Abstract
A new micelle drug carrier that consists of a diblock polymer of propylene sulfide (PS) and N,N-dimethylacrylamide (poly(PS74−b-DMA310)) has been synthesized and characterized for site-specific release of hydrophobic drugs to sites of inflammation. Propylene sulfide was first polymerized using a thioacyl group transfer (TAGT) method with the RAFT chain transfer agent (CTA) 4-cyano-4-(ethylsulfanylthiocarbonylsulfanyl) pentanoic acid (CEP), and the resultant poly(PS74−CEP) macro-CTA was used to polymerize a second polymer block of DMA using reversible addition-fragmentation chain transfer (RAFT). The formation of the poly(PS74−b-DMA310) diblock polymer was confirmed by 1H NMR spectra and gel permeation chromatography (GPC). poly(PS74−b-DMA310) formed 100 nm micelles in aqueous media as confirmed by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Micelles loaded with the model drugs Nile red and DiO were used to demonstrate the ROS-dependent drug release mechanism of these micelles following treatment with hydrogen peroxide (H2O2), 3-morpholinosydnonimine (SIN-1), and peroxynitrite. These oxidants were found to oxidize the micelle PPS core, making it more hydrophilic and triggering micelle disassembly and cargo release. Delivery of poly(PS74−b-DMA310) micelles dual-loaded with the Förster Resonance Energy Transfer (FRET) fluorophore pair DiI and DiO was used to prove that endogenous oxidants generated by lipopolysaccharide (LPS)-treated RAW 264.7 macrophages significantly increased release of nanocarrier contents relative to macrophages that were not activated. In vitro studies also demonstrated that the poly(PS74−b-DMA310) micelles were cytocompatible across a broad range of concentrations. These combined data suggest that the poly(PS74−b-DMA310) micelles synthesized using a combination of TAGT and RAFT have significant potential for site-specific drug delivery to tissues with high levels of oxidative stress.
Keywords: Poly(propylene sulfide) (PPS), Reversible addition fragmentation chain transfer (RAFT) polymerization, Reactive oxygen species (ROS), Inflammation, Smart polymer micelles, Targeted drug delivery
1.0 Introduction
Rheumatoid arthritis, neurodegenerative diseases, atherosclerosis, diabetes, and many cancers are among the pathologies characterized by hyper-activation of enzymes (i.e. NADPH oxidase, iNOS, etc.) that create high concentrations of reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radicals, singlet oxygen, superoxide anions, nitric oxide, nitroxyl, and nitrogen dioxide [1]. Nitric oxide and superoxide anion can also react with each other to generate other more potent reactive oxygen and nitrogen species such as peroxynitrite [2]. Excessive levels of these ROS, known as oxidative stress, can cause DNA mutations and can alter function of proteins, leading to apoptosis or other aberrant cell activities than can cause or exacerbate disease [1-3].
Because of the role of imbalanced ROS activity in the etiology of numerous diseases, delivery platforms that enable targeted release of antioxidants or other drugs at sites of high ROS activity have the potential for high therapeutic impact [4-8]. Stimuli-responsive, “smart” polymer-based micelles have attracted considerable attention for drug delivery because they can stably package their cargo until disassembly and drug release is triggered in response to changes in environmental signals such as temperature [9-11], pH [12], oxidation [13-15], light [16, 17], or other specific molecules [18]. The aim of the current study was to investigate a new smart micelle for targeted drug release in regions of high ROS activity.
Sulfur(II)-containing materials undergo a phase transition from a hydrophobic to hydrophilic state under oxidative environments [19], with poly(propylene sulfide) (PPS) being an example synthetic polymer known to exhibit oxidation responsiveness [14]. Specifically, hydrophobic PPS has a two-stage transition to more hydrophilic poly(propylene sulphoxide) and ultimately poly(propylene sulphone) upon oxidation [14]. Block copolymers of PEG and PPS have been explored by Hubbell et al for engineering of polymersomes for vaccine delivery and micelles for encapsulation of hydrophobic drugs [20, 21]. PEG-b-PPS micelles were shown to enable sustained, slow release of the hydrophobic drug cycolsporin A, but, to our knowledge, these or similar polymers have not been rigorously investigated for triggered release of hydrophobic drug cargo in response to different ROS. Furthermore, a thioacyl group transfer (TAGT) method [22] was utilized here that enables pairing of the anionic ring opening polymerization of PPS with the RAFT polymerization of the hydrophilic polymer block. RAFT is amenable to use with a wide variety of monomers [23], and here, DMA was chosen for the corona forming polymer block due to its solubility in water and low toxicity [24, 25]. The focus of the current study was on an amphiphilic diblock copolymer of poly(PS74−b-DMA310). This diblock polymer self-assembled into micelles, and its oxidation, disassembly, and cargo release was thoroughly characterized under H2O2, 3-Morpholinosydnonimine (SIN-1), peroxynitrite, and endogenous ROS generated by lipopolysaccharide (LPS)-activated macrophages.
2.0 Materials and Methods
2.1 Materials
Propylene sulfide (PS), N,N-dimethylacrylamide (DMA), ethanethiol, carbon disulfide (CS2), sodium hydride (NaH), 4,4′-Azobis(4-cyanovaleric acid) Tetraphenylphosphonium chloride (TPPCl), 2,2′-azobis(isobutyronitrile) (AIBN), Nile red, DiO (3,3′-dioctadecyloxacarbocyanine perchlorate), DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate),and hydrogen peroxide (H2O2) were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). SIN-1 was purchased from Invitrogen (San Diego, CA, USA) as packages of 1 mg plastic vials. Peroxynitrite was purchased from EMD Millipore (Billerica, MA, USA). 4-Cyano-4-(ethylsulfanyltiocarbonyl)sulfanylpentanoic acid (CEP) was synthesized following the previously reported procedure [26] (1H NMR (400 MHz CDCl3) δ: 1.35 (t, −S−CH2−CH3); δ 1.85 (s, −C(CN)−CH3); δ 2.4-2.67 (m, −CH2−CH2−); δ 3.42 (q, −S−CH2−CH3)). Propylene sulfide was dried and distilled over calcium hydride (CaH2) before use, and N,N-dimethylacrylamide (DMA) was purified by distillation under reduced pressure just before polymerization. 1-Methyl-2-pyrrolidone (NMP) was dried and distilled over CaH2.
