Abstract
Enhanced omega-3 fatty acid (n-3) intake benefits cardiovascular disease (CVD) risk reduction. Increasing consumption at a population level may be better addressed by diet than through supplementation. However, limited data are available on the effect of the dose response to fish intake on plasma levels of n-3 fatty acids. To compare the effects of different doses of farmed Atlantic salmon on plasma phospholipid fatty acid (PLFA) proportions and CVD risk biomarkers (glucose, insulin, HOMAIR, hsCRP, and IL-6) in healthy subjects we performed a randomized 3-period cross-over designed trial (4 wk treatment, 4-8 wk washout) to compare the effects of twice/wk consumption of farmed Atlantic salmon at doses of 90, 180, and 270 g in 19 apparently healthy men and women with a mean age of aged 40-65 years and a BMI between 25-34.9 kg/m2. All study visits were conducted at the USDA, ARS Grand Forks Human Nutrition Research Center. EPA and total n-3 were increased (p<0.05) by all treatments in a dose response manner, with total n-3 of 8.03 ± 0.26 and 9.21 ± 0.26 % for 180 and 270 g doses, respectively. Linoleic acid did not change in response to treatment while arachidonic acid (P<0.05) and total omega-6 fatty acids (n-6) decreased dose dependently (<0.0001). The addition of farmed Atlantic salmon to the diet twice/wk for 4 wk at portions of 180g and 270g modifies PLFA proportions of n-3 and n-6 in a level associated with decreased risk for CVD.
Keywords: omega-3 fatty acids, salmon, phospholipid fatty acids
INTRODUCTION
The intake of long chain omega 3(n-3) fatty acids in the Western diet is suboptimal.1, 2 Although the Institute of Medicine (IOM) has not established a recommended dietary allowance for eicosapentaenoic acid (EPA; 20:5n-3) or docosahexaenoic acid (DHA; 22:6n-3), the Dietary Guidelines for Americans recommends an intake of 8 ounces of seafood weekly.3 This is similar to the American Heart Association recommendation of fish intake twice/wk for risk reduction of cardiovascular disease (CVD).4, 5, 6 In spite of these recommendations, fish intake is low in the US with less than 13 g consumed daily.7
A combined analysis of prospective cohort studies demonstrated that a total intake of 250-500 mg/day of EPA and DHA is associated with a significant reduction in CVD risk.8 Another analysis focusing on only prospective studies in the US suggests that approximately 500 mg of combined EPA and DHA provides the most protection.9 The International Life Sciences Institute workshop on establishing a Dietary Reference Intake for EPA/DHA suggests 250-500 mg/day of combined EPA and DHA as the appropriate intake for CVD risk reduction8; however, greater intake may impart additional protective effects.10
Increasing EPA and DHA consumption at a population level will be better addressed by dietary modification than through supplementation6. However, there are limited data describing the effect of various portions of high n-3 fish intake on plasma proportions of n-3 fatty acids. Therefore, a clinical trial was performed to evaluate the effects of various levels of high n-3 fish intake on incorporation of n-3 fatty acids into phospholipid fatty acids (PLFA).
We hypothesized that farmed Atlantic salmon (Salmo salar) intake would result in increased EPA, DHA, and total n-3 in PLFA in a dose-dependent manner. To test this hypothesis we performed a randomized, cross-over designed, dose-response feeding trial using farmed Atlantic salmon, a fish high in EPA and DHA, as the n3 source to evaluate the effect on PLFA composition over four wk interventions.
METHODS
Experimental Protocol
A randomized, crossover design was employed to compare the effects of different portions of high n-3 fish consumption. All participants consumed farmed Atlantic salmon in doses of 90, 180, or 270 g twice/wk in random order. Each treatment period lasted for 28 days (4 wks). Upon completion of the first and second treatment, participants returned to their usual diet for a washout period of 28-56 days and then crossed over to the second or third treatment in the randomization. The salmon was prepared in the metabolic kitchen and all study visits were performed at the Grand Forks Human Nutrition Research Center (GFHNRC), Grand Forks, ND. Participants picked up their salmon portions once weekly. Compliance was monitored with the use of a weekly questionnaire administered by the research staff.
Approval for the study was obtained from the University of North Dakota Institutional Review Board. Informed consent was obtained from all study participants. The study was registered at clinicaltrial.gov as NCT01183520.
