Abstract
In the intestine, bacterial components activate innate responses that protect the host. We hypothesize that bacterial components reduce Interleukin-8 (IL-8) production in intestinal epithelial cells stimulated by flagellin via the Toll-like receptor (TLR) signaling pathway. Caco-2 cells were pretreated with various doses of lipopolysaccharide (LPS), lipoteichoic acid (LTA), or low-dose flagellin (LDFL) for 24 hours. Cells were then treated with flagellin (FL) 500 ng/mL (HDFL) for another 48 hours. IL-8 production was measured in the cell culture medium by ELISA. Eighty-four genes in the TLR pathway were evaluated by RT Profiler PCR Array. Pathway Studio 8.0 software was used for altered pathway analysis. HDFL induced IL-8 production by 19 fold (p<0.01). Pre-treatment with LDFL at 20, 10 or 1 ng /ml reduced HDFL-induced IL-8 production by 61%, 52% and 40%, respectively (p<0.05). LPS at 50 µg/ml decreased HDFL–induced IL-8 production by 38% (p<0.05). HDFL up-regulated CXCL10, IL-1B, IL-8, IRAK2, NF-κB1 and I-κB (all p< 0.05). Pathway Studio analysis showed that HDFL induced cell processes including inflammation, cell death and apoptosis. Pre-treatment with LDFL at 10 ng/mL down-regulated FADD, FOS, MAP4K4, MyD88, TLR2, TLR3 and TNFERSF1A compared to HDFL (all p<0.05). These down-regulated genes are integral for numerous cell functions including inflammatory response, cell death, apoptosis and infection. These results demonstrate that LPS and LDFL provoke tolerance to HDFL-induced IL-8 production. This tolerance effect was accompanied by a complex interaction of multiple genes related to inflammatory as well as other responses in the TLR pathway rather than a single gene alteration.
Keywords: Tolerance, Toll-Like Receptors, Flagellin, Intestinal Epithelial Cells, systems biology
1. Introduction
The human gastrointestinal tract is a home to trillions of commensal microorganisms that play an important role in intestinal physiology, as well as the development and functions of the intestinal epithelium [1–3]. Intestinal epithelial cells also contribute to the regulation of inflammatory conditions and create an intestinal barrier against invading pathogens [4]. As a result, the intestinal epithelium must maintain homeostasis by differentiating between resident microorganisms and invading pathogens [5]. Toll-like receptors (TLRs) in the gut epithelium are pattern recognition receptors (PPRs) that help maintain homeostasis by recognizing ligands known as microbial-associated molecular patterns (MAMPs) from both pathogenic and non-pathogenic bacteria [6–12]. They subsequently create signals that promote epithelial cell proliferation, secretion of Immunoglobulin A into the gut lumen, and antimicrobial peptide expression [3]. In turn, this helps maintain a healthy epithelial barrier, making TLRs key components for the innate defense against pathogens.
Some ligands recognized by TLRs include lipoteichoic acid (LTA) by TLR2, lipopolysaccharide (LPS) byTLR4 and flagellin by TLR5 [13]. Specific MAMPs recognized by TLR1, TLR2, TLR4, TLR5, and TLR6 mainly stimulate the production of pro-inflammatory cytokines [6,14]. Once TLRs recognize a particular PAMP, they recruit adaptor proteins containing Toll/Interleukin-1 receptor (TIR) domains that initiate downstream signaling cascades [15]. Eventually, the transcription factor Nuclear Factor kappa B (NF-κB), dissociates from inhibitor kappa B (I-κB) and translocates into the nucleus, where it controls the transcription of inflammatory mediators [6,16]. It seems that the gastrointestinal tract is tolerant to certain commensal bacteria because they inhibit I-κB degradation, preventing NF-κB translocation [17].
