Summary
Type I Interferons (IFN-I) promote antiviral CD8+T cell responses, but the contribution of different IFN-I sources and signaling pathways are ill-defined. While plasmacytoid dendritic cells (pDCs) produce IFN-I upon TLR stimulation, IFN-I are induced in most cells by helicases like MDA5. Using acute and chronic lymphocytic choriomeningitis virus (LCMV) infection models, we determined that pDCs transiently produce IFN-I that minimally impacts CD8+T cell responses and viral persistence. Rather, MDA5 is the key sensor that induces IFN-I required for CD8+T cell responses. In the absence of MDA5, CD8+T cell responses to acute infection rely on CD4+T cell help, and loss of both CD4+T cells and MDA5 results in CD8+T cell exhaustion and persistent infection. Chronic LCMV infection rapidly attenuates IFN-I responses, but early administration of exogenous IFN-I rescues CD8+T cells, promoting viral clearance. Thus, effective antiviral CD8+T cell responses depend on the timing and magnitude of IFN-I responses.
Introduction
Type I interferon (IFN-I), i.e. IFN-α/β, plays dual roles during the host response to viruses: it provides innate defense and promotes adaptive CD8 T cell responses (Garcia-Sastre and Biron, 2006; Trinchieri, 2010). IFN-I impacts CD8 T cells at several levels: it promotes priming by enhancing the capacity of dendritic cells (DC) to process and present viral antigens (Le Bon et al., 2003; Le Bon and Tough, 2008); it boosts the capacity of CD8 T cells to secrete mediators such as perforin and granzyme that lyse infected cells; and it promotes the survival of memory CD8 T cells (Kolumam et al., 2005; Marrack et al., 1999; Thompson et al., 2006; Tough et al., 1996).
Viruses trigger an IFN-I response in most cells by stimulating sensors that detect viral nucleic acids. Among these, the RIG-like receptors (RLR), including RIG-I and MDA5, are helicases that detect distinct forms of viral RNA in the cytosol of infected cells and activate the adaptor IPS1, which initiates a downstream signaling pathway that leads to IFN-I secretion (Pichlmair and Reis e Sousa, 2007; Takeuchi and Akira, 2010). IFN-I is also produced by specialized antigen-presenting cells, known as plasmacytoid dendritic cells (pDC). pDCs sense viruses through Toll-like receptor (TLR) 7 and TLR9 (Gilliet et al., 2008). TLR7 and TLR9 detect viral RNA and DNA, respectively and transduce downstream signals through the adaptor MyD88, triggering secretion of IFN-I (Pichlmair and Reis e Sousa, 2007; Takeuchi and Akira, 2010). In contrast to cytosolic RLRs, TLR7 and TLR9 are located in a specialized endosomal compartment (Barbalat et al., 2011; Blasius and Beutler, 2010). Thus, pDCs detect external viral particles that have been captured by endocytosis (Wang et al., 2007) or cytosolic viral particles that become enclosed in a vacuole during autophagy (Lee et al., 2007).
While it has been established that IFN-I is critical for the generation of CD8 T cell responses (Garcia-Sastre and Biron, 2006; Haring et al., 2006; Mescher et al., 2006; Trinchieri, 2010), the impact of disparate sources and signaling pathways of IFN-I during this process remains poorly understood. pDCs facilitate the accumulation of virus-specific CD8 T cells during vesicular stomatitis virus (VSV) infection (Swiecki et al., 2010). The generation of a CD8 T cell response during lymphocytic choriomeningitis virus (LCMV) infection requires MyD88 (Jung et al., 2008; Zhou et al., 2005), but it is not clear whether this requirement reflects the involvement of pDCs. In contrast, antiviral CD8 T cells are not dependent on either the MyD88 or IPS-1 signaling pathways during influenza A virus infection (Koyama et al., 2007), excluding a role for pDCs and RLRs. Thus, it is likely that pDCs and/or RLRs influence on CD8 T cell responses may vary during diverse viral infections.
To more precisely define the impact of disparate sources of IFN-I on the generation of antiviral CD8 T cell responses, we chose to study LCMV infection in the mouse. LCMV is a non-cytopathic RNA virus that elicits acute or chronic infection depending on the viral strain (Oldstone, 2002; Zinkernagel, 2002).
Infection with LCMV Armstrong (ARM) elicits a strong CD8 T cell response that controls viral replication, resulting in clearance of the infection. LCMV CL13 (CL13) replicates faster than ARM and has broader tropism for target cells, which include DCs, hematopoietic progenitor cells and fibroblasts (Bergthaler et al., 2010; Borrow et al., 1995; Macal et al., 2012; Mueller et al., 2007; Sevilla et al., 2000; Sevilla et al., 2004). Thus, the CD8 T cell response during infection with CL13 is insufficient to curb viral replication, resulting in persistent infection and exhaustion of CD8 T cells, which are either deleted or unable to produce cytolytic mediators and cytokines (Moskophidis et al., 1993; Wherry, 2011; Zajac et al., 1998).
It has been shown that IFN-I is essential for the generation of effective anti-LCMV CD8 T cells (Kolumam et al., 2005; Moskophidis et al., 1994; Muller et al., 1994; Ou et al., 2001; Thompson et al., 2006; van den Broek et al., 1995a). Thus, LCMV provides the opportunity to evaluate the impact of disparate viral sensors and sources of IFN-I on CD8 T cell responses in settings of acute or chronic viral infection. Results presented here demonstrate that the impact of pDCs and RLRs on anti-LCMV CD8 T cell responses depends on the timing and magnitude of the IFN-I response they mediate.
Results
pDCs are an early and transient source of IFN-I during LCMV infection
The role of pDCs during LCMV infection is controversial. It has been shown that LCMV stimulates pDC production of IFN-I (Jung et al., 2008; Montoya et al., 2005) by activating a pathway that depends upon MyD88 and, in part, TLR7 and TLR9 (Jung et al., 2008). However, another study indicated that IFN-I response to LCMV is largely pDC-independent (Dalod et al., 2002). Moreover, it has been shown that CL13 impairs pDC function, contributing to immunosuppression (Bergthaler et al., 2010; Lee et al., 2009a; Zuniga et al., 2008). We assessed the role of pDCs in anti-LCMV defense using BDCA2-DTR transgenic mice, in which pDCs can be specifically depleted by administration of diphtheria toxin (DT) (S1A) (Swiecki et al., 2010). Mice were treated with DT or PBS one day before inoculation with ARM or CL13 and every other day throughout the infection. Serum IFN-α and viral titers were determined at various time points post-infection (p.i.). Both ARM and CL13 infection induced an IFN-α response that was detectable 16 h p.i., optimal at 24-48 h p.i. (Figure 1A-B) and rapidly declined to undetectable levels 4-5 days p.i. (data not shown). Depletion of pDCs reduced serum IFN-α levels at 16 h p.i. but not at later time points (Figure 1A-B). Consistent with the transient effect of pDCs on systemic IFN-I responses, we observed a marked reduction in pDC frequencies in the spleen of ARM and CL13 infected mice 48 h p.i. (Figure 1C). This reduction in pDC numbers most likely reflects pDC death due to IFN-I-induced apoptosis that we recently reported in other systemic viral infections (Swiecki et al., 2011).