Participants
Participants were recruited using newspaper advertisements, fliers and email announcements distributed within the University of North Dakota and general community. Inclusion criteria included men and women aged 40-65 years, BMI between 25-34.9 kg/m2, low intake of fish (≤ 1 serving monthly), and free of major medical conditions. Exclusionary criteria included smoking, lipid modifying drugs or supplements, steroid use, weight loss within the past 3-months, pregnancy or lactation, and use of fish oil of flax supplements. Potential participants were screened through an online application or phone interview and invited to attend an informational meeting in which the study staff described the study in detail. Eligible participants were then scheduled for an examination of height, weight, blood pressure and fasting blood glucose by fingerstick (Accu-Chek Compact Plus, Roche). Body weight (kg) was measured (to 0.1 kg) using a calibrated digital scale (Fairbanks model 50735; Kansas City, MO) with subjects wearing light clothing and no shoes. Stature (to 1 mm) was measured with a free-standing stadiometer (Seca model S-214, Birmingham, UK). Blood pressure was measured with a BP Tru (model BPM-300, BP Medical Devices, Coquitlam, BC) after being seated quietly for 5 minutes. Health status was determined by a medical history questionnaire. All potential participants completed a questionnaire designed to assess their usual n-3 fatty acid intake.
Dietary Intervention
Filleted salmon was provided by Cooke Aquaculture (Blacks Harbor, New Brunswick, Canada; Machiasport, ME). Cooked fish entrees were analyzed for fatty acids and the n-3 content is illustrated in Table 1. Complete details of the fish handling, preparation and analysis were previously described.11 Six individual entrée recipes were developed and participants chose the entrees they were to consume. Participants were asked to maintain their habitual diet while consuming the assigned salmon portion. They were directed to replace meal entrees with the salmon and were given direction on the storage and reheating of the salmon from the research dietitians. Dietary treatment compliance was assessed weekly by questionnaire. During each wash-out period, participants kept a 3-day record of their food consumption which was analyzed by a research dietitian with the USDA National Nutrient Database for Standard Reference, Release 2212 using a customized GFHNRC nutrient analysis program.
Table 1.
n-3 fatty acid content of farmed Atlantic salmon treatments (baked salmon)
| 90g Portion |
180 g Portion |
270 g Portion |
||||
|---|---|---|---|---|---|---|
| 2x/wk | Average/day | 2x/wk | Average/day | 2x/wk | Average/day | |
| 18:3n-3 (mg) | 196.2 | 28.0 | 392.4 | 56.1 | 588.6 | 84.1 |
| 18:4n-3 (mg) | 628.2 | 89.7 | 1256.4 | 179.5 | 1884.6 | 269.2 |
| 20:3n-3 (mg) | 37.8 | 5.4 | 75.6 | 10.8 | 113.4 | 16.2 |
| 20:4n-3 (mg) | 554.4 | 79.2 | 1108.8 | 158.4 | 1663.2 | 237.6 |
| 20:5n-3 (mg) | 1108.8 | 158.4 | 2217.6 | 316.8 | 3326.4 | 475.2 |
| 22:5n-3 (mg) | 511.2 | 73.0 | 1022.4 | 146.1 | 1533.6 | 219.1 |
| 22:6n-3 (mg) | 1045.8 | 149.4 | 2091.6 | 298.8 | 3137.4 | 448.2 |
| Total n-3 (mg) | 4082.4 | 583.1 | 8164.8 | 1166.5 | 12247.2 | 1749.6 |
Blood Collection
Fasting blood samples were obtained at day 0 and 28 of each treatment and used to determine glucose, insulin and PLFA proportions. Samples were obtained on day 28 only for high sensitivity C-reactive protein (hsCRP), and interleukin-6 (IL-6) concentrations. Whole blood samples were centrifuged to obtain serum and plasma; samples were aliquoted and stored at −80°C until analysis.
Biomarker Analysis
Serum glucose concentrations were measured by the COBAS INTEGRA 400 PLUS with Glucose HK Gen.3 kit (Cat# 04404483, Roche Diagnostics, Indianapolis, IN, USA). Serum insulin and hsCRP concentrations were determined by the IMMULITE 1000 System with the insulin kit (Cat# LKIN1) and the hsCRP kit (Cat# LKCRP0) of Siemens Healthcare (Llanberis, Gwynedd, UK). Serum IL-6 concentrations were measured using a by Spectra MAX-190 (Molecular Device Corporation, Sunnyvale, CA, USA) with Quantikine HS kit (Cat# HS600B, R&D Systems, Minneapolis, MN). All the procedures were conducted following the manufacturer’s instructions. Insulin resistance was determined by the calculation of HOMAIR.13 The CVs for these samples for glucose, insulin, hsCRP, and IL-6 were <1.1%, <6.4%, <6.0% and <7.8%, respectively.