2.2 Polymer synthesis and characterization
2.2.1 Synthesis of PPS macro CTA poly(PS74−CEP)
Poly(propylene sulfide) was synthesized using the RAFT chain transfer agent CEP through the TAGT polymerization method [22]. Briefly, PS (0.30 mL, 3.8 mmol), CEP (24.9 mg, 0.095 mmol), TPPCl (7.1 mg, 0.019 mmol), and NMP (0.95 mL) were placed into a flame-dried ampoule equipped with a three-way stopcock and degassed for 30 min with three freeze-pump-thaw cycles. The ampoule was immersed in an oil bath at 50 °C for 20 h, and afterwards, cooled in a liquid nitrogen bath. The crude polymerization mixture was precipitated twice from NMP into hexane, which is a nonsolvent for PPS. The resulting polymer was dissolved into chloroform and precipitated into tenfold excess of cold methanol to better remove the TPPCl catalyst [22]. The product was dried at 60 °C under vacuum to yield a yellow viscous oil (0.15 g) (poly(PS74−CEP)), Mn = 5,800 g/mol, PDI = 1.32).
2.2.2 Synthesis of poly(PS74−b-DMA310) diblock polymer
RAFT chain extension of poly(PS74−CEP) macro-CTA was done to prepare poly(PS74−b-DMA310). Poly(PS74−CEP) (0.58 g, 1 mmol), DMA (4.0 ml, 403 mmol), AIBN (0.8 mg, 0.05 mmol), and dioxane (10.52 mL) were placed in a dry ampoule, and the solution was degassed by purging with nitrogen for 30 minutes. The polymerization was conducted at 60 °C for 3 h and then quenched by cooling the reaction vessel in an ice bath and exposure to air. The final polymer (poly(PS74−b-DMA310), Mn 36,700 g/mol, PDI=1.29) was purified by precipitation into diethyl ether.
2.2.3 Polymer characterization
1H NMR spectra were recorded in CDCl3 and D2O with a Brüker 400 MHz spectrometer. For molecular weight determination, gel permeation chromatography (GPC) was performed using dimethylformamide (DMF) + 0.1 M LiBr mobile phase at 60 °C through three serial Tosoh Biosciences TSKGel Alpha columns (Tokyo, Japan). A Shimadzu RID-10A refractive index detector and a Wyatt miniDAWN Treos multi-angle light scattering detector was used to calculate absolute molecular weight based on dn/dc values experimentally determined through offline injections into the RI detector.
2.3 Micelle preparation and characterization
To prepare micelle solutions, 10 mg of purified poly(PS74−b-DMA310) was dissolved in 250 μl of THF. PBS (10 ml, pH 7.4) was added dropwise into the THF polymer solution under vigorous stirring to dilute the sample to a final polymer concentration of 1 mg/mL. The solution was pushed through a 0.45 μm syringe filter, and dynamic light scatter (DLS) measurements were done to measure hydrodynamic diameter using a Malvern Zetasizer Nano-ZS (Malvern Instruments Ltd, Worcestershire, U.K) equipped with a 4 mW He–Ne laser operating at λ = 632.8 nm. Transmission electron microscopy (TEM) samples were prepared by placing one drop of solution (micellar dispersion at 1.0 mg/mL concentration) onto copper grids (400 mesh, carbon coated, Ted Pella Inc. Redding, CA). The grids were dried overnight in a desiccator under vacuum before imaging with a Philips CM20 HR-TEM (Philips, EO, Netherlands).
2.4 Determination of critical micelle concentration (CMC)
The critical micelle concentration was assessed fluorescently using Nile red [27] and by identifying particle morphological changes with DLS. The Nile Red dye is hydrophobic and exhibits strong fluorescence in the presence of intact micelles but is poorly soluble and minimally fluorescent if released into an aqueous environment when micelles destabilize. Different dilutions were prepared from a 1 mg/mL stock solution to obtain micelle samples ranging in concentration from 0.0001 to 1 mg/mL. Then, 10 μL of a 1 mg/mL Nile red stock solution in THF were added to 1 mL of each micelle sample and incubated overnight in the dark at room temperature. The next day, samples were filtered with a 0.45 μm syringe filter and their Nile red fluorescence was measured in 96 well plates using a micro plate reader (Tecan Infinite 500, Tecan Group Ltd., Mannedorf, Switzerland) at an excitation wavelength of 535±20 nm and an emission wavelength of 612±25 nm. The CMC was defined, as previously described [28], as the intersection point on the plot of the Nile red fluorescence versus the copolymer concentration. For DLS measurements, a range of micelle concentrations were prepared by serial dilutions of a 1 mg/mL stock solution to obtain samples of concentration ranging from 0.001 to 1 mg/mL in PBS. DLS was then used to assess micelle hydrodynamic size and to determine the concentration at which the micelles exhibited morphological changes [29].
2.5 Measuring Micelle Loading with Nile Red and DiO
A fluorescence-based method was used to calculate drug loading and encapsulation efficiency of Nile red and DiO across a range of weight ratios from 6.25 – 200 μg dye per mg poly(PS74−b-DMA310) based on a reported method [30]. After removal of any free dye through centrifugation, the loaded dyes were extracted from the micelles by diluting the samples into 90% DMF. Nile red (excitation at 535±20 nm, emission 612±25 nm) or DiO (excitation at 485±20 nm, emission 535±25 nm) content was quantified in the respective samples relative to fluorescence standard curves of the molecules measured in 90% DMF. Encapsulation efficiency (EE) was defined as the weight percent of the drug loaded versus what was added to the micelle solution, and drug loading (DL) was calculated as the weight percent of dye relative to polymer.
2.6 ROS-mediated release of Nile red and DiO
To assess ROS-dependent release from the poly(PS74−b-DMA310) micelles, Nile red and DiO were utilized as model small molecule drugs. To prepare 1% Nile red loaded micelle solution, 50 μL of a 1 mg/mL Nile red stock solution in THF was added to 5 mL of micelle solution (1mg/ml). The residual THF was removed through rotary evaporation and the micelle solution incubated overnight in the dark at room temperature. The next day, samples were filtered using a 0.45 μm syringe filter prior to use. Nile red-loaded micelles were exposed to a range of concentrations (0 to 3.3 volume %) of hydrogen peroxide. Fluorescence intensity of Nile red was monitored in a 96 well plate using a micro plate reader (Tecan Infinite 500) at an excitation wavelength of 535±20 nm and an emission wavelength of 612±25 nm. Release of the dye due to micelle oxidation and destabilization was assessed over time based on disappearance of Nile red fluorescence. The loss of fluorescence for each sample at each time point was determined by subtracting the fluorescent value from that of the sample prior to H2O2 addition, and the percent fluorescence remaining was determined by normalization to the same value (before addition of H2O2). This value for percent fluorescence remaining was subtracted from 100% and expressed as a percent release for each sample at each time point. An analogous experiment was completed to assess the H2O2-dependent release of DiO from micelles prepared using the same technique. A similar study was carried out to assay the release of Nile red from the micelles upon treatment with SIN-1, a molecule known to generate superoxide, nitric oxide, and peroxynitrite [31]. Nile red loaded micelles were treated with a range of SIN-1 concentrations (1 to 100 mM), and a fresh dose of the respective concentration of SIN-1 was added every 24h due to the short half life of SIN-1 [31]. Nile red release was quantified as described for H2O2 experiments. Because SIN-1 generates nitric oxide and superoxide that then react to form peroxynitrite, micelle release was also studied following direct peroxynitrite treatment. A range of final concentrations of peroxynitrite (1-100 μM) were tested by addition of a basic peroxynitrite stock solution into buffered (PBS) solutions of Nile red-loaded micelles. The final pH of the samples was 7.4 after this addition, and fluorescent intensities were measured after 15 minutes to quantify release as described above. The full time course was not done with peroxynitrite because of its very short half-life at physiologic pH.
