Abstract
Type I interferons (IFN-I) were first described over 50 years ago as factors produced by cells that interfere with virus replication and promote an antiviral state. Innate and adaptive immune responses to viruses are also greatly influenced by IFN-I. In this article we discuss the diversity of cellular sources of IFN-I and the pathways leading to IFN-I production during viral infections. Finally, we discuss the effects of IFN-I on cells of the immune system with emphasis on dendritic cells.
Keywords: interferon, virus, dendritic cell, monocyte, macrophage, Toll-like receptor, RIG-I-like receptor
Multiplicity of cellular sources of IFN-I
Type I interferons (IFN-I, i.e. IFN-α and IFN-β) were first described over 50 years ago as cytokines that confer resistance to viral infections [1-3]. Although most cells can produce IFN-I, there is emerging evidence that cellular sources can vary during different viral infections. In the following section we discuss the diversity of IFN-I sources and their importance in antiviral immunity.
pDCs are a source of IFN-I that is limited to specific infections
pDCs are bone marrow-derived cells that secrete large amounts of IFN-I and thus are considered a primary source of IFN-I for antiviral responses. pDCs detect RNA and DNA viruses with two endosomal sensors, Toll-like receptors (TLR) 7 and TLR9, which induce secretion of IFN-I as well as proinflammatory cytokines (i.e. IL-12, IL-6 and TNF-α) through the myeloid differentiation primary response gene 88 (MyD88) signaling pathway [4-6]. Viruses reach endosomal compartments in pDCs through endocytosis or autophagy [7,8]. Because many viruses can induce pDC activation and IFN-I secretion through TLR7/9 (reviewed in [9,10]), it has been thought that pDC may be a crucial player in antiviral responses.
However, recent evidence suggests that the capacity of pDCs to produce IFN-I and control virus infections in vivo is more restricted than anticipated. In mucosal infections, such as influenza virus infection, cells lining the airways, like epithelial cells and alveolar macrophages, provide the primary source of IFN-I, whereas pDC secrete IFN-I when the virus bypasses the local barrier and becomes systemic [11]. pDCs do provide a primary source of IFN-I in systemic infections like murine cytomegalovirus (MCMV) and vesicular stomatitis virus (VSV), but their impact appears to be limited in time and capacity [12•]. Why are pDCs restricted in their ability to control systemic infections? Our recent data suggests that splenic pDCs upregulate pro-apoptotic molecules and undergo apoptosis in a IFN-I-dependent manner during certain systemic virus infections in vivo [13•]. Sustained pDC survival during an infection might result in more efficient antiviral responses but also lead to immunopathology.
Recent studies have suggested that pDCs may contribute to antiviral responses against selective viral infections of the skin. pDC infiltrate human skin lesions during virus infections caused by Molluscum contagiosum virus (MCV) [14] and Varicella zoster virus (VZV) [15] and pDC accumulation has been associated with lesion regression [14]. What mediates pDC recruitment to infected areas and how they contribute to antiviral responses in the skin is under investigation [16]. Localization of pDCs in virus-induced skin lesions coincides with the presence of dendritic cells (DC) that show the signature of IFN-induced activation (IFN-DC), suggesting that pDCs may promote the differentiation of monocytes into activated DCs, which contribute to antiviral immunity in the skin.
Finally, pDC survival and antiviral functions may be severely compromised if they become infected with viruses. pDCs are normally refractory to viral replication because they constitutively express machinery such as interferon regulatory factor (IRF) 7 which enables them to rapidly secrete IFN-I and exist in an antiviral state [17,18]. Certain viruses such lymphocytic choriomeningitis virus (LCMV) clone 13, which establishes a chronic infection in mice, are relatively insensitive to IFN-I and can replicate in pDCs as well as classical DCs (cDC) [19-22]. There is also evidence that chronically stimulated pDCs become hypofunctional, which may contribute to the persistence of virus infections [23,24]. Thus, the impact of pDC on antiviral defense may also vary depending on the tropism of the virus for pDCs and the duration of pDC activation.
Cellular sources of IFN-I vary with the type of viral infection
Given that IFN-I production by pDCs is finite and transient during certain systemic virus infections in vivo, other cell types may critical for promoting IFN-I-mediated antiviral immunity (Figure 1). Epithelial cells in the gut and lung produce IFN-I in response to mucosal infections caused by rotavirus and influenza, respectively [25,26] while neurons are critical sources of IFN-I during brain infections caused by Theiler's virus, La Crosse virus and West Nile virus [27,28]. In systemic infection with encephalomyocarditis virus (EMCV), non-hematopoietic stromal cells have a prominent role in IFN-I production, limiting viral replication [29].
Figure 1. Cellular sources of IFN-I during virus infections.
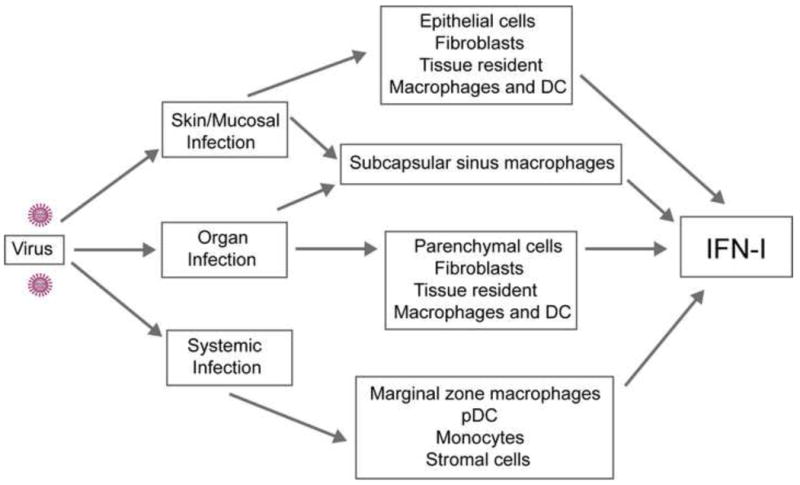
The cells involved in the production of IFN-I depend on the route of infection and tissue tropism of the virus. During skin and mucosal infections, epithelial cells, fibroblasts, tissue resident macrophages and DCs secrete IFN-I and restrict viral replication. In infected organs, IFN-I is produced by parenchymal cells, fibroblasts, tissue resident macrophages and DCs. In draining lymph nodes, subcapsular sinus macrophages have a major role in the secretion of IFN-I and restriction of viral spread. During systemic virus infections, marginal zone macrophages, pDCs, monocytes and non-hematopoietic stromal cells contribute to IFN-I production and viral containment.