Plasma Phospholipid Fatty Acid Analysis
Plasma PLFA proportion was used to evaluate salmon intake effects as they respond readily to dietary changes14 and provide a surrogate measure of tissue membrane fatty acid content based on plasma fatty acid exchange.15 PLFA analysis was performed by gas chromatography as previously described.11 Plasma PLFA results for baseline and 4 wk results on each salmon dose are presented as mol%.
Statistics
Sample size was based on the expected mean difference of DHA using data from Calzada et al.16 Using a repeated measures analysis of variance 17 participants give 80% power to detect a mean difference in DHA of 0.2 mol%. Data are reported as means ± standard error of the mean unless otherwise stated. SAS V9.3 for Windows (SAS Institute, Inc., Cary, NC) was used for all statistical analyses. The mixed model procedure (Proc Mixed) in SAS was used to test for effects of treatment (90, 180 or 270g salmon), time (day 0 and 28), treatment by time interaction, feeding sequence, and time period on each outcome. Participant was treated as a random effect. Feeding sequence and time period were not significant in any of the models. When the interaction between treatment and time was statistically significant (p < 0.05), Tukey contrasts were used to perform pairwise comparisons of all group means.
RESULTS AND DISCUSSION
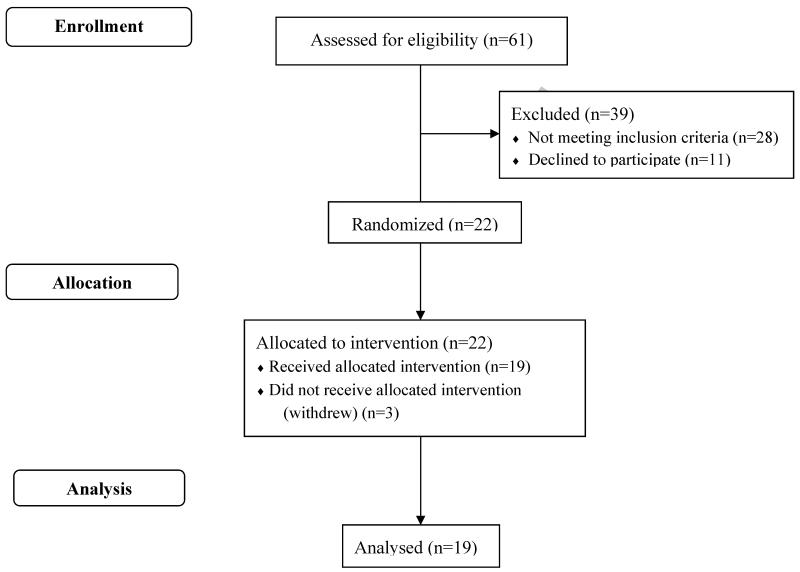
A total of 61 participants were screened and 22 were enrolled and allocated to treatment. Three participants withdrew from study participation and the remaining 19 (11 female, 8 male) completed all aspects of the trial. The flow of participants through the trial is demonstrated in Figure 1. The mean age was 51.6 ± 1.5 years and mean BMI was 29.2 ± 0.6 kg/m2 (mean ± SEM). Participants reported low n-3 intake at baseline (0.04g EPA, 0. 08g DHA) and on the 3-day dietary records kept during the washout periods between treatments (0.01g and 0.01g EPA, 0.03g and 0.02g DHA, washout periods 1 and 2, respectively). Compliance with the treatments was high with participants reporting >99% consumption of provided salmon portions. The food diaries recorded during the wash-out periods demonstrated that participants returned their habitual diet and baseline PLFAs at the beginning of each treatment were not different demonstrating that the length of the washout period was adequate. There were no significant differences in body weight by time or treatment.
Figure 1.
Participants from recruitment to data analysis.
No changes were observed in glucose, insulin, HOMAIR, hsCRP, and IL-6 in response to any level of salmon either within or between treatments. The inability to detect any change may be attributable to a number of factors. Although overweight, recruited participants were apparently healthy individuals. The treatment period was limited to 4 wks which may be an inadequate to affect changes in biomarkers. It will be important to evaluate salmon consumption in individuals at increased risk of chronic disease who present with abnormal biomarker status at study onset.
