstay of treatment for OAB is medical therapy with anticholinergics. However, adverse events have been reported with this class of drugs including cognitive changes.
Objective
To investigate the effect of an anticholinergic medication on cognitive function in postmenopausal women being treated for OAB.
Study Design
Prospective cohort study conducted from January to December 2010, with 12-week follow-up after medication initiation.
Setting
Urogynecology clinic at one academic medical center.
Patients
Women age 55 or older seeking treatment for OAB and opting for anticholinergic therapy were recruited.
Intervention
Baseline cognitive function was assessed via the Hopkins Verbal Learning Test – Revised Form (HVLT-R) (and its 5 subscales), the Orientation, Memory & Concentration (OMC) short form, and the Mini-Cog evaluation. After initiation of trospium chloride extended release, cognitive function was reassessed at Day 1, Week 1, Week 4 and Week 12. Bladder function was assessed via three condition-specific quality of life questionnaires. Secondary outcomes included change in bladder symptoms, correlation between cognitive and bladder symptoms, and overall medication compliance.
Main Outcome Measure
Change in HVLT-R score at Week 4 after medication initiation, compared to baseline (pre-medication) score.
Results
Of 50 women enrolled, 35 completed the assessment. Average age was 70.4 years and 77.1% had previously taken anticholinergic medication for OAB. At enrollment 65.7% had severe overactive bladder and 71.4% had severe urge incontinence. Cognitive function showed an initial decline on Day 1 in HVLT-R total score (p=0.037), HVLT-R Delayed Recognition subscale (p=0.011) and HVLT-R Recognition Bias subscale (p=0.01). At Week 1 the HVLT-R Learning subscale declined from baseline (p=0.029). All HVLT-R scores normalized by Week 4. OMC remained stable throughout. The Mini-Cog nadired at a 90.9% pass rate at Week 4. OAB symptoms did not improve until Week 4, based on questionnaire scores (p<0.05).
Conclusion
Cognitive function exhibited early changes after initiation of trospium chloride but normalized within four weeks. Cognitive changes occurred weeks prior to OAB symptom improvement. Surveillance for cognitive changes with anticholinergic use should be part of OAB management.
Keywords: Anticholinergic, Cognitive Function, Elderly, Overactive Bladder
INTRODUCTION
Overactive bladder (OAB) is a common condition affecting the elderly population.[1] OAB is defined by the International Continence Society (ICS) as “urinary urgency, usually accompanied by frequency and nocturia, with or without urge urinary incontinence”.[2] OAB afflicts more than 38 million Americans, and 1 in every 3 elderly adults.[1,2] OAB has far-reaching consequences for both physical and mental health: skin breakdown due to leakage, sleep disturbance, fall-related injuries, depression, prolonged hospital stays, admission to a nursing home and lower quality of life.[1, 3–5] OAB is an embarrassing condition that interferes with not only social functioning but also a wide range of activities of daily living.[4] Annual direct costs of OAB in the US -- including diagnostic tests, physician visits, medications, treatment procedures, supplies such as pads, diapers and home care -- totaled over $12 billion in 2005.[6] This is comparable to breast cancer ($12.7 billion) and osteoporosis ($13.8 billion). There are also indirect costs: loss of work productivity, time off from work for treatment, and inability to maintain employment due to OAB. [7,8] We can decrease the significant healthcare and economic burdens associated with OAB by investing more healthcare resources in effective and safe treatments.
The mainstay of treatment for OAB is pharmacotherapy -- the primary class of drugs is anticholinergics. Because anticholinergic receptors are found throughout the body, the side effects of these drugs are common and widespread.[9–11] One of the most serious adverse effects is cognitive impairment. The central nervous system (CNS) contains all five anticholinergic receptors, which influence a wide array of cognitive function: learning, memory, language, arousal/attention, and visuospatial/sensory/motor processing.[9,10,12] It is therefore not surprising that anticholinergic medications have been linked to cognitive impairment.[13,14] Multiple cognitive effects have been reported with anticholinergic use: memory changes, blurred vision, somnolence, hallucinations, confusion, and delirium. All of these cognitive impairments are more prevalent in the elderly, possibly due to the increased CNS absorption of anticholinergics in this population.[9, 15–18] Given that 1 in 3 elderly adults suffers from OAB, the proportion of elderly using anticholinergics for OAB alone is high. Because anticholinergics are also used to treat many other medical conditions, there is potential for compounding of drug-induced cognitive impairment. One study of almost 6000 elderly patients found that 59% were taking at least one anticholinergic; and 10% were taking up to three.[3] Therefore, it is becoming increasingly critical to understand the magnitude of adverse anticholinergic effects on cognitive function. Currently, there is no standard of practice for OAB management. Management of anticholinergic use in the elderly must involve monitoring of: functional status, comorbidities, concurrent medication use, and active surveillance for adverse events – including the development of cognitive impairment.