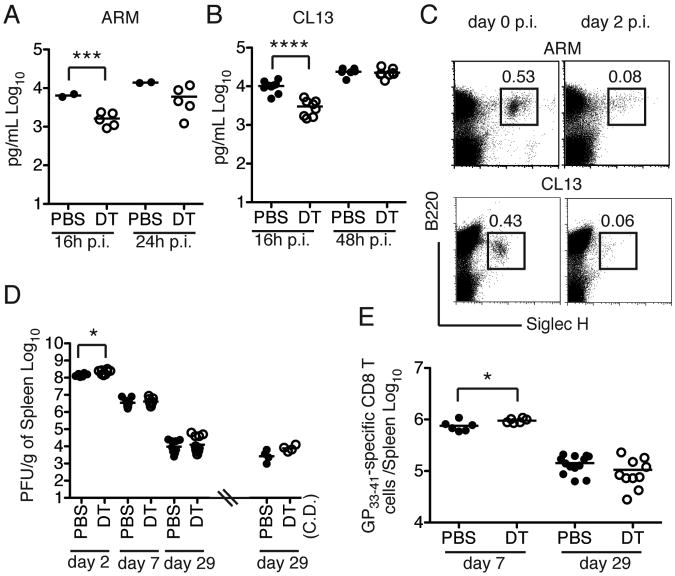
Figure 1. (see also S1). Contribution of pDCs to IFN-I and CD8 T cell responses to LCMV.
BDCA2-DTR (DTR) mice treated with PBS or DT were infected with ARM (A) or CL13 (B) and serum IFN-α was measured at different time points p.i. (C) Frequencies of pDCs in spleens of WT mice infected with either ARM or CL13 on day 2 p.i. were compared to frequencies of pDCs in naive mice (day 0). (D) DTR mice were injected with PBS or DT during the first week of infection with CL13 to deplete pDCs and viral titers in the spleen were determined. Dots on the right side of the hash mark indicate CL13 titers on day 29 p.i. in spleens of mice treated with PBS or DT for the entire duration of the experiment (chronic depletion, CD.). (E) Total number of GP33–41 specific CD8 T cells in spleens of PBS or DT-treated mice infected with CL13. Data are representative of experiments that were repeated 2-4 times. **** = p<0.0001; *** = p<0.001. * = p<0.05, Student's t-test.
In agreement with the rapid disappearance of pDCs during LCMV infection, pDC depletion had no major impact on LCMV titers during chronic infection except for a slight increase of viral titers at 48 h p.i. (Figure 1D). Moreover, pDC depletion did not affect the generation of GP33–41 specific CD8 T cells in the spleens of CL13-infected mice (Figure 1E and S1B). In fact, absolute numbers of GP33–41 specific CD8 T cells were slightly increased on day 7 p.i., probably reflecting the transient increase in viral titers and antigen availability. We also extended pDC depletion throughout days 28-30 p.i. during chronic CL13 infection, assuming that pDCs may recover and produce low amounts of IFN- α that are not detectable in the serum. Prolonged pDC depletion had no major effect on viral titers or GP33–41 specific CD8 T cells (Figure 1D and S1B), although we did note a more pronounced reduction in spleen cellularity than that observed in untreated mice (Figure S1C). These results demonstrate that pDCs have an early but limited impact on the amplitude and duration of IFN-α responses to LCMV and no significant effect on CD8 T cell responses or viral persistence at least until day 30 p.i.
Sustained IFN-I responses to acute and chronic LCMV infection depend on MDA5
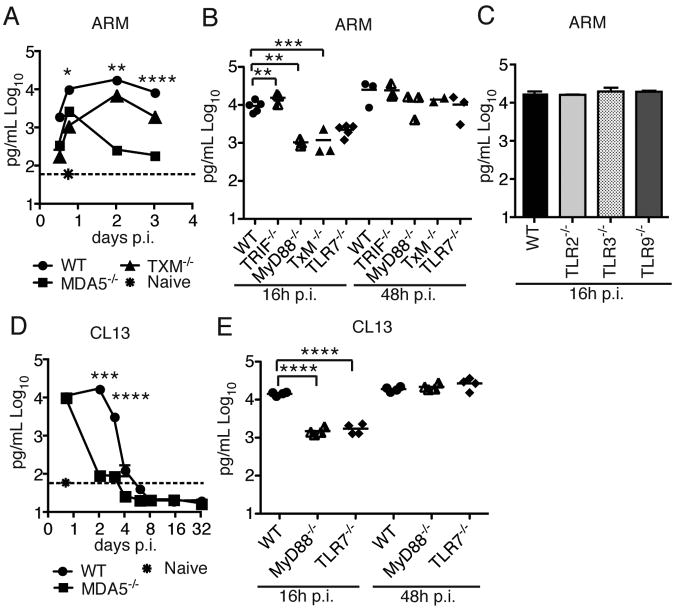
A MyD88-dependent (Jung et al., 2008) and an MDA5-dependent pathway (Zhou et al., 2010) have been implicated in the IFN-I response to LCMV infection; however, the relative importance of each is not known. We first investigated this issue in acute LCMV infection. We inoculated WT, TRIF −/− × MyD88−/− (TxM)−/− and MDA5−/− mice with ARM and determined serum IFN-α at different time points p.i. In WT mice, serum IFN-α peaked 48 h p.i. and was sustained up to 3 days p.i. (Figure 2A). In TxM−/− mice, serum IFN-α was markedly reduced 16-24 h p.i. but was only slightly lower than in WT mice at later time points. On the contrary, IFN-α was significantly diminished at all time points and most severely at 2-3 days p.i. in MDA5−/− mice. These data suggest that MDA5 is the major sensor of ARM, whereas TLRs play an early, but limited, role. We next determined which TLR is responsible for the early IFN-I response to ARM. We infected WT, TRIF−/−, MyD88−/−, TLR7−/−, TLR2−/−, TLR3−/− and TLR9−/− mice with ARM and assessed serum IFN-α at different time points p.i. Only TLR7−/− and MyD88−/− mice had a significant reduction in serum IFN-α, indicating that early production of IFN-I depends on the TLR7/MyD88 pathway (Figure 2B-C).
Figure 2. Contribution of TLR7/MyD88 and MDA5 pathways to IFN-I production during LCMV infection.
(A-E) Serum IFN-α in response to ARM (A-C) or CL13 (D,E) at various time points p.i. 2-10 mice from multiple experiments were analyzed for each time point. The dotted lines in (A) and (D) indicate IFN-α baseline in serum from uninfected mice. Error bars represent the mean +/- SEM. **** = p<0.0001; *** = p<0.001. ** = p<0.01. * = p<0.05, Student's t-test.
Since CL13 has a broader cell tropism and replicative capacity than ARM, we first asked whether the same sensors are required and have a similar impact during the host response to this LCMV strain. We infected WT, MDA5−/−, MyD88−/− and TLR7−/− mice with CL13 and determined serum IFN-α at various time points p.i. Similar to what we observed following ARM infection, MDA5−/− mice lacked a sustained IFN-I response (Figure 2D) whereas TLR7−/− and MyD88−/− mice had a noticeable defect only in the early IFN-I response (Figure 2E). Thus, we conclude that MDA5 has a major role in the IFN-I response to LCMV. The more limited and temporary impact of TLR7 on IFN-α production is consistent with the transient role of pDCs, which detect LCMV through TLR7.
MDA5-deficiency delays CD8 T cell responses to acute LCMV and prolongs viral infection
Acute LCMV infection normally elicits a robust effector CD8 T cell response followed by contraction and generation of a pool of memory CD8 T cells (Butz and Bevan, 1998). Given its major impact on IFN-I production, we asked whether the lack of MDA5 affects generation of LCMV-specific CD8 T cells. We infected WT and MDA5−/− mice with ARM and monitored the induction of LCMV-specific CD8 T cells in the spleen and peripheral blood over a 64-day time span.
