Abstract
The recent analysis of the first successful RV144 vaccine trial revealed that a high titer of plasma anti-V2 antibodies (Abs) correlated with a decreased risk of HIV-1 infection in vaccine recipients. To understand the mechanism of immune correlates, we studied seven anti-V2 monoclonal Abs (mAbs) developed from HIV-1 infected individuals. The V2 mAbs target conserved epitopes, including the binding site for α4β7 integrin, and are broadly cross-reactive with various gp120 proteins. Preferential usage of the VH1-69 gene by V2 mAbs may depend on selection by the same antigenic structure. Six of seven V2 mAbs weakly neutralized four to eight of the 41 pseudoviruses tested and resistance to neutralization was correlated with longer V2 domains. The data suggest the presence of shared, conserved structural elements in the V2 loop, and these can be used in the design of vaccine immunogens inducing broadly reactive Abs with anti-viral activities.
Keywords: HIV-1, V2 domain, Envelope proteins, Human monoclonal antibodies, HIV neutralizing antibodies, Glycosylation
Introduction
The development of an effective HIV vaccine has remained an enormous challenge even after 30 years of intensive research. The first moderate success was reported in 2009 with the RV144 clinical vaccine trial which demonstrated a 31.2% reduction of infection in vaccine recipients (Rerks-Ngarm et al., 2009). As vaccine-elicited antibody (Ab) and CD4 T cell responses were noted without a significant effect on viral load, it suggested that protection was mediated by anti-HIV-1 Abs (Tomaras and Haynes, 2010). This hypothesis was recently confirmed by data showing that vaccinees with high titers of plasma anti-V2 Abs had significantly lower risk of HIV-1 infection (www.hivvaccineenterprise.org/conference/2011/webcasting) (Karasavvas et al., 2011; Zolla-Pazner et al., 2011a). For the first time, the correlates of immunity in the RV144 trial were determined, which is an essential step in the design of an efficient HIV vaccine.
The most intriguing question is the mechanism of protection mediated by anti-V2 Abs and whether this is dependent on their fine specificity, titer, or immunogenetics. The V2 region is immunogenic, inducing V2-specific Abs in approximately 20–45% of HIV-1 infected individuals (Israel et al., 1997; Kayman et al., 1994; McKeating et al., 1996). Cryo-electron microscopy has revealed that the V2 loop appears at the tip of the envelope (Env) trimer (White et al., 2010; Wu et al., 2010a), and human anti-V2 monoclonal Abs (mAbs) can access the epitope as they can bind to intact virions (Nyambi et al., 1998, 2000). Both polyclonal serum Abs and human anti-V2 mAbs display immunologic cross-reactivity (Corti et al., 2010; Gorny et al., 1994; Israel et al., 1997; Shotton et al., 1995) which suggests that the V2 region contains conserved structural elements. This finding is supported by recent studies of the sequence of the V2 loop showing that approximately 75% of the residues, excluding the highly variable area just downstream from the LDV/I α4β7 binding motif, are relatively or strictly conserved (Zolla-Pazner and Cardozo, 2010). Mapping of the conformational epitope recognized by the human V2-specific mAb 697 shows that it targets residues in V2 between amino acids 164 and 194 (HxB2 numbering) (Gorny et al., 1994). This segment includes two highly conserved sequences, RDK and the area surrounding the LDV/I α4β7 binding motif, which explains the cross-reactivity of mAb 697 (Gorny et al., 1994). Structural conservation in the V2 region was further confirmed by the identification of the broadly neutralizing mAbs PG9 and PG16, both of which target a quaternary neutralizing epitope composed of portions of V2 and V3 (Walker et al., 2009). Together, the data from several sources clearly demonstrates that there are conserved structural elements and shared epitopes in the V2 loop (Changela et al., 2011; Spurrier et al., 2011; Walker et al., 2009).
V2-specific mAbs have been isolated from immunized animals and from HIV-infected humans; they display neutralizing activity with different potency and breadth (Corti et al., 2010; He et al., 2002; Kayman et al., 1994; McKeating et al., 1993; Pinter et al., 1998, 2004, 2005; Warrier et al., 1994; Wu et al., 1995). Monoclonal Abs produced from immunized animals are isolate-restricted in their neutralizing activity (McKeating et al., 1993); a chimpanzee mAb, C108g, neutralized only two viruses but with high potency (Warrier et al., 1994). The first human V2-specific mAb, 697, was found to neutralize two heterologous primary isolates and one pseudovirus (SF162) but failed to neutralize HIV-1IIIB virus (Gorny et al., 1994; He et al., 2002), while the recently developed human mAb HGP68 displayed cross-clade neutralizing activity against few Tier 1 pseudoviruses (Corti et al., 2010). The present study extends these previous analyses and is the first to characterize the immunochemical, functional and genetic aspects of a panel of seven human V2-specific mAbs generated in our laboratory. This characterization was undertaken based on the hypothesis that anti-V2 Abs may contribute to protection and therefore should be a target of prophylactic vaccines.
Results
Epitopes of anti-V2 mAbs
All seven anti-V2 mAbs (Table 1) were generated using standard cellular methods (Gorny et al., 1991) based on selection of cells reactive with one of the following antigens: oligomeric gp140451, recombinant gp120LAI or V1V2-gp70 (Gorny et al., 1994, 2000; Nyambi et al., 2000; Pinter et al., 2004). Monoclonal Ab 2297, which is described for the first time in this study, was selected with V1V2-gp70.
Table 1.
Human anti-V2 mAbs developed from the cells of HIV-1-infected individuals.
| # | mAbs | Selection | Isotype | Epitope | Subtype of the infecting virus | Reference |
|---|---|---|---|---|---|---|
| 1 | 1361a | gp140451 | IgG1 k | Discontinuous | Bb | Gorny et al. (2000) |
| 2 | 1393Aa | gp140451 | IgG1 k | Discontinuous | Bb | Nyambi et al. (2000) |
| 3 | 1357a | gp140451 | IgG1 k | Discontinuous | Bb | Gorny et al. (2000) |
| 4 | 697 | gp120LAI | IgG1λ | Discontinuous | B | Gorny et al. (1994) |
| 5 | 830A | gp120LAI | IgG3 k | Discontinuous | B | Pinter et al. (2004) |
| 6 | 2158 | V1V2-gp70 | IgG1 k | Discontinuous | B | Pinter et al. (2004) |
| 7 | 2297 | V1V2-gp70 | IgG1 λ | Discontinuous | B | This study |
Three mAbs were derived from the same subject; two of these mAbs (1361 and 1393A) are clonal as shown in Table 2.
An HIV-1 virus was isolated from this one volunteer and classified as clade B on the basis of the C2-V5 Env sequence; no viruses were isolated from the other four donors; the donors were presumably infected with clade B based on their residency in the US (New York).
The specificities of six V2 mAbs were compared to that of mAb 697 which was previously mapped using gp120HxB2 with one, two or three mutated residues in an area spanning positions 168 to 194 of the envelope region (Gorny et al., 1994). The potential epitope residues for mAb 697 are shown on the surface of the V1V2 structure recently described (McLellan et al.,2011) (Supplementary Fig. 1). All the mutated residues which abrogated binding of mAb 697 to gp120 were found to be well conserved in the V2 domain of 41 pseudoviruses tested in the TZM-bl neutralization assay. The percent of conservation of mutated residues (yellow boxes in Fig. 1) from the N- to C-terminal side is as follows: K–90%, F–95%, Y–98%, L–76%, D–100%, P–78%, I–61%, Y–98%, R–80% and L–98% (Fig. 1). Notably, two of the residues targeted, LD (179/180), are partofthe α4β7 integrin binding site expressed on activated T cells and are thought to be involved in the binding of virions to these cells (Arthos et al., 2008; Nawaz etal., 2011).
Fig. 1.