Macrophages are also important filters that produce IFN-I and contain viral spread. Microglial cells and alveolar macrophages are essential sources of IFN-I during brain or respiratory viral infections, respectively [11,30] while tissue resident macrophages in the liver control viral replication in LCMV-infected mice [31]. Marginal zone (MZM) and metallophilic macrophages in the spleen produce IFN-I and contain viral spread during systemic Herpes simplex 1 (HSV-1) [32] and LCMV [33] infections. The function of MZM as viral filters was recently corroborated in CD11c-DTR transgenic mice. These mice have been used for several years to address the impact of DCs on immune responses; however, treatment of these mice with diphtheria toxin also eliminates MZM and other macrophage subsets [34]. Using CD11c-DTR transgenic mice, it has been shown that macrophages block the spreading of EMCV [29]. Lymph node subcapsular sinus macrophages, which are similar to MZM, produce IFN-I following footpad infection with VSV and prevent viral spread to the central nervous system [35,36••]. CD169-DTR transgenic mice have been described and will be useful for assessing the impact of CD169+ subcapsular sinus macrophages and MZM during viral infections [37]. Myeloid cells providing a source of IFN-I also include inflammatory monocytes and DCs. While inflammatory monocytes are necessary for the restriction of systemic Vaccinia virus (VACV) replication [38••], splenic DCs produce IFN-I in response to systemic infections with adenoviruses [39] and MCMV [40].
IFN-I production during bacterial infections
Bacterial infections can also trigger the secretion of IFN-I. Splenic macrophages [41], tumor necrosis factor (TNF) alpha- and inducible nitric oxide synthase-producing DCs (Tip-DC) [42] and a subset of B cells [43] are major sources of IFN-I during L. monocytogenes infection. Splenic DCs secrete IFN-I in response to group B streptococcus [44••] while both macrophages and cDCs produce IFN-I following S. pyogenes infection [45]. Epithelial cells can also produce IFN-I in response to bacterial pathogens such as L. pneumophila, P. aeruginosa and S. aureus[46-48]. In contrast to viral infections, IFN-I production during bacterial infections appears to be deleterious, promoting pathogenesis [48-52]. The mechanisms behind this are incompletely understood, however, it has been shown that IFN-I inhibits inflammasome activation [53••] and IL-12 production [54], which aid in the clearance of bacterial infections.
Molecular sensors for IFN-I production
The multiplicity of cellular sources of IFN-I is paralleled by a broad spectrum of molecules that sense viruses in different cell types and trigger IFN-I secretion (Figure 2). Some of these sensors, like the Toll-like receptors (TLR), are expressed by cells such as pDCs, macrophages, DCs as well as some stromal cells. Other sensors are expressed in virtually all tissues, either constitutively or after IFN-I-mediated induction. In this section we discuss the specificity and distribution of these sensors.
Figure 2. Molecular sensors involved in virus recognition and IFN-I production.
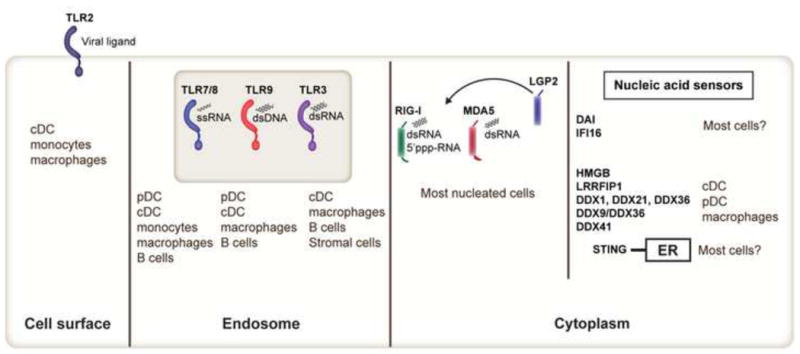
Toll-like receptors (TLR), RIG-I-like receptors (RIG-I, MDA5, LGP2) and newly identified cytoplasmic nucleic acid sensors detect microbial products and initiate IFN-I secretion. TLR2 is expressed by cDCs, monocytes and macrophages and senses viral ligands such as hemagglutinin. TLR7/8 detects ssRNA and are expressed by pDCs, cDCs, monocytes, macrophages and B cells. TLR9 is expressed by pDCs, cDCs (in mouse, not human), macrophages and B cells and recognizes dsDNA. TLR3 detects dsRNA and is expressed mainly in cDCs, macrophages, B cells and stromal cells. RLRs are induced by IFN-I and sense distinct forms of RNA. LGP2 lacks signaling domains and may regulate MDA5 and RIG-I. Unlike TLRs, RLRs have a more broad cellular and tissue distribution. Cytoplasmic nucleic acid sensors such as DDX1, DDX21, DDX36, DDX9, DDX36 and DDX41 have been recently identified and appear to be more restricted in their expression patterns (i.e. cDC or pDC).
Toll-like receptors
In pDCs, IFN-I is produced via the TLR7/9-MyD88-interferon regulatory factor (IRF) 7 pathway. MyD88 associates with Interleukin-1 receptor-associated kinase 1/4 (IRAK1/4), TNF-receptor-associated factor (TRAF) 3, inhibitor of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) kinase α (IKKα) and osteopontin leading to the phosphorylation/nuclear translocation of IRF7 and transcription of IFN-I genes [6,55-58••]. The PI(3)K-mTOR-p70S6K pathway positively regulates IRF7 [59••] while eukaryotic initiation factor 4E-binding proteins repress it at the translation level [60••].
Production of inflammatory cytokines in pDCs requires association of MyD88 with IRAK1/4, which triggers the TRAF6-transforming growth factor β-activated kinase (TAK1) pathway, leading to activation of NF-κB and mitogen-activated protein kinase (MAPK). MyD88 also recruits IRF5, which induces inflammatory cytokine/chemokine production in concert with NF-κB [61]. pDC production of IFN-I or inflammatory cytokine following TLR7/9 engagement depends on the type of endosomal compartment where TLRs meet their ligands [62,63].
IFN-I secretion occurs when TLR7/9 are translocated from the endoplasmic reticulum (ER) to a specialized lysosome-related organelle. This process requires UNC93B ([64,65], adaptor protein 3 (AP-3) as well as Slc15a4, BLOC-1, and BLOC-2 [66••,67••]. TLR9 activation also requires proteolytic cleavage and cofactors in the endosome [68-73] such that DNA can efficiently bind to TLR9. TLR8 also signals through the MyD88 pathway and senses viral ssRNA in human monocytes [74]. The murine homolog was thought to be nonfunctional, however, it was recently shown that VACV DNA activates pDCs via TLR8 [75].