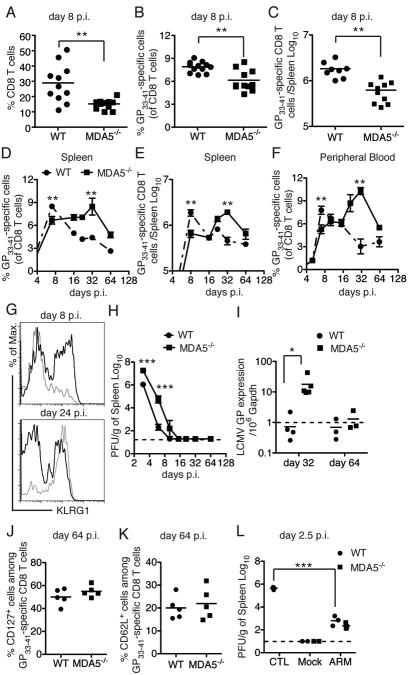
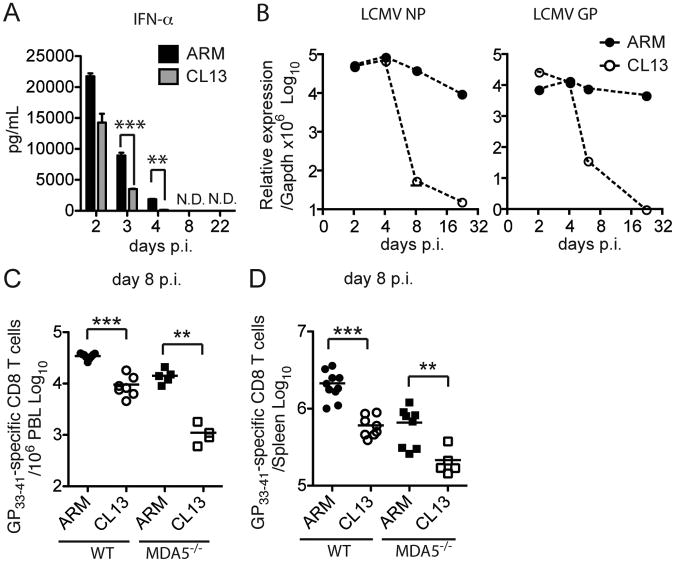
On day 8 p.i., WT mice had a substantial expansion of total CD8 T cells, which represented, on average, 30% of splenocytes, whereas CD8 T cells comprised approximately 10% of splenocytes in MDA5−/− mice (Figure 3A). In parallel with a reduced percentage of total CD8 T cells, MDA5−/− mice had significantly lower percentages and numbers of LCMV GP33–41 specific CD8 T cells than did WT mice (Figure 3B-C). MDA5−/− splenocytes killed EL4 cells pulsed with the GP33–41 peptide ex vivo (S2), although less efficiently than WT splenocytes, consistent with a lower representation of LCMV GP33–41 specific CD8 T cells in the spleen. Thus, MDA5-deficiency affected the number but not the function of virus-specific CD8 T cells at early time points.
Figure 3. (see also S2). Impact of MDA5 deficiency on the CD8 T cell response and viral clearance during ARM infection.
(A) Frequencies of splenic CD8 T cells on day 8 p.i. (B) Frequencies and (C) absolute numbers of splenic LMCV GP33–41 specific CD8 T cells on day 8 p.i. (D) Frequencies and (E) absolute numbers of splenic LMCV GP33–41 specific CD8 T cells at various time points during ARM infection. (F) Frequencies of LMCV GP33–41 specific CD8 T cells in peripheral blood during ARM infection. (G) KLRG1 expression on GP33–41 specific CD8 T cells in spleens of WT (black histogram) and MDA5−/− (gray histogram) mice. (H, I) Viral burden in spleens was examined by plaque assay (H) and qPCR for glycoprotein (GP) RNA (I) at various time points p.i. (J, K) Frequencies of CD127+ cells (J) and CD62L+ cells (K) among LMCV GP33–41 specific CD8 T cells in spleens on day 64 p.i. (L) 2×106 purified CD8 T cells from ARM-infected WT and MDA5−/− mice on day 64 p.i. were transferred into naïve WT mice. Mice were infected (ARM) or not (Mock) with 3×105 ARM 16 h post transfer; control mice (CTL), which did not receive T cells, were also infected. Viral burden in spleens was analyzed on day 2.5 p.i. The dashed lines represent the minimum level of detection. 3-8 mice were used for analyses at each time point. Error bars represent the mean +/- SEM. *** = p<0.001. ** = p<0.01. * = p<0.05, Student's t-test.
In WT mice, GP33–41 specific CD8 T cells waned in the spleen and peripheral blood after day 8 p.i., and comprised approximately 3% of total CD8 T cells on day 64 p.i. (Figure 3D-F). In contrast, in MDA5−/− mice, the frequency and absolute numbers of GP33–41 specific CD8 T cells gradually increased over time in both blood and spleen peaking between day 16 and day 32 p.i.. Contraction of GP33–41 specific CD8 T cells in MDA5−/− mice occurred only after day 32 p.i., and reached levels similar to those of WT mice on day 64 p.i. (Figure 3D-F). Delayed expansion of CD8 T cells in MDA5−/− mice was associated with deferred expression of the Killer Cell Lectin-like Receptor (KLRG1), a marker for T cell activation (Blaser et al., 1998) (Figure 3G). In parallel, viral infection was also prolonged in MDA5−/− mice. While no virus was detectable in the spleen of WT mice by plaque assay on day 10 p.i., viral elimination was delayed until day 16 p.i. in MDA5−/− mice (Figure 3H). To detect possible ongoing viral replication below the limit of detection of the plaque assay at later time points, we measured LCMV GP RNA in the spleen. While LCMV GP RNA was below the level of detection in the spleens of infected WT mice on day 32 p.i., spleens of MDA5-/- mice contained on average 20 copies of GP RNA per 106 copies of GAPDH RNA (Figure 3I). However, by day 64 p.i., LCMV GP RNA was not detected in either group.
Finally, we asked whether MDA5 deficiency alters generation of the memory CD8 T cell pool at late time points after ARM infection. On day 64 p.i. WT and MDA5−/− mice had similar percentages of splenic GP33–41 specific CD8 T cells expressing the memory markers CD127, CD62L and CD44 (Figure 3J-K and data not shown), suggesting that MDA5 deficiency did not affect the size of the memory CD8 T cell pool. Moreover, we asked whether memory CD8 T cells from MDA5−/− mice are as protective as their WT counterparts in mice challenged with LCMV. CD8 T cells were purified from the spleens of WT and MDA5−/− mice on day 62 p.i. and adoptively transferred into naïve mice, which were subsequently challenged with ARM. Mice that received CD8 T cells from either WT or MDA5−/− mice were equally effective in reducing viral titers, in comparison to control mice that did not receive CD8 T cells (Figure 3L). These results indicate that MDA5 deficiency did not significantly alter the generation of memory CD8 T cells or their ability to protect mice from secondary challenge. Altogether, our data suggest that lack of MDA5 delays priming and expansion of virus-specific CD8 T cells and clearance of ARM infection. However, lack of MDA5 does not induce long-term CD8 T cell defects and viral persistence, indicating that alternative mechanisms compensate for the lack of MDA5; CD8 T cell responses are preserved and the virus is cleared.