Probiotics are microorganisms that confer health benefits to their hosts. However, unwanted side effects have been associated with the administration of probiotics [18–27]. A study from our lab suggests that there might be safer alternatives to live probiotics in the prevention and treatment of gastrointestinal conditions [28]. In this study, Caco-2 cells were pre-treated with live and ultraviolet inactivated Lactobacillus Rhamnosus GG, a type of probiotic. Results showed that both treatments significantly blunted Interleukin-8 (IL-8) production after stimulation with flagellin [28]. These altered probiotics may produce the desired health benefits of live probiotics, with minimal to no side-effects to immune-compromised individuals [28,29].
The tolerance effects caused by pre-exposing different kinds of cells to microbial cell components have been reported in several investigations [1,30–33]. One study supports the theory of the beneficial effects that microbial cell components might have in regulating inflammatory responses within intestinal epithelial cells through TLRs by creating tolerance to harmful exposures [1]. Another study showed that pre-treating polarized intestinal epithelial cells with flagellin for a prolonged period of time causes these cells to be insensitive to flagellin-induced stimulation [31]. The findings of this study suggest that the tolerance seen by pre-exposing the cells to flagellin could be attributed to a lack of activity of the downstream signaling molecule IRAK-4.
The purpose of this study is to determine if pre-treating Caco-2 cells with TLR ligands LTA, LPS, and flagellin would blunt the production of IL-8 after inducing IL-8 production with flagellin. We further hypothesize that bacterial components reduce IL-8 production in intestinal epithelial cells stimulated by flagellin via the TLR signaling pathway.
2. Materials and Methods
2.1. Caco-2 cell culture
Caco-2 cells obtained from the American Type Culture Collection (Manassas, VA., USA) were cultured at 37°C under 95% air and 5% carbon dioxide. The cell medium contained minimum essential medium (MEM, Invitrogen Life Technologies), 15% fetal bovine serum (FBS, Invitrogen Life Technologies), MEM Nonessential Amino Acid, and antibiotic-antimycotic solution (ABAM, Invitrogen Life Technologies). The culture medium was changed every other day. Upon reaching confluency, the cells were seeded onto two 24-well plates at 1×105/well (Costar, Corning, Corning, NY., USA) and designated into different treatment groups, in triplicates. Experiments were initiated at 2 weeks after seeding. Previous studies in our laboratory have shown this is a time at which the cells begin to express alkaline phosphatase activity and are in an early stage of differentiation, corresponding to the upper crypt-lower villus stage of differentiation.
Cells were pre-treated with various concentrations of microbial cell components, LTA from Staphylococcus aureus (Sigma, St. Louis, MO, 50µg/ml, 10µg/ml and 1µg/ml), LPS from Escherichia coli O26:B6 (Sigma, St. Louis, MO, 50µg/ml, 10µg/ml, 1 µg/ml) or flagellin (20ng/ml, 10ng/ml and 1ng/ml), respectively, for 24 hrs. The cells were then stimulated by flagellin at 500 ng/ml, a concentration fully inducing IL-8 production based on our previous study [28], for another 24 hrs. The cell medium only group served as a control.
2.2. Expression and purification of flagellin
Flagella were purified from Pseudomonas aeruginosa strains grown overnight in Luria- Bertani broth. Flagella were mechanically sheared from the surface of the bacteria and collected by ultracentrifugation. The purification and extraction process was performed as previously described [34].
2.3. ELISA for IL-8
The cell medium were collected and stored at −20°C. Human IL-8 ELISA (BD Biosciences Pharmingen, San Diego, CA,) was performed on the cells medium per manufacturer’s instructions. Immulon 4HBX-extra High Binding 96-well microtiter plates (Dynex Technologies Inc., Alexandria, VA., USA) were used to quantify the cytokines. The PowerWaveX microplate reader (Bio-TEK Instruments Inc., Winooski, VT., USA) was used to read the plates at 450 nm.