TLR3 has a prominent role in virus recognition and IFN-I production in cDCs, macrophages and stromal cells [55,76,77]. TLR3 detects viral dsRNA and the synthetic analog polyinosinic:polycytidylic acid [poly(I:C)], which gain access to the endosomal compartment by phagocytosis or endocytosis [78-80]. TLR3 signals through the Toll/IL-1 receptor (TIR) domain–containing adaptor-inducing IFN-β (TRIF) pathway, which results in the phosphorylation and nuclear translocation of IRF3 and the transcription and secretion of IFN-β [81,82]. TLR3-TRIF signaling also activates NF-κB and the transcription of inflammatory cytokine genes. Association of TRAF3 with TRIF or MyD88 results in different modes of TRAF3 ubiquitination (degradation or activating) that regulate inflammatory cytokine and IFN-I production [83••]. TLR3 has been implicated in the recognition of many RNA viruses (reviewed in [9,10]). However, in human, TLR3 has proven essential for the recognition of a DNA virus, herpes simplex virus 1 (HSV-1), in the brain most likely though the generation of RNA intermediates that occur during viral replication [84-89••].
TLR2 may also contribute to virus recognition and IFN-I production in DCs and inflammatory monocytes. TLR2 is located on the cell surface and senses viral hemagglutinin as well as other unknown viral components [38••,90-98]. Monocytes produce IFN-I in a TLR2-dependent manner during VACV infection and are essential for viral clearance [38••]. However, in an earlier study, it was shown that TLR2 was important for cytokine responses to VACV but not IFN-I production [97]. Thus, the roles of TLR2 and/or monocytes in promoting antiviral immunity await further clarification.
RIG-I-like receptors
The retinoic acid inducible gene-I–like (RIG-I–like) receptors (RLRs): RIG-I, melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology-2 (LGP2) detect RNA intermediates that accumulate in the cytosol during viral replication [55,77,99-102]. In contrast to the selective expression of TLRs in certain cell types, RLR expression is induced in most cells by IFN-I. Ligand binding to RLRs induces conformational changes allowing interaction with the adapter molecule IFN-β-promoter stimulator 1 (IPS-1, also known as MAVS, VISA or Cardif) [103-106]. IPS-1 is localized on the mitochondria and recruits TRAF3, which activates TANK-binding kinase 1 (TBK1) and IKKε, leading to the phosphorylation and nuclear translocation of IRF3 and IRF7 and production of both IFN-β and IFN-α [107-109]. Additionally, IPS-1 associates with FAS-associated death domain protein (FADD) and receptor-interacting protein-1 (RIP-1), which activate caspase-8 and caspase-10, resulting in NF-κB activation and production of inflammatory cytokines [103,110,111]. IPS-1 is also located on peroxisomes and facilitates rapid antiviral responses through IRF1 [112••].
RLRs recognize RNA structures that are highly specific to viral RNA and distinct from endogenous 5′-capped mRNA [113-126••]. RIG-I preferentially binds to 5′-triphosphorylated ssRNA as well as short dsRNA while MDA5 recognizes long dsRNA like poly(I:C) and does not require 5′-triphosphorylation. The distinct ligand preferences of MDA5 and RIG-I permit recognition of disparate viruses (reviewed in [9,10]). Recent studies have shown that RIG-I also recognizes DNA viruses by detecting RNA intermediates generated through the RNA polymerase III-mediated transcription of dsDNA [127••,128••].
Laboratory of genetics and physiology-2 (LGP2) is an RLR that detects dsRNA [129-131]. LGP2 does not contain any signaling domains and was initially thought to negatively regulate MDA5 and RIG-I [131]. Accordingly, LGP2-deficient mice have more robust IFN-I responses following poly(I:C) stimulation and VSV infection compared to WT mice [130]. However, recent data has demonstrated that LGP2 may positively influence antiviral responses, as RLR-mediated IFN-I responses were impaired in mice lacking LGP2 or the LGP2 ATP-binding site [129].
The RLR signaling pathway is tightly controlled. RIG-I activation is regulated by Lys63-linked polyubiquitination and E3 ubiquitin ligases such as RNF125, TRIM25 and Riplet [132-134]. Caspase-12 positively regulates E3 ubiquitin ligase TRIM25-mediated ubiquitination of RIG-I [135] while caspase-8 cleavage of RIP-1 negatively regulates RIG-I activation [136]. Small ubiquitin-like modifier-1 (SUMO-1) may also impact RIG-I- and MDA5-mediated IFN-I responses [137,138]. NLRX1 localizes to the mitochondrial outer membrane and interacts with IPS-1 to inhibit IFN-I responses [139,140]. Mir-146a, a microRNA, was found to negatively regulate RIG-I-mediated IFN-I production in macrophages [141]. Moreover, viruses have evolved mechanisms to counter RIG-I and MDA5 signaling. Ebola virus VP35 protein binds to dsRNA and blocks RLR-mediated IFN-I production [142,143] while paramyxovirus V proteins bind to MDA5, but not RIG-I, and inhibit dsRNA-induced activation of IFN-I genes [144,145].
Other cytoplasmic virus sensors
The presence of viral DNA in the cytosol also leads to IFN-I production through cytoplasmic sensors [146]. DNA sensors, like RLRs, seem to be broadly distributed and mediate IFN-I production in most cell types. DNA-dependent activator of IFN-regulatory factors (DAI) (also known as ZBP1 or DLM-1) was the first DNA-binding protein shown to respond to cytosolic DNA [147,148]. DAI-deficient mice respond normally to DNA-based vaccines [149,150], suggesting redundancy in detection of cytosolic viral DNA. IFI16 (p204 in mice), a member of the PYHIN protein family, detects non-AT-rich dsDNA like VACV DNA in vitro [151••]; however, due to the lack of in vivo evidence for its impact on antiviral responses, the physiological importance of IFI16 is unknown.
Stimulator of interferon genes (STING) is an ER-associated protein required for the cytosolic DNA-sensing pathway [152-154••]. Given the close proximity between mitochondria and ER, it has been proposed that STING assists RIG-I recognition of viral RNA from ER-attached ribosomes and association with IPS-1 [146]. High mobility group box (HMGB) proteins also have a role in sensing nucleic acids. Both intracellular DNA and poly(I:C)-mediated IFN-I responses are defective in cells lacking HMGB1, while only intracellular DNA-mediated IFN-I production is impaired in HMGB2-deficient cells [155]. In pDCs, DNA-containing immune complexes elicit TLR9-mediated IFN-I production in an HMGB1-RAGE-dependent manner [156]. LRRFIP1, a cytosolic nucleic acid sensor, modulates IFN-I production via the β-catenin pathway in macrophages exposed to VSV or L. monocytogenes[157••].