Blockade of IL-12 does not exacerbate the delayed anti-ARM CD8 T cell response in MDA5−/− mice
Although lack of MDA5 delays the CD8 T cell response to acute LCMV infection, the infection is eventually cleared, suggesting that other signals compensate for lack of MDA5. It has been shown that in the absence of IFN-I signaling, IL-12 production is elevated, providing an alternative signal that facilitates CD8 T cell responses (Cousens et al., 1999). Therefore, we first investigated the impact of IL-12 on CD8 T cell response against ARM in MDA5−/− mice. We compared the levels of IL-12p35 mRNA in the spleen and IL-12p40 protein in the serum of WT and MDA5−/− mice early during ARM infection. TxM−/− mice were also included as controls, as TLRs are known to promote IL-12 responses to viral infection (Pichlmair and Reis e Sousa, 2007; Takeuchi and Akira, 2010). We found no differences in IL-12 levels between WT and MDA5−/− mice. In contrast TxM−/− mice had markedly reduced amounts of IL-12p40 in the serum as well as very low IL-12p35 mRNA expression in the spleen (Figure S3A-B). Further indicating that IL-12 does not compensate for IFN-I deficiency in MDA5−/− mice, antibody-mediated blockade of IL-12 in MDA5−/− mice did not augment the disparity between MDA5−/− and WT mice in terms of their capacity to generate LCMV-specific CD8 T cells (data not shown). Similarly, IL-12 blockade did not impair the long-term capacity of virus-specific CD8 T cells to produce TNF-α and IFN-γ in either MDA5−/− or WT mice (Figure S3C-D).
Loss of CD4 T cell help and MDA5-mediated IFN-I production leads to CD8 T cell exhaustion during ARM infection
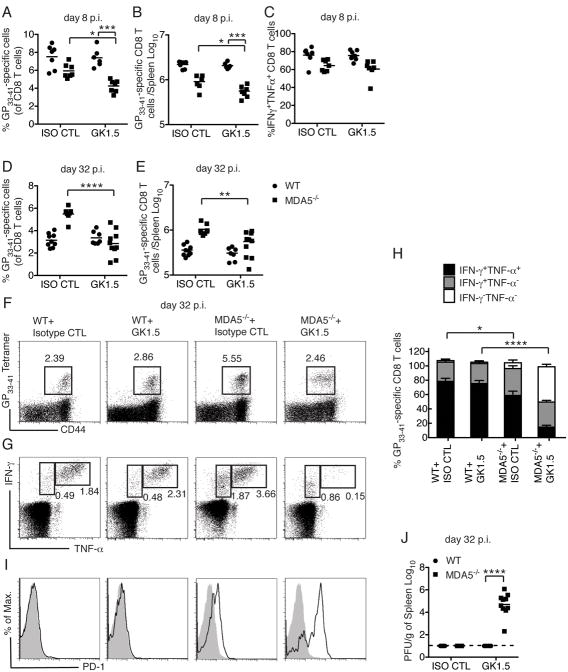
We next asked whether CD4 T celI help compensates for lack of MDA5 in inducing anti-LCMV CD8 T cell responses and viral clearance. It has been shown that CD8 T cell responses to ARM do not require CD4 T cell help, since CD4−/− mice can clear infection (Ahmed et al., 1988; Battegay et al., 1994; Matloubian et al., 1994; Rahemtulla et al., 1991). Consistent with this, depletion of CD4 T cells with the GK1.5 antibody in WT mice had little impact on the priming and expansion of LCMV-specific CD8 T cells on day 8 p.i. or their cytokine production (Figure 4A-C). In the short term (8 days p. i.) CD4 T cell-depletion in MDA5−/− mice only caused a minor but statistically significant reduction of GP33–41 specific CD8 T cells compared to non-depleted MDA5−/− mice. Additionally, the percentage of IFN-γ+TNF-α+ CD8 T cells was similar in CD4 T cell-depleted and non-depleted MDA5−/− mice (Figure 4C).
Figure 4. (see also Figure S3). Impact of CD4 T cell help on CD8 T cells during ARM infection in the absence of MDA5.
Mice were treated with isotype control mAb (ISO CTL) or GK1.5 mAb and infected with ARM. (A-C) Analysis on day 8 p.i. Frequencies (A) and absolute numbers (B) of splenic GP33–41 specific CD8 T cells. (C) Frequencies of IFN-γ-and TNF-α-producing CD8 T cells after ex vivo GP33–41 peptide stimulation. (D-J) Analysis on day 32 p.i. Frequencies (D) and absolute numbers (E) of splenic GP33–41 specific CD8 T cells. (F, G) Flow cytometric determination and (H) Percentages of splenic IFN-γ- and TNF-α-producing CD8 T cells afterex vivo GP33–41 peptide stimulation. (I) PD-1 expression (black histogram) among GP33–41 specific CD8 T cells. Gray histograms show PD-1 expression on naïve CD8 T cells. (J) Viral burden in spleens was also measured. Data are representative of two independent experiments with 3-5 mice/group in each experiment. Error bars represent the mean +/- SEM. **** = p<0.0001; *** = p<0.001; * = p<0.05, Student's t-test.
However, in the long term, CD4 T cell depletion in MDA5−/− had a major impact on CD8 T cells. On day 32 p.i., GK1.5 treatment led to a further reduction of GP33–41 specific CD8 T cells in MDA5−/− mice (Figure 4D-F). More importantly, a marked proportion of GP33–41 specific CD8 T cells in GK1.5 treated MDA5−/− mice had lost the ability to produce TNF-α in response to peptide stimulation (Figure 4G-H), indicating that those CD8 T cells were functionally exhausted (Wherry, 2011). In contrast, TNF-α and IFN-γ production was only slightly reduced in non-depleted MDA5−/− mice. CD8 T cells in MDA5−/− depleted of CD4 T cells displayed other characteristics associated with exhaustion (Wherry, 2011), such as PD-1 expression (Figure 4I). Finally, while WT mice treated with GK1.5 and MDA5-/- mice were able to clear infection, GK1.5-treated MDA5−/− mice had relatively high viral burdens in the spleen on day 32 p.i. (Figure 4J). Thus, while an isolated defect in MDA5 results in a delayed CD8 T cell response with limited functional consequences, the lack of both MDA5 and CD4 T cell help converts an acute LCMV infection into a chronic infection associated with marked CD8 T cell exhaustion.
Early blockade of IFN-I signaling impaired antiviral CD8 T cells beyond MDA5 deficiency
Because the MDA5 pathway stimulates production of both IFN-I and inflammatory cytokines, we wanted to dissociate the contribution of IFN-I from the potential contribution of inflammatory cytokines on the induction of anti-ARM CD8 T cells. Therefore, to mimic the lack of early IFN-I response, we treated WT mice with a single injection of an antibody that blocks the receptor for IFN-I (IFNAR) at 16 h p.i. Early IFNAR blockade was carried out with or without CD4 T cell depletion. Whereas CD4 depletion alone had little effect, combined blockade of IFNAR signaling and CD4 depletion resulted in reduced numbers of GP33–41 specific CD8 T cells, almost complete abrogation their capacity to produce both IFN-γ and TNF-α, and increase in PD-1 expression (Figure S3E-H). This provides further evidence that CD8 T cell exhaustion in ARM infection results from a lack of early IFN-I response during the induction of CD8 T cell response combined with absence of CD4 T cell help. Interestingly, although MDA5 deficiency alone did not induce obvious functional exhaustion of CD8 T cells, IFNAR blockade induced a detectable reduction of IFN-γ and TNF-α production and modest increase in PD-1 expression (Figure S3E-H). These results suggest that in the absence of MDA5, LCMV infection elicits residual amounts of IFN-I, which, combined with CD4 T cell help, prevent functional exhaustion of virus-specific CD8 T cells.