Our primary aim was to investigate the effect of a specific anticholinergic, trospium chloride extended release, on cognitive function in postmenopausal women in a clinic setting. Secondary aims included understanding the severity of OAB in this clinic population, the timing of changes in cognitive function and OAB symptom improvement, and medication compliance.
METHODS
After approval by the Institutional Review Board at the University of North Carolina at Chapel Hill (UNC), we conducted a prospective cohort study assessing cognitive function in women initiating an anticholinergic medication for the treatment for OAB. STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines were followed.[19] Women age 55 or older seeking treatment for OAB were recruited from the Female Pelvic Medicine and Reconstructive Surgery clinic between January and December 2010. Initially the age of recruitment was 65 and older but we amended this within the first three months of the study after finding it difficult to meet recruitment goals. After being counseled by their physician on treatment options for OAB, women opting for anticholinergic medication were offered enrollment in the study, which would entail treatment with trospium chloride extended release. After complete description of the study to the subjects, written informed consent was obtained. Subjects received a small monetary compensation for completing the follow-up assessments but medication costs were not covered.
Baseline cognitive function was assessed prior to anticholinergic start via the Hopkins Verbal Learning Test – Revised Form (HVLT-R), the Orientation, Memory & Concentration (OMC) short form, and the Mini-Cog evaluation.[20–22] This battery of tests comprise validated measures of cognitive function commonly used in the geriatric population. The Mini-Cog test is a very brief three-minute cognitive screening test that is useful in detecting mild cognitive impairment, dementia and early stage Alzheimer's, and is not dependent on education level or language. It includes a drawing test and verbal recall. Both the accuracy of the clock face and the verbal recall are scored. The HVLT-R is a verbal memory test that involves reading subjects a list of 12 words and having them recall the words both immediately and after a delay of 25 minutes. There are 6 versions which provide differing sets of 12 words. The HVLT-R generates a total score and seven subscale scores, including Total Recall, Delayed Recall, Delayed Recognition, Learning, Retention, Discrimination Index and Recognition Bias. The HVLT-R has a low risk of ceiling effects (even for the non-dementia population) and is not affected by gender or education. It is easy to administer, quick and a well tolerated tool for the screening of dementia. The OMC is a mini mental status assessment, asking questions regarding current date and time, ability to recall a random name and address and the ability to count backwards from 20 and name the months in reverse. Both the HVLT-R and OMC provide raw numerical scores, while the Mini-Cog provides a dichotomous pass/fail score.
After this baseline testing was performed, women were instructed to start trospium chloride extended release. Date and time of initiation was recorded. Subjects completed a medication diary, recording the date and time of each pill taken on a grid chart for the entire study period. Cognitive function was then reassessed using the Mini-Cog, HVLT-R and OMC at Day 1, Week 1, Week 4 and Week 12 of medication usage. These time periods were determined based on the pharmacokinetics of trospium chloride extended release. We also assessed pelvic floor function, specifically focusing on OAB symptoms. Pelvic floor and bladder function were measured via three validated condition-specific quality of life questionnaires: the Pelvic Floor Distress Inventory – Short Form 20 (PFDI-20)[23], the Pelvic Floor Impact Questionnaire – Short Form 7 (PFIQ-7)[23] and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire – Short Form 12 (PISQ-12)[24]. Both the PFDI-20 and PFIQ-7 contain urinary subscales: the UDI-6 and UIQ-7, respectively. Urge urinary incontinence was defined as a positive response to question 3 on the PFDI-20 indicating urine leakage with urge. The severity of OAB symptoms were defined as a positive response to questions 1 and/or 3 on the PFDI-20 indicating severity of urgency (with or without leakage) and frequency.