Recently, DExD/H box-containing helicases, DHX1, DDX21 and DDX36, were found to form a viral sensor with TRIF that recognizes cytosolic dsRNA in cDCs [158••]. DDX36 and DDX9 selectively bind to synthetic oligonucleotides that mimic microbial DNA (CpGA and CpGB), promoting either IFN-I or inflammatory cytokine secretion in human pDCs, respectively [159••]. DDX41 is another sensor that depends on STING to sense intracellular DNA in cDCs [160••]. Finally, two inflammasomes, AIM2 and NLRP3, detect cytosolic viral nucleic acids [161-170] triggering the activation of caspase-1 and maturation of IL-1β rather than IFN-I production (reviewed in [171-173]). In conclusion, over the past few years many novel molecules have been described in addition to TLRs and RLRs that participate in viral recognition, IFN-I responses and immunity. Future studies will reveal whether these sensors have prominent roles in innate and adaptive immune responses in vivo and if they can be exploited for therapeutic purposes.
The effects of IFN-I: cell resistance and immune responses
IFN-I limit viral spreading by inducing apoptosis of virus-infected cells and inducing an antiviral state in uninfected cells through the induction of interferon stimulated genes (ISG). ISGs have been reviewed elsewhere in this issue of Current Opinion in Virology and will not be discussed in this article. More recently, it has been appreciated that IFN-I has immunoregulatory roles, stimulating both innate and adaptive immunity [174]. In this section we review the impact of IFN-I on immune responses.
The impact of IFN-I on immune responses
While it has been known for many years that IFN-I promote resistance to viral infections, the impact of IFN-I on immune cell functions is becoming increasingly appreciated (Figure 3). IFN-I together with IL-12 augment NK cell and CD8+ T cell cytolytic activity and IFN-γ production in vitro and in vivo [175], promote T helper 1 (TH1) polarization of CD4+ T cells, as well as long-term T cell survival and memory [176-179]. IFN-I together with IL-6 induce the differentiation of B cells into immunoglobulin secreting plasma cells [180,181]. Thus, mice unable to respond to IFN-I, such as IFNAR-/- mice, often have profound defects in their antiviral immune responses. CD8+ T cell and NK cell responses that are essential for the resolution of LCMV and MCMV infections, respectively, are impaired in IFNAR-/- mice [178,182]. Moreover, the production of neutralizing antibodies that are critical for the clearance of VSV is also weakened in IFNAR-/- mice [183,184].
Figure 3. Effects of IFN-I on cells during virus infections.
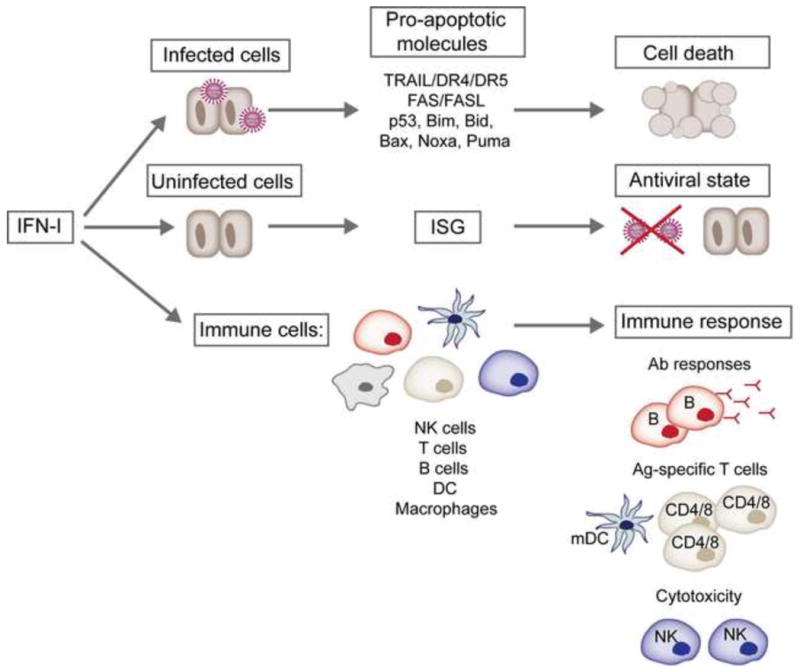
IFN-I promote the death of virus-infected cells through the induction of pro-apoptotic molecules involved in the extrinsic (TRAIL/DR4/DR5 or Fas/FasL) and intrinsic (i.e. p53, Bim, Bid, Bax, Noxa, Puma) apoptosis pathways. In addition, IFN-I act on uninfected cells by inducing hundreds of interferon-stimulated genes (ISGs) that restrict viral replication and confer an antiviral state. The maturation, expansion and effector functions of NK cells, T and B cells, DCs and macrophages are also profoundly influenced by IFN-I. mDC, mature DC.
IFN-I have multiple and complex effects on DCs (Figure 4). By preventing viral infection of DCs, IFN-I allow DCs to acquire viral antigens and the appropriate maturation signals from the infection site. Moreover, IFN-I enhance the antigen presenting machinery of DCs [185-187] and their migratory capacity [188], facilitating priming of naïve T cells [189]. IFN-I also promote the differentiation of monocytes to DCs [190]. Several studies have revealed that DC turnover is strongly influenced by IFN-I in vivo. IFN-I regulate cDC and pDC numbers in vivo by inducing the downregulation of anti-apoptotic molecules, upregulation of pro-apoptotic molecules and caspase activation [13•,191-193•]. Furthermore, IFN-I induce the proliferation and differentiation of dormant hematopoietic stem cells (HSC) [194], which may replace activated or dying DCs in the periphery during virus infections. A tightly regulated turnover of cDCs and pDCs during an IFN-I response may prevent excessive immune responses, immunopathology and perhaps autoimmunity.
Figure 4. Effects of IFN-I on dendritic cell subsets.