IFN-I responses are more rapidly impaired by CL13 infection than by ARM infection
Our data show that when IFN-I responses are impaired, as they are in MDA5−/− mice, host responses become reliant on CD4 T cell help to prevent persistent ARM infection. Since CL13 infection impairs CD4 T cell help (Brooks et al., 2005), we asked whether IFN-I responses are concomitantly reduced, such that the combined defect results in viral persistance. Although we had observed that IFN-α responses to ARM and CL13 have similar kinetics (see Figure 2), we hypothesized that the magnitude of IFN-α response was different during the two infections. This possibility could not be easily excluded since mice are usually infected with different inoculi of ARM and CL13 (3×105 versus 3×106 PFU/mouse respectively) and different routes (i.p. versus i.v.); therefore, the amounts of IFN-α induced in the serum cannot be directly compared. Thus, we infected WT mice i.v. with 3×106 PFU of ARM or CL13 and measured serum IFN-α at different time points. As previously observed, IFN-α peaked on day 2 in both infections and then declined. However, serum IFN-α declined more rapidly in CL13- than ARM-infected mice (Figure 5A).
Figure 5. IFN-I production, viral replication and CD8 T cell responses during ARM and CL13 infections.
Serum IFN- α (A) and LCMV NP and GP RNA load (B) in spleens of WT mice infected with 3×106 PFU of ARM or CL13 i.v. were measured. (C, D) GP33–41 specific CD8 T cells in peripheral blood (C) and spleen (D) were analyzed on day 8 p.i. Data are representative of at least two independent experiments with a minimum of 3 mice/group. Error bars represent the mean +/- SEM. *** = p<0.001. ** = p<0.01. * = p<0.05, Student's t-test.
It has been shown that LCMV nucleoprotein binds to and inhibits MDA5 and RIG-I in vitro (Zhou et al., 2010). Thus, it seemed possible that CL13 is more efficient than ARM at blocking MDA5 because of its higher rate of replication. However, the RNA levels of nucleoprotein and glycoprotein were similar in CL13 and ARM infected spleens on day 4 p.i. (Figure 5B). Thus, it is likely that CL13 down-modulates the IFN-I response through alternative mechanisms.
In parallel with a more rapid decline in IFN-α, WT mice infected with CL13 had fewer LCMV-specific CD8 T cells than mice infected with ARM on day 8 p.i. in both blood and spleen (Figure 5C and 5D). Moreover, the generation of anti-LCMV-specific CD8 T cells was more severely reduced in CL13- than in ARM-infected MDA5−/− mice, indicating that generation of CD8 T cells is more IFN-I-dependent during CL13 than during ARM infection. We speculate that while reduction of the IFN-I response during ARM infection in MDA5−/− mice is compensated by CD4 T cell help (see Figure 4), this is less likely to occur during CL13 infection because the CD4 T cell response is known to be partially impaired in this situation (Brooks et al., 2005). Indeed, during CL13 infection, activated CD4 T cells become susceptible to NK cell-mediated lysis, which dampens CD4 T cell responses (Waggoner et al., 2012).
Early administration of IFN-I during chronic LCMV infection prevents viral persistence and CD8 T cell exhaustion
Because the early IFN-I response was lower in mice infected with CL13 than in those infected with ARM, we asked whether IFN-I treatment early during CL13 infection can improve subsequent CD8 T cell responses. We administered 15,000 U of recombinant IFN-β and IFN-α5 to WT mice i.v. on day 3 and day 5 after infection with CL13 and analyzed peripheral blood and splenic CD8 T cells on day 8 p.i.. IFN-I treatment significantly augmented the frequency and absolute numbers of GP33–41 specific CD8 T cells in WT mice (Figure 6A and 6B). Administration of IFN-I also improved CD8 T cell responses to CL13 in MDA5−/− mice, amplifying GP33–41 specific CD8 T cells to the levels seen in IFN-I-treated WT mice (Figure 6C). Exogenous IFN-I was equally effective when administered on day 3 and day 4 or on day 2 through day 5, but less effective on day 2 and day 3 (Figure 6C) and was completely ineffective beyond one week of infection (data not shown), consistent with a recent study (Audige et al, 2011). Interestingly, the window of IFN-I effectiveness coincides with IFN-I decline following infection with CL13 and the priming of CD8 T cells, suggesting that priming of CD8 T cell responses requires sustained IFN-I during the initial phase of the infection. Depletion of CD4 T cells did not diminish the efficacy of IFN-I treatment in enhancing CD8 T cell response (Figure 6D and 6E), further confirming that CD4 T cell help is not necessary in the presence of optimal levels of IFN-I. Administration of heat-inactivated IFN-I had no effect on CD8 T cells (Figure S4A), excluding a putative adjuvant effect of bacterial components contaminating IFN-I preparations. In addition, IFN-I treatment of TCRβδ−/− mice did not affect viral titers in the spleen, indicating that the amount of IFN-I administered was not sufficient to directly inhibit viral replication (Figure S4B).
Figure 6. (see also Figure S4). IFN-I treatment boosts the CD8 T cell response during CL13 infection.
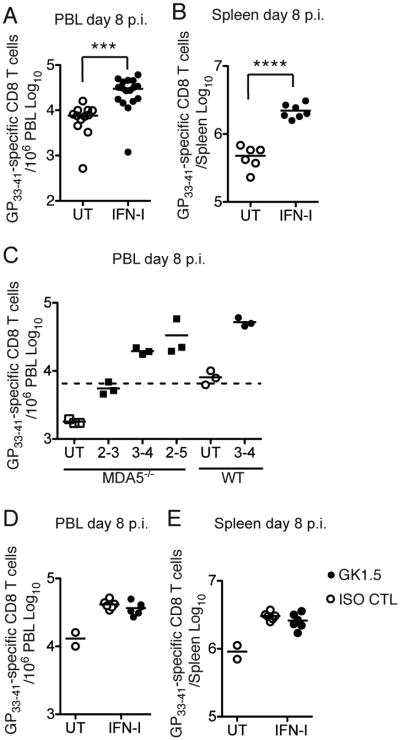
(A, B) WT mice were treated with IFN-I or not (UT) on days 3 and 5 p.i. with CL13. Numbers of GP33–41 specific CD8 T cells were determined in peripheral blood (PBL) (A) and spleen (B) on day 8 p.i. (C) MDA5−/−and WT mice were UT or treated with IFN-I on the indicated days p.i. GP33–41 specific CD8 T cells were analyzed in PBL on day 8 p.i. (D, E) WT mice were treated with control (ISO CTL) or GK1.5 mAb on days −1 and +1 p.i. IFN-I was administered or not on days 3 and 5 p.i. GP33–41 specific CD8 T cells were analyzed in PBL (D) and spleen (E) on day 8 p.i. Data represent at least two independent experiments with 2-5 mice/group. **** = p<0.0001; *** = p<0.001, Student's t-test.