2.7 FRET-based Imaging of Micelle Release by Activated Macrophages
Micelle release of the Förster Resonance Energy Transfer (FRET) dye pair DiO and DiI was used as a readout for model drug release in vitro [32]. To prepare FRET micelles, 10 μl of DiO solution (1 mg/ml solution in THF) and 10 μl of DiI solution (1 mg/ml solution in THF) were added into 1 ml of micelle solution (1 mg/ml). The THF was then removed by rotary evaporation and the samples were left overnight in the dark. To remove any unloaded dye, the micelle solution was diluted four fold in sterile DI water and reconcentrated using a centrifuge filtration tube with 10,000 MW cutoff for 30 minutes. This process was repeated four times. Control micelles loaded with just DiO or DiI were prepared in the same manner as FRET micelles. Generation of the FRET effect within the dual loaded micelles was confirmed using a Jobin Yvon/Horiba Fluorolog-3 FL3-111 Spectrophotofluorometer at an excitation wavelength of 484 nm.
To image micelle release mediated by endogenously-produced ROS in vitro, RAW 264.7 mouse macrophages were seeded in phenol-red free DMEM at 5,000 cells/well onto multi-chambered #1 borosilicate cover-glass slides (Fisher Scientific) and allowed to adhere overnight. After 24 hours, the media was replaced with phenol-red free DMEM supplemented with 100 ng/ml lipopolysaccharide (LPS) or vehicle control. After 24 hours, LPS-activated and control cells were treated with vehicle control or with DiO/DiI FRET micelle solutions to achieve a final concentration to 1.67 μg/ml for each dye. Images were acquired 24h after micelle treatment with a Zeiss LSM 510 META confocal microscope using a 63× oil immersion lens equipped with a 488 nm Argon laser with 505-550 band-pass filters for green emission and 560-615 band-pass filter for red (FRET) emission. The exposure time, gain, and all other microscope settings were held constant during fluorescent imaging of all treatment groups. Image J was used to quantitatively assess cellular fluorescence. Cells were also seeded onto 96 well plates and treated as explained above to confirm quantitative FRET data using a SpectraMax M5 (Molecular Devices, Sunnyvale, CA,) plate reader (see additional details in Figure S-8).
2.8 Cell Viability Assay
RAW 264.7 macrophages (RAWs) were plated at 8,000 cells/well in a 96-well plate and incubated at 37 °C in Alpha MEM supplemented with 10% FBS and 1% penicillin-streptomycin. After 24h, the medium was replaced with fresh medium containing a range of polymer micelle concentrations (62.5-1000 μg/ml). The cells were then incubated at 37 °C for 24 h or 48 h. At the respective endpoints, the cells were lysed and analyzed for intracellular LDH with a Cytotoxicity Detection Kit (Roche Applied Science) as previously reported [33]. With this assay, a colorimetric readout (absorbance at 492 nm with reference at 595 nm) is acquired that represents relative cell number. Each group was assayed in triplicate, and LDH quantities in the micelle treatment groups were normalized to samples receiving no treatment (NT).
3. Results and Discussion
3.1 Synthesis and characterization of poly(PS74−b-DMA310)
The AB diblock copolymer poly(PS74−b-DMA310) was successfully prepared using the TAGT and RAFT methods described (Scheme 1). The final diblock copolymer poly(PS74−b-DMA310) displayed relatively low polydispersity (Mw/Mn = 1.29) and showed the expected decrease in the elution time following chain extension from poly(PS74−CEP) (Figure 1).
Scheme 1.
Synthetic route for preparation of ROS-responsive poly(PS74−b-DMA310) diblock copolymer via a combination of TAGT and RAFT polymerization.
Figure 1.
Confirmation of RAFT polymerization of a DMA second block from a poly(PS74−CEP) macro-CTA. The GPC elugram showed a characteristic shift to a decreased elution time following RAFT polymerization of the larger molecular weight poly(PS74−b-DMA310) diblock from the poly(PS74−CEP) macro-CTA.
Successful diblock polymerization was also confirmed by 1H NMR spectra of the poly(PS74−CEP) macro-CTA and the poly(PS74−b-DMA310) diblock copolymer (Figure 2). The 1H NMR spectra of the resulting poly(PS74−b-DMA310) taken in CDCl3, a solvent in which micelles do not form, displayed characteristic signals attributed to both PPS and PDMA. The use of RAFT for polymerization of the corona-forming polymer block provides synthetic flexibility because it enables the possibility of using other monomers or even random copolymer compositions (i.e., for incorporation of pendant groups that can be used to attach cell-specific targeting moieties within the micelle corona).
Figure 2.
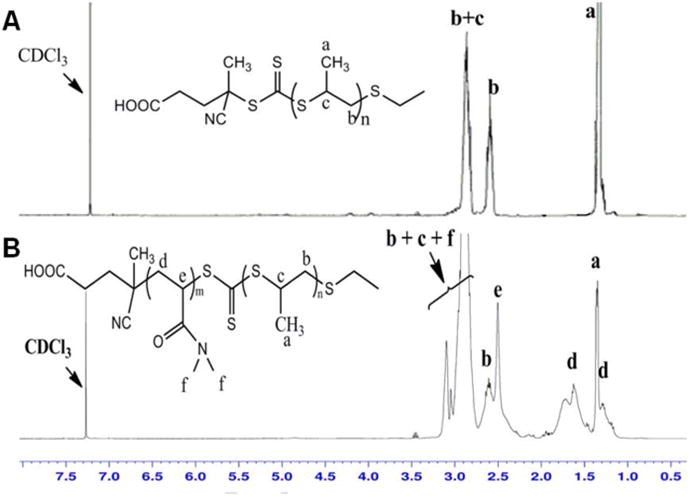
1H NMR spectra of (a) poly(PS74−CEP) and (b) poly(PS74−b-DMA310) in CDCl3 show characteristic peaks for both PS and DMA, providing additional evidence of diblock polymer formation.