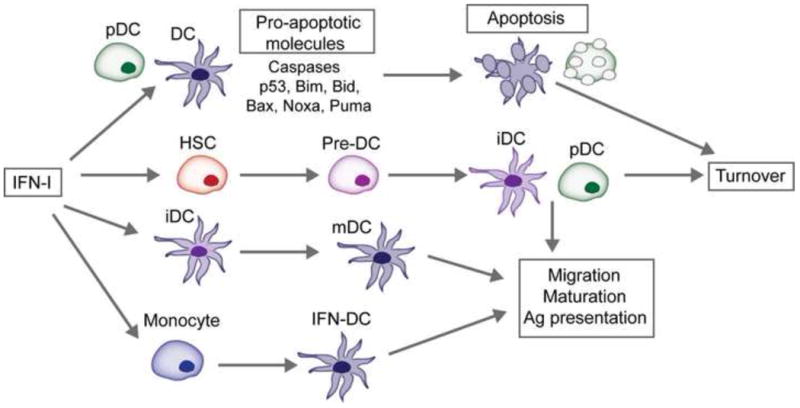
IFN-I promote the migration, maturation and antigen (Ag) presenting capabilities of DCs. IFN-I also induces the differentiation of monocytes into a specialized subset of DC called interferon-induced DC (IFN-DC). Recent evidence suggests that IFN-I regulates DC turnover. Both splenic cDCs and pDCs upregulate caspases and molecules involved in the intrinsic apoptosis pathway following exposure to IFN-I. Furthermore, dormant hematopoietic stem cells (HSC) are activated by IFN-I to proliferate and differentiate which may replace activated or dying DCs during antiviral responses. The regulated turnover of cDCs and pDCs during IFN-I-mediated responses may be a mechanism to prevent excessive immune stimulation and immunopathology. iDC, immature DC; mDC, mature DC.
On the other hand, chronic IFN-I production may impair immune responses and promote disease progression. Prolonged exposure of HSC to IFN-I impairs their functions [194], such that the turnover of cells might be delayed or severely compromised. During Human immunodeficiency virus 1 (HIV-1) infection, chronic IFN-I production has been associated with the upregulation of markers of exhaustion on CD8+ T cells and progressive CD4+ T cell depletion through apoptotic mechanisms [195,196]. Thus, while IFN-I enhance innate and adaptive immune defense against viruses, the duration of their secretion need to be tightly controlled.
Concluding Remarks
For several years, pDCs were presumed to be critical for antiviral responses because of their propensity to rapidly produce IFN-I in vitro after exposure to viruses. However, recent evidence suggests that pDCs have a limited capacity to control viral burden and are a very early and transient source of IFN-I during certain systemic virus infections in vivo [12•]. Given the restricted ability of pDCs to control such viral infections, a variety of cell types, and viral sensors seem necessary for effective IFN-I-mediated antiviral responses. Clearly, distinct sensors differ in their specificity for viruses, viral components and mechanism of action. Furthermore, the expression of sensors may differ in cellular and tissue distribution, such that sensors required for responses to a given virus in one anatomical location may vary in another. The kinetics of sensor expression might also vary with cell type, virus and site of infection. Emerging studies should yield a better understanding of how cellular sources of IFN-I and viral sensors complement, synergize or antagonize each other during antiviral immune responses. New viral sensors inducing IFN-I production are constantly being identified. Future work will shed light on how these newly described sensors influence viral replication in vivo and roles they may play in innate and adaptive immune responses.
Highlights.
-
-
A variety of cell types produce IFN-I during virus infections.
-
-
Cells use multiple molecular pathways leading to IFN-I secretion.
-
-
IFN-I impact the immune system, particularly DC.
Acknowledgments
These studies were supported by the Juvenile Diabetes Research Foundation (JDRF) grant 24-2007-420 and National Institutes of Health grant CA109673 (to M. Colonna). M. Swiecki was supported by the NRSA training grant 5T32DK007296 from NIDDK.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagano Y, Kojima Y. Immunizing property of vaccinia virus inactivated by ultraviolets rays. C R Seances Soc Biol Fil. 1954;148:1700–1702. [PubMed] [Google Scholar]
- 2.Lindenmann J, Burke DC, Isaacs A. Studies on the production, mode of action and properties of interferon. Br J Exp Pathol. 1957;38:551–562. [PMC free article] [PubMed] [Google Scholar]
- 3.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 4.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 6.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- 8.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Swiecki M, McCartney SA, Colonna M. dsRNA sensors and plasmacytoid dendritic cells in host defense and autoimmunity. Immunol Rev. 2011;243:74–90. doi: 10.1111/j.1600-065X.2011.01049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swiecki M, McCartney SA, Wang Y, Colonna M. TLR7/9 versus TLR3/MDA5 signaling during virus infections and diabetes. J Leukoc Biol. 2011;90:691–701. doi: 10.1189/jlb.0311166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, Morii E, Aozasa K, Kawai T, Akira S. Alveolar macrophages are the primary interferon-alpha producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- l2•.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. This is the first study to show the impact of pDC on antiviral responses in vivo using a genetically modified mouse strain where pDC can be selectively depleted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011 doi: 10.1084/jem.20110654. These studies (13, 191-193) indicate that IFN-I promote the turnover of DC subsets by inducing cell death via the upregulation of pro-apoptotic molecules and caspase activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermi W, Fisogni S, Salogni L, Scharer L, Kutzner H, Sozzani S, Lonardi S, Rossini C, Calzavara-Pinton P, Leboit PE, et al. Spontaneous Regression of Highly Immunogenic Molluscum contagiosum Virus (MCV)-Induced Skin Lesions Is Associated with Plasmacytoid Dendritic Cells and IFN-DC Infiltration. J Invest Dermatol. 2011;131:426–434. doi: 10.1038/jid.2010.256. [DOI] [PubMed] [Google Scholar]
- 15.Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J Invest Dermatol. 2006;126:507–509. doi: 10.1038/sj.jid.5700052. [DOI] [PubMed] [Google Scholar]
- 16.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 18.Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting Edge: TLR-Dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{alpha} production in plasmacytoid dendritic cells. J Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 19.Moskophidis D, Battegay M, Bruendler MA, Laine E, Gresser I, Zinkernagel RM. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergthaler A, Flatz L, Hegazy AN, Johnson S, Horvath E, Lohning M, Pinschewer DD. Viral replicative capacity is the primary determinant of lymphocytic choriomeningitis virus persistence and immunosuppression. Proc Natl Acad Sci U S A. 2010;107:21641–21646. doi: 10.1073/pnas.1011998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4:374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol. 2009;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- 25.Broquet AH, Hirata Y, McAllister CS, Kagnoff MF. RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J Immunol. 2011;186:1618–1626. doi: 10.4049/jimmunol.1002862. [DOI] [PubMed] [Google Scholar]
- 26.Opitz B, Rejaibi A, Dauber B, Eckhard J, Vinzing M, Schmeck B, Hippenstiel S, Suttorp N, Wolff T. IFNbeta induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. 2007;9:930–938. doi: 10.1111/j.1462-5822.2006.00841.x. [DOI] [PubMed] [Google Scholar]
- 27.Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103:7835–7840. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, Calderon B, Gilfillan S, Diamond MS, Unanue ER, Colonna M. RNA sensor-induced type I IFN prevents diabetes caused by a beta cell-tropic virus in mice. J Clin Invest. 2011;121:1497–1507. doi: 10.1172/JCI44005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth-Cross JK, Bender SJ, Weiss SR. Murine coronavirus mouse hepatitis virus is recognized by MDA5 and induces type I interferon in brain macrophages/microglia. J Virol. 2008;82:9829–9838. doi: 10.1128/JVI.01199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang PA, Recher M, Honke N, Scheu S, Borkens S, Gailus N, Krings C, Meryk A, Kulawik A, Cervantes-Barragan L, et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52:25–32. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