Our primary outcome was a change in cognitive function, as determined by HVLT-R, at four weeks after starting the anticholinergic medication. Secondary outcomes included the severity of OAB symptoms in this clinic population, the time of onset of any change in cognitive function, the time of onset of OAB symptom improvement and medication compliance. Inclusion criteria consisted of English-literate women age 55 or older opting for medical management of OAB. Exclusion criteria were the presence of pre-existing comorbidities such as dementia, depression, and any other psychiatric diagnoses that may affect cognitive function as determined by patient history and medical records; current use of other anticholinergic medications or other agents known to affect cognitive function; and medical conditions known to be adversely affected by anticholinergic therapy, such as narrow-angle glaucoma, severe urinary retention and obstructive bowel disease. Furthermore, women who met criteria for dementia on the baseline Mini-Cog were excluded from the study. Prior or current use of an anticholinergic other than trospium chloride for the treatment of OAB was not a reason for exclusion. Any women currently using a different anticholinergic but electing to enroll in the study underwent a two-week washout period prior to medication start.
Data were analyzed using SPSS 16.0 (Chicago, IL). Analysis was undertaken with univariate analysis for descriptive variables and bivariate analysis using paired t test for continuous variables, with a Bonferroni correction for multiple comparisons. The patient’s baseline cognitive function served as the control for which variables at Day 1, Week 1, Week 4 and Week 12 were each compared individually. This research was designed as a pilot study to investigate the relationship between anticholinergic initiation and changes in cognitive function as well as to answer questions regarding study design such as baseline prevalence of OAB in our clinic population and the feasibility of assessing cognitive function in this setting as part of the management of OAB. Therefore a control group was not used. Rather, the subjects’ baseline scores served as the comparison for post-medication cognitive performance using paired t-tests as described above. A convenience sample of 50 subjects was chosen. A p value <0.05 was considered significant.
RESULTS
During the study period 50 women were enrolled and 35 completed the primary assessment at Week 4. Fifteen women dropped out prior to the primary assessment period, with the main reason being discontinuation of the medication due to lack of efficacy. No subject reported any subjective cognitive effects during the study period. Average age at enrollment was 70.4 years and the majority of women were Caucasian (Table 1). More than three-quarters of subjects had previously taken anticholinergic medication for the treatment of OAB, with an average of 1.4 previous medications. Two-thirds of subjects reported severe overactive bladder symptoms and almost three-quarters reported severe urge incontinence based on validated questionnaire responses. At the time of enrollment, 13 of 35 women (37.1%) were taking a different anticholinergic for the treatment of OAB and underwent a washout period. Baseline cognitive function was not impaired, as per protocol, based on an intact Mini-Cog assessment, HVLT-R total score and OMC score. We then compared these baseline cognitive function scores to post-treatment scores (Table 2). On Day 1 of anticholinergic use, cognitive function showed a decline in the HVLT-R total score (p=0.037) (Figure 1), the HVLT-R Delayed Recognition subscale (p=0.011) and the HVLT-R Recognition Bias subscale (p=0.01). There were no changes seen in the other HVLT-R subscales at that time. At Week 1 of anticholinergic use, there was a decline in the HVLT-R Learning subscale (p=0.029) only. The other HVLT-R scores were unchanged from baseline at Week 1, including those that had seen a decline on Day 1. At Week 4 of anticholinergic use, the HVLT-R Total Recall subscale score was improved over baseline (p=0.02), with no differences in the other HVLT-R scores. At Week 12, there were no statistical differences in cognitive function compared to baseline HVLT-R scores. These analyses were based on paired t-tests used to compare each follow-up point to its baseline score. When we instituted a Bonferroni correction for multiple comparisons the changes in the HVLT-R subscales of Delayed Recognition (p=0.011) and Recognition Bias (p=0.01) remained significant. The OMC remained stable throughout the testing period, with no significant changes seen at any time points. The Mini-Cog assessment showed a decline as early as Day 1, with a nadir at Week 4 of 90.9%. It was noted to improve at Week 12 in the women who were still on the anticholinergic medication. We also compared all cognitive outcomes between those women who underwent a washout period at the time of enrollment due to current anticholinergic use and those not currently taking an anticholinergic at the time of enrollment. There were no differences in cognitive function outcomes at any time point between these two groups.
Table I.