Salmon intake resulted in marked changes in PLFA (Table 2). Arachidonic acid (ARA; 20:4-n6) decreased significantly (p = 0.0002) with salmon consumption, regardless of the amount consumed. No change was seen in linoleic acid (LA; 18:2n-6) across treatments although a significant reduction was observed in total n-6 (p<0.0001). EPA (20:5n-3) increased in a dose responsive manner (p < 0.0001) while DHA (22:6n-3) was enhanced by all treatments (p<0.0001). Total n-3 was also increased in a dose response manner (p<0.0001) as was the sum of EPA and DHA (22:6n-3) (p<0.0001). The observed reduction in the n-6 to n-3 ratio (p<0.0001) was dose dependent.
Table 2.
Plasma phospholipid n-3 and n-6 fatty acid proportions (mol %) in response to salmon consumption
| 90 g Salmon (n=19) | 180 g Salmon (n=19) | 270 g Salmon (n=19 ) | Analysis of Variance p values3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 Weeks | Baseline | 4 Weeks | Baseline | 4 Weeks | Treatment | Draw | Treatment × Draw |
|
| Polyunsaturated | |||||||||
| 18:2n-6 | 16.68 ± 0.60 | 17.73 ± 0.62 | 17.42 ± 0.68 | 18.31 ± 0.70 | 17.26 ± 0.53 | 17.68 ± 0.74 | 0.55 | 0.10 | 0.85 |
| 18:3n-6 | 0.79 ± 0.02ab | 0.66 ± 0.03b | 0.77 ± 0.03ab | 0.91 ± 0.08a | 0.72 ± 0.03b | 0.80 ± 0.04ab | 0.01 | 0.41 | 0.003 |
| 20:3n-6 | 4.93 ± 0.19 | 4.02 ± 0.15 | 4.96 ± 0.23 | 4.21 ± 0.18 | 4.99 ± 0.21 | 4.39 ± 0.17 | 0.45 | <0.0001 | 0.64 |
| 20:4n-6 | 18.75 ± 0.90 | 17.76 ± 0.34 | 18.22 ± 0.91 | 16.39 ± 0.55 | 17.63 ± 0.79 | 14.88 ± 0.50 | 0.005 | 0.0002 | 0.33 |
| 20:5n-3 | 0.85 ± 0.04a | 0.93 ± 0.04a | 0.90 ± 0.04a | 1.88 ± 0.12b | 0.88 ± 0.03a | 2.60 ± 0.14c | <0.0001 | <0.0001 | <0.0001 |
| 22:5n-3 | 1.64 ± 0.21 | 1.30 ± 0.1 | 1.71 ± 0.18 | 1.50 ± 0.15 | 1.32 ± 0.15 | 1.43 ± 0.05 | 0.21 | 0.15 | 0.18 |
| 22:6n-3 | 3.21 ± 0.11 | 4.70 ± 0.17 | 3.06 ± 0.15 | 4.65 ± 0.23 | 3.40 ±0.18 | 5.17 ± 0.25 | 0.03 | <0.0001 | 0.71 |
| Σ n-3 | 5.64 ± 0.22a | 6.94 ± 0.24b | 5.53 ± 0.22a | 8.03 ± 0.26c | 5.51 ± 0.24a | 9.21 ± 0.26d | <0.0001 | <0.0001 | <0.0001 |
| Σ n-6 | 45.23 ± 0.45 | 43.02 ± 0.74 | 44.24 ± 0.50 | 42.42 ± 0.55 | 44.64 ± 0.75 | 41.09 ± 0.73 | 0.07 | <0.0001 | 0.26 |
| Σ 20:5n-3, 22:6n-3 | 4.1 ± 0.1a | 5.6 ± 0.2b | 4.0 ± 0.2a | 6.5 ± 0.2c | 4.3 ± 0.2 a | 7.8 ± 0.2d | <0.0001 | <0.0001 | <0.0001 |
| 20:4n-6/20:5n-3 | 22.5 ± 1.3a | 19.9 ± 1.1a | 20.7 ± 1.2a | 9.4 ± 0.7b | 20.6 ± 1.1a | 6.1 ± 0.4c | <0.0001 | <0.0001 | <0.0001 |
| n-6/n-3 | 8.3 ± 0.3a | 6.4 ± 0.3b | 8.3 ± 0.3a | 5.4 ± 0.3c | 8.4 ± 0.3a | 4.5 ± 0.3d | 0.0003 | <0.0001 | <0.0001 |
Values are means ± SEM.