- 32.Eloranta ML, Alm GV. Splenic marginal metallophilic macrophages and marginal zone macrophages are the major interferon-alpha/beta producers in mice upon intravenous challenge with herpes simplex virus. Scand J Immunol. 1999;49:391–394. doi: 10.1046/j.1365-3083.1999.00514.x. [DOI] [PubMed] [Google Scholar]
- 33.Louten J, van Rooijen N, Biron CA. Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection. J Immunol. 2006;177:3266–3272. doi: 10.4049/jimmunol.177.5.3266. [DOI] [PubMed] [Google Scholar]
- 34.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 35.Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 36••.Iannacone M, Moseman EA, Tonti E, Bosurgi L, Junt T, Henrickson SE, Whelan SP, Guidotti LG, von Andrian UH. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. This study shows that subcapsular sinus macrophages produce IFN-I and prevent the spread of vesicular stomatitis virus (VSV) to the central nervous system after subcutaneous infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–1207. doi: 10.1038/ni.1792. This is the first paper demonstrating that TLR2 signaling induces IFN-I production in response to a virus and that monocytes are critical for controlling systemic vaccinia virus (VACV) infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fejer G, Drechsel L, Liese J, Schleicher U, Ruzsics Z, Imelli N, Greber UF, Keck S, Hildenbrand B, Krug A, et al. Key role of splenic myeloid DCs in the IFN-alphabeta response to adenoviruses in vivo. PLoS Pathog. 2008;4:e1000208. doi: 10.1371/journal.ppat.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 41.Stockinger S, Kastner R, Kernbauer E, Pilz A, Westermayer S, Reutterer B, Soulat D, Stengl G, Vogl C, Frenz T, et al. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 2009;5:e1000355. doi: 10.1371/journal.ppat.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dresing P, Borkens S, Kocur M, Kropp S, Scheu S. A fluorescence reporter model defines “Tip-DCs” as the cellular source of interferon beta in murine listeriosis. PLoS One. 2010;5:e15567. doi: 10.1371/journal.pone.0015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao Y, Han Y, Chen Z, Xu S, Cao X. IFN-alpha-producing PDCA-1(+) Siglec-H(-) B cells mediate innate immune defense by activating NK cells. Eur J Immunol. 2011;41:657–668. doi: 10.1002/eji.201040840. [DOI] [PubMed] [Google Scholar]
- 44••.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G. Beninati C: Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. This paper is the first to show that cDCs produce IFN-I in response to phagosomal but not cytosolic bacteria in a TLR7-dependent manner. [DOI] [PubMed] [Google Scholar]
- 45.Gratz N, Hartweger H, Matt U, Kratochvill F, Janos M, Sigel S, Drobits B, LiXD, Knapp S, Kovarik P. Type I interferon production induced by Streptococcus pyogenes-derived nucleic acids is required for host protection. PLoS Pathog. 2011;7:e1001345. doi: 10.1371/journal.ppat.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Opitz B, Vinzing M, van Laak V, Schmeck B, Heine G, Gunther S, Preissner R, Slevogt H, N'Guessan PD, Eitel J, et al. Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J Biol Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- 47.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of Type I Interferon Signaling by Pseudomonas aeruginosa is Diminished in Cystic Fibrosis Epithelial Cells. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0080OC. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin FJ, Gomez MI, Wetzel DM, Memmi G, O'Seaghdha M, Soong G, Schindler C, Prince A. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest. 2009;119:1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Connell RM, Saha SK, Vaidya SA, Bruhn KW, Miranda GA, Zarnegar B, Perry AK, Nguyen BO, Lane TF, Taniguchi T, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bauler TJ, Chase JC, Bosio CM. IFN-beta mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J Immunol. 2011;187:1845–1855. doi: 10.4049/jimmunol.1100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, Decker T, Du Pasquier RA, Romero P, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. These findings contribute to our understanding of why IFN-I is an effective treatment against inflammatory diseases and why humans are more susceptible to bacterial infections following viral infections. [DOI] [PubMed] [Google Scholar]
- 54.Biron CA. Interferons alpha and beta as immune regulators--a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 55.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 56.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 58.Honda K, Yanai H, Takaoka A, Taniguchi T. Regulation of the type I IFN induction: a current view. Int Immunol. 2005;17:1367–1378. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- 59••.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Colina R, Costa-Mattioli M, Dowling RJ, Jaramillo M, Tai LH, Breitbach CJ, Martineau Y, Larsson O, Rong L, Svitkin YV, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452:323–328. doi: 10.1038/nature06730. These three studies (57, 59, 60) demonstrate the importance of IRF7 in antiviral immune responses and show that IRF7 is regulated by the mTOR pathway and 4E-binding proteins. [DOI] [PubMed] [Google Scholar]
- 61.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, Honda K, Ohba Y, Mak TW, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 62.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 63.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 65.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66••.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. The experiments in these two studies (66, 67) show that TLR7/9 trafficking from endosomes to a specialized lysosome-related organelle is required for IFN-I and inflammatory cytokine production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park B, Brinkmann MM, Spooner E, Lee CC, Kim YM, Ploegh HL. Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol. 2008;9:1407–1414. doi: 10.1038/ni.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ewald SE, Engel A, Lee J, Wang M, Bogyo M, Barton GM. Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J Exp Med. 2011;208:643–651. doi: 10.1084/jem.20100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoshizaki M, Tazawa A, Kasumi E, Sasawatari S, Itoh K, Dohi T, Sasazuki T, Inaba K, Makrigiannis AP, Toyama-Sorimachi N. Spatiotemporal regulation of intracellular trafficking of Toll-like receptor 9 by an inhibitory receptor, Ly49Q. Blood. 2009;114:1518–1527. doi: 10.1182/blood-2008-12-192344. [DOI] [PubMed] [Google Scholar]