Baseline demographics
| Study Population (n=35) |
|
|---|---|
| Age | 70.4±8.4 |
| Race | |
| White | 33 (94.3) |
| Black | 2 (5.7) |
| Prior Anticholinergic Use | 27 (77.1) |
| Number Previous Anticholinergics |
1.4±1.2 |
| Baseline OABa Symptoms | |
| Mild | 11 (31.4) |
| Severe | 23 (65.7) |
| Baseline Urge Leakage | |
| None | 3 (8.6) |
| Mild | 7 (20.0) |
| Severe | 25 (71.4) |
| Baseline HVLT-Rb Total Score | 60.3±6.0 |
| Baseline OMCc Score | 25.1±3.1 |
| Baseline Mini-Cog Pass Rate | 35 (100) |
Data presented as mean ± SD or n (%).
OAB = overactive bladder
HVLT-R = Hopkins Verbal Learning Test – Revised; maximum possible score is 76.
OMC = Orientation, Concentration and Memory test; maximum possible score is 28.
Table II.
Cognitive Function with Anticholinergic Use
| Baseline (n=35) |
Day 1 (n=35) |
Week 1 (n=35) |
Week 4 (n=35) |
Week 12 (n=15) |
|
|---|---|---|---|---|---|
| HVLT-Ra Total Score (best score 72) | 60.3±6.0 | 56.1±12.2 b | 59.2±13.3 | 60.0±11.9 | 57.4±17.3 |
| Total Recall (best score 36) | 26.7±4.3 | 25.4±4.9 | 27.9±5.9 | 28.3±3.9 b | 27.8±5.6 |
| Delayed Recall (best score 12) | 10.2±2.0 | 9.5±2.5 | 9.8±2.4 | 10.1±1.9 | 9.9±2.7 |
| Delayed Recognition (best score 24) | 23.5±0.8 | 22.8±1.4 b | 23.2±1.1 | 23.4±0.8 | 23.6±0.8 |
| Learning (best score 0) % | 3.9±1.7 | 3.8±1.8 | 3.1±1.4 b | 3.4±1.3 | 2.7±1.3 |
| Retention (best score 100) | 90.4 | 97.8 | 92.9 | 91.2 | 92.1 |
| Discrimination Index (best score 24) | 23.4±1.3 | 22.4±2.1 | 23.0±1.6 | 22.3±3.4 | 23.3±1.5 |
| Recognition Bias (best score 0) | 0.02±0.05 | 0.07±0.10 b | 0.03±0.05 | 0.03±0.04 | 0.02±0.04 |
| OMCc (best score 28) | 25.1±3.1 | 26.0±2.0 | 26.0±2.4 | 25.9±2.2 | 26.4±2.5 |
| Mini-Cog % Pass (best score 100) | 100 | 96.7 | 96.8 | 90.9 | 100 |
Data presented as mean ± SD or (%)
All analyses performed with paired t-test. Only significant p values reported.
HVLT-R = Hopkins Verbal Learning Test – Revised; maximum possible score is 76.
p<0.05 versus baseline
OMC = Orientation, Concentration and Memory test; maximum possible score is 28.
Figure 1.
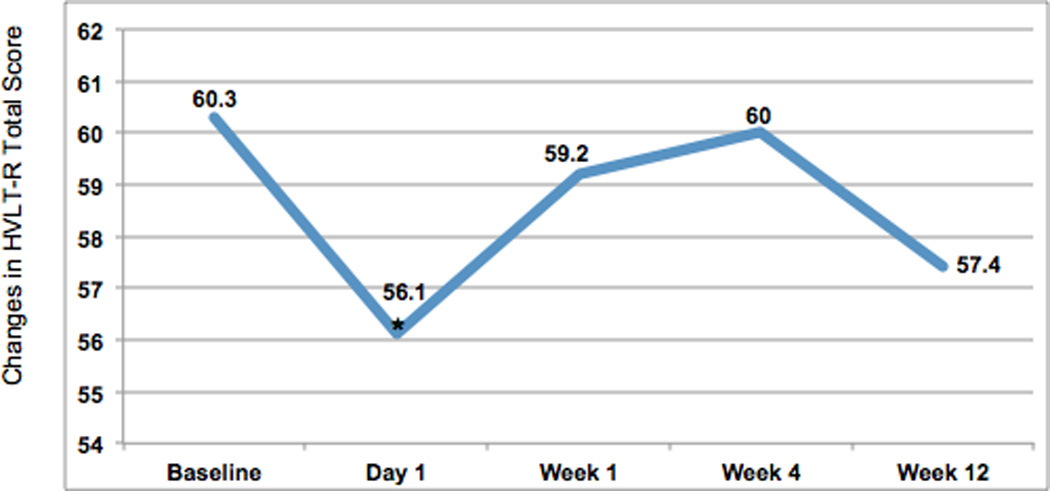
Changes in HVLT-R Total Score, plotted points are mean values
* p<.005
HVLT-R = Hopkins Verbal Learning Test - Revised
When assessing pelvic floor and bladder symptoms, we did not see a significant improvement until Week 4, based on both PFDI-20 and PFIQ-7 total scores and UDI-6 and UIQ-7 subscale scores. (Table 3) These changes remained significant after a Bonferroni correction for multiple comparisons. Pelvic floor and bladder symptoms improved further at Week 12 based on PFDI-20, PFIQ-7 and UDI-6 scores. There was no change in sexual function based on PISQ-12 scores, but only 15 subjects were sexually active at the time of enrollment, with no change in sexual status during the study period.