Means within a row not sharing a common superscript are significantly different (p < 0.05) by Tukey contrasts.
The treatment effect tests whether the response to the salmon differed depending on the amount of salmon consumed. The draw effect tests whether subjects responded to the salmon, regardless of the amounts consumed. The treatment × draw effect tests for a differential response (i.e. dose-response) to the amount of salmon amounts consumed.
While EPA in PLFA showed a clear dose-response relationship, the proportion of DHA was elevated and appeared to reach saturated at the lowest level of salmon intake. A similar response has been observed following consumption of n-3 supplements.14 Likely, this phenomenon is the summation of multiple physiologic processes. As PLFA are a surrogate measure of cellular membrane phospholipid, these data imply that cellular pools of DHA became saturated where as those of EPA did not. The lack of EPA increase in the PLFA at the lowest salmon intake suggests that the EPA consumed in the 90 g portion was converted to DHA, but that at greater intake the conversion of EPA to DHA was saturated. It is possible that at the higher salmon intake, that retroconversion of DHA to EPA occurred although human data suggest that this contribution is minimal.17 Thus the increase in EPA is likely owing to elevated consumption of EPA. Human trials with fish oil supplements indicate that plasma EPA and DHA pools are not identical with incorporation of EPA into phospholipid and cholesterol ester and DHA into phospholipid and triglyceride lipid fractions.15 Our study did not examine these other lipid pools, however. None the less, our data indicate that PLFA pools of EPA and DHA behave differently and that this EPA pool is labile. These data have significant impact for understanding the physiologic relationship of EPA to CVD risk.
Both the absolute quantity and the bioavailability of the n-3 fatty acids affect incorporation into circulation and target tissues. Most of the n-3 fatty acids in supplemental fish oil and that in fish are in the triglyceride (TG) form with lesser quantities present in phospholipid. There appears to be much better bioavailability of n-3 in the TG form compared to the ethyl ester form.18. 19 The bioavailability of n-3 fatty acids from whole fish appears to be greater than that from supplements.18 Elvevoll et al.20 demonstrated enhanced incorporation of EPA and DHA from both smoked salmon and cooked salmon compared to almost triple the dose provided by cod liver oil. Provision of salmon twice/wk resulted in dramatic increases in PLFA EPA and DHA with relatively small doses.
These data have direct relevance to the recommendations for fish consumption to meet dietary guidelines and in CVD risk reduction. Twice/wk portions of 180g and 270g salmon over 4 wks was effective in modifying PLFA proportions to near optimal levels of EPA and DHA for CVD risk reduction.19, 8, 21 Interestingly, increases in PLFA EPA, DHA, and total n-3 were enhanced in an identical manner to that of a fully controlled dietary intervention.22 While the 90 g portion of salmon did not have the same magnitude of effect in increasing EPA, this portion of fish enhanced PLFA DHA and total n-3. Thus, even small portions of salmon contribute significantly to n-3 status. While more study is needed, these data suggest that incorporation of 180g total per wk of n3-rich fish may yield optimum n-3 status.
It is unknown whether continued consumption beyond that shown here would result in continued elevation of PLFA n-3 levels. There was a dose response to intake over 4wks and washout PLFA proportions returned to baseline reflective of a diet with minimal n-3. Whether continued intake at each of these portions would ultimately result in attainment of saturation levels of both EPA and DHA in tissue and circulation has not been evaluated. Interventions are required to determine the long-term effects of various doses of high n-3 fish.
The primary strength of our study is the well controlled manner in which the treatments were prepared and distributed to participants. The cross-over design of the trial, the very high reported compliance, and the fact that participants returned to their usual diet between treatments, and maintained their body weight throughout the trial strengthens the outcome data.
Limitations of the study include the small sample size, the fact that recruited participates were apparently healthy individuals and no effect of salmon intake was observed on biomarkers of CVD risk. Additionally, as participants’ diets were not monitored while they were on the treatments, their total nutrient intake is unknown.
CONCLUSIONS
The results of this study demonstrate the effect of consuming various portions of farmed Atlantic salmon on PLFA n-3 proportions in healthy men and women. Randomly assigned treatments resulted in marked changes in PLFA over the four wk test periods in a differential manner. The proportion of DHA increased on all treatment levels while EPA responded in a dose-dependent manner. Total n-3 was enhanced and total n-6 and ARA were significantly reduced. The addition of farmed Atlantic salmon to the diet twice/wk at portions of 180g and 270g modifies PLFA proportions of n-3 and n-6 fatty acids to levels associated with decreased risk for CVD.