2.4. Human Toll-Like Receptor (TLR) signaling pathway RT2 Profiler™ PCR array
From experiment above, the total RNA of Caco-2 cells was isolated using TRIzol® Reagent (Invitrogen, Carlsbad, CA., USA) in untreated control group, flagellin 500 ng/ml only group and pretreatment groups, including flagellin 10 ng/ml pretreatment + flagellin 500 ng/ml and pretreatment of LPS 50 µg/ml + flagellin 500 ng/ml. The RNA was purified using the RNeasy Mini Kit (Qiagen, Valencia, CA., USA), per manufacturers’ instructions. RNA quantification and quality were obtained by a Nanodrop device and analyzed using bioanalyzer. RNA was reverse transcribed to cDNA using the RT2 First Strand Kit (SABiosciences, Valencia, CA). A real time PCR was performed on the cDNA using the Human TLR Signaling Pathway RT2 Profiler™ PCR Array (SABiosciences, Valencia, CA) by a ABI HT7900 real time PCR system (Applied BiosystemsFoster City, CA). The expression of 84 genes in the TLR signaling pathway for each condition was profiled using this method. Three “housekeeping” genes (B2M, RPL13A and ACTB) served as internal controls. To normalize gene expression (2−ΔCt) and determine the fold change between groups (2−ΔΔCt), the average of the housekeeping genes Ct values was used.
2.5. Systems biology analysis
The altered pathway relevant to different treatment was analyzed using Pathway Studio 8.0 software (Ariadne Genomics, Rockville, MD, USA). This software helps to interpret biological meaning from gene (protein) expression, build and analyze pathways, and find relationships among genes, proteins, cell processes, and diseases. PathwayStudio comes with a built-in resource named ResNet, which is a database of molecular interactions based on natural language processing of scientific abstracts in PubMed. Using ResNet, a researcher can simply drag his favorite gene product/protein list onto a new pathway diagram and build a pathway using well-known interactions discussed in existing literature. In our study, we first imported a gene list including altered genes after different concentrations of treatment. The network was generated using the “direct interaction” algorithm to map cellular process and interactions between altered proteins. The program searches the current pathway database and ResNet for interactions with the selected entities, and adds them to the pathway. After the new pathway was built, we were able to obtain more detailed information of the altered gene components.
2.6. Statistical analysis
Values are given as means ± SEM of triplicate measurements. One-way ANOVA were performed to determine whether the varying pretreatments of microbial cell components differed in their ability to lower the flagellin-induced IL-8 production by intestinal epithelial cells. We performed multiple comparisons of means using post-hoc Tukey’s test. Student T-test was used for qRT-PCR array results. GraphPad Prism was used for all analyses. Differences among means were considered significant at < 0.05.
3. Results
3.1. Pretreatment effects of the cell components Flagellin, LTA and LPS on IL-8 production
The treatment of the Caco-2 cells with flagellin 500 ng/ml (HDFL) produced a 19-fold increase in IL-8 production (p<0.05). The pretreatment with LPS at 50 µg/ml, 10 µg/ml and 1 µg/ml decreased HDFL-induced IL-8 production compared to HDFL group by 38%, 34% and 62%, respectively (p<0.05). The pretreatment with LDFL at 20 ng/ml, 10 ng/ml and 1 ng/ml decreased HDFL-induced IL-8 production compared to HDFL group, 61%,52% and 40%, respectively (p<0.05). The pretreatment with LTA 50 µg/ml, LTA 10 µg/ml and LTA 1 µg/ml did not reduce HDFL-induced IL-8 production (Fig 1).
Figure 1. Effect of pretreatment of the cell components Flagellin, LTA and LPS on IL-8 production.
The IL-8 production was measured using ELISA in Caco-2 cell culture medium. Flagellin at 500 ng/ml (HDFL) increased IL-8 production (p<0.05). The pretreatment with various dosed of LPS and flagellin decreased HDFL-induced IL-8 production (P<0.05). The pretreatment with LTA did not show the effects on HDFL-induced IL-8 production. Values are given as means ± SEM. n=3. *: p< 0.05 vs. control; §: p<0.05 vs. HDFL.