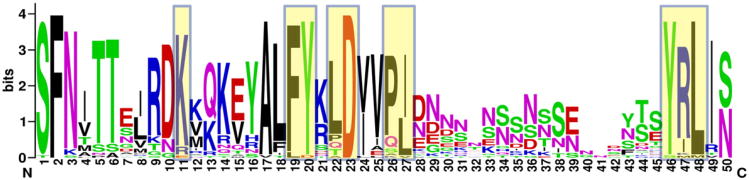
Conservation of the mAb 697 epitope in the V2 of pseudotyped viruses tested in the neutralization assay. Sequence logo was created by alignment of 41 V2 sequences from Table 5 using http://weblogo.berkeley.edu. The logo consists of stacks of letters for each position in the sequence. The overall height of the stack indicates the sequence conservation at that position, while the height of letter within the stack indicates the relative frequency of each amino acid at that position. The highly conserved V2 N- and C-terminal cysteines are not shown. The V2 residues in highlighted boxes are targeted by V2-specific mAb 697. The hypervariable region is in area spanning positions 28 to 45. Amino acids are colored according to their chemical properties: basic amino acids (K,R,H) are blue, acidic amino acids (D,E) are red, hydrophobic amino acids (A,V,L,I,P,W,F,M) are black and polar amino acids (G,S,T,Y,C,Q,N) are green.
The epitopes of other anti-V2 mAbs, with the exception of mAb 2297, are similar or overlapping the 697 epitope on the basis of competition assays. An experiment shown in Fig. 2 indicates that five anti-V2 mAbs (830A, 1357, 1361, 1393A and 2158), as well as unlabeled 697 serving as a positive control, inhibit the binding of biotinylated mAb 697 to V1V2-gp70 in a dose-response manner. Inhibition by the V2 mAbs, except 2297, was very efficient at 10 μg/ml, blocking biotinylated 697 to V1V2-gp70 in the range of 93% to 96%. In contrast, mAb 2297 was much less competitive and inhibited only 47% of the binding of biotinylated 697. Negative controls, mAb 447-52D (anti-V3) and irrelevant mAb 1418 (anti-parvovirus B19) were not reactive with V1V2-gp70 and did not compete with the V2 mAbs. In addition, all seven V2 mAbs bound to the V1V2Zm109 scaffolded protein while control mAbs, 654 (anti-CD4bs) and 1418 (anti-parvovirus B19) were not reactive (Table 2). These results indicate that six of seven V2 mAbs recognize a similar region in V2, while mAb 2297 is directed to a different, but overlapping, epitope.
Fig. 2.
Competition of V2mAbs with biotinylated anti-V2 mAb 697 for binding to a V1V2-gp70 fusion protein. Unlabeled V2 and control mAbs were titrated at final concentrations ranging from 10 to 0.01 μg/ml, while biotinylated mAb 697 was used at 0.2 μg/ml. Anti-V3 mAb 447 (447-52D) and anti-parvovirus B19 mAb 1418 were used as negative controls.
Table 2.
ELISA cross-reactivity of human anti-V2 mAbs with recombinant gp120 molecules derived from viruses from clades A, B and Ca.
| V2 mAbs | CD4bs B19 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| # | gp120s | Subb | 697 | 1393A | 830A | 2158 | 1357 | 1361 | 2297 | 654 | 1418 |
| 1 | MN | B | 4.1 | 3.9 | 3.9 | 4.0 | 4.1 | 4.0 | 4.1 | 3.4 | 0.1 |
| 2 | IIIB | B | 4.0 | 4.2 | 4.1 | 4.2 | 4.0 | 3.9 | 4.4 | 4.2 | 0.1 |
| 3 | JR-FL | B | 4.0 | 4.0 | 3.9 | 3.9 | 4.0 | 3.9 | 4.1 | 3.9 | 0.1 |
| 4 | REJO4541 | B | 3.9 | 3.8 | 3.8 | 3.9 | 4.0 | 4.0 | 4.0 | 3.8 | 0.1 |
| 5 | C.ZA1197 | C | 3.7 | 3.7 | 2.4 | 2.6 | 2.3 | 3.4 | 2.4 | 3.5 | 0.1 |
| 6 | DU172 | C | 3.6 | 3.8 | 3.7 | 3.8 | 3.7 | 3.8 | 3.7 | 3.7 | 0.1 |
| 7 | TRO | B | 3.9 | 4.0 | 0.9 | 4.1 | 3.9 | 3.9 | 4.3 | 2.1 | 0.1 |
| 8 | ZM53M | C | 3.3 | 3.6 | 3.5 | 3.4 | 3.3 | 4.0 | 1.4 | 3.5 | 0.1 |
| 9 | ZM109 | C | 3.5 | 3.5 | 3.6 | 3.6 | 3.6 | 3.8 | 1.2 | 1.3 | 0.1 |
| 10 | ZM233M | C | 3.6 | 3.7 | 3.7 | 1.8 | 1.4 | 3.9 | 0.5 | 3.5 | 0.1 |
| 11 | p1058 | B | 3.7 | 3.8 | 3.8 | 3.7 | 3.7 | 3.8 | 3.5 | 0.1 | 0.1 |
| 12 | MSC5007 | AG | 4.2 | 3.9 | 4.0 | 3.9 | 4.1 | nt | 0.2 | 3.9 | 0.1 |
| 13 | ZM197M | C | 3.5 | 3.6 | 3.6 | 3.7 | 3.6 | 4.0 | 0.4 | 3.6 | 0.1 |
| 14 | DU422 | C | 3.7 | 3.8 | 3.6 | 3.7 | 0.5 | 3.8 | 0.3 | 3.6 | 0.1 |
| 15 | CAP210 | C | 3.6 | 3.7 | 3.5 | 1.7 | 1.0 | 4.0 | 0.1 | 3.8 | 0.1 |
| 16 | DU156 | C | 3.5 | 3.5 | 0.9 | 3.6 | 0.6 | 4.1 | 0.2 | 3.2 | 0.1 |
| 17 | 89.6 | B | 4.0 | 1.4 | 0.1 | 4.4 | 1.0 | nt | 4.2 | 0.3 | 0.1 |
| 18 | KER2018 | A | 0.2 | 0.2 | 2.2 | 0.8 | 0.9 | nt | 0.1 | 3.9 | 0.1 |
| 19 | DU151 | C | 3.6 | 1.5 | 0.3 | 0.3 | 0.2 | 3.9 | 0.4 | 0.5 | 0.1 |
| 20 | PVO | B | 2.3 | 0.1 | 0.1 | 0.5 | 0.1 | 0.1 | 0.1 | 1.3 | 0.1 |
| 21 | 92RW020 | A | 0.1 | 0.2 | 1.7 | 0.2 | 0.1 | nt | 0.1 | 0.3 | 0.1 |
| Controlsc | |||||||||||
| V1V2-gp70 | B | 3.5 | 3.8 | 3.7 | 3.6 | 3.5 | 4.0 | 3.7 | 0.1 | 0.1 | |
| gp70 | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
| V1V2ZM109 | C | 3.4 | 4.2 | 4.1 | 4.5 | 3.1 | 3.9 | 0.5 | 0.1 | 0.1 | |
| BSA | - | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | |
A standard ELISA was used to determine the binding activity of seven V2 mAbs and two control mAbs, 654 (anti-CD4bs) and 1418 (anti-parvovirus B19). Monoclonal Abs were tested at concentration 10 μg/ml against gp120s, V1V2-gp70, V1V2ZM109 and BSA coated onto ELISA plate at 1 μg/ml. The numbers are O.D. values, and shading shows O.D. values: >2 (bold, dark), 0.5–2 (bold, gray); no binding <0.5 (white);
Sub–subtype of HIV-1;
Controls–include two positive controls: V1V2-gp70 fusion protein containing V1V2Case-A2 (clade B) sequence (Kayman et al., 1994) and V1V2ZM109 clade C sequence in 1FD6 scaffold (McLellan et al., 2011), and two negative controls: gp70 protein (Kayman et al., 1994) and bovine serum albumin (BSA); nt–not tested.