Further work is needed to establish the optimal dose and duration of high n-3 fish for the modification of cardiometabolic biomarkers with individuals who at enhanced risk for or exhibit frank CVD. Identification of the appropriate dose of n-3 rich fish in promoting optimal n-3 status is required.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005. 81(2):341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 2.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93(5):950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Agriculture and the U.S. Department of Health and Human Services . Dietary Guidelines for Americans. 7th Edition. U.S. Government Printing Office; Washington, DC: Dec, 2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, et al. on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.Kris-Etherton P, Harris WS, Appel LJ, for the Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 7.Dahl TE. Status and trends of wetlands in the conterminous United States 1998 to 2004. U.S. Department of the Interior, Fish and Wildlife Service; Washington, DC: 2006. http://wetlandsfws.er.usgs.gov/status_trends/ [Google Scholar]
- 8.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139(4):804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008;10(6):503–509. doi: 10.1007/s11883-008-0078-z. [DOI] [PubMed] [Google Scholar]
- 10.Makhoul Z, Kristal AR, Gulati R, Bersamin A, Boyer B, Mohatt GV. Associations of very high intakes of eicosapentaenoic and docosahexaenoic acids with biomarkers of chronic disease among the Yup’ik Eskimos. Am J Clin Nutr. 2010;91(3):777–785. doi: 10.3945/ajcn.2009.28820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raatz SK, Golovko MY, Brose SA, Rosenberger TA, Burr GS, Wolters WR, Picklo MJ. Baking reduces prostaglandin, resolvin, and hydroxy-fatty acid content of farm-raised Atlantic salmon (Salmo salar) J Ag Food Chem. 2011;59(20):11278–11286. doi: 10.1021/jf202576k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.USDA, Agricultural Research Service USDA National Nutrient Database for Standard Reference, Release 22. 2009 http://www.ars.usda.gov/ba/bhnrc/ndl.
- 13.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 14.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids. Am J Clin Nutr. 2006;3(suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 15.Vidgren HM, Agren JJ, Schwab U, Rissanen T, Hanninen O, Uusitupa MIJ. Incorporation of n-3 fatty acids into plasma lipid fractions, and erythrocyte membranes and platelets during dietary supplementation with fish, fish oil, and docosahexaenoic acid-rich oil in healthy young men. Lipids. 1997;32(7):697–705. doi: 10.1007/s11745-997-0089-x. [DOI] [PubMed] [Google Scholar]
- 16.Calzada C, Colas R, Guillot N, Guichardant M, Laville M, Vericel E, Lagarde M. Subgram daily supplementation with docosahexaenoic acid protects low density lipoproteins from oxidation in healthy men. Atherosclerosis. 2010;208(2):467–472. doi: 10.1016/j.atherosclerosis.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 17.Brossard N, Croset M, Pachiauadi C, Riou JP, Tayot JL. Retroconversion and metabolism of [13C]22:6n-3 in humans and rats after intake of a single dose of [13C]22:6n-3-triacylglycerols. Am J Clin Nutr. 1996;64(4):577–586. doi: 10.1093/ajcn/64.4.577. [DOI] [PubMed] [Google Scholar]
- 18.Visioli F, Rise P, Barassi MC, Marangoni F, Galli C. Dietary intake of fish vs. formulations leads to higher plasma concentrations of n-3 fatty acids. Lipids. 2003;38(4):415–418. doi: 10.1007/s11745-003-1077-x. [DOI] [PubMed] [Google Scholar]
- 19.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish oil capsules on the and free fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr. 2007;86(6):1621–1625. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 20.Elvevoll EO, Barstad H, Breimo ES, Brox J, Eilertsen K-E, Lund T, Olsen JO, Østerud B. Enhanced incorporation on n-3 fatty acids from fish compared with fish oils. Lipids. 2006;41(12):1109–1114. doi: 10.1007/s11745-006-5060-3. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FA, Rimm EB, Siscovick DS. Circulating long chain ω-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study. Ann Intern Med. 2011;155(3):160–170. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young LR, Kurzer MS, Thomas W, Redmon JB, Raatz SK. Effects of dietary fat and omega-3 fatty acid intake on urinary eicosanoids and sex hormone levels in postmenopausal women: A randomized controlled feeding trial. Nutr Cancer. 2011;63(6):930–939. doi: 10.1080/01635581.2011.589957. [DOI] [PubMed] [Google Scholar]