3.2 Micelle preparation and characterization
Formation of poly(PS74−b-DMA310) micelles with a PPS core and a PDMA corona were successfully prepared by a solvent evaporation method using THF. Verification that the poly(PS74−b-DMA310) existed in water as micelles stabilized by a hydrophilic poly(DMA) block was done by recording 1H NMR spectra in CDCl3 and D2O (Figure S-1). The 1H NMR spectrum of the diblock copolymer in CDCl3 showed all peaks associated with both the PPS and PDMA blocks (Figure S-1a). Under these conditions, both blocks are solvated and show free segmental motion, which is consistent with a molecularly dissolved unimeric polymer. In contrast, 1H NMR of poly(PS74−b-DMA310) in D2O (Figure S1-b) showed suppression of the PPS peaks (note suppression of peak ‘a’ in Figure S-1b D2O spectrum versus S-1a CDCl3 spectrum) and resembled the spectrum for a homopolymer of PDMA (Mn=20 KDa) taken in D2O (Figure S-1d). These combined results indicate that, in aqueous conditions, the poly(PS74−b-DMA310) assembles into micelles with the PPS polymer block confined in the micelle core away from the D2O solvent, and thus unable to generate 1H NMR signal.
1H NMR spectra of micelles were also compared in D2O before and after H2O2 treatment to verify that ROS could oxidize the PPS in the micelle core and increase its water solubility. For reference, 1H NMR spectra were first recorded for a PPS homopolymer in D2O before and after treatment with H2O2 (Figure S-1e, S-1f). The appearance of a new peak at 1.53 ppm in the 1H NMR spectra of poly(PS74−b-DMA310) in D2O following treatment with H2O2 is a characteristic shift [14] indicating that, in the micelle form, the H2O2 was still able to oxidize the PPS from a sulfide into a more hydrophilic sulfone (Figure S-1c). The shifting of the PPS peaks (CH2 and CH protons) from 2.5-3.0 ppm to 3.2-4.2 ppm (circled and labled ‘b+c’) was also observed (Figure S-1c) and further confirmed PPS conversion into a more hydrophilic sulfone, which also matched observations for PPS homopolymer H2O2 oxidation by us (Figure S-1e, S-1f) and others [14].
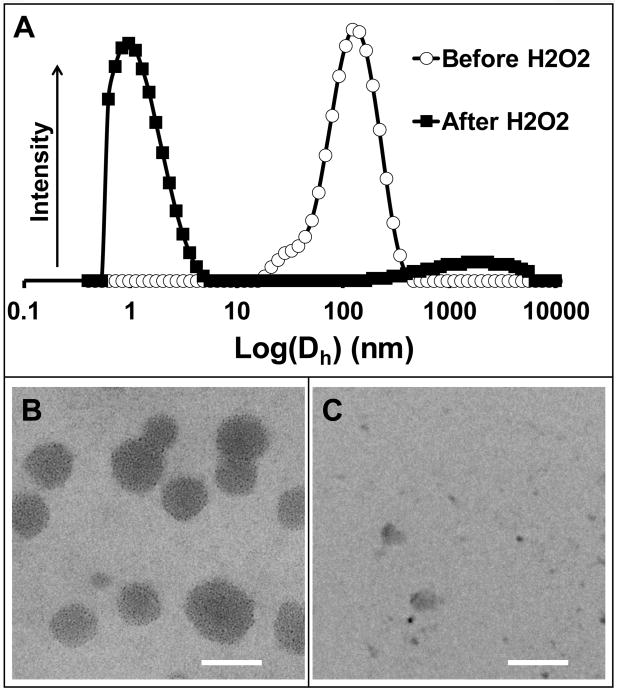
Physical characterization of the micelles was also done using DLS and TEM. The average hydrodynamic diameter of the poly(PS74−b-DMA310) micelles was measured by DLS to be 99 nm (Figure 3a). This size, along with the spherical morphology of the micelles, was confirmed using TEM (Figure 3b). These micelles fall in a desirable size range for nanotherapeutics and may be well-suited for tumor targeting through the enhanced permeation and retention (EPR) effect [34].
Figure 3.
poly(PS74−b-DMA310) micelles disassembled following exposure to H2O2. (a) DLS measurement of poly(PS74−b-DMA310) demonstrated transition from nanoparticulate micelles to smaller polymer unimers following H2O2 treatment. TEM imaging (b) before and (c) after H2O2 treatment visually confirmed the presence of micelles that disassembled following oxidation. TEM scale bars = 100 nm.
DLS and TEM were also utilized to confirm the H2O2-responsive disassembly of the micelles. Figure 3a shows the change in hydrodynamic diameter of micelles before and after H2O2 (3.3 volume % for 24h) treatment. The average size of micelles was found to change from 99 nm to 5 nm after treatment with H2O2, and this behavior was also supported by absence of visible micelles in TEM images of H2O2 treated micelle samples (Figure 3c). These data indicate that the poly(PS74−b-DMA310) micelles may be useful for site-specific cargo delivery to sites with high ROS through environmentally-triggered micelle disassembly.
3.3 Determination of critical micelle concentration (CMC)
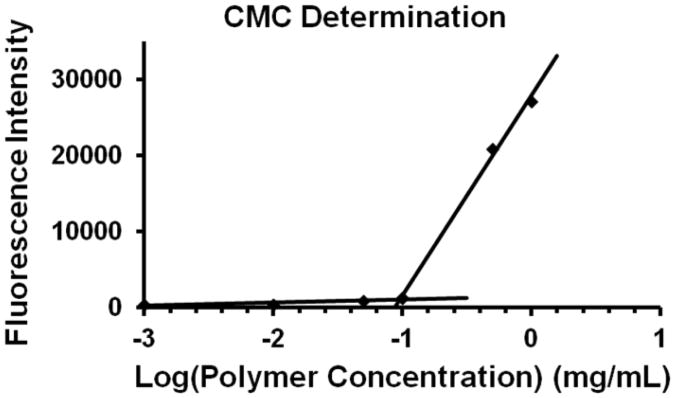
The critical micelle concentration (CMC) was determined using Nile red as a fluorescent probe [35]. Nile red is hydrophobic and does not fluoresce in an aqueous environment but is strongly fluorescent when it partitions itself into the hydrophobic core of intact micelles. The fluorescence intensity of Nile red in poly(PS74−b-DMA310) aqueous solutions of various concentrations were recorded, and the maximum fluorescence intensity of the Nile red emission is shown in Figure 4 as a function of micelle concentration. The CMC of the diblock copolymer was determined using the crossing point of the plot and was estimated to be 0.09 mg/mL. The CMC value of the micelles was also estimated using a DLS-based dilution method [29]. Table 1 shows the hydrodynamic diameter (Dh) of poly(PS74−b-DMA310) in PBS from concentrations ranging 1.0 mg/mL to 0.001 mg/mL, and the full DLS spectra for these samples are also shown in Figure S-2. The hydrodynamic diameter of micelles was stable down to 0.1 mg/mL, and dilution to concentrations below this value caused the micelles to become increasingly unstable, resulting in the formation of aggregates. This indicated that the CMC value fell in the range from 0.05-0.1 mg/mL, which was in agreement with the Nile red based assay.