Cross-reactivity of V2 mAbs
Several studies have reported that polyclonal serum V2-specific Abs and mAbs are cross-reactive with recombinant and solubilized Env proteins from different viruses (Gorny et al., 1994; Israel et al., 1997; Kayman et al., 1994). The seven anti-V2 mAbs studied here were screened against 21 recombinant gp120 proteins from viruses representing subtypes A, AG, B and C (Table 2). Each V2 mAb displayed broad cross-reactivity, reacting with 12 to 19 of the 21 gp120 molecules. The most cross-reactive was mAb 697 and the least cross-reactive was mAb 2297. All V2 mAbs reacted with the positive controls, V1V2-gp70 and V1V2ZM109, but failed to react with the negative control, BSA. The control mAb 654 (anti-CD4bs) reacted with the majority of gp120s but did not bind to V1V2 fusion proteins while the irrelevant mAb 1418 did not react with any tested antigens.
Use of immunoglobulin (Ig) variable genes by anti-V2 mAbs
Previous studies by us and others show biased usage of Ig genes and gene families by anti-V3, anti-CD4i, anti-gp41 and other mAbs (Gorny et al., 2009; Huang et al., 2004; Scheid et al., 2009). Therefore, the seven anti-V2 mAbs were sequenced and analyzed to determine whether some Ig genes were similarly used in a dominant fashion by anti-V2 mAbs. The sequence analysis revealed that two of three mAbs, 1361 and 1393A, generated from one individual but at different times (6 months apart), were derived from the same B cell clone. Both mAbs use the same VH and VK genes and the CDR3 of the heavy and light chain amino acid sequences are very similar with three and two residue differences, respectively (Table 3). Analysis of all V2 mAbs showed that only two VH family genes were used, VH1 and VH4. Notably, the VH1-69 gene was particularly dominant, encoding for four of six V2 mAbs targeting the 697 epitope (mAb 2297 recognized a different epitope) including the two clonal mAbs (1361 and 1393A). Thus three of five unique mAbs used the same gene segment. This preferential gene usage suggests that the VH1-69 encoded mAbs may recognize the same V2 structural element. The light chain genes were different for each V2 mAb with the exception of the clonal mAbs, 1361 and 1393A, which used the same VK3-20 gene (Table 3).
Table 3.
Immunoglobulin gene usage by human anti-V2 mAbs.
| mAbs | IGHV | % muta | CDR H3 | H3 length AA | IGLV | % muta | CDR L3 |
|---|---|---|---|---|---|---|---|
| 1361b | 1-69 | 4.5 | ARSRDHFFDTRVFQG | 15 | K3-20 | 1.8 | QHYAGSRT |
| 1393Ab | 1-69 | 2.4 | ARSRGHYFDTRVFEG | 15 | K3-20 | 0.4 | QQYGGSRT |
| 1357b | 1-2 | 5.9 | VRRAVLTALPPRYYFDF | 17 | K1-39 | 2.9 | QQSHSTSWT |
| 697 | 1-69 | 5.9 | ATSGVGLHFGYFDY | 14 | L1-40 | 1.0 | QSYDSSLSGYV |
| 2158 | 1-69 | 8.7 | ARDKSDVVVVTSRPAYYYGMDV | 23 | K1-5 | 3.9 | QQYNSSPET |
| 830A | 4-34 | 13.0 | ARAPSGYPGVSLYQYYGLDV | 20 | K3-15 | 9.0 | QQYKHWPPYT |
| 2297 | 4-61 | 3.4 | ATHSYQLAMIRGIIMKEDAFDI | 22 | L1-51 | 1.1 | GTWDNSLSAGW |
Percentage of nucleotide mutations determined using the IMGT database (http://imgt.cines.fr).
Monoclonal Abs generated from the same HIV-1 infected donor at different time points; two of these mAbs (1361 and 1393A) are derived from one B cell clone as they use the same Ig genes and have only few different residues (bold) in the CDRs H3 and L3.
The length of CDR H3, which is most actively involved in the interaction with antigens, ranged between 14 and 23 amino acids. Three mAbs, 830A, 2297 and 2158, had unusually long CDR H3 regions of 20, 22 and 23 amino acids, respectively.
Mutations in the VH and VL genes, which reflect the differences in the nucleotide sequence compared to germline, were in the range of 2.4% to 13.0% (mean 6.2%) for the VH genes, and 0.4% to 9.0% (mean 2.9%) for the VL genes (Table 3). The rate of the heavy chain mutation is relatively low compared to broadly cross-neutralizing mAbs: 19% and 21% for mAbs PG9 and PG16, respectively, specific to quaternary epitopes (Walker et al., 2009) and >30% for mAbs VRC01, VRC02 and VRC03 against CD4 binding site (Wu et al., 2010b).
Crystal structure of Fab 697's antigen-binding site
We further characterized V2 mAb 697 by crystallizing its Fab and determining the crystal structure to 2.5 Å resolution (Fig. 3 and Supplementary Fig. 2). The antigen-binding site of 697 is relatively flat and neutral in charge (Figs. 3A and B). This is very different from all the anti-V3 mAbs we have studied, which almost always have the antigen binding site negatively charged with a binding pocket (Burke et al., 2009; Jiang et al., 2010). Optical docking area (ODA) analyzed by molecular modeling using the software ICM (Molsoft LLC, La Jolla, CA) (Abagyan et al., 1994) predicted that the CDR H2 and H3 loops of the heavy chain likely play a dominant role in interaction with the antigen (Fig. 3C). The antigen binding site consists of roughly two distinct regions: (1) a shallow groove running across the antigen binding site between the heavy and light chains and formed by large aromatic residues which can accommodate residues with large side chains, such as Tyr, in the epitope identified by previous functional studies (Gorny et al., 1994) and (2) a convex hydrophobic surface comprised of a cluster of CDR H2/H3 residues (Fig. 3D).
Fig. 3.
Structural characteristics of the antigen-binding site of mAb 697. (A) A side view of the crystal structure of Fab 697 (only the Fv region is shown). The frame regions of the light chain and heavy chain are colored cyan and green, respectively, while CDR loops (Kabat definition) are colored individually. (B) The electrostatic potential is displayed on the surface of the antigen-binding site viewed from the top (same view for panels C and D). Note the relatively flat and hydrophobic surfaces of the antigen-binding site. (C) Optical docking area (ODA) analysis of Fab 697. This analysis uses desolvation energy to predict sites of antigen–antibody interactions, and spheres are placed at the surface. The redness and size of the spheres are proportional to the region's likelihood to be involved in antigen–antibody interactions. ODA analysis suggested that the heavy chain plays a dominant role in antigen binding and hot spots of binding are concentrated at CDRs H2 and H3. (D) Residues identified by ODA analysis that may play key roles in antigen binding. Note the hydrophobic cluster of residues from CDRs H2 and H3.
The V1V2 structure, in the context of a scaffold and mAb PG9, folds as four anti-parallel beta strands according to recently published data (McLellan et al., 2011) and is similar to the bridging sheet region targeted by anti-CD4i antibodies. The majority of anti-CD4i mAbs are encoded by the VH1-69 gene (Gorny et al., 2009; Huang et al., 2004) and two of these mAbs, 412d and 17b, have been compared with mAb 697 (Supplementary Figs. 3 and 4). The amino acid sequence of 697 CDR H2 is highly homologous with the corresponding domain of 412d and 17b and, like these anti-CD4i mAbs, its hydrophobic residues may play a similar role in its interaction with the V2 epitope consistent with the ODA prediction (Kwong et al., 1998) (Supplementary Fig. 4). Structural alignments of the CDRs H1 and H2 backbones of the 697 heavy chain (excluding CDR H3) with that of 412d and 17b showed a pairwise root mean square deviation (RMSD) of 1.02 Å and 0.67 Å, respectively, which indicate a close superimposition, while light chain L1 and L2 show an RMSD of 1.4 Å and 1.7 Å, respectively, as they are encoded by different VL genes. Generally the antigen binding sites of the three Fabs have similar backbone conformations except CDR H3 (Supplementary Fig. 3). Furthermore, since both 412d and 17b mAbs bind to one side of a four stranded bridging sheet (Supplementary Fig. 5), mAb 697 may use a similar binding mode consistent with its relatively flat antigen binding surface (Fig. 3B).