Table III.
Pelvic Floor Symptoms with Anticholinergic Use
| Baseline (n=35) |
Week 1 (n=35) |
P Value |
Week 4 (n=35) |
P Value | Week 12 (n=15) |
P Value | |
|---|---|---|---|---|---|---|---|
| PFDI-20c | 107.1±54.6 | 101.0±57.1 | 0.37 | 90.5±61.1 | 0.007 | 58.5±41.6a | <0.001 |
| UDI-6d | 58.0±32.1 | 55.0±26.8 | 0.46 | 48.5±24.7 | 0.001 | 32.1±23.1b | <0.001 |
| PFIQ-7e | 62.2±65.5 | 65.0±73.0 | 0.62 | 49.8±60.0 | 0.37 | 35.0±31.3 | 0.02 |
| UIQ-7f | 38.1±27.1 | 39.8±30.8 | 0.68 | 29.1±27.5 | 0.03 | 22.2±19.3 | 0.07 |
| PISQ-12g | 28.5±5.8 | 29.2±8.0 | 0.60 | 30.6±7.1 | 0.19 | 29.3±6.2 | 0.08 |
Data presented as mean ± SD
All analyses performed with paired t-test, with outcomes compared to baseline.
p=0.01 when comparing Week 4 to Week 12
p=0.03 when comparing Week 4 to Week 12
PFDI-20 = Pelvic Floor Distress Inventory, short form
UDI-6 = Urinary Distress Inventory, short form
PFIQ-7 = Pelvic Floor Impact Questionnaire, short form
UIQ-7 = Urinary Impact Questionnaire, short form
PISQ-12 = Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire, short form
When assessing compliance, the overall medication discontinuation rate was 57.1% (20/35) by 12 weeks, with an average time to discontinuation of 29.9 days as determined by medication diary and interview. Leading reasons included lack of efficacy (28.6%), expense of medication (8.6%), constipation (8.6%), dry mouth (2.9%), headache (2.9%), and nausea (2.9%). No subjects cited cognitive dysfunction as a reason for discontinuation and no subjects were withdrawn from the study for adverse events.
DISCUSSION
The mainstay of treatment for the highly prevalent condition OAB is anticholinergic medication, a class of drugs known to interfere with cognitive function. OAB is more prevalent in the elderly population - a group known to have increased cognitive dysfunction. Therefore, it is essential to understand the effect of anticholinergics on cognitive function. In our group of post-menopausal women, we found observed fluctuations in cognitive function after beginning a new anticholinergic medication. While some cognitive changes were seen as early as one day after medication start, other changes did not appear until one week of medication use. By four weeks of medication use, cognitive function returned to baseline and did not fluctuate after that time, up to 12 weeks after medication initiation. No subjects reported any subjective changes in cognitive function.