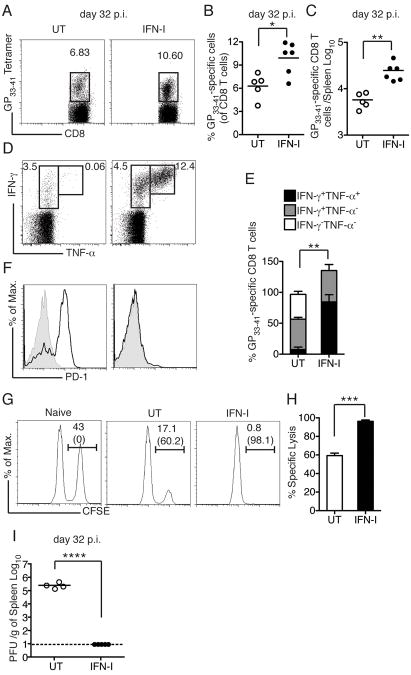
Finally, we analyzed CD8 T cell responses in IFN-I treated mice on day 32 after CL13 infection. Similar to the primary response on day 8, IFN-I-treated mice had consistently higher frequencies and absolute numbers of GP33–41 specific CD8 T cells in the spleen (Figure 7A-C). Moreover, while GP33–41 specific CD8 T cells in WT mice were unable to produce TNF-α and expressed PD-1, GP33–41 specific CD8 T cells from IFN-I treated mice remained poly-functional and did not express PD-1 (Figure 7D-F), confirming that early IFN-I treatment effectively prevented CD8 T cell exhaustion. We also asked whether IFN-I treatment improved the ability of GP33–41 specific CD8 T cells to kill target cells in vivo.Cytotoxicity was evaluated in CL13-infected mice with or without IFN-I treatment on day 32 p.i. For target cells, naïve splenocytes were either pulsed with the GP33–41 peptide or left untreated and each population was labeled with a different concentration of carboxyfluorescein diacetate succinimidyl ester (CFSE) in order to distinguish the two. IFN-I-treated mice were more efficient at clearing peptide-pulsed cells than were untreated mice (Figure 7G-H). Furthermore, IFN-I-treated mice had completely cleared infection on day 32 p.i., while high viral loads were still detectable in the spleens of untreated mice (Figure 7I). Altogether, our data suggest that therapeutic administration of IFN-I within a critical time frame after infection (day 3-5 p.i.) effectively prevents CD8 T cell exhaustion and establishment of chronic infection.
Figure 7. IFN-I treatment prevents CD8 T cell exhaustion and chronic LCMV infection.
(A-E) CL13-infected WT mice were treated with IFN-I or not (UT) on days 3 and 5 p.i. Spleens of UT and IFN-I-treated mice were analyzed on day 32 p.i. for frequencies (A, B) and absolute numbers (C) of GP33–41 specific CD8 T cells. (D, E) IFN-γ and TNF-α production by CD8 T cells afterex vivoGP33–41 peptide stimulation was also analyzed. (F) PD-1 expression (black histogram) among GP33–41 specific CD8 T cells was determined. Gray histograms indicate PD-1 expression of naïve CD8 T cells. (G, H) In vivo cytotoxicity of virus-specific CD8 T cells was performed in naïve or CL13-infected mice with or without IFN-I treatment. (I) Spleen viral load in CL13-infected mice with or without IFN-I treatment. Data represent at least two independent experiments with 2-5 mice/group. Error bars represent the mean +/- SEM. **** = p<0.0001; *** = p<0.001; ** = p<0.01; * = p<0.05, Student's t-test.
Discussion
In this study, we defined the pathways that elicit an IFN-I response during acute and chronic LCMV infection and their relative impact on CD8 T cell responses. As yet, there is no consensus regarding the role of pDCs in LCMV infection. An early report indicated that the IFN-I response to LCMV is pDC-independent (Dalod et al., 2002). However, a more recent study placed emphasis on the role of pDCs in inducing both IFN-I and CD8 T cell responses to acute LCMV (Jung et al., 2008). Mice with a conditional deletion of the transcription factor E2-2 in CD11c+ cells lack pDCs, which results in impaired control of chronic LCMV infection due to defective antiviral CD4 T cell responses (Cervantes-Barragan et al., 2012). Taking advantage of a mouse model of inducible pDC depletion, we found that neither transient nor chronic depletion of pDCs had an appreciable effect on CD8 T cell responses or viral burden during either acute or chronic LCMV infection. pDCs made an early but transient contribution to the systemic IFN-I response to LCMV probably because they rapidly disappeared from the spleen perhaps from IFN-I-induced apoptosis (Swiecki et al., 2011). The early contribution of pDCs to the IFN-I response to LCMV is in agreement with a report by Macal et al. in this issue of Cell Host & Microbe (Macal et al., 2012). Similar to another report in this issue of Cell Host & Microbe, the TLR7/MyD88 pathway was responsible for early production of IFN-I, but had no major impact on the magnitude of the IFN-I response and viral burden in the spleen until day 30 p.i. (Walsh et al., 2012). Interestingly, the lack of TLR7 facilitates persistence of CL13 infection in brain and kidney at later timepoints (Walsh et al., 2012). Thus, lack of TLR7 may have broader impact than lack of pDCs on anti-LCMV responses, especially in chronic infection.
In contrast, MDA5 was the major trigger of IFN-I in response to both acute and chronic LCMV infection, made evident by markedly reduced IFN-I production in infected MDA5−/− mice. Because activation of MDA5 requires the presence of viral RNA in the cytosol, it is likely that LCMV-infected cells are the major source of IFN-I. Supporting this conclusion, depletion of macrophages, which are a preferential target of ARM, results in almost complete abrogation of IFN-I (Borrow et al., 1995; Louten et al., 2006; Muller et al., 2002). Since CL13 infects not only macrophages but also hematopoietic progenitors, DCs and fibroblasts (Bergthaler et al., 2010; Borrow et al., 1995; Macal et al., 2012; Mueller et al., 2007; Sevilla et al., 2000; Sevilla et al., 2004), IFN-I is probably generated by a wide array of cells during CL13 infection. Consistent with previous studies (Borrow et al., 2010; Lee et al., 2009a; Zuniga et al., 2008), we found that the systemic IFN-I response to LCMV rapidly declined within the first week of infection, whether the virus was cleared (ARM) or persisted (CL13). Thus, waning of the systemic IFN-I response did not reflect viral clearance, but rather the capacity of LCMV to actively inhibit the IFN-I response. Inhibition may be mediated by NP, which was recently reported to interfere with IRF3 activation (Martinez-Sobrido et al., 2006) as well as bind to and inhibit MDA5 and RIG-I (Zhou et al., 2010). Moreover, CL13 may down-modulate IFN-I responses through additional immunosuppressive mechanisms.
The MDA5 pathway was not only important for IFN-I production but also for CD8 T cell responses. Because IFN-I has been shown to promote the expansion of LCMV-ARM-specific CD8 T cells (Kolumam et al., 2005; Thompson et al., 2006; van den Broek et al., 1995b), it was not unexpected to observe an initial reduction in CD8 T cells in MDA5−/− mice. However, we were surprised to see that CD8 T cells expanded even more in MDA5−/− than in WT mice at later time-points and ultimately controlled viral infection. This prolonged CD8 T cell response is probably due to sustained viral replication and viral antigen presentation and is reminiscent of what has been reported in mice infected with murine cytomegalovirus (MCMV) and lacking efficient NK cell responses (Andrews et al., 2010; Lee et al., 2009b).
The ultimate control of viral infection in MDA5−/− mice suggested compensation by other mechanisms. While we excluded a role for IL-12, CD4 T cell depletion combined with MDA5 deficiency did result in CD8 T cell exhaustion and persistent infection characteristic of CL13 infection. Thus, IFN-I and CD4 T cell help synergize to promote effective CD8 T cell responses against ARM and prevent viral persistence. Corroborating this, a recent report showed that IFN-I can compensate for CD4 help in inducing CD8 T cell responses during vaccinia infection (Wiesel et al., 2011). Because IL-12 did not compensate for the lack of IFN-I, we surmise that CD4 T cell help for CD8 T cells is independent of IL-12, but might be mediated by IL-2 (Ridge et al., 1998; Williams et al., 2006).