Neutralization of pseudoviruses (psVs)
The anti-V2 mAbs were tested for their ability to neutralize a panel of 41 cloned pseudoviruses (psVs) using the TZM-bl cell assay. The panel included 15 neutralization-sensitive Tier 1 psVs (subtype A, AG, B, and C), 22 moderately resistant Tier 2 psVs (subtypes B and C) and four resistant Tier 3 psVs (subtype B) (Table 4) (Seaman et al., 2010). Generally, the neutralizing activity of V2 mAbs is weak and limited to neutralization-sensitive psVs. Only four to eight Tier 1 psVs of 41 tested were neutralized by V2 mAbs with the exception of mAb 2297 which showed no neutralizing activity. The IC50 values which express the concentration (μg/ml) of mAb needed to achieve 50% neutralization showed a broad range between <0.4 and 88.7 μg/ml.
Table 4.
Neutralization of pseudotyped viruses by human anti-V2 mAbsa.
| Virus | Tier | clade | 1361 | 1393A | 2158 | 697 | 1357 | 830A | 2297 | 1418 |
|---|---|---|---|---|---|---|---|---|---|---|
| MW965.26 | 1A | C | <0.4 | 0.8 | 0.8 | 1.8 | 0.8 | <0.4 | >50 | >50 |
| SF162.LS | 1A | B | 5.4 | 12.5 | 6.4 | 19.9 | 33.5 | 6.2 | >50 | >50 |
| DJ263.8 | 1B | A | 11.8 | 11.1 | 9.1 | 38.6 | 29.2 | 7.7 | >50 | >50 |
| BaL.26 | 1B | B | 5.8 | 8.6 | 7.3 | 20.2 | 7.9 | 36.1 | >50 | >50 |
| Bx08.16 | 1B | B | 4.8 | 7.2 | 7.9 | 11.2 | 9.5 | >50 | >50 | >50 |
| SS1196.1 | 1B | B | 23.8 | 88.7 | 27.6 | 16.8 | >100 | >50 | >50 | >50 |
| HXB2.DG | 1B | B | 18.6 | 19.8 | 17.8 | >100 | 36.9 | >50 | >50 | >50 |
| 6535.3 | 1B | B | 12.1 | 18.5 | >100 | >100 | >100 | >50 | >50 | >50 |
| BZ167.12 | 1B | B | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| HO29.12 | 1B | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| ZM109F.PB4 | 1B | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| ZM197M.PB7 | 1B | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| 25710-2.43 | 1B | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| 242-14 | 1B | AG | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| 271-11 | 1B | AG | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| HO30.7 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| HO31.7 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| QH0692.42 | 2 | B | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| REJO4541.67 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| RHPA4259.7 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| WITO4160.33 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| SC422661.8 | 2 | B | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| TRO.11 | 2 | B | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| AC10.0.29 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| THRO4156.18 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| CAAN5342.A2 | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| JRFLJB | 2 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| CAP45.2.00.G3 | 2 | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| CAP210.2.00.E8 | 2 | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| Du156.12 | 2 | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| Du172.17 | 2 | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| Du422.1 | 2 | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| ZM53M.PB12 | 2 | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| ZM135M.PL10 | 2 | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| ZM214M.PL15 | 2 | C | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| ZM233M.PB6 | 2 | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| ZM249M.PL1 | 2 | C | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| HO35.18 | 3 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| HO61.14 | 3 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
| PVO.4 | 3 | B | >50 | >100 | >100 | >100 | >100 | >50 | >50 | >50 |
| TRJO4551.58 | 3 | B | >50 | >100 | >100 | >100 | nt | >50 | >50 | >50 |
Neutralization of psVs was performed using the TZM-bl cell assay with mAbs titrated from maximum concentrations of either 50 or 100 μg/ml. The numbers above represent the IC values and are color-coded from the most potent (red), to medium (orange), to weak (yellow) neutralization. Monoclonal Ab 1418 was included as negative control. nt–not tested. 50
Dose–response curves for the six V2 mAbs with neutralizing activity are shown for each of the eight anti-V2 mAb-sensitive pseudoviruses (Fig. 4). These curves are generally parallel and overlapping indicating, again, that the V2 mAbs recognize similar or identical epi-topes. Monoclonal Ab 1357, from the same individual whose cells generated the clonal mAbs 1361 and 1393A, has comparable activity except that it does not neutralize pseudoviruses 6535.3 and SS1196.1, suggesting that its epitope is slightly different (Fig. 4, Table 4).
Fig. 4.
Neutralization of eight Tier 1 pseudoviruses by anti-V2 mAbs using the TZM-bl cell assay. The IC50 values for these mAbs are shown in Table 4. The closed symbols indicate three mAbs derived from one infected individual, including two clonal mAbs 1361 and 1393A. Human mAb 1418, specific for parvovirus B19, was used as a negative control.
Length and N-linked glycosylation sites in V2 domain
The V2 sequences of all 41 tested pseudoviruses were aligned (Table 5) to analyze the length and the presence of N-linked glycosylation sites in order to test whether these characteristics correlated with neutralization sensitivity. The V2 loops studied here are shown to vary in length between 35 and 48 amino acids (Table 5). For the neutralization-sensitive psVs, the V2 lengths varied between 38 and 41 amino acids, with a mean length of 38.5. In contrast, the V2 domains of the neutralization-resistant psVs was significantly longer and varied between 35 and 48 amino acids, with a mean length of 41.3 (p=0.0292).
Table 5.
Sequence alignment of the V2 domain of 41 pseudotyped viruses tested in the TZM-bl neutralization assaya.
Alignment and potential N-linked glycosylation sites (red) were determined using clustal X program and N-glycosite program from Los Alamos HIV-1 Database;
Number of amino acids (AA) in the V2 domain;
N–number of potential N-linked glycosylation sites;
Underlined residues (black) are involved in the 697 epitope.
To determine the number of potential N-linked glycosylation sites within V2, the “N-glycosite” program provided by the Los Alamos HIV-1 Database was used (www.hiv.lanl.gov). There was an increased number of V2 glycosylation sites in resistant psVs (2.42) compared to sensitive psVs (1.88), however, the difference did not reach statistical significance (p = 0.0695).
Generally, according to published studies, a longer V2 region is accompanied by an increased number of N-linked glycosylation sites (Owens et al., 2007; Sagar et al., 2006; van Gils et al., 2011). As shown in Fig. 5, there is a significant correlation between the length and number of N-linked glycosylation sites in 41 psVs tested (p<0.0001) and the sensitive viruses form a cluster with shorter lengths and fewer glycosylation sites. The additional glycosylation sites are included mainly in the hypervariable fragment of the V2 domain which is responsible for the varied length of V2 (Fig. 1, Table 5).
Fig. 5.
Correlation between the length of the V2 domain and the number of potential N-linked glycosylation sites. The potential N-linked glycosylation sites in the V2 domains of 41 psVs were determined using the N-glycosite program from Los Alamos HIV-1 database. Statistical analysis was determined using GraphPad Prism, version 4.01, for Windows. Solid points (●), psVs sensitive to neutralization; open circles (○), psVs resistant to neutralization.
These results suggest that the resistance of some pseudoviruses to neutralization by anti-V2 mAbs may depend on masking of the corresponding epitopes by glycans associated with longer V2 domains. In order to exclude the possibility that a lack of V2 epitopes in the virus envelope is the reason for resistance, we compared the neutralization and binding of V2 mAbs to gp120 derived from the pseudoviruses tested in the neutralization assay. Twelve pairs of neutralization (Table 4) and binding data (Table 2) were analyzed and all 12 gp120s were recognized by the majority of V2 mAbs but, none of the corresponding pseudoviruses were neutralized by these mAbs (Supplementary Table 2). It indicates that the epitopes for the anti-V2 mAbs were present in the envelope of the pseudoviruses but they were still resistant to neutralization, possibly due to the masking effect of carbohydrates on the virus surface.
