Abstract
The first herpesviruses described in association with serious elephant disease were referred to as endotheliotropic herpesviruses (EEHV) because of their ability to infect capillary endothelial cells and cause potentially fatal disease. Two related viruses, EEHV1 and EEHV2, have been described based on genetic composition. This report describes the similarities and differences in clinicopathologic features of 2 cases of fatal endotheliotropic herpesvirus infections in Asian elephants caused by a previously unrecognized virus within the betaherpesvirus subfamily that is markedly divergent from the 2 previously studied fatal probosciviruses, based on polymerase chain reaction sequence analysis of 2 segments of the viral genome. In addition to ascites, widespread visceral edema, petechiae, and capillary damage previously reported, important additional findings with EEHV3 infection were the presence of grossly visible renal medullary hemorrhage, a tropism for larger veins and arteries in various tissues, relatively high density of renal herpetic inclusions, and involvement of the retinal vessels. These findings indicate a less selective organ tropism, and this may confer a higher degree of virulence for EEHV3.
Keywords: electron microscopy, endotheliotropic herpesviruses, elephants, herpesvirus, histopathology, polymerase chain reaction
Elephants are charismatic megavertebrates and, as exhibit animals at zoos, are cherished by employees and the visiting public. Both species are listed by the World Conservation Union as critically endangered and no longer can be removed from wild habitats for human benefit. Thus, captive breeding is the sole source for replacing elephants at zoos and for maintaining the genetic diversity of captive populations. Unfortunately, the mortality rates in captive bred elephants are considered unacceptably high in zoologic facilities, and it is estimated that the current zoo populations will be extinct within 48 years, based on current conception and attrition rates.5
The first herpesviruses described in association with serious elephant disease were referred to as endotheliotropic herpesviruses (EEHV) because of their morphologically visible affects on infected endothelial cells.12 Phylogenetically, they are primitive betaherpesviruses recently assigned to a new genus Proboscivirus9 and are not known to exist outside of elephantid hosts. EEHVs are considered a significant cause of reproductive failure and death in captive elephants from Europe, the United States, and Asia.1–3,6,10,13 Two related viruses, EEHV 1 and EEHV 2 were described based on genetic composition,13 and there appear to be 2 major variants of EEHV1 that differ greatly in several but not all genes.4,5 All EEHVs that have been associated with systemic endotheliolytic disease in Asian elephants (Elephas maximus) belong to the EEHV1 group, whereas the only 2 known similar cases in African elephants (Loxodonta africana) involved EEHV2. The EEHVs are also found within pulmonary lymphoid nodules and cutaneous papillomas of African elephants, where they are considered subclinical and low-grade pathogens; however, EEHV has potential for causing fatal endotheliolytic lesions in Asian and African elephants, especially juvenile and young adult elephants. DNA-based polymerase chain reaction (PCR) tests on whole blood exist and are sensitive for the diagnosis of EEHV 1 and 2 in viremic animals but are insensitive for the diagnosis of latently infected elephants that are not viremic.13 Overall, EEHVs have either been confirmed (by PCR DNA sequencing from necropsy tissue or blood) or implicated (because of focal lesions with cells that contain typical herpesvirus inclusion bodies) in causing nearly 40 cases worldwide of fatal systemic endotheliolytic disease, especially in captive-born juvenile and young adult elephants. Comingling of species or mechanical transfer between species are among the suspected routes of exposure, although cases have occurred in Asian elephants not in direct contact with African elephants.13
Elephants with endotheliolytic herpesvirus infections have an acute onset of lethargy, generalized edema of head and limbs, oral ulceration and cyanosis of the tongue, tachycardia, and death after a disease course of 1–7 days. Affected animals may have lymphopenia and thrombocytopenia. Several affected animals have survived the infection when treated with the antiherpesvirus drug famciclovir. Necropsy findings include pericardial effusion, intestinal hemorrhage, and mucosal ulcerations. Target tissues include heart, tongue, liver, and large intestine. Histologic findings include microhemorrhages, edema and mild inflammation. Lesions are accompanied by intranuclear herpesvirus inclusions in capillary endothelial cells.12 This report describes the similarities and differences in clinicopathologic features of 2 cases of fatal endotheliotropic herpesvirus infections in Asian elephants caused by a previously unrecognized virus within the betaherpesvirus subfamily that is markedly divergent from the 2 previously studied fatal probosciviruses.
Materials and Methods
Animals
Case No. 1 was a 5-year-old, female captive bred Asian elephant that had a 2-day history of illness before death. No antemortem testing was performed. Case No. 2 was a 6.5-year-old, 2,332-kg female captive-bred Asian elephant that died after an 8-day history of abdominal discomfort, lethargy, and partial anorexia. Complete blood cell count, fibrinogen, and biochemistry panel obtained at day 3 and day 5 of illness was normal based on International Species Information System (ISIS, 2002, 2600 Eagan Woods Drive, Suite 50, Eagan, MN 55121–1170 USA) values and in-house data for this animal and concurrently housed conspecifics. Symptomatic treatment was initiated and included flunixin meglumine (Banamine paste, 50 mg/g, Schering-Plough Animal Health Corp., Union, New Jersey USA, 07083; 2500–3000 mg PO, SID), sulfadiazine-trimethoprim 5 : 1 (Tucoprim powder, 400 mg/g, Pfizer, Inc., New York, NY, 10017 USA; 28,800 mg PO, SID), psyllium powder, bran mash, oral mineral oil administration, and warm-water enemas but only mild-to-moderate improvement was noted and symptoms appeared to wax and wane. Leptospirosis serology was negative for antibodies, fecal cytology was normal, and no pathogens were isolated on fecal cultures. Blood PCR was negative for EEHV1 and EEHV2 on day 3 of illness and on a postmortem sample (National Elephant Endotheliotropic Herpesvirus Laboratory, National Zoological Park, 3001 Connecticut Ave, NW, Washington DC, 20008 USA).