3.2. Effect of HDFL on TLR pathways in intestinal epithelial cells
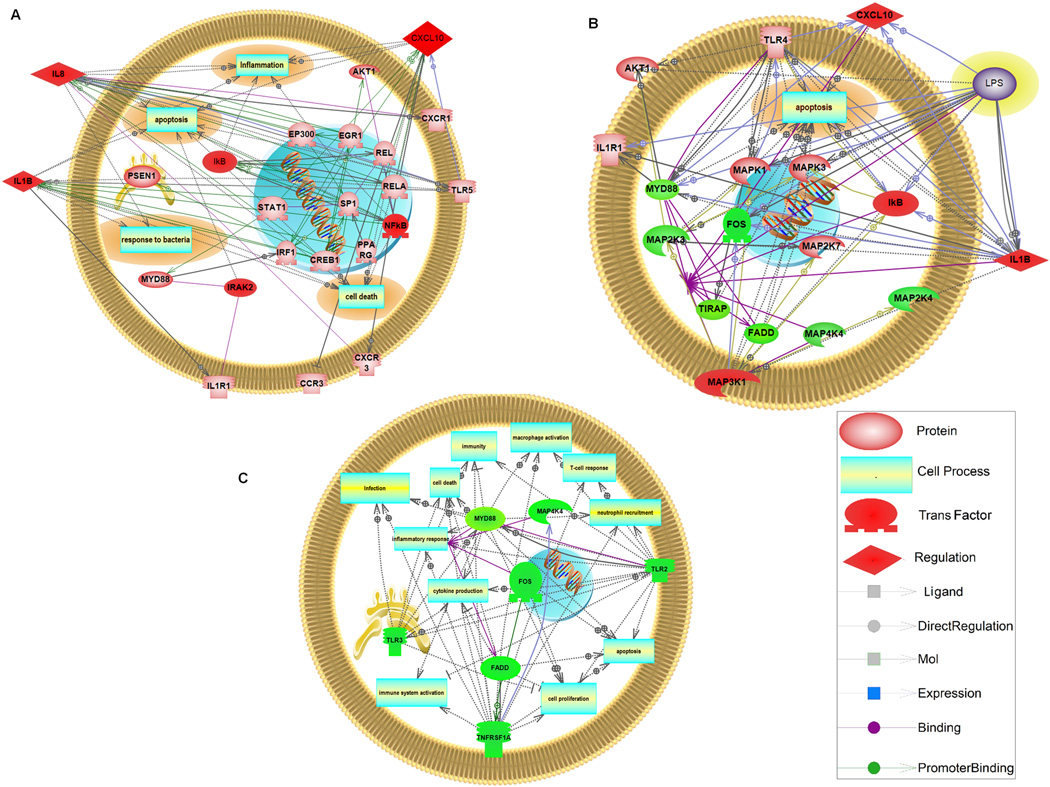
RT real time PCR array results showed that high dose of flagellin at 500 ng/ml significantly up-regulated 6 genes in TLR signaling pathway, including CXCL10, IL1B, IL8, IRAK2, NF-κB1 and I-κB compared to untreated cells ( P<0.05, Table 1). A Pathway Studio analysis showed that HDFL induced cell processes including inflammation, response to bacteria, apoptosis and cell death (Fig 2A).
Table 1.
The genes in TLR pathway regulated by stimulation with flagellin 500ng/ml (p<0.05).
| Gene | Fold Regulation | P−Value |
|---|---|---|
| IRAK2 | 1.56 | 0.046 |
| NFKB1 | 1.13 | 0.049 |
| IκBα | 1.79 | 0.004 |
| CXCL10 | 2.61 | 0.004 |
| IL1B | 1.67 | 0.048 |
| IL8 | 1.58 | 0.028 |
Figure 2. Systems Biology Analysis of altered genes in TLR Pathway and Networks.
Using Pathway Studio 8.0, altered genes in TLR pathway relevant to different treatments were analyzed. The network was generated using the “direct interaction” algorithm to map cellular process and interactions between altered genes. The program searches the current pathway database and ResNet for interactions with the selected entities, and adds them to the pathway. The up-regulated genes are in red and down-regulated genes are in green.