Discussion
The V2 region of gp120 has long been known to induce Abs in HIV-infected individuals, but only a few human V2-specific mAbs have been described (Corti et al., 2010; Gorny et al., 1994, 2000; He et al., 2002; Nyambi et al., 2000; Pinter et al., 2004), and only one has been characterized in detail (Gorny et al., 1994). Therefore, the goal of this study was to fully characterize a panel of human V2-specific mAbs in terms of specificity, immunogenetics and function, particularly in the context of the RV144 vaccine data which showed the protective ability of the V2 antibodies (www.hivvaccineenterprise.org/conference/2011/webcasting).
The most interesting element of the presented studies is the immunochemical and functional cross-reactivity of the V2 mAbs (Table 2 and (Corti et al., 2010; Gorny et al., 1994)) which demonstrates that these mAbs recognize conserved immunologic features in V2, despite its sequence variability. A competition ELISA of a V2 mAb panel against mAb 697, whose epitope was previously mapped (Fig. 1) (Gorny et al., 1994), showed that all but one target the same epitope recognized by mAb 697,orvery closely overlappingV2 epitopes. The one exception was mAb 2297 which competed weakly with mAb 697, suggesting that the 2297 epitope is different, but still overlaps that of the other V2 mAbs. The 697 epitope is conserved and its residues are present in a high percentage of the sequences of the 41 psVs tested and analyzed here (Fig. 1), as well as in the larger panel of V2 sequences previously studied (Zolla-Pazner and Cardozo, 2010). The epitope includes two residues contained in the highly conserved LDV/I motif, which is the binding site for the α4β7 integrin, expressed on activated T cells (Arthos et al., 2008). Presumably, these anti-V2 mAbs may block binding of HIV-1 to integrin which is supposed to be a prior step to the binding of the virus to CD4 receptor.
The finding that six of seven anti-V2 mAbs target similar epitopes may be related to the observed preferential usage of the VH1-69 gene by these mAbs; four of these six mAbs (or three of five if two clonal mAbs are counted as one unique mAb) are encoded by this VH gene. Interestingly, mAb 2297, which targets a more distant epitope, is not encoded by this gene. Preferential Ig gene usage by Abs specific for particular epitopes is now well established (Gorny et al., 2009; Huang et al., 2004; Scheid et al., 2009). For example, human anti-V3 mAbs which preferentially use VH5-51 and VL lambda genes target one conserved cluster of epitopes in the crown of the V3 loop as determined by crystallographic studies (Gorny et al., 2009, 2011). Similarly, V2 mAbs encoded by the VH1-69 gene paired with VK genes recognize a closely overlapping cluster of epitopes which have a conserved character and near identical structures.
VH1-69 is frequently utilized by anti-CD4i and anti-gp41 mAbs (Gorny et al., 2009; Huang et al., 2004) as well as by anti-influenza HA mAbs (Ekiert et al., 2009; Luftig et al., 2006). It is characteristic for these mAbs that CDR H2 displayed a particular interaction of hydrophobic residues at the tip of the CDR H2 domain with helical elements of the HA and gp41 antigens (Ekiert et al., 2009; Luftig et al., 2006) or with the hydrophobic four-stranded β-sheet in the case of anti-CD4i mAbs 412d and 17b (Huang et al., 2007; Kwong et al., 1998). It was recently reported that the V2 domain has, also, the four-stranded β-sheet (McLellan et al., 2011) and possibly it has a selective effect on VH1-69 gene usage by 3 of 5 anti-V2 mAbs. Thus, structural features of either the helical or beta sheet antigens containing the hydrophobic residues interact with similarly charged residues of the CDR H2 mAbs encoded by the VH1-69 genes. The preferential use of VH1-69 suggests that conserved epitopes in V2 best conform to the Ab binding site encoded by this particular gene. Data of this nature, elucidating conserved structures in sequence-variable epitopes, can be useful in the development of rationally-designed immunogens that induce cross-reactive, functional Abs (Moseri et al., 2010; Zolla-Pazner et al., 2011b).
Final evidence that anti-V2 mAbs recognize conserved epitopes is provided by the binding assay which showed extensive cross-reactivity of these mAbs with various recombinant gp120s representing the sequences of clades A, AG, B and C HIV-1. The epitopes of anti-V2 serum antibodies from RV144 vaccines have linear characteristics and overlap with the conserved epitopes of the V2 mAbs (www.hivvaccineenterprise.org/conference/2011/webcasting). These data support the observation that a high titer of serum anti-V2 Abs is correlated with the decreased risk of HIV-1 infection in RV144 vaccines, as only cross-reactive Abs can contribute to protection.
The mechanism of protection mediated by plasma anti-V2 Abs is unknown but is not dependent on their neutralizing activity as no such correlation was found in the RV144 vaccines (www.hivvaccineenterprise.org/conference/2011/webcasting). The neutralizing activity of V2 mAbs is weak, as shown here, possibly resulting from the presence of N-linked glycosylation which may mask the V2 domain on the virus surface as well as the V3 and CD4-binding sites. Analysis of V2 sequences revealed that resistant pseudoviruses displayed a significantly increased number of residues in the V2 domain which is accompanied by an increased number of N-linked glycosylation sites. Other factors also contribute to the phenomenon of resistance, as only a few Tier 2 and Tier 3 psVs, with short V2 domains and a low number of N-linked glycosylation sites, were not neutralized by V2 mAbs (Table 5 and Fig. 5).
The alternate hypothesis is that V2 mAbs may block virus binding to α4β7 integrin, as expressed on activated T cells. The LDV/I motif of integrin binding site in the V2 domain is involved in the epitope for V2 mAbs and plasma V2 Abs from RV144 vaccines (www.hivvaccineenterprise.org/conference/2011/webcasting) (Karasavvas et al., 2011; Zolla-Pazner et al., 2011a) and thus they may mediate an inhibitory function, by blocking viral access to integrin. This is supported by recently published data which showed that anti-V2 mAb 697 inhibits gp120 binding to Vγ2Vσ2 T cells which express high levels of both α4β7 and CCR5 in the absence of CD4 expression (Li and Pauza, 2011). Another experiment showed, also, that anti-V2 mAbs 697 and 2158 block the binding of gp120 to α4β7 integrin which was expressed on the surface of retinoic-stimulated CD8 cells (Mary A. Marovitch, unpublished data).
This additional inhibitory function of V2 mAbs was not measured in the neutralization assay used in the study because the TZM-bl target cells do not express α4β7 integrin. Thus, in the TZM-bl cell assay, the anti-V2 mAbs mediate neutralization by affecting the co-receptor binding site which is formed by the stem of the V1V2, V3 and the bridging sheet upon virus binding to the CD4 receptor (Kwong et al., 1998; Rizzuto et al., 1998; Wu et al., 1996). Evidently, this mechanism of neutralization, which has a post-binding character exhibited after the virus binds to cellular CD4, is responsible for the neutralizing activity of anti-V2 mAbs in the TZM-bl cell assay. The blocking of virus binding to α4β7 integrin by anti-V2 Abs would precede the binding of the virus to CD4 receptor.
In summary, the study presented in this paper is the first to characterize a panel of seven V2-specific human mAbs. We show that these mAbs target a conserved cluster of epitopes which include the α4β7 integrin binding site. Most of them are encoded by VH1-69 and are cross-reactive with various gp120s, suggesting that they may recognize identical or similar epitopes. Their neutralizing activity is weak and limited to neutralization-sensitive pseudotyped viruses, suggesting that this function alone may not have contributed to the protection which was mediated by plasma anti-V2 antibodies detected in the RV144 vaccines. We hypothesize that the protective function of plasma V2 Abs may depend on their ability to block virus binding to the α4β7 integrin, and possibly, along with the neutralizing activity. Given that V2-specific antibodies mediate anti-viral functions, including potentially inhibitory activity, their elicitation with a vaccine may contribute to protection. The elicitation of such V2-specific Abs may be facilitated by the rational design of immunogens that focus the Ab response on the conserved V2 structures whose existence is strongly implied by the data presented above.