Figure 4.
Changes in the fluorescence intensity of Nile red (1.0 × 10−6 M) with different concentrations of poly(PS74−b-DMA310) indicated that the CMC was approximately 0.09 mg/mL.
Table 1.
DLS measurements of poly(PS74−b-DMA310) demonstrating that the polymer CMC falls below 0.1 mg/mL.
| Polymer Concentration | Size (nm) |
|---|---|
| poly(PS74−b-DMA310) - 1 mg/mL | 99.45 |
| poly(PS74−b-DMA310) - 0.5 mg/mL | 97.55 |
| poly(PS74−b-DMA310) - 0.1 mg/mL | 99.28 |
| poly(PS74−b-DMA310) - 0.05 mg/mL | 506.6 |
| poly(PS74−b-DMA310) - 0.01 mg/mL | 175.00 |
| poly(PS74−b-DMA310) - 0.001 mg/mL | 407.2 |
3.4 Assessment of Micelle Loading with Nile red and DiO
The drug loading and encapsulation efficiency poly(PS74−b-DMA310) micelles were determined using both Nile red and DiO. For the drug to polymer ratios tested, the maximum drug loading content and encapsulation efficiency of Nile red were calculated to be 1.76 wt % (Figure S-3a) and 63.1% ((Figure S-3b)), respectively. The values were found consistent with the previously reported literature [30]. DiO also showed similar maximum drug loading content (1.48 wt%, (Figure S-3c)) and encapsulation efficiency (63%, (Figure S-3d)).
3.5 ROS dependent release of Nile red
Nile red was chosen as a model hydrophobic drug for the investigation of drug release [27] in response to H2O2, which was used to mimic the presence of pathophysiologic oxidative stress [36]. Figure 5 shows in vitro release kinetics of Nile red mediated by different concentrations of H2O2. The fluorescence intensity of Nile red-loaded micelles treated with H2O2 was found to decrease over time, and the rate of this decrease correlated to the concentration of H2O2 present. The loss of Nile red fluorescence intensity under oxidative environments can be explained by the conversion of the PPS block from a hydrophobic sulfide to more hydrophilic sulfone. This triggers disassembly of the micelle into unimeric polymers and releases the Nile red into the more polar aqueous environment where its fluorescence is no longer apparent. The fluorescence intensity of the negative control samples that were not treated with H2O2 remained relatively constant over the timeframe studied, indicating that the micelles were stable in the absence of ROS. This observation suggests that the poly(PS74−b-DMA310) micelles would have little nonspecific drug “leak” in vivo prior to targeted disassembly and drug release at sites of oxidative stress.
Figure 5.
H2O2 concentration-dependent release of the model drug Nile red from poly(PS74−b-DMA310) micelles. Figure legend indicates volume % of H2O2 in PBS (n=3).
To verify that the decrease in fluorescence intensity of Nile red during the release experiments was due to micelle release and not H2O2 degradation of the dye itself, the free Nile red molecule was dissolved in a mixture of THF/water (15/85) and treated with a range of concentrations of H2O2. The fluorescence intensity was found to remain constant over the period of 170 h, confirming that loss of signal was due to dye released from the micelles due to destabilization of the micelle structure (Figure S-4). Furthermore, because subsequent cell experiments were done using the dyes DiO and DiI, an additional experiment was done to confirm that H2O2 dose dependent micelle release of these model drugs was similar to the release profile for Nile red-loaded micelles (Figure S-5).
To assess the responsiveness of the poly(PS74−b-DMA310) micelles to other ROS, the in vitro release kinetics of Nile red were measured over a period of 92 h following treatment with a range of concentrations (1-100 mM) of the peroxynitrite generator SIN-1 (Figure S-6). The rate of release of Nile red from the micelles was found to be dependent on the concentration of SIN-1. To confirm that release was mediated by peroxynitrite, Nile red release in response to treatment with peroxynitrite alone (1-100 μM) was also tested. Use of exogenous peroxynitrite to model endogenous production is difficult to mimic in vitro because it has a very short (∼1 s) half-life at physiological pH. However, it was found that 20% Nile Red release was triggered from micelles after a single treatment with 100 μM peroxynitrite (Figure S-7), further validating the multi-faceted response of the (PS74−b-DMA310) micelles to various ROS species. These results indicate that poly(PS74−b-DMA310) may be broadly applicable for in vivo delivery to pathological sites with high oxidative stress.
Direct comparison of hydrogen peroxide or peroxynitrite concentrations in vivo versus those used in the cell-free experiments reported here are difficult because cells at sites of inflammation in vivo continuously generate ROS (many of which have very short half-lives), whereas, for practical reasons, bolus dose delivery is utilized in cell free studies It has been postulated, for example, that much higher concentrations of exogenous peroxynitrite (half-life <1 s at physiologic pH) may be required to achieve biologic responses similar to those produced by much lower concentrations of continuously produced endogenous peroxynitrite in vivo [37]. Here, we have compared cell-free bolus doses to the total quantity of H2O2 or peroxyntitrite that would be generated within a given tissue volume during a defined timeframe. These calculations are based on an average (spherical)macrophage diameter of 21 μm [38] and published values of 0.63 - 1.258 nmol/(106 cells)/min and 0.11 nmol/(106 cells)/min for activated macrophage production of H2O2 and peroxynitrite, respectively [39-41]. Using these values, one can estimate that, over a brief 6 hour period, a concentration of 47-93 mM H2O2 or 8 mM peroxynitrite would be accumulated locally in vivo if there was no diffusional loss or degradation of these species. Note that the intermediate H2O2 concentration of 0.33 vol% tested in the Nile red release study corresponds to 97 mM and correlates well to the higher end values in this calculation, while the calculated value for peroxynitrite exceeds the highest dose (100 μM) tested in the peroxynitrite Nile red release study by 80-fold. It is estimated that the peroxynitrite generation rate of SIN-1 is 1μM/min/(mM SIN-1), and an approximately 1 mM dose has been used to mimic pathological conditions on cultured cells [42]. Over 6 hours, the 1 mM SIN-1 would generate a concentration of 360 μM peroxynitrite under the no-loss assumption, which is 3.6-fold higher than the highest dose tested in our peroxynitrite bolus delivery experiment (Figure S-7).