3.3. Effect of Flagellin 10 ng/ml pre-treatment on TLR pathways in HDFL challenged intestinal epithelial cellsh
Flagellin pretreatment at 10 ng/ml up-regulated 4 genes, including CXCL10, IL1B, MAP3K1 and I-κB and down-regulated 6 others, including FADD, FOS, MAP2K3, MAP4K4, MyD88, and TIRAP compared to the untreated control group (P<0.05, table 2a). These altered genes showed major changes in apoptosis pathways, but not inflammation process using Pathway Studio analysis (Fig 2B). The effects on MAP3K1, FOS and MAP4K4 suggest that the pretreatment with flagellin 10 ng/ml acts through both NF-κB and AP-1 pathways.
Table 2.
The genes in TLR pathway regulated by flagellin 10ng/ml pretreatment (p<0.05).
| (a) The effects of flagellin 10ng/ml pretreatment followed by flagellin 500ng/ml compared to the untreated control group. | ||
|---|---|---|
| Gene | Fold Regulation | P−Value |
| MYD88 | −1.59 | 0.000 |
| TIRAP | −1.24 | 0.020 |
| FADD | −1.32 | 0.048 |
| MAP3K1 (MEKK1) | 1.31 | 0.036 |
| MAP2K3 (MKK3) | −1.26 | 0.039 |
| MAP4K4 (NIK) | −1.50 | 0.049 |
| FOS | −1.79 | 0.020 |
| NFKB1A (IκBα) | 1.81 | 0.002 |
| CXCL10 | 3.01 | 0.021 |
| IL 1 B | 1.61 | 0.038 |
| (b) The effects of flagellin 10ng/ml pretreatment followed by flagellin 500ng/ml compared to flagellin 500ng/ml only. | ||
|---|---|---|
| Gene | Fold Regulation | P−Value |
| TNFRSF1A | −1.18 | 0.026 |
| TLR 2 | −1.27 | 0.021 |
| TLR 3 | −1.34 | 0.034 |
| MyD88 | −1.54 | 0.016 |
| FADD | −1.26 | 0.018 |
| FOS | −1.52 | 0.048 |
| MAP4K4 (NIK) | −1.55 | 0.001 |
When compared to HDFL only cells, the pretreatment with flagellin 10 ng/ml only caused a significant down-regulation in 7 genes, including FADD, FOS,MAP4K4, MyD88, TLR 2, TLR 3 and TNFRSF1A (P<0.05, table 2b). The rest of genes remained unchanged. These down-regulated genes are integral for numerous cell functions including inflammatory response, cell death, apoptosis and infection (Fig 2C).
3.4. Effect of LPS 50 µg/ml pre-treatment on TLR pathways in HDFL challenged intestinal epithelial cells
When compared to the untreated control group, LPS 50 µg/ml pretreatment caused a significant up-regulation of I-κB and CXCL10 (p<0.05, Table 3a). When compared to HDFL group, pretreatment with LPS 50 µg/ml significantly up-regulated TLR 6 (p<0.05, Table 3b), which plays a fundamental role in pathogen recognition and activation of innate immunity [35]. This suggests that TLR 6 may be involved in the tolerance effect.
Table 3.
The genes in TLR pathway regulated by LPS 50µg/mL pre-treatment (p<0.05).
| (a) The effect of LPS 50µg/mL pre-treatment followed by flagellin 500ng/ml compared to the untreated control group | ||
|---|---|---|
| Gene | Fold Regulation | P-Value |
| CXCL10 | 2.86 | 0.006 |
| IκBα | 1.49 | 0.029 |
| (b) The effect of LPS 50µg/ml pre-treatment followed by flagellin 500ng/ml compared to flagellin 500ng/ml only. | ||
|---|---|---|
| Gene | Fold Regulation | P-value |
| TLR 6 | 11.74 | 0.034 |
4. Discussion
Our findings show that the pretreatment of Caco-2 cells with the cell components LPS and low dose flagellin decreases the high dose flagellin-induced IL-8 production. These results prove that low dose of LPS and LDFL induce a tolerance to the stimulation of HDFL in intestinal epithelial cells. However, this tolerance effect is not seen with the pretreatment of LTA.