Anticholinergic drug exposure in the elderly has been shown to have variable effects. Han et al. demonstrated that anticholinergic exposure with multiple medications has a cumulative effect on cognitive function.[25] This cumulative effect was confirmed in a recent longitudinal study of over 13,000 elderly adults.[26] Ancelin et al. found that elderly adults taking various anticholinergics had multiple areas of cognitive dysfunction: reaction time, attention, memory, recall, visuospatial construction, and language tasks.[27] In contrast, a randomized trial of healthy elderly volunteers showed no short-term differences in cognitive function between OAB anticholinergics and placebo.[28] Staskin et al. found no difference in cognitive testing between trospium chloride and placebo, and no measurable trospium chloride in the CNS.[29] Because cognitive function is not routinely evaluated by practitioners treating OAB, the true rate of cognitive impairment associated with anticholinergic use is unknown.[4]
Evaluation of baseline urinary symptoms in our clinic population demonstrated that most women are severely affected by OAB and have tried at least one prior anticholinergic medication prior to seeking care with a specialist. This may be helpful in understanding baseline disease severity in women seeking specialty care for management of OAB. We also found that there was a delay between the onset of changes in cognitive function and OAB symptom improvement after medication start. While cognitive changes occurred early, OAB symptoms were not found to improve until 4 weeks after starting anticholinergic medication. Therefore, it is important to realize that side effects, specifically in cognitive function, may be occurring prior to symptom improvement, and normalization of cognitive function may be found at time of peak effects of anticholinergic medications. In regards to compliance, we found that the discontinuation rate for this anticholinergic medication was high, with over half of women stopping the medication by the end of the study period. Average time to discontinuation was 4 weeks, which was the same time when we first observed an improvement in OAB symptoms. Among those who continued therapy to 12 weeks, we observed further improvement of symptoms. Clinicians may consider advising their patients to try medication for at least 4 weeks, as symptom improvement may not take place until that time. We also described leading reasons for medication discontinuation, which may provide further help in counseling patients as to medication expectations.
We were interested to see an improvement in the HVLT-R total score at Week 4 and the Mini-Cog at Week 12. Our repeated testing likely led to these improved cognitive scores due to a learning effect, which has been reported previously.[14] While the HVLT-R offers a different set of 12 words with each administration, the OMC and Mini-Cog have repetitive elements. Frequent administration of all three tests may have been an intervention in itself, by providing memory exercises for the subjects. In designing an expanded version of this study we plan to have less frequent cognitive testing and the use of a placebo group to avoid this learning effect.
We chose trospium chloride extended release as our anticholinergic medication for this study for a number of reasons. We chose a single agent for all subjects in order to avoid the effect of variation in drug absorption, action and efficacy. Due to its molecular properties, this drug has a relatively low capacity to penetrate the blood-brain barrier (BBB) and exert cognitive effects. A drug’s ability to cross the BBB is based on not only its size but also its polarity and lipophilic properties.[17] Trospium chloride, as a quarternary amine, is large, highly polar, and hydrophilic and thus is relatively impermeable to the BBB. Furthermore, trospium chloride is a known P-glycoprotein ligand and is actively removed from the central nervous system via efflux.[30] There are abundant data demonstrating cognitive effects of non-selective anticholinergics that do cross the BBB.[9,17,31] However, there is less direct evidence of whether trospium chloride exerts these effects due to its unique properties. trospium chloride does have some drawbacks as well: 1) it has reduced oral bioavailability, thus requiring that it be taken with a full glass of water one hour before meals, 2) it requires a dose reduction for subjects with decreased renal function and 3) it may compete with other drugs that are excreted in the kidneys, including digoxin and metformin, although studies have not shown this effect.[11,32] At the time of study recruitment trospium chloride was a relatively new medication, and it was more likely that our study population was naïve to it.
The principal limitation of this study is the lack of a control group, which may lead to misclassification bias. This project was designed as a pilot study to gather information on the effect of a unique anticholinergic on cognitive function in postmenopausal women in our clinic population, as well as the timing of onset of these changes in relation to improvement in OAB symptoms and the baseline severity of OAB. A clinically meaningful change in HVLT-R total score has been reported to be 6 points.[29] Our findings approached this cut-off, with a mean and standard deviation decline of 4.2 ± 12.2 points at Day 1. Because we used a convenience sample of subjects, we did not aim to have an adequate sample size to meet power in the current study, which would have required significantly more subjects. Based on our data, we can now design an adequately powered placebo-controlled study to further address the effect of anticholinergics on cognitive function in this population. There is also potential for selection bias due to the use of subjects that were naïve to anticholinergics and those who had previously tried these medications. However, no subjects had previously taken trospium chloride and we found no difference in outcomes between these two groups. Another limitation is the accuracy of the chosen cognitive function tests to discriminate small but potentially meaningful changes in cognitive function. We used validated tools in our study but this remains a potential confounding variable. A final limitation is the fact that we did not assess pelvic floor and bladder symptoms between Weeks 1 and 4, so we cannot say exactly when the time to improvement in symptoms occurred. However, this was not the main aim of the study.