Materials and methods
Human monoclonal antibodies
Ten human mAbs, produced in our laboratory using cellular methods as described (Gorny et al., 1991), were used; these included seven mAbs specific for V2 (697, 830A, 1357, 1361, 1393A, 2158, and 2297) (Gorny et al., 1994, 2000; Nyambi et al., 2000; Pinter et al., 2004) (see Table 1), one specific for the CD4 binding site (CD4bs, mAb 654) (Nyambi et al., 1998), one specific for V3 (mAb 447-52D) (Gorny et al., 1993), and one specific for parvovirus B19 (mAb 1418) (Gigler et al., 1999). Monoclonal Ab 2297, a new anti-V2 mAb, is described above.
This study has been reviewed and approved by the New York University School of Medicine Institutional Review Board.
Recombinant HIV-1 proteins
Nineteen recombinant gp120s were purchased from Immune Technology Corp. (www.immune-tech.com); gp120MN and gp120IIIB were purchased from Immunodiagnostics, Inc. (Woburn, MA). Fusion protein V1V2Case-A2-gp70 (V1V2-gp70) with clade B sequence and gp70 protein were provided by Dr. Abraham Pinter (Kayman et al., 1994) and V1V2ZM109 clade C sequence (residues 126–196) in 1FD6 scaffold was provided by Dr. Peter D. Kwong (McLellan et al., 2011),
ELISA
A standard ELISA was used to determine the binding of anti-V2 mAbs to various recombinant gp120s as previously described (Gorny et al., 1997). Briefly, gp120 proteins were coated directly onto ELISA plates, blocked with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS), and incubated with mAbs at a concentration 10 μg/ml or at concentrations ranging from 10 to 0.01 μg/ml. The plates were washed, and the bound mAbs were detected by incubation with alkaline phosphatase-conjugated goat anti-human IgG (Fc) (Southern Biotech, Birmingham, AL). After washing, the substrate p-nitrophenyl phosphate was added and the plates were read at 410 nm.
The epitopes of the anti-V2 mAbs were determined by a competition ELISA in which unlabeled V2 mAbs competed with biotinylated V2-specific mAb 697 for binding to V1V2-gp70, as previously described (Gorny et al., 2002). Briefly, unlabeled anti-V2 or control mAbs at final concentrations ranging from 10 to 0.01 μg/ml were mixed at a 1:1 ratio with biotinylated anti-V2 mAb 697 at a concentration of 0.2 μg/ml. Binding of biotinylated mAb 697 to V1V2-gp70 was detected using alkaline phosphatase-conjugated streptavidine followed by incubation with p-nitrophenyl phosphate substrate.
Sequencing the immunoglobulin (Ig) variable fragment for heavy and light chains
Sequencing of the Ig variable genes was performed as described (Gorny et al., 2009). Briefly, the messenger RNA was extracted from the hybridoma cell lines and reverse transcribed into cDNA using an oligo dT primer. Amplification of the variable fragment of the heavy chains (VH) was performed by PCR using gene family-specific primers sets with a cDNA template (Gorny et al., 2009; Marks et al., 1991). The variable domains of the light chain genes (VL) were amplified from poly-C tailed cDNA by PCR using deoxyinosine-containing anchor primer as a forward primer (5′RACE Abridged Anchor primer, 5′-GGC CAC GCG TCG ACT AGT ACG GGI IGG GII GGG IIG-3′) (Invitrogen) and gene-specific primer as the reverse primer, located in the constant region of κ (5′-AAC ACT CWY YCC TGT TGA AGC TCT T-3′) or λ (5′-CAC TGT CTT CTC CAC GGT GCT CCC TTC-3′). PCR products for both the VH and VL genes were separately cloned into 2.1-TOPO TA cloning vectors (Invitrogen). For each chain, 6 to 12 colonies were screened. Plasmids with the appropriate inserts were sequenced in both directions using the M13 primers. All sequencing reactions were performed by Macrogen in Rockville, MD. The sequence data were analyzed using Pregap4, BioEdit software and the International ImMunoGene Tics (IMGT) information system (http://imgt.cines.fr).
Neutralization assay with TZM.bl target cells
The seven V2-specific human mAbs V2 and parvovirus B19-specific mAb 1418, used as a negative control, were tested for neutralizing activity against 41 pseudoviruses (psVs) using the TZM-bl cell line as target cells. The psVs carried Envs from clades A, AG, B and C and included two psVs classified as Tier 1A, 13 from Tier 1B, 21 from Tier 2, four from Tier 3, and one classified as “chronic” (Hioe et al., 2010; Li et al., 2005; Seaman et al., 2010). Monoclonal Abs were tested with seven 2-fold serial dilutions starting at concentrations of 50 or 100 μg/ml. Each mAb dilution was pre-incubated with each psV, and subsequently each mAb/psV mixture was incubated 48 h with TZM-bl cells expressing CD4, CXCR4 and CCR5. Pseudovirus infectivity was determined by measuring the luciferase activity in the cell lysates. The reduction of infectivity was expressed as percent neutralization by comparing the enzyme activity, as relative light units, in the presence of mAbs versus absence of mAbs (Hioe et al., 2010; Seaman et al., 2010).
HIV-1 sequences
The sequences of the 41 psVs were aligned using Clustal X program. Potential N-linked glycosylation sites were determined using N-glycosite program from Los Alamos HIV-1 database.
Crystallization and structure determination of Fab 697
Fab of mAb 697 was generated by papain digestion and purified using size exclusion chromatography (Burke et al., 2009). Welldiffracting crystals of Fab 697 were obtained in a solution of 18% polyethylene glycol 8000 and 0.1 M Tris pH 8.5 by the vapor diffusion hanging drop method. Selected crystals were briefly soaked in their respective mother liquor with additional 20% glycerol (v/v) added prior to mounting onto the X-ray beam. Data were collected at the synchrotron beamline GM/CA-CAT of Advanced Photo Source, Ar-gonne National Laboratory. The data set was processed using the HKL2000 package (Otwinowski and Minor, 1997), and the structure was solved by molecular replacement using MOLREP (Vagin and Teplyakov, 1998) in the CCP4 software package with the structure of mAb m396 (PDB: 2G75) as the starting model. Cycles of refinement were carried out using COOT (Emsley and Cowtan, 2004) and Phenix (Adams et al., 2002). Final structural analysis was performed using the ICM molecular modeling software package (Molsoft LLC, La Jolla, CA) (Abagyan et al., 1994) and the figures were created using ICM and PyMOL (DeLano, 2002).
Statistical analysis
The Student's t tests and correlation analysis were performed with GraphPad Prism, version 4.01, for Windows (GraphPad software, San Diego, CA).
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants AI077451, HL59725, AI082274 and AI084119, by the Immunology Core of the NYU Center for AIDS Research (NIH grant AI27742), and by research funds from the Bill and Melinda Gates Foundation and the Department of Veterans Affairs. B.S. is supported by NIH training grant GM088118. The authors thank Dr. Abraham Pinter for providing the V1V2-gp70 fusion protein and gp70 protein, and Drs. Marie Pancera, Jason McLellan and Peter D. Kwong for the V1V2ZM109 scaffolded protein.
Footnotes
Appendix A. Supplementary data: Supplementary data to this article can be found online at doi:10. 1016/j.virol.2012.02.003.
Contributor Information
Miroslaw K. Gorny, Email: mirek.gorny@med.nyu.edu.
Ruimin Pan, Email: ruimin.pan@nyumc.org.
Constance Williams, Email: constance.williams@nyumc.org.