The concurrent roles for IFN-I and CD4 T cell help in effectively controlling ARM infection provide insight into the mechanisms responsible for viral persistence and CD8 T cell exhaustion during CL13 infection in WT mice. We found that CL13 inhibits the IFN-I response more effectively than does ARM. This may reflect the high replicative capacity of CL13 as well as its ability to infect and inhibit a broader array of cells than ARM, including DCs and stromal cells (Bergthaler et al., 2010; Borrow et al., 1995; Macal et al., 2012; Mueller et al., 2007; Sevilla et al., 2000; Sevilla et al., 2004). Additionally, CL13 impairs CD4 T cell responses, due partly to induction of IL-10 (Brooks et al., 2005) and partly to NK cell-mediated lysis of activated CD4 T cells (Waggoner et al., 2012). Thus, we envision that CL13 may promote CD8 T cell exhaustion and establish chronic infection by jeopardizing both early IFN-I responses and CD4 T cell help to CD8 T cells. Other factors, such as the increased viral loads and the presence of inhibitory cytokines may also contribute to the dysfunctional antiviral CD8 T cell response in CL13 infection.
Importantly, IFN-I treatment on days 3 and 4 p.i. bolstered the CD8 T cell response and led to clearance of CL13 infection. Reconstitution of optimal levels of IFN-I was equally effective in WT and MDA5−/− mice, probably because exogenous IFN-I can compensate for the CL13-mediated inhibition of IFN-I as well as for MDA5-deficiency. IFN-I may also boost CD4 T helper cells in both WT and MDA5−/− mice (Havenar-Daughton et al., 2006). IFN-I treatment during CL13 infection was effective during a narrow window of time that coincides with the decline of the IFN-I response and priming of CD8 T cells. Thus, the impact of disparate cellular and molecular sources of IFN-I on CD8 T cell-mediated control of viral infection depends on the timing and duration of the IFN-I response engendered and the rate of viral replication. IFN-I production by pDCs is too little and too transient to enable CD8 T cell responses to LCMV, but may be sufficient to control viruses that replicate more slowly than LCMV or are inoculated in lower amounts. The magnitude and duration of MDA5-induced IFN-I is adequate to elicit effective CD8 T cell responses and control infection by ARM, but not CL13, which replicates more rapidly and effectively than ARM, impairing both MDA5 and CD4 T cell function. Thus, a supplementary source of IFN-I is capable of promoting CD8 T cell-mediated control of CL13 infection.
Recombinant IFN-α is currently used to treat chronic hepatitis C virus and hepatitis B virus infections because of its direct antiviral properties (Gonzalez and Keeffe, 2011). Our study indicates that IFN-I also promotes the generation of antiviral CD8 T cells that facilitate viral clearance during chronic infection. We predict that IFN-I would be particularly effective if administered during the acute phase of infection, concurrent with CD8 T cell priming and expansion. Moreover, IFN-I treatment may be effective in conjunction with the administration of “therapeutic” vaccines to induce CD8 T cells that reduce the viral set point. Thus, the rescue of CD8 T cell responses may be a benchmark for IFN-I treatment of certain viral infections.
Experimental Procedures
Mice, viral infections, treatment with DT and recombinant IFN-I
Knockout mice (listed in Supplemental Experimental Procedures), BDCA2-DTR transgenic mice and WT C57BL/6 control mice were age- and gender-matched and bred in the same room. DT (Sigma-Aldrich) was injected i.p. into BDCA2-DTR transgenic mice as previously described (Swiecki et al., 2011). IFN-I treatment consisted of i.v. injections of 15,000 U of recombinant mouse IFN-β and IFN-α5 (kindly provided by D. Fremont). Mice were infected with 3×105 PFU of ARM i.p. or 3×106 PFU of CL13 i.v. For the comparison of IFN-I production, mice were infected i.v. with 3×106 PFU of ARM or CL13. Viral burden in the spleen was determined by plaque assay in Vero cells and qPCR. Animal studies were approved by the Washington University Animal Studies Committee.
Quantitative PCR and ELISA
Relative levels of IL-12p35, LCMV NP and GP RNA expression were determined by qPCR, using SYBR Green PCR master mix (Bio-Rad Laboratories) and an ABI7000 machine (Applied Biosystems). Primers are described in Supplemental Experimental Procedures. IFN-α and IL-12p40 were determined by ELISA (PBL Interferon Source, eBioscience).
Flow cytometry
LCMV-specific CD8 T cells were detected with H-2Db GP33–41 tetramers (Beckman-Coulter). Additional reagents used for flow cytometry are listed in Supplemental Experimental Procedures.
Determination of CD8 T cell poly-functionality
Spleen cells from LCMV-infected mice were cultured for 6 h in the presence of GP33–41 peptide (5 μg/mL) and GolgiPlug (BD Biosciences). Intracellular IFN-γ and TNF-α production by CD8 T cells was determined by flow cytometry. Formulas for calculation of poly-functional CD8 T cells are included in Supplemental Experimental Procedures.
Cytotoxicity assays
In vitro assays consisted of a standard 51 Cr release assay. Effector and target cells are specified in Supplemental Experimental Procedures. In vivo cytotoxicity of CD8 T cells was determined by adoptively transferring 5×106 GP 33–41 peptide pulsed naïve splenocytes labeled with CFSE at 5 mM and 5×106 unpulsed naïve splenocytes labeled with CFSE at 1 mM. Spleens were harvested 7 h post transfer and the ratio between GP 33–41 pulsed cells (CFSEhi) and unpulsed cells (CFSElo) was determined by flow cytometry.
Antibody mediated depletion and neutralization
Purified GK1.5 mAb and neutralizing IL-12 mAb (10F6) were used as described previously (Ahmed et al., 1988; Mattner et al., 1997). MAR1 or isotype control mAb was administered i.p. 16 h p.i. (2 mg/mouse).
Statistical analysis
All statistical analyses were performed using GraphPad Prism (Graphpad software). The statistical significance of differences in mean values was analyzed with an unpaired, two-tailed Student's t-test.
Supplementary Material
Research highlight.
MDA5 is the key trigger of IFN-I that promotes CD8+T cell responses to LCMV
In MDA5−/− mice infected with acute LCMV, CD8+T cells are sustained by CD4+T cells
Chronic LCMV inhibits production of IFN-I, which facilitates CD8+T cell exhaustion
Given early during chronic LCMV, IFN-I rescues CD8+T cells and clears the virus
Acknowledgments
We would like to thank D. Fremont, M. Epperson, K. Sheehan, A. French (Washington University, St. Louis, MO), S. Akira (Osaka University, Osaka, Japan), R. Flavell (Yale University, New Heaven), R. Ahmed (Emory University, Atlanta, GA) and M. Oldstone (The Scripps Research Institute, La Jolla, CA) for reagents, mice and viruses. This project was supported by the Pulmonary and Critical Care training grant 2T32HL007317-31 from the National Heart, Lung and Blood Institute (to Y.W.); the NRSA training grant 5T32DK007296 from the National Institute of Diabetes and Digestive and Kidney Diseases (to M. S.); the NIAID Center for HIV/AIDS Vaccine Immunology grant A1067854 (to M. C.).