The major strength of this study is the prospective design, with baseline cognitive function obtained for comparison to post-treatment time-points. Anyone displaying baseline cognitive dysfunction was excluded from the study. We used a single anticholinergic medication for all subjects in order to avoid any variation in pharmacokinetics, efficacy or side effects that could be due to multiple medications. In addition, all subjects were either naïve to anticholinergic or had undergone a washout period prior to obtaining baseline cognitive testing. All medications were reviewed prior to enrollment to confirm this. When we compared the naïve group to the washout group, we found no differences in any of the cognitive or pelvic floor outcomes. Finally, the interdisciplinary collaboration involved in this study allowed us to look at multiple facets of medication effects, including the pharmacokinetics of the drug (timing of effects seen), and the interplay between onset of cognitive changes and the improvement in OAB symptoms.
CONCLUSION
OAB is a wide-spread condition with far-reaching consequences. As our population ages and the prevalence of OAB increases, anticholinergic medication will remain a mainstay in the management of this disease. Understanding the side effects of the anticholinergic medications that we prescribe is critical. The data obtained from this study will facilitate the design of a large-scale longitudinal clinical trial, which will expand on our understanding of the current topic. As providers, we must continue to provide treatment options for OAB that are both effective and safe.
Acknowledgments
This project was supported by the IBM Fund Award (Junior Faculty Development Grant, University of North Carolina). The content is solely the responsibility of the authors and does not necessarily represent the official views of the grant sponsors.
Footnotes
This research was an oral presentation at the American Urogynecologic Society on September 16, 2011 and a poster presentation at the Society for Urodynamics in Female Urology from February 28-March 2, 2013.
There are no conflicts of interest to disclose.
REFERENCES
- 1.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 2.Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi: 10.1007/s00192-009-0976-9. Epub 2009/11/26. [DOI] [PubMed] [Google Scholar]
- 3.Scheife R, Takeda M. Central nervous system safety of anticholinergic drugs for the treatment of overactive bladder in the elderly. Clin Ther. 2005;27(2):144–153. doi: 10.1016/j.clinthera.2005.02.014. Epub 2005/04/07. [DOI] [PubMed] [Google Scholar]
- 4.Liberman JN, Hunt TL, Stewart WF, Wein A, Zhou Z, Herzog AR, et al. Health-related quality of life among adults with symptoms of overactive bladder: results from a U.S. community-based survey. Urology. 2001;57(6):1044–1050. doi: 10.1016/s0090-4295(01)00986-4. Epub 2001/05/30. [DOI] [PubMed] [Google Scholar]
- 5.Sexton CC, Coyne KS, Thompson C, Bavendam T, Chen CI, Markland A. Prevalence and effect on health-related quality of life of overactive bladder in older americans: results from the epidemiology of lower urinary tract symptoms study. J Am Geriatr Soc. 2011;59(8):1465–1470. doi: 10.1111/j.1532-5415.2011.03492.x. Epub 2011/07/02. [DOI] [PubMed] [Google Scholar]
- 6.Hu TW, Wagner TH. Health-related consequences of overactive bladder: an economic perspective. BJU Int. 2005;96(Suppl 1):43–45. doi: 10.1111/j.1464-410X.2005.05654.x. [DOI] [PubMed] [Google Scholar]
- 7.Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63(3):461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Sexton CC, Coyne KS, Vats V, Kopp ZS, Irwin DE, Wagner TH. Impact of overactive bladder on work productivity in the United States: results from EpiLUTS. Am J Manag Care. 2009;15(4 Suppl):S98–S107. Epub 2009/04/16. [PubMed] [Google Scholar]
- 9.Kay GG, Abou-Donia MB, Messer WS, Jr, Murphy DG, Tsao JW, Ouslander JG. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc. 2005;53(12):2195–2201. doi: 10.1111/j.1532-5415.2005.00537.x. Epub 2006/01/10. [DOI] [PubMed] [Google Scholar]