Xiao-Hong Wang, Email: xiao-hong.wang@nyumc.org.
Barbara Volsky, Email: barbara.volsky@nyumc.org.
Timothy O'Neal, Email: timothy.oneal@nyumc.org.
Brett Spurrier, Email: brett.spurrier@med.nyu.edu.
Jared M. Sampson, Email: jared.sampson@nyumc.org.
Liuzhe Li, Email: liliuzhe@yahoo.com.
Michael S. Seaman, Email: mseaman@bidmc.harvard.edu.
Xiang-Peng Kong, Email: kong@saturn.med.nyu.edu.
Susan Zolla-Pazner, Email: susan.zolla-pazner@nyumc.org.
References
- Abagyan R, Totrov M, Kuznetsov D. ICM–a new method for protein modeling and design: Applications to docking and structure prediction from the distorted native conformation. J Comput Chem. 1994;15(5):488–506. [Google Scholar]
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9(3):301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- Burke V, Williams C, Sukumaran M, Kim SS, Li H, Wang XH, Gorny MK, Zolla-Pazner S, Kong XP. Structural basis of the cross-reactivity of genetically related human anti-HIV-1 monoclonal antibodies: implications for design of V3-based immunogens. Structure. 2009;17(11):1538–1546. doi: 10.1016/j.str.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changela A, Wu X, Yang Y, Zhang B, Zhu J, O'Dell S, Pancera M, Gorny MK, Phogat SK, Robinson JE, Stamatatos L, Zolla-Pazner S, Mascola JR, Kwong PD. Crystal structure of human antibody 2909 reveals conserved features of quaternary-specific antibodies that potentially neutralize HIV-1. J Virol. 2011;85(6):2524–2535. doi: 10.1128/JVI.02335-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Langedijk JP, Hinz A, Seaman MS, Vanzetta F, Fernandez-Rodriguez BM, Silacci C, Pinna D, Jarrossay D, Balla-Jhagjhoorsingh S, Willems B, Zekveld MJ, Dreja H, O'Sullivan E, Pade C, Orkin C, Jeffs SA, Montefiori DC, Davis D, Weissenhorn W, McKnight A, Heeney JL, Sallusto F, Sattentau QJ, Weiss RA, Lanzavecchia A. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One. 2010;5(1):e8805. doi: 10.1371/journal.pone.0008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL User's Manual. DeLano Scientific; Palo Alto: 2002. [Google Scholar]
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Gigler A, Dorsch S, Hemauer A, Williams C, Kim S, Young NS, Zolla-Pazner S, Wolf H, Gorny MK, Modrow S. Generation of neutralizing human monoclonal antibodies against parvovirus B19 proteins. J Virol. 1999;73(3):1974–1979. doi: 10.1128/jvi.73.3.1974-1979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Xu JY, Gianakakos V, Karwowska S, Williams C, Sheppard HW, Hanson CV, Zolla-Pazner S. Production of site-selected neutralizing human monoclonal antibodies against the third variable domain of the HIV-1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1991;88:3238–3242. doi: 10.1073/pnas.88.8.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Xu JY, Karwowska S, Buchbinder A, Zolla-Pazner S. Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol. 1993;150(2):635–643. [PubMed] [Google Scholar]
- Gorny MK, Moore JP, Conley AJ, Karwowska S, Sodroski J, Williams C, Burda S, Boots LJ, Zolla-Pazner S. Human anti-V2 monoclonal antibody that neutralizes primary but not laboratory isolates of HIV-1. J Virol. 1994;68:8312–8320. doi: 10.1128/jvi.68.12.8312-8320.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, VanCott TC, Hioe C, Israel ZR, Michael NL, Conley AJ, Williams C, Kessler JA, 2nd, Chigurupati P, Burda S, Zolla-Pazner S. Human monoclonal antibodies to the V3 loop of HIV-1 with intra- and interclade cross-reactivity. J Immunol. 1997;159(10):5114–5122. [PubMed] [Google Scholar]
- Gorny MK, VanCott TC, Williams C, Revesz K, Zolla-Pazner S. Effects of oligomerization on the epitopes of the Human Immunodeficiency Virus Type 1 envelope glycoproteins. Virology. 2000;267:220–228. doi: 10.1006/viro.1999.0095. [DOI] [PubMed] [Google Scholar]
- Gorny MK, Williams C, Volsky B, Revesz K, Cohen S, Polonis VR, Honnen WJ, Kayman SC, Krachmarov CP, Pinter A, Zolla-Pazner S. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize HIV-1 primary isolates from various clades. J Virol. 2002;76(18):9035–9045. doi: 10.1128/JVI.76.18.9035-9045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Wang XH, Williams C, Volsky B, Revesz K, Witover B, Burda S, Urbanski M, Nyambi P, Krachmarov C, Pinter A, Zolla-Pazner S, Nadas A. Preferential use of the VH5-51 gene segment by the human immune response to code for antibodies against the V3 domain of HIV-1. Mol Immunol. 2009;46(5):917–926. doi: 10.1016/j.molimm.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang XH, Williams C, O'Neal T, Volsky B, Li L, Cardozo T, Nyambi P, Zolla-Pazner S, Kong XP. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure. PLoS One. 2011;6(12):e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Honnen WJ, Krachmarov CP, Burkhart M, Kayman SC, Corvalan J, Pinter A. Efficient isolation of novel human monoclonal antibodies with neutralizing activity against HIV-1 from transgenic mice expressing human Ig loci. J Immunol. 2002;169(1):595–605. doi: 10.4049/jimmunol.169.1.595. [DOI] [PubMed] [Google Scholar]
- Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, Williams C, Gorny MK, Zolla-Pazner S. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PLoS One. 2010;5(4):e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Venturi M, Majeed S, Moore MJ, Phogat S, Zhang MY, Dimitrov DS, Hendrickson WA, Robinson J, Sodroski J, Wyatt R, Choe H, Farzan M, Kwong PD. Structural basis of tyrosine sulfation and VH-gene usage in antibodies that recognize the HIV type 1 coreceptor-binding site on gp120. Proc Natl Acad Sci U S A. 2004;101(9):2706–2711. doi: 10.1073/pnas.0308527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lam SN, Acharya P, Tang M, Xiang SH, Hussan SS, Stanfield RL, Robinson J, Sodroski J, Wilson IA, Wyatt R, Bewley CA, Kwong PD. Structures of the CCR5 N terminus and of a tyrosine-sulfated antibody with HIV-1 gp120 and CD4. Science. 2007;317(5846):1930–1934. doi: 10.1126/science.1145373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel ZR, Gorny MK, Palmer C, McKeating JA, Zolla-Pazner S. Prevalence of a V2 epitope in clade B primary isolates and its recognition by sera from HIV-1 infected individuals. AIDS. 1997;11(1):128–130. [PubMed] [Google Scholar]
- Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, Zolla-Pazner S, Kong XP. Conserved structural elements in the V3 crown of HIV-1 GP120. Nat Struct Mol Biol. 2010;17(8):955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- Karasavvas N, Billings E, Rao M, Currier J, Michael N, Rerks-Ngarm S, Pitisuttithum P, Kaewkungwal J, Ngauy V, Nitayaphan S, Madnote S, Arworn D, Kim J, de Souza M. The Thai phase iii clinical trial (RV144) induces the generation of antibodies that target a conserved region within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2011;27:A–29. [Google Scholar]
- Kayman SC, Wu Z, Revesz K, Chen H, Kopelman R, Pinter A. Presentation of native epitopes in the V1/V2 and V3 regions of human immunodeficiency virus type 1 gp120 by fusion glycoproteins containing isolated gp120 domains. J Virol. 1994;68(1):400–410. doi: 10.1128/jvi.68.1.400-410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Pauza CD. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood. 2011;118(22):5824–5831. doi: 10.1182/blood-2011-05-356535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human Immunodeficiency Virus Type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig MA, Mattu M, Di Giovine P, Geleziunas R, Hrin R, Barbato G, Bianchi E, Miller MD, Pessi A, Carfi A. Structural basis for HIV-1 neutralization by a gp41 fusion intermediate-directed antibody. Nat Struct Mol Biol. 2006;13(8):740–747. doi: 10.1038/nsmb1127. [DOI] [PubMed] [Google Scholar]
- Marks JD, Tristem M, Karpas A, Winter G. Oligonucleotide primers for polymerase chain reaction amplification of human immunoglobulin variable genes and design of family-specific oligonucleotide probes. Eur J Immunol. 1991;21(4):985–991. doi: 10.1002/eji.1830210419. [DOI] [PubMed] [Google Scholar]