In a previous study by Sun et al, flagellin induced tolerance of the TLR 5 signaling pathway was seen in all polarized epithelial cell lines tested, suggesting that the ability to become tolerant is common to epithelial cells [31]. It was also suggested that flagellin tolerance occurs at an early step in the TLR5 signaling pathway because the tolerant cells remained responsive to TNF-α stimulation but not to flagellin [31].
Our study demonstrated that the tolerance effect is regulated by the genes in the TLR signaling pathway when cells are pretreated with the microbial cell components, LPS and flagellin. Numerous genes were up- or down-regulated by the treatment of microbial components. Multiple pathways are involved in decreasing the IL-8 production by the pretreatment of the microbial cell components compared to the HDFL group. In addition, the tolerance occurs as a result of “reversing” the effects HDFL. When the cells are stimulated with Flagellin 500 ng/ml, there is an absolute up-regulation of specific gene expression, including CXCL10, IL1B, IL8, IRAK2, NF-κB1 and I-κB compared to untreated cells. However, when the cells are pretreated with Flagellin 10 ng/ml, there is an absolute down-regulation of specific gene expression, including FADD, FOS, MAP4K4, MyD88, TLR 2, TLR 3 and TNFRSF1A compared to the cells treated with HDFL. Interestingly, this reversing effect involved genes that are not common for these two treatments. These results suggest that multiple genes are associated with the tolerance process. It is possible that some other factors may also play a role [36].
In this study, a high dose of flagellin induced an overall increase in both inflammatory cytokines and the genes involved in the NF-κB pathway, which is consistent with prior studies that show the role of the NF-κB/ I-κB complex in the inflammatory response (Fig 3) [1,28,29]. Pretreatment with either LPS or LDFL up-regulated I-κB compared to untreated cells, suggesting a decrease in a translocation of NF-κB to the nucleus. In particular, when there is flagellin induced IL-8 production, Iκ-B becomes phosphorylated which signals it for ubiquitination [29]. Iκ-B is then no longer attached to NF-κB, allowing the NF-κB to translocate into the nucleus of the cell, which ultimately results in inflammation [28]. Our findings suggest that the pretreatment of the cells with LDFL followed by stimulation with HDFL causes significant down-regulation in the genes involved in both NF-κB and JNK/p38 pathways.
Figure 3. Toll-Like Receptors Signaling Pathway.
The diagram of the signaling cascades in TLR pathway. Affected signals by treatments were shaded.
In vitro and in vivo studies have shown that exposure of cells to the TLR4 ligand LPS induces tolerance toward a second exposure to LPS and induces cross-tolerance to some other TLR ligands [37– 39]. Using human peripheral blood mononuclear cells, LPS-induced tolerance caused by the pretreatment of LPS showed attenuated or inhibited expression of TRIF, NF-κB, ERK and JNK cascade-related genes [37]. A Study by Vartanian KB et al also demonstrated that LPS preconditioning redirects TLR4 signaling in response to stroke through inhibition of NFκB activity, enhanced IRF3 activity, and increased anti-inflammatory/type I IFN gene expression in mouse model [38]. In the present study, the genes involved in upstream of NF-κB pathway were not regulated in LPS pretreated cells, suggesting that LPS-induced tolerance might not affect this part of the pathway.
In this study, LTA pretreatment did not show tolerance to HDFL stimulation. Previous studies from other groups have reported that prolonged LPS or LTA treatments induced tolerance and cross-tolerance in human promonocytic THP-1 cells [40]. LPS-tolerized cells develop cross-tolerance and no longer respond to LTA associated with down-regulation of IRAK protein level and kinase activity. However, prolonged LTA treatment induced tolerance to LTA and did not cause IRAK degradation. LTA-tolerized cells can still respond to LPS stimulation [40]
We employed the systems biology approach by using Pathway Studio to evaluate the altered genes to build and analyze pathways, and find relationships among genes, cell processes, and diseases. Our results demonstrated that HDFL induced cell processes including inflammation, response to bacteria, apoptosis and cell death, while LDFL pretreatment altered genes majorly associated with apoptotic changes but not inflammation process compared to untreated control cells. Furthermore, LDFL pretreatment down-regulated genes that are important for numerous cell processes including inflammatory response, cell death, apoptosis and infections compared to the HDFL-only group. To our knowledge, this is the first time a systems biology approach was used to analyze the signaling cascade of gene regulation associated with intracellular tolerance in intestinal epithelial cells. These changes in gene expression were seen in mRNA level. Further studies could be done to detect the signaling pathway in the protein level by using Western blots.