- 72.Park B, Buti L, Lee S, Matsuwaki T, Spooner E, Brinkmann MM, Nishihara M, Ploegh HL. Granulin is a soluble cofactor for toll-like receptor 9 signaling. Immunity. 2011;34:505–513. doi: 10.1016/j.immuni.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Avalos AM, Ploegh HL. Competition by inhibitory oligonucleotides prevents binding of CpG to C-terminal TLR9. Eur J Immunol. 2011;41:2820–2827. doi: 10.1002/eji.201141563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 75.Martinez J, Huang X, Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci U S A. 2010;107:6442–6447. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–224. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 77.Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 79.Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–892. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 80.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 82.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, anadaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 83••.Tseng PH, Matsuzawa A, Zhang W, Mino T, Vignali DA, Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat Immunol. 2010;11:70–75. doi: 10.1038/ni.1819. This manuscript shows that degradative ubiquitination of TRAF3 is required for MyD88-mediated inflammatory cytokine production while TRAF3 association with TRIF leads to noncanonical TRAF3 self-ubiquitination and IFN-I production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang SY, Jouanguy E, Sancho-Shimizu V, von Bernuth H, Yang K, Abel L, Picard C, Puel A, Casanova JL. Human Toll-like receptor-dependent induction of interferons in protective immunity to viruses. Immunol Rev. 2007;220:225–236. doi: 10.1111/j.1600-065X.2007.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86••.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. This is the first study to show that in humans, TLR3 expression in the brain is essential for recognition and control of a DNA virus, HSV-1. [DOI] [PubMed] [Google Scholar]
- 87.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in Host Defense: Natural Insights from Evolutionary, Epidemiological, and Clinical Genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 88.Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, Casanova JL. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 89.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006;80:4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. J Leukoc Biol. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 92.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Tolllike receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 95.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bieback K, Lien E, Klagge IM, Avota E, Schneider-Schaulies J, Duprex WP, Wagner H, Kirschning CJ, Ter Meulen V, Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 99.Loo YM, Gale M., Jr Immune Signaling by RIG-I-like Receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65. doi: 10.1111/j.1600-065X.2008.00727.x. [DOI] [PubMed] [Google Scholar]
- 101.Wilkins C, Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 103.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 104.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 105.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 106.Kumar H, Kawai T, Kato H, Sato S, Takahashi K, Coban C, Yamamoto M, Uematsu S, Ishii KJ, Takeuchi O, et al. Essential role of IPS-1 in innate immune responses against RNA viruses. J Exp Med. 2006;203:1795–1803. doi: 10.1084/jem.20060792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 108.Saha SK, Pietras EM, He JQ, Kang JR, Liu SY, Oganesyan G, Shahangian A, Zarnegar B, Shiba TL, Wang Y, et al. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. Embo J. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 110.Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. doi: 10.1038/nature03124. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- 112••.Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell. 2010;141:668–681. doi: 10.1016/j.cell.2010.04.018. The study shows that peroxisomal MAVS (IPS-1) induce rapid IFN-I independent expression of defense factors through IRF1 that provide short term antiviral protection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113••.Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 114••.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 115••.Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, Akira S, Way M, Schiavo G, Reis e Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J Virol. 2009;83:10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116••.Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G, et al. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117••.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••118.Cui S, Eisenacher K, Kirchhofer A, Brzozka K, Lammens A, Lammens K, Fujita T, Conzelmann KK, Krug A, Hopfner KP. The C-terminal regulatory domain is the RNA 5′-triphosphate sensor of RIG-I. Mol Cell. 2008;29:169–179. doi: 10.1016/j.molcel.2007.10.032. [DOI] [PubMed] [Google Scholar]
- ••119.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120••.Rehwinkel J, Tan CP, Goubau D, Schulz O, Pichlmair A, Bier K, Robb N, Vreede F, Barclay W, Fodor E, et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 121••.Wang Y, Ludwig J, Schuberth C, Goldeck M, Schlee M, Li H, Juranek S, Sheng G, Micura R, Tuschl T, et al. Structural and functional insights into 5′-ppp RNA pattern recognition by the innate immune receptor RIG-I. Nat Struct Mol Biol. 2010;17:781–787. doi: 10.1038/nsmb.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••122.Lu C, Xu H, Ranjith-Kumar CT, Brooks MT, Hou TY, Hu F, Herr AB, Strong RK, Kao CC, Li P. The structural basis of 5′ triphosphate double-stranded RNA recognition by RIG-I C-terminal domain. Structure. 2010;18:1032–1043. doi: 10.1016/j.str.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••123.Lu C, Ranjith-Kumar CT, Hao L, Kao CC, Li P. Crystal structure of RIG-I C-terminal domain bound to blunt-ended double-strand RNA without 5′ triphosphate. Nucleic Acids Res. 2011;39:1565–1575. doi: 10.1093/nar/gkq974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••124.Kowalinski E, Lunardi T, McCarthy AA, Louber J, Brunel J, Grigorov B, Gerlier D. Cusack S: Structural Basis for the Activation of Innate Immune Pattern-Recognition Receptor RIG-I by Viral RNA. Cell. 2011;147:423–435. doi: 10.1016/j.cell.2011.09.039. [DOI] [PubMed] [Google Scholar]
- ••125.Luo D, Ding SC, Vela A, Kohlway A, Lindenbach BD, Pyle AM. Structural Insights into RNA Recognition by RIG-I. Cell. 2011;147:409–422. doi: 10.1016/j.cell.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126••.Jiang F, Ramanathan A, Miller MT, Tang GQ, Gale M, Patel SS, Marcotrigiano J. Structural basis of RNA recognition and activation by innate immune receptor RIG-I. Nature. 2011 doi: 10.1038/nature10537. Epub ahead of print. Collectively, these studies (113-126) indicate that RIG-I and MDA5 recognize distinct forms of RNA and provide insight into how RIG-I binds to dsRNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127••.Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald KA, Hornung V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat Immunol. 2009;10:1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128••.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. These two studies (127, 128) show that RIG-I detects dsRNA intermediates transcribed by RNA pol III during infection with DNA viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Satoh T, Kato H, Kumagai Y, Yoneyama M, Sato S, Matsushita K, Tsujimura T, Fujita T, Akira S, Takeuchi O. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc Natl Acad Sci U S A. 2010;107:1512–1517. doi: 10.1073/pnas.0912986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Venkataraman T, Valdes M, Elsby R, Kakuta S, Caceres G, Saijo S, Iwakura Y, Barber GN. Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J Immunol. 2007;178:6444–6455. doi: 10.4049/jimmunol.178.10.6444. [DOI] [PubMed] [Google Scholar]