- 10.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148(5):565–578. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staskin DR, Harnett MD. Effect of trospium chloride on somnolence and sleepiness in patients with overactive bladder. Curr Urol Rep. 2004;5(6):423–426. doi: 10.1007/s11934-004-0064-0. [DOI] [PubMed] [Google Scholar]
- 12.Kaufer DI. Cholinesterase-inhibitor therapy for dementia: novel clinical substrates and mechanisms for treatment response. CNS Spectr. 2002;7(10):742–750. doi: 10.1017/s1092852900008737. Epub 2004/03/23. [DOI] [PubMed] [Google Scholar]
- 13.Callegari E, Malhotra B, Bungay PJ, Webster R, Fenner KS, Kempshall S, et al. A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br J Clin Pharmacol. 2011;72(2):235–246. doi: 10.1111/j.1365-2125.2011.03961.x. Epub 2011/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–870. doi: 10.1111/j.1532-5415.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 15.Pakulski C, Drobnik L, Millo B. Age and sex as factors modifying the function of the blood-cerebrospinal fluid barrier. Med Sci Monit. 2000;6(2):314–318. Epub 2001/02/24. [PubMed] [Google Scholar]
- 16.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug metabolism reviews. 2009;41(2):67–76. doi: 10.1080/03602530902722679. Epub 2009/06/12. [DOI] [PubMed] [Google Scholar]
- 17.Pak RW, Petrou SP, Staskin DR. trospium chloride: a quaternary amine with unique pharmacologic properties. Current urology reports. 2003;4(6):436–440. doi: 10.1007/s11934-003-0023-1. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 18.Biastre K, Burnakis T. trospium chloride treatment of overactive bladder. Ann Pharmacother. 2009;43(2):283–295. doi: 10.1345/aph.1L160. [DOI] [PubMed] [Google Scholar]
- 19.STROBE statement--checklist of items that should be included in reports of observational studies (STROBE initiative) Int J Public Health. 2008;53(1):3–4. doi: 10.1007/s00038-007-0239-9. Epub 2008/06/05. [DOI] [PubMed] [Google Scholar]
- 20.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- 21.Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 22.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983;140:734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 23.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193(1):103–113. doi: 10.1016/j.ajog.2004.12.025. Epub 2005/07/16. [DOI] [PubMed] [Google Scholar]
- 24.Rogers RG, Coates KW, Kammerer-Doak D, Khalsa S, Qualls C. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(3):164–168. doi: 10.1007/s00192-003-1063-2. discussion 8. Epub 2003/09/05. [DOI] [PubMed] [Google Scholar]
- 25.Han L, Agostini JV, Allore HG. Cumulative anticholinergic exposure is associated with poor memory and executive function in older men. Journal of the American Geriatrics Society. 2008;56(12):2203–2210. doi: 10.1111/j.1532-5415.2008.02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. doi: 10.1111/j.1532-5415.2011.03491.x. Epub 2011/06/29. [DOI] [PubMed] [Google Scholar]
- 27.Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–459. doi: 10.1136/bmj.38740.439664.DE. Epub 2006/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173(2):493–498. doi: 10.1097/01.ju.0000148963.21096.5d. [DOI] [PubMed] [Google Scholar]
- 29.Staskin D, Kay G, Tannenbaum C, Goldman HB, Bhashi K, Ling J, et al. trospium chloride has no effect on memory testing and is assay undetectable in the central nervous system of older patients with overactive bladder. Int J Clin Pract. 2010;64(9):1294–1300. doi: 10.1111/j.1742-1241.2010.02433.x. Epub 2010/06/22. [DOI] [PubMed] [Google Scholar]
- 30.Ieiri I, Takane H, Otsubo K. The MDR1 (ABCB1) gene polymorphism and its clinical implications. Clin Pharmacokinet. 2004;43(9):553–576. doi: 10.2165/00003088-200443090-00001. Epub 2004/06/26. [DOI] [PubMed] [Google Scholar]
- 31.Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57(3):414–421. doi: 10.1016/s0090-4295(00)01113-4. Epub 2001/03/15. [DOI] [PubMed] [Google Scholar]
- 32.FDA. Sanctura XR ® (trospium chloride) Extended Release - full prescribing information. 2011 http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022103s004lbl.pdf, editor.