The preceding analysis suggests that the peroxynitrite and SIN-1 concentrations used in our drug release experiments span physiologically-relevant ranges, while the hydrogen peroxide concentrations may have been higher than what would be present in inflamed tissues. Because sites of inflammation in vivo consist of a complex milieu of different ROS and reactive nitrogen species, it is potentially advantageous that poly(PS74−b-DMA310) micelles were responsive to multiple reactive species, and it is not necessarily a problem if the micelles do not sufficiently respond to H2O2 alone to trigger rapid release. The release behavior of the poly(PS74−b-DMA310) carriers may in fact be ideally tuned to remain stable “in transit” and slowly respond to achieve sustained release of cargo once a pathophysiological environment is encountered. Though more switch-like release from the nanocarriers may be ideal for some applications, it is conceivable that the sustained release profile that these nanocarriers display may be therapeutically advantageous in some scenarios, and in vivo testing will need to be done to better validate the behavior of these carriers for different pathologies.
3.6 FRET-based Imaging of Micelle Release by Activated Macrophages
Proof-of concept studies in LPS-activated macrophages indicated successful use of poly(PS74−b-DMA310) micelles for environmentally-targeted drug release in response to endogenously-produced ROS. To do so, release from micelles co-loaded with DiO and DiI was measured using FRET. A FRET signal (emission of DiI, a red fluorophore, upon excitation of DiO, a green fluorophore) occurs only when the two dyes are in close proximity, i.e., co-loaded into the micelle core. Fluorimetry measurements following excitation at 484 nm was utilized to confirm FRET signal in dual DiO/DiI-loaded Poly(PS74−b-DMA310) micelles (Figure 6a). Tenfold dilution of dual DiO/DiI-loaded micelles into DMF, an organic solvent that disrupts the micelle core, disrupted the FRET signal, but a similar tenfold dilution into PBS did not (Figure 6b), further confirming successful generation of FRET micelles.
Figure 6.
Demonstration of FRET effect in Poly(PS74−b-DMA310) micelles co-loaded with DiO and DiI. (a) Fluorescence emission spectra of PPS74−b-PDMA310 FRET micelles (1% DiI and 1% DiO), 1% DiO micelles, and 1% DiI micelles with 484-nm excitation. (b) Fluorescence spectra of micelles diluted tenfold into PBS or DMF. FRET signal is indicated by the quenching of emission at 510 nm and increase in emission at 580 nm in intact, dual-loaded micelle samples.
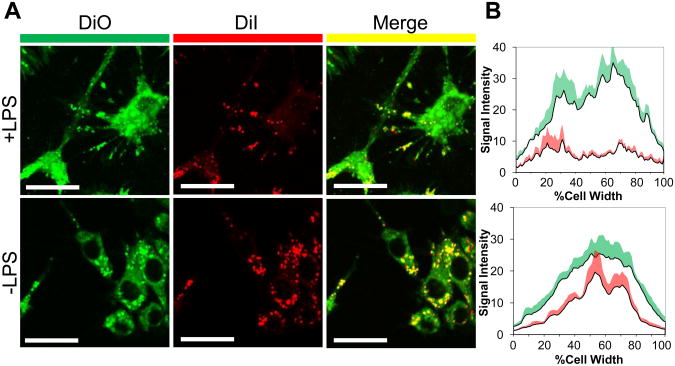
ROS endogenously produced by LPS-activated macrophages were shown to trigger release of the DiO/DiI dual-loaded poly(PS74−b-DMA310) micelles in vitro using this FRET-based readout. Bacterial LPS was used to activate RAW macrophages since it models inflammatory disease states, activates ROS-producing enzymes, and stimulates production of ROS and peroxynitrite in macrophage [43-45]. Confocal microscopy imaging of control cells showed the presence of the FRET signal (quenched DiO/green, bright DiI/red) in control cells excited at 488 nm, suggesting cell internalization of the intact DiO/DiI dual-loaded micelles. In LPS-activated macrophages, a decrease in DiI/red signal and increase in DiO/green signal suggested that endogenously-produced ROS had triggered micelle disruption and intracellular dye release (Figure 7a). Quantitative analysis of the macrophage images confirmed this change in the fluorescence profile between LPS-treated and control cells (Figure 7b). Macroscopic plate reader data also indicated that there was a significant reduction in FRET signal for cells treated with LPS relative to vehicle controls (p<.005) (Figure S-8). These combined FRET microscopy and plate reader data strongly indicate that “inflamed” macrophages generate sufficient ROS to facilitate release of poly(PS74−b-DMA310) micelle cargo. This proof-of-principle experiment confirmed the potential feasibility in using this system for environmentally-targeted delivery of hydrophobic drugs to sites of inflammation.
Figure 7.
Cell-mediated micelle release of FRET pair DiO and DiI by LPS-activated macrophages. (a) Representative FRET confocal microscopy images of RAW 264.7 cells delivered poly(PS74−b-DMA310) micelles co-loaded with DiI and DiO with and without pre-activation with LPS. Upon excitation at 485 nm, decreased red emission (560-615 nm) and increased green emission (505-550 nm) was visible in cells treated with LPS relative to controls, indicating that LPS-driven ROS generation triggered release of micelle cargo. Scale bars = 25 μm. (b) Green and red fluorescent emission intensity integrated across the longest dimension of macrophages (n= 4) showed quantitative differences, with a significant drop in the red (FRET-based) signal in the cell populations treated with LPS. Standard error is indicated by the shaded region overlaying each data set.