- 131.Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA. The RNAhelicase Lgp2 inhibits TLR-independent sensing of viral replicationby retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 132.Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–7505. doi: 10.1073/pnas.0611551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 134.Oshiumi H, Miyashita M, Inoue N, Okabe M, Matsumoto M, Seya T. The ubiquitin ligase Riplet is essential for RIG-I-dependent innate immune responses to RNA virus infection. Cell Host Microbe. 2010;8:496–509. doi: 10.1016/j.chom.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Wang P, Arjona A, Zhang Y, Sultana H, Dai J, Yang L, LeBlanc PM, Doiron K, Saleh M, Fikrig E. Caspase-12 controls West Nile virus infection via the viral RNA receptor RIG-I. Nat Immunol. 2010;11:912–919. doi: 10.1038/ni.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA helicase activation of IRF3 transcription factoris negatively regulated by caspase-8-mediated cleavage of the RIP1 protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 137.Mi Z, Fu J, Xiong Y, Tang H. SUMOylation of RIG-I positively regulates the type I interferon signaling. Protein Cell. 1:275–283. doi: 10.1007/s13238-010-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fu J, Xiong Y, Xu Y, Cheng G, Tang H. MDA5 is SUMOylated by PIAS2beta in the upregulation of type I interferon signaling. Mol Immunol. 2011;48:415–422. doi: 10.1016/j.molimm.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 140.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hou J, Wang P, Lin L, Liu X, Ma F, An H, Wang Z, Cao X. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol. 2009;183:2150–2158. doi: 10.4049/jimmunol.0900707. [DOI] [PubMed] [Google Scholar]
- 142.Cardenas WB, Loo YM, Gale M, Jr, Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits alpha/beta interferon production induced by RIG-I signaling. J Virol. 2006;80:5168–5178. doi: 10.1128/JVI.02199-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Prins KC, Cardenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol. 2009;83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc Natl Acad Sci U S A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Childs KS, Andrejeva J, Randall RE, Goodbourn S. Mechanism of mda-5 Inhibition by paramyxovirus V proteins. J Virol. 2009;83:1465–1473. doi: 10.1128/JVI.01768-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 148.Wang Z, Choi MK, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proc Natl Acad Sci U S A. 2008;105:5477–5482. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ishii KJ, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C, et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451:725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- 150.Lippmann J, Rothenburg S, Deigendesch N, Eitel J, Meixenberger K, van Laak V, Slevogt H, N'Guessan PD, Hippenstiel S, Chakraborty T, et al. IFNbeta responses induced by intracellular bacteria or cytosolic DNA in different human cells do not require ZBP1 (DLM-1/DAI) Cell Microbiol. 2008;10:2579–2588. doi: 10.1111/j.1462-5822.2008.01232.x. [DOI] [PubMed] [Google Scholar]
- 151••.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. This study is the first to demonstrate that the PYHIN family member IFI16 (p204 in mouse) interacts with STING and is involved in the induction of IFN-β in response to intracellular DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152••.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, et al. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 153••.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154••.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. These three studies (152-154) identify stimulator of interferon genes (STING) as a critical regulator of IFN-I-mediated responses to intracellular DNA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, et al. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature. 2009;462:99–103. doi: 10.1038/nature08512. [DOI] [PubMed] [Google Scholar]
- 156.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 157••.Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a beta-catenin-dependent pathway. Nat Immunol. 2010;11:487–494. doi: 10.1038/ni.1876. This is the first study to show that leucine-rich repeat flightless-interacting protein 1 (LRRFIP1) binds to cytosolic nucleic acids and signals through the β-catenin pathway to induce IFN-I production. [DOI] [PubMed] [Google Scholar]
- 158••.Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 Helicases Form a Complex with the Adaptor Molecule TRIF to Sense dsRNA in Dendritic Cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159••.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160••.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12:959–965. doi: 10.1038/ni.2091. Collectively, these are the first studies (158-160) to identify novel DExD/H box-containing helicases that sense cytoplasmic nucleic acids in human cDCs and pDCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323:1057–1060. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- 162.Fernandes-Alnemri T, Yu JW, Datta P, Wu J. Alnemri ES: AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 164.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 169.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 170.Kanneganti TD, Body-Malapel M, Amer A, Park JH, Whitfield J, Franchi L, Taraporewala ZF, Miller D, Patton JT, Inohara N, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281:36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 171.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity. 2011;34:665–679. doi: 10.1016/j.immuni.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 172.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 173.Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol. 2010;10:688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;207:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 176.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le Bon A, Tough DF. Links between innate and adaptive immunity via type I interferon. Curr Opin Immunol. 2002;14:432–436. doi: 10.1016/s0952-7915(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 178.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 180.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 181.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–3064. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 182.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Steinhoff U, Muller U, Schertler A, Hengartner H, Aguet M, Zinkernagel RM. Antiviral protection by vesicular stomatitis virus-specific antibodies in alpha/beta interferon receptor-deficient mice. J Virol. 1995;69:2153–2158. doi: 10.1128/jvi.69.4.2153-2158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fink K, Lang KS, Manjarrez-Orduno N, Junt T, Senn BM, Holdener M, Akira S, Zinkernagel RM, Hengartner H. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006;36:2094–2105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 185.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 186.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 188.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–256. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 190.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191••.Mattei F, Bracci L, Tough DF, Belardelli F, Schiavoni G. Type I IFN regulate DC turnover in vivo. Eur J Immunol. 2009;39:1807–1818. doi: 10.1002/eji.200939233. [DOI] [PubMed] [Google Scholar]
- 192••.Yen JH, Ganea D. Interferon beta induces mature dendritic cell apoptosis through caspase-11/caspase-3 activation. Blood. 2009;114:1344–1354. doi: 10.1182/blood-2008-12-196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193••.Fuertes Marraco SA, Scott CL, Bouillet P, Ives A, Masina S, Vremec D, Jansen ES, O'Reilly LA, Schneider P, Fasel N, et al. Type I Interferon Drives Dendritic Cell Apoptosis via Multiple BH3-Only Proteins following Activation by PolyIC In Vivo. PLoS One. 2011;6:e20189. doi: 10.1371/journal.pone.0020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 195.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–128. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