Overall, our results demonstrate that LPS and LDFL provoke tolerance to HDFL-induced IL-8 production. This tolerance effect was accompanied by a complex interaction of multiple genes related to inflammatory as well as other responses in the TLR pathway rather than a single gene alteration.
Highlights.
LPS and low dose of Flagellin induced tolerance to Flagellin-stimulated IL-8 production.
The tolerance is associated with TLR-IκB/NFκB and JNK/p38.
The mechanism of the tolerance is through down-regulation of genes in TLR pathway.
The tolerance has a systemic interaction of multiple genes related to inflammation.
Abbreviations
- HDFL
FL 500 ng/mL
- I-κB
inhibitor kappa B
- IL-8
Interleukin-8
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- LDFL
low-dose flagellin
- MAMPs
microbial-associated molecular patterns
- NF-κB
Nuclear Factor kappa B
- PPRs
pattern recognition receptors
- TLRs
Toll-like receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SH, Keates A, Moyer MP, Pothoulakis C. MEK is a key modulator for TLR5-induced interleukin-8 and MIP3 gene expression in non-transformed human colonic epithelial cells. J Biol Chem. 2004;279:25179–25188. doi: 10.1074/jbc.M400967200. [DOI] [PubMed] [Google Scholar]
- 3.Abreu M, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–4460. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 4.Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Toll-like receptors-2, -3 and-4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–574. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansonetti PJ, Medzhitov R. Learning tolerance while fighting ignorance. Cell. 2009;138:416–420. doi: 10.1016/j.cell.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S. Pathogen recognition by innate immunity and its signaling. Proc Jpn Acad Ser B. 2009;85:143–156. doi: 10.2183/pjab.85.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 9.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 10.Thompson AJV, Locarnini SA. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol Cell Biol. 2007;85:435–445. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 11.Sharma R, Young C, Neu J. Molecular modulation of intestinal epithelial barrier: contribution of microbiota. J Biomed Biotechnol. 2010;2010:305879. doi: 10.1155/2010/305879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu MT. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–141. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 13.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 14.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 15.Wells JM, Loonen L, Karczewski JM. The role of innate signaling in the homeostasis of tolerance and immunity in the intestine. Zentralbl Bakteriol. 2010;300:41–48. doi: 10.1016/j.ijmm.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Neish AS. The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect. 2002;4:309–317. doi: 10.1016/s1286-4579(02)01543-5. [DOI] [PubMed] [Google Scholar]
- 17.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellin-induced inflammation. Proc Natl Acad Sci USA. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? AmJ Clin Nutr. 2006;83:1256–1264. doi: 10.1093/ajcn/83.6.1256. [DOI] [PubMed] [Google Scholar]
- 19.Besselink MG, van Santvoort HC, Buskens E, et al. Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomized, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659. doi: 10.1016/S0140-6736(08)60207-X. [DOI] [PubMed] [Google Scholar]
- 20.Wassenaar TM, Klein G. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J Food Prot. 2008;71:1734–1741. doi: 10.4315/0362-028x-71.8.1734. [DOI] [PubMed] [Google Scholar]
- 21.Honeycutt TC, El Khashab M, Wardrop RM, III, et al. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: a randomized placebo-controlled trial. Pediatr Crit Care Med. 2007;8:452–458. doi: 10.1097/01.PCC.0000282176.41134.E6. [DOI] [PubMed] [Google Scholar]
- 22.Liong MT. Safety of probiotics: translocation and infection. Nutr Rev. 2008;66:192–202. doi: 10.1111/j.1753-4887.2008.00024.x. [DOI] [PubMed] [Google Scholar]