3.7 Cell viability
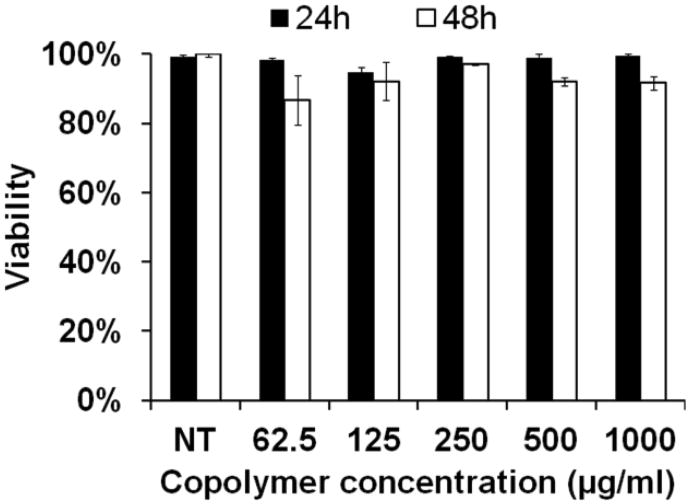
The cytotoxicity of the poly(PS74−b-DMA310) micelles was evaluated in RAW 264.7 cells for a range of concentrations (0-1000 μg/mL) using the LDH assay. This assay offers a simple way to measure LDH, a stable cytoplasmic enzyme present in most cells that provides an accurate marker for cell viability [46]. Figure 8 shows that viability of the RAW cells remained high in the presence of micelles across the entire range of concentrations tested after both 24h and 48h incubation times.
Figure 8.
Poly(PS74−b-DMA310) cytocompatibility in RAW 264.7 macrophages was found to be high across the entire range of micelle concentrations tested at both 24 and 48 h. The cell viability was determined by LDH assay and each treatment group was normalized to no treatment (NT) controls (n = 3).
4. Conclusion
This report presents novel, ROS-responsive polymeric micelles that have the potential to be applied to preferentially release entrapped hydrophobic drug cargo under pro-inflammatory, oxidative environments. Thorough characterization of the “smart” poly(PS74−b-DMA310) micelles demonstrated responsiveness to H2O2, SIN-1, and peroxynitrite, indicating that multiple reactive species would contribute to cargo release at sites of inflammation. The reaction of PPS-containing polymers with SIN-1/peroxyntitrite is a novel finding to our knowledge, and it is anticipated that response to oxidants other than H2O2 is important for accomplishing drug release under pathophysiologically-relevant conditions. The importance of this finding is further supported by the fact that other sulfides have been shown to react three times faster with peroxynitrite than to H2O2[47]. To this end, it was found that LPS-activated macrophages, a model system known to robustly produce peroxynitrite [44], preferentially triggered cell-mediated micelle release of the FRET pair DiO and DiI. It was also shown that the micellar carrier did not cause any in vitro cytotoxicity, and it is aniticipated that conversion of polypropylene sulfide to more water soluble forms (i.e., polypropylene sulfoxide) would result in safe removal of the micelle constituents from body after exposure to ROS rich environments. These combined data indicate that poly(PS74−b-DMA310) micelles provide a promising platform for targeted drug therapy at sites of oxidative stress and that this carrier may be especially good candidate for delivery to pathological sites with high peroxynitrite production.
Supplementary Material
Acknowledgments
This research was supported by Vanderbilt University School of Engineering startup funds. Dynamic light scattering and TEM were conducted through the use of the core facilities of the Vanderbilt Institute of Nanoscale Sciences and Engineering (VINSE). Confocal Microscopy was performed in part through the use of the VUMC Cell Imaging Shared Resource, (supported by NIH grants CA68485, DK20593, DK58404, HD15052, DK59637, and Ey008126.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Archives of Biochemistry and Biophysics. 2003;417(1):3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- 2.Pacher Pl, Beckman JS, Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiological Reviews. 2007;87(1):315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal Tract Complications of Nonsteroidal Anti-inflammatory Drug Treatment in Rheumatoid Arthritis: A Prospective Observational Cohort Study. Arch Intern Med. 1996;156(14):1530–1536. [PubMed] [Google Scholar]
- 5.Ma N, Li Y, Ren H, Xu H, Li Z, Zhang X. Selenium-containing block copolymers and their oxidation-responsive aggregates. Polymer Chemistry. 2010;1(10):1609–1614. [Google Scholar]
- 6.Han P, Ma N, Ren H, Xu H, Li Z, Wang Z, Zhang X. Oxidation-Responsive Micelles Based on a Selenium-Containing Polymeric Superamphiphile. Langmuir. 2010;26(18):14414–14418. doi: 10.1021/la102837a. [DOI] [PubMed] [Google Scholar]
- 7.Ma N, Li Y, Xu H, Wang Z, Zhang X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. Journal of the American Chemical Society. 2009;132(2):442–443. doi: 10.1021/ja908124g. [DOI] [PubMed] [Google Scholar]
- 8.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoshima S, Sugihara S. Syntheses of stimuli-responsive block copolymers of vinyl ethers with side oxyethylene groups by living cationic polymerization and their thermosensitive physical gelation. Journal of Polymer Science Part A: Polymer Chemistry. 2000;38(21):3962–3965. [Google Scholar]
- 10.Qin S, Geng Y, Discher DE, Yang S. Temperature-Controlled Assembly and Release from Polymer Vesicles of Poly(ethylene oxide)-block- poly(N-isopropylacrylamide) Advanced Materials. 2006;18(21):2905–2909. [Google Scholar]
- 11.Bae Y, Fukushima S, Harada A, Kataoka K. Design of Environment-Sensitive Supramolecular Assemblies for Intracellular Drug Delivery: Polymeric Micelles that are Responsive to Intracellular pH Change. Angewandte Chemie International Edition. 2003;42(38):4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]
- 12.Dimitrov I, Trzebicka B, Müller AHE, Dworak A, Tsvetanov CB. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Progress in Polymer Science. 2007;32(11):1275–1343. [Google Scholar]
- 13.Ghosh S, Irvin K, Thayumanavan S. Tunable Disassembly of Micelles Using a Redox Trigger. Langmuir. 2007;23(15):7916–7919. doi: 10.1021/la700981z. [DOI] [PubMed] [Google Scholar]
- 14.Napoli A, Valentini M, Tirelli N, Muller M, Hubbell JA. Oxidation-responsive polymeric vesicles. Nat Mater. 2004;3(3):183–189. doi: 10.1038/nmat1081. [DOI] [PubMed] [Google Scholar]
- 15.Broaders KE, Grandhe S, Fréchet JMJ. A Biocompatible Oxidation-Triggered Carrier Polymer with Potential in Therapeutics. Journal of the American Chemical Society. 2010;133(4):756–758. doi: 10.1021/ja110468v. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Tong X, Zhao Y. Preparation of Azobenzene-Containing Amphiphilic Diblock Copolymers for Light-Responsive Micellar Aggregates. Macromolecules. 2004;37(24):8911–8917. [Google Scholar]
- 17.Jiang Y, Wang Y, Ma N, Wang Z, Smet M, Zhang X. Reversible Self-Organization of a UV-Responsive PEG-Terminated Malachite Green Derivative: Vesicle Formation and Photoinduced Disassembly. Langmuir. 2007;23(7):4029–4034. doi: 10.1021/la063305l. [DOI] [PubMed] [Google Scholar]