Footnotes
Contact: Marco Colonna, Department of Pathology and Immunology, BJC Institute of Health at Washington University, 8th Floor Room 8107, 425 S. Euclid, St Louis MO, 63110, USA. Tel: 314-362-0367; FAX: 314-747-0809; mcolonna@pathology.wustl.edu
The authors have no further conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed R, Butler LD, Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews DM, Estcourt MJ, Andoniou CE, Wikstrom ME, Khong A, Voigt V, Fleming P, Tabarias H, Hill GR, van der Most RG, et al. Innate immunity defines the capacity of antiviral T cells to limit persistent infection. J Exp Med. 2010;207:1333–1343. doi: 10.1084/jem.20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audige A, Hofer U, Dittmer U, van den Broek M, Speck RF. Evaluation of the immunomodulatory and antiviral effects of the cytokine combination IFN-alpha and IL-7 in the lymphocytic choriomeningitis virus and Friend retrovirus mouse infection models. Viral Immunol. 2011;24:375–385. doi: 10.1089/vim.2011.0006. [DOI] [PubMed] [Google Scholar]
- Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- Battegay M, Moskophidis D, Rahemtulla A, Hengartner H, Mak TW, Zinkernagel RM. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J Virol. 1994;68:4700–4704. doi: 10.1128/jvi.68.7.4700-4704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergthaler A, Flatz L, Hegazy AN, Johnson S, Horvath E, Lohning M, Pinschewer DD. Viral replicative capacity is the primarydeterminant of lymphocytic choriomeningitis virus persistence and immunosuppression. Proc Natl Acad Sci USA. 2010;107:21641–21646. doi: 10.1073/pnas.1011998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser C, Kaufmann M, Pircher H. Virus-activated CD8 T cells and lymphokine-activated NK cells express the mast cell function-associated antigen, an inhibitory C-type lectin. J Immunol. 1998;161:6451–6454. [PubMed] [Google Scholar]
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–315. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Borrow P, Evans CF, Oldstone MB. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J Virol. 1995;69:1059–1070. doi: 10.1128/jvi.69.2.1059-1070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Martinez-Sobrido L, de la Torre JC. Inhibition of the type I interferon antiviral response during arenavirus infection. Viruses. 2010;2:2443–2480. doi: 10.3390/v2112443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Teyton L, Oldstone MB, McGavern DB. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol. 2005;79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz E, Bevan MJ. Dynamics of the CD8+ T cell response during acute LCMV infection. Adv Exp Med Biol. 1998;452:111–122. doi: 10.1007/978-1-4615-5355-7_13. [DOI] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Lewis KL, Firner S, Thiel V, Hugues S, Reith W, Ludewig B, Reizis B. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci USA. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, Biron CA. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, Briere F, Trinchieri G, Biron CA. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- Gonzalez SA, Keeffe EB. Chronic viral hepatitis: epidemiology, molecular biology, and antiviral therapy. Front Biosci. 2011;16:225–250. doi: 10.2741/3685. [DOI] [PubMed] [Google Scholar]
- Haring JS, Badovinac VP, Harty JT. Inflaming the CD8+ T cell response. Immunity. 2006;25:19–29. doi: 10.1016/j.immuni.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, Takeuchi O, Akira S. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and induces a cytotoxic T-cell response via MyD88. J Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, Kawai T, Akira S. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Etchart N, Rossmann C, Ashton M, Hou S, Gewert D, Borrow P, Tough DF. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- Le Bon A, Tough DF. Type I interferon as a stimulus for crosspriming. Cytokine Growth Factor Rev. 2008;19:33–40. doi: 10.1016/j.cytogfr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J Immunol. 2009a;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim KS, Fodil-Cornu N, Vidal SM, Biron CA. Activating receptors promote NK cell expansion for maintenance, IL-10 production, and CD8 T cell regulation during viral infection. J Exp Med. 2009b;206:2235–2251. doi: 10.1084/jem.20082387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louten J, van Rooijen N, Biron CA. Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection. J Immunol. 2006;177:3266–3272. doi: 10.4049/jimmunol.177.5.3266. [DOI] [PubMed] [Google Scholar]
- Macal M, Lewis GM, Kunz S, Flavell R, Harker JA, Zuniga EI. Early host responses upon in vivo infection with a plasmacytoid dendritic cell-tropic arenavirus. Cell Host Microbe. 2012 This Issue. [Google Scholar]
- Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sobrido L, Zuniga EI, Rosario D, Garcia-Sastre A, de la Torre JC. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner F, Ozmen L, Podlaski FJ, Wilkinson VL, Presky DH, Gately MK, Alber G. Treatment with homodimeric interleukin-12 (IL-12) p40 protects mice from IL-12-dependent shock but not from tumor necrosis factor alpha-dependent shock. Infect Immun. 1997;65:4734–4737. doi: 10.1128/iai.65.11.4734-4737.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid activation of spleen dendritic cell subsets following lymphocytic choriomeningitis virus infection of mice: analysis of the involvement of type 1 IFN. J Immunol. 2005;174:1851–1861. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- Moskophidis D, Battegay M, Bruendler MA, Laine E, Gresser I, Zinkernagel RM. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J Virol. 1994;68:1951–1955. doi: 10.1128/jvi.68.3.1951-1955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci USA. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Hunziker L, Enzler S, Buhler-Jungo M, Di Santo JP, Zinkernagel RM, Mueller C. Role of an intact splenic microarchitecture in early lymphocytic choriomeningitis virus production. J Virol. 2002;76:2375–2383. doi: 10.1128/jvi.76.5.2375-2383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr Top Microbiol Immunol. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- Ou R, Zhou S, Huang L, Moskophidis D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J Virol. 2001;75:8407–8423. doi: 10.1128/JVI.75.18.8407-8423.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, et al. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, Holz A, Lewicki H, Homann D, Yamada H, Campbell KP, de La Torre JC, Oldstone MB. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J Exp Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011;208:2367–2374. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Type I interferon: friend or foe? J Exp Med. 2010;69:2053–2063. doi: 10.1084/jem.20101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both alpha/beta and gamma interferon receptors. J Virol. 1995a;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek MF, Muller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995b;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature. 2012;481:394–398. doi: 10.1038/nature10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Teijaro JR, Zuniga EI, Welch MJ, Fremgen DM, Blackburn SD, von Tiehl K, Wherry EJ, Flavell RA, Oldstone MB. Toll-like receptor 7 deficiency is associated with multiple defects in the adaptive immune response and lifelong persistent virus infection. Cell Host Microbe. 2012 doi: 10.1016/j.chom.2012.04.016. This Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Asher DR, Chan M, Kurt-Jones EA, Finberg RW. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J Immunol. 2007;178:3363–3367. doi: 10.4049/jimmunol.178.6.3363. [DOI] [PubMed] [Google Scholar]
- Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- Wiesel M, Kratky W, Oxenius A. Type I IFN substitutes for T cell help during viral infections. J Immunol. 2011;186:754–763. doi: 10.4049/jimmunol.1003166. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Cerny AM, Zacharia A, Fitzgerald KA, Kurt-Jones EA, Finberg RW. Induction and inhibition of type I interferon responses by distinct components of lymphocytic choriomeningitis virus. J Virol. 2010;84:9452–9462. doi: 10.1128/JVI.00155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Kurt-Jones EA, Mandell L, Cerny A, Chan M, Golenbock DT, Finberg RW. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur J Immunol. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. Lymphocytic choriomeningitis virus and immunology. Curr Top Microbiol Immunol. 2002;263:1–5. doi: 10.1007/978-3-642-56055-2_1. [DOI] [PubMed] [Google Scholar]
- Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4:374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.