- 23.Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2008;4:CD006135. doi: 10.1002/14651858.CD006135.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Lin HC, Hsu CH, Chen HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants:a multicenter, randomized, controlled trial. Pediatrics. 2008;122:693–700. doi: 10.1542/peds.2007-3007. [DOI] [PubMed] [Google Scholar]
- 25.Neu J. Perinatal and neonatal manipulation of the intestinal microbiome: a note of caution. Nutr Rev. 2007;65:282–285. doi: 10.1301/nr.2007.jun.282-285. [DOI] [PubMed] [Google Scholar]
- 26.Singhi S. Probiotics in the critically ill: handle with care. Pediatr Crit Care Med. 2007;8:499–501. doi: 10.1097/01.PCC.0000282165.04260.59. [DOI] [PubMed] [Google Scholar]
- 27.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–191. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 28.Lopez M, Li N, Kataria J, Russell M, Neu J. Live and ultraviolet-inactivated lactobacillus rhamnosus gg decrease flagellin-induced interleukin-8 production in caco-2 cells. J Nutr Immunol. 2008;138:2264–2268. doi: 10.3945/jn.108.093658. [DOI] [PubMed] [Google Scholar]
- 29.Kataria J, Li N, Wynn JL, Neu J. Probiotic microbes: do they need to be alive to be beneficial? Nutr Rev. 2009;67:546–550. doi: 10.1111/j.1753-4887.2009.00226.x. [DOI] [PubMed] [Google Scholar]
- 30.Mizel SB, Snipes JA. Gram-negative flagellin-induced self-tolerance is associated with a block in interleukin-1 receptor associated kinase release from toll-like receptor 5. J Biol Chem. 2002;277:22414–22420. doi: 10.1074/jbc.M201762200. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Fegan PE, Desai AS, Madara JL, Hobert ME. Flagellin-induced tolerance of the Toll-like receptor 5 signaling pathway in polarized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G767–G778. doi: 10.1152/ajpgi.00447.2006. [DOI] [PubMed] [Google Scholar]
- 32.McCall CE, Grosso-Wilmoth LM, LaRue K, Guzman RN, Cousart SL. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest. 1993;91:853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 34.Verma A, Arora SK, Kuravi SK, Ramphal R. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect Immun. 2005;73:8237–8246. doi: 10.1128/IAI.73.12.8237-8246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajava AV, Vasselon T. A Network of Hydrogen Bonds on the Surface of TLR2 Controls Ligand Positioning and Cell Signaling. J Biol Chem. 2010;285:6227–6234. doi: 10.1074/jbc.M109.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oshima N, Ishihara S, Rumi MA, Aziz MM, Mishima Y, Kadota C, Moriyama I, Ishimura N, Amano Y, Kinoshita Y. A20 is an early responding negative regulator of Toll-like receptor 5 signalling in intestinal epithelial cells during inflammation. Clin Exp Immunol. 2010;159:185–198. doi: 10.1111/j.1365-2249.2009.04048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendes ME, Baggio-Zappia GL, Coló Brunialti M. Differential expression of toll-like receptor signaling cascades in LPS-tolerant human peripheral blood mononuclear cells. Immunobiology. 2011;216:285–295. doi: 10.1016/j.imbio.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Vartanian KB, Stevens SL, Marsh BJ, Williams-Karnesky R, Lessov NS, Stenzel-Poore MP. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J Neuroinflammation. 2011;8:140. doi: 10.1186/1742-2094-8-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vos AF, Pater JM, van den Pangaart PS, de Kruif MD, van 't Veer C, van der Poll T. In vivo lipopolysaccharide exposure of human blood leukocytes induces cross-tolerance to multiple TLR ligands. J Immunol. 2009;183:533–542. doi: 10.4049/jimmunol.0802189. [DOI] [PubMed] [Google Scholar]
- 40.Jacinto R, Hartung T, McCall C, Li L. Lipopolysaccharide- and lipoteichoic acid-induced tolerance and cross-tolerance: distinct alterations in IL-1 receptor-associated kinase. J Immunol. 2002;168:6136–6141. doi: 10.4049/jimmunol.168.12.6136. [DOI] [PubMed] [Google Scholar]