- 18.Rijcken CJF, Soga O, Hennink WE, Nostrum CFv. Triggered destabilisation of polymeric micelles and vesicles by changing polymers polarity: An attractive tool for drug delivery. Journal of Controlled Release. 2007;120(3):131–148. doi: 10.1016/j.jconrel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Vo CD, Kilcher G, Tirelli N. Polymers and Sulfur: what are Organic Polysulfides Good For? Preparative Strategies and Biological Applications. Macromolecular Rapid Communications. 2009;30(4-5):299–315. doi: 10.1002/marc.200800740. [DOI] [PubMed] [Google Scholar]
- 20.Velluto D, Demurtas D, Hubbell JA. PEG-b-PPS Diblock Copolymer Aggregates for Hydrophobic Drug Solubilization and Release: Cyclosporin A as an Example. Molecular Pharmaceutics. 2008;5(4):632–642. doi: 10.1021/mp7001297. [DOI] [PubMed] [Google Scholar]
- 21.Cerritelli S, ONeil CP, Velluto D, Fontana A, Adrian M, Dubochet J, Hubbell JA. Aggregation Behavior of Poly(ethylene glycol-bl-propylene sulfide) Di- and Triblock Copolymers in Aqueous Solution. Langmuir. 2009;25(19):11328–11335. doi: 10.1021/la900649m. [DOI] [PubMed] [Google Scholar]
- 22.Nagai A, Koike N, Kudo H, Nishikubo T. Controlled thioacyl group transfer (TAGT) polymerization of cyclic sulfide: Novel approach to AB diblock copolymers by the combination of RAFT and TAGT polymerizations. Macromolecules. 2007;40(23):8129–8131. [Google Scholar]
- 23.Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH. Living Free-Radical Polymerization by Reversible Addition Fragmentation Chain Transfer: The RAFT Process. Macromolecules. 1998;31(16):5559–5562. [Google Scholar]
- 24.Šprincl L, Vacík J, Kopeček J, Lím D. Biological tolerance of poly(N-substituted methacrylamides) Journal of Biomedical Materials Research. 1971;5(3):197–205. doi: 10.1002/jbm.820050307. [DOI] [PubMed] [Google Scholar]
- 25.Kopeček J, Šprincl L, Bažilová H, Vacík J. Biological tolerance of poly(N-substituted acrylamides) Journal of Biomedical Materials Research. 1973;7(1):111–121. doi: 10.1002/jbm.820070109. [DOI] [PubMed] [Google Scholar]
- 26.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. Development of a novel endosomolytic diblock copolymer for siRNA delivery. J Control Release. 2009;133(3):221–229. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler SD, Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33(8):833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 28.Coutinho PJG, Castanheira EMS, Céu Rei M, Real Oliveira MECD. Nile Red and DCM Fluorescence Anisotropy Studies in C12E7/DPPC Mixed Systems. The Journal of Physical Chemistry B. 2002;106(49):12841–12846. [Google Scholar]
- 29.Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, Stayton PS. pH-Responsive Polymeric Micelle Carriers for siRNA Drugs. Biomacromolecules. 2010 doi: 10.1021/bm100652w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu P, Tirelli N. Scavenging ROS: Superoxide Dismutase/Catalase Mimetics by the Use of an Oxidation-Sensitive Nanocarrier/Enzyme Conjugate. Bioconjugate Chemistry. 2012;23(3):438–449. doi: 10.1021/bc200449k. [DOI] [PubMed] [Google Scholar]
- 31.Hogg N, Darley-Usmar VM, Wilson MT, Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by Forster resonance energy transfer imaging. Proc Natl Acad Sci U S A. 2008;105(18):6596–6601. doi: 10.1073/pnas.0707046105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duvall CL, Convertine AJ, Benoit DSW, Hoffman AS, Stayton PS. Intracellular Delivery of a Proapoptotic Peptide via Conjugation to a RAFT Synthesized Endosomolytic Polymer. Molecular Pharmaceutics. 2009;7(2):468–476. doi: 10.1021/mp9002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discovery Today. 2006;11(17-18):812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Trimaille T, Mondon K, Gurny R, Moller M. Novel polymeric micelles for hydrophobic drug delivery based on biodegradable poly(hexyl-substituted lactides) Int J Pharm. 2006;319(1-2):147–154. doi: 10.1016/j.ijpharm.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Seaver LC, Imlay JA. Are respiratory enzymes the primary sources of intracellular hydrogen peroxide? J Biol Chem. 2004;279(47):48742–48750. doi: 10.1074/jbc.M408754200. [DOI] [PubMed] [Google Scholar]
- 37.Beckman JS, Chen J, Ischiropoulos H, Crow JP, Lester P. Methods in Enzymology. Vol. 233. Academic Press; 1994. pp. 229–240. [DOI] [PubMed] [Google Scholar]
- 38.Krombach F, Munzing S, Allmeling AM, Gerlach JT, Behr J, Dorger M. Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect. 1997;105(5):1261–1263. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan CF, Root RK. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. The Journal of Experimental Medicine. 1977;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johansson A, Jesaitis AJ, Lundqvist H, Magnusson KE, Sjölin C, Karlsson A, Dahlgren C. Different Subcellular Localization of Cytochrome b and the Dormant NADPH-Oxidase in Neutrophils and Macrophages: Effect on the Production of Reactive Oxygen Species during Phagocytosis. Cellular Immunology. 1995;161(1):61–71. doi: 10.1006/cimm.1995.1009. [DOI] [PubMed] [Google Scholar]
- 41.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Archives of Biochemistry and Biophysics. 1992;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 42.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: Implications for uncoupling endothelial nitric oxide synthase. Biochemical Pharmacology. 2005;70(3):343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Emre Y, Hurtaud C, Nübel T, Criscuolo Fo, Ricquier D, Cassard-doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402(2):271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zingarelli B, O'Connor M, Wong H, Salzman AL, Szabà C., 3 Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. The Journal of Immunology. 1996;156(1):350–358. [PubMed] [Google Scholar]
- 45.Adams DO, Hamilton TA. The Cell Biology Of Macrophage Activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 46.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of Immunological Methods. 1988;115(1):61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 47.Lobachev V, Zimtseva G, Rudakov E. Oxidation of Diethyl Sulfide in Aqueous Solutions by Peroxynitrite and the H2O2-NO. Theoretical and Experimental Chemistry. 2005;41(5):302–309. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.