- McKeating JA, Shotton C, Cordell J, Graham S, Balfe P, Sullivan N, Charles M, Page M, Bolmstedt A, Olofsson S, Kayman SC, Wu Z, Pinter A, Dean C, Sodroski J, Weiss RA. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1993;67:4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating JA, Shotton C, Jeffs S, Palmer C, Hammond A, Lewis J, Oliver K, May J, Balfe P. Immunogenicity of full length and truncated forms of the human immunodeficiency virus type I envelope glycoprotein. Immunol Lett. 1996;51:101–105. doi: 10.1016/0165-2478(96)02562-x. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O'Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang ZY, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang LX, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480(7377):336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseri A, Tantry S, Sagi Y, Arshava B, Naider F, Anglister J. An optimally constrained V3 peptide is a better immunogen than its linear homolog or HIV-1 gp120. Virology. 2010;401(2):293–304. doi: 10.1016/j.virol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. The genotype of earlytransmitting HIV gp120s promotes alphabeta-reactivity, revealing alphabetaCD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 2011;7(2):e1001301. doi: 10.1371/journal.ppat.1001301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Gorny MK, Bastiani L, van der Groen G, Williams C, Zolla-Pazner S. Mapping of epitopes exposed on intact human immunodeficiency virus type 1 (HIV-1) virions: a new strategy for studying the immunologic relatedness of HIV-1. J Virol. 1998;72(11):9384–9391. doi: 10.1128/jvi.72.11.9384-9391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyambi PN, Mbah HA, Burda S, Williams C, Gorny MK, Nadas A, Zolla-Pazner S. Conserved and exposed epitopes on intact, native, primary human immunodeficiency virus type 1 virions of group M. J Virol. 2000;74(15):7096–7107. doi: 10.1128/jvi.74.15.7096-7107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276A:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Owens GP, Winges KM, Ritchie AM, Edwards S, Burgoon MP, Lehnhoff L, Nielsen K, Corboy J, Gilden DH, Bennett JL. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol. 2007;179(9):6343–6351. doi: 10.4049/jimmunol.179.9.6343. [DOI] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine. 1998;16(19):1803–1811. doi: 10.1016/s0264-410x(98)00182-0. [DOI] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, He Y, Gorny MK, Zolla-Pazner S, Kayman SC. The V1/V2 domain of gp120 is a global regulator of sensitivity of primary human immunodeficiency virus type 1 isolates to neutralization by antibodies commonly induced upon infection. J Virol. 2004;78(10):5205–5215. doi: 10.1128/JVI.78.10.5205-5215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A, Honnen WJ, D'Agostino P, Gorny MK, Zolla-Pazner S, Kayman SC. The C108g epitope in the V2 domain of gp120 functions as a potent neutralization target when introduced into envelope proteins derived from human immunodeficiency virus type I primary isolates. J Virol. 2005;79(11):6909–6917. doi: 10.1128/JVI.79.11.6909-6917.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman S, Vernon R, Thompson J, Tyka M, Sadreyev R, Pei J, Kim D, Kellogg E, DiMaio F, Lange O, Kinch L, Sheffler W, Kim BH, Das R, Grishin NV, Baker D. Structure prediction for CASP8 with all-atom refinement using Rosetta. Proteins. 2009;77(Suppl 9):89–99. doi: 10.1002/prot.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- Rizzuto CD, Wyatt R, Hernandez-Ramos N, Sun Y, Kwong PD, Hendrickson WA, Sodroski J. A conserved HIV gp120 glycoprotein structure involved in chemokine receptor binding. Science. 1998;280(5371):1949–1953. doi: 10.1126/science.280.5371.1949. [DOI] [PubMed] [Google Scholar]
- Sagar M, Wu X, Lee S, Overbaugh J. Human immunodeficiency virus type 1 V1–V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol. 2006;80(19):9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84(3):1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotton C, Arnold C, Sattentau Q, Sodroski J, McKeating JA. Identification and characterization of monoclonal antibodies specific for polymorphic antigenic determinants within the V2 region of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1995;69(1):222–230. doi: 10.1128/jvi.69.1.222-230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrier B, Sampson J, Totrov M, Li H, O'Neal T, Williams C, Robinson J, Gorny MK, Zolla-Pazner S, Kong XP. Structural analysis and computational modeling of human and macaque monoclonal antibodies provide a model for the quaternary neutralizing epitope of HIV-1 gp120. Structure (London, England: 1993) 2011;19(5):691–699. doi: 10.1016/j.str.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaras GD, Haynes BF. Strategies for eliciting HIV-1 inhibitory antibodies. Curr Opin HIV AIDS. 2010;5(5):421–427. doi: 10.1097/COH.0b013e32833d2d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. A translation-function approach for heavy-atom location in macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 3):400–402. doi: 10.1107/s0907444997014923. [DOI] [PubMed] [Google Scholar]
- van Gils MJ, Bunnik EM, Boeser-Nunnink BD, Burger JA, Terlouw-Klein M, Verwer N, Schuitemaker H. Longer V1V2 region with increased number of potential N-linked glycosylation sites in the HIV-1 envelope glycoprotein protects against HIV-specific neutralizing antibodies. J Virol. 2011;85(14):6986–6995. doi: 10.1128/JVI.00268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Miiro G, Serwanga J, Pozniak A, McPhee D, Manigart O, Mwananyanda L, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Allen S, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrier SV, Pinter A, Honnen WJ, Girard M, Muchmore E, Tilley SA. A novel, glycan-dependent epitope in the V2 domain of human immunodeficiency virus type 1 gp120 is recognized by a highly potent, neutralizing chimpanzee monoclonal antibody. J Virol. 1994;68(7):4636–4642. doi: 10.1128/jvi.68.7.4636-4642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJV, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JLS, Subramaniam S. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog. 2010;6(12):e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kayman SC, Honnen W, Revesz K, Chen H, Vijh-Warrier S, Tilley SA, McKeating J, Shotton C, Pinter A. Characterization of neutralization epitopes in the V2 region of human immunodeficiency virus type 1 gp120: role of glycosylation in the correct folding of the V1/V2 domain. J Virol. 1995;69(4):2271–2278. doi: 10.1128/jvi.69.4.2271-2278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384(6605):179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010a;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SR, LÃℐving R, Lindqvist B, Hebert H, Koeck PJB, SjÃℐberg M, Garoff H. Single-particle cryoelectron microscopy analysis reveals the HIV-1 spike as a tripod structure. Proc Natl Acad Sci U S A. 2010b;107(44):18844–18849. doi: 10.1073/pnas.1007227107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cardozo T. Structure–function relationships of HIV-1 envelope sequence-variable regions provide a paradigm for vaccine design. Nat Rev Immunol. 2010;10(7):527–535. doi: 10.1038/nri2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Kong X, Jiang X, Cardozo T, Nadas A, Cohen S, Totrov M, Seaman MS, Wang S, Lu S. Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA. J Virol. 2011a;85(19):9887–9898. doi: 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla-Pazner S, Cardozo T, deCamp A, Haynes B, Kim J, Kong X, Michael N, Rerks-Ngarm S, Williams C. V2-reactive antibodies in RV144 vaccinees' plasma. AIDS Res Hum Retroviruses. 2011b;27:A–21. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