Necropsy
Necropsy findings were not recorded for case No. 1. Necropsy protocol was performed in accordance with Species Survival Plan guidelines for case No. 2 (http://www.elephanttag.org/Professional/professional_Medical_Health_Care.html).
Histopathology
For case No. 1, kidney, liver, spleen, small intestine, brain, heart, lung, and lymph node were collected. For case No 2, representative sections of all tissues were collected. For both cases, tissues were fixed in 10% neutral buffered formalin and were trimmed at day 5 of fixation for case No. 1, and day 2 for case No. 2. Tissues were routinely processed, sectioned at 5 µm, mounted on frosted-glass microscopy slides, and stained with hematoxylin and eosin (HE). Warthin-Starry and Goodpasture’s stains were applied to select tissue sections.
Electron microscopy
Formalin-fixed wet spleen from case No. 1 and wet kidney, liver, and lung from case No. 2 were examined by electron microscopy. Tissues were retrimmed and placed into modified (1/2 strength) Karnovsky’s fixative. 8 After Karnovsky’s immersion tissues were further postfixed in 2% osmium tetroxide reduced with 2.5% potassium ferrocyanide.16 After osmification, the tissue was rinsed in 0.2 M sodium cacodylate, dehydrated through a graded ethanol series, transitioned through propylene oxide and infiltrated and embedded in Eponate-12 epoxy formulation (Eponate-12, Ted Pella Inc., Redding, CA 96049). Thick sections were cut, mounted on glass slides, and stained with toluidine blue O and examined by light microscopy. Thin sections were mounted on bare 150-mesh copper grids, stained in 4% uranyl acetate in 75% ethanol, followed by poststaining in Reynold’s lead citrate.11 The sections were then examined in a Zeiss 906E transmission electron microscope at 60 kV accelerating voltage (Carl Zeiss SMT, Peabody, MA 01960 USA).
Detection of herpesvirus by PCR
The templates for PCR amplification were total DNA samples purified from minced frozen necropsy tissue (Medimachine, Dako, Carpinteria, CA, USA) and/or from whole blood by using a Gentra Capture Columnkit (GC-0050). The redundant terminase (TER) PCR amplification used the following conditions: 34 cycles of 94°C for 1 minute, 50°C for 1 minute, and 72°C for 1 minute followed by 72°C for 7 minutes, by using Platinum PCR Supermix (Invitrogen). The Codehops POL PCR amplification (Promega reagents) used the following conditions: 95°C for 2 minutes, then 45 cycles of 95°C for 40 seconds, 50°C for 45 seconds, and 73°C for 1 minute after by 73°C for 5 minutes.
The following 2 sets of redundant consensus herpesvirus DNA PCR primers were used for initial attempts at detection of novel herpesvirus genomes:
TER7
B1 LGH2425 5′-ACAGCCACGCCNGTNCCNGANGC-3′
A3 LGH2426 5′-GCAAGATCATNTTYRTNTCNTC-3′
B2 LGH2427 5′-TGTTGGTCGTRWANGCNGGRTC-3′
A2 LGH2428 5′-TTGTGGACGAGRSNMAYTTYAT-3′
A3SEQ 5′-CCCCATCTGAGCAAGATCAT-3′
B2SEQ 5′-GGCTGACAAATGTTGGTCGT-3′
First round PCR B1/A2 575 base pair (bp); second round A3/B2 415 bp; A3SEQ/B2SEQ = 360 bp.
DNA polymerase (POL) Codehops15
DFASA(Mod) LGH2595 5′-GTGTTCGACTTYGCNAGYYTNTAYCC-3′
GDTD1B LGH2597 5′-CGGCATGCGACAAACACGGAGTCNGTRTCNCCRRA-3′
VYGA LGH2596 5′-AGTGCAACGCGGTGTAYGGNKTNACNGG-3′
First round DFASA/GDTD1B 550 bp; second round VYGA/GDTD1B 250 bp.
The following EEHV3 species-specific nonredundant second-generation primer sets were used to obtain greatly increased sensitivity of detection:
EEHV3 TER
A1 LGH6707 5′-GTGCTGTAGCGGATCATGTC -3′
B1 LGH6708 5′-CGTGCAACACGAGCACGCAAAG -3′
A2 LGH6727 5-CACGAGCACGCAAAGTACGTC -3′
B2 LGH6728 5′-CGGATCATGTCGAACTCCGTG-3′
First round A1/B1 = 310 bp, second A1/B2 = 290 bp, third A2/B2 = 270 bp
Sequencing and sequence analysis
All DNA sequencing was carried out by direct cycle sequencing on both strands of agarose gel electrophoresis purified PCR DNA products from either first-round or second-round PCR amplification. The correct-sized PCR products were purified with a Qiagen Gel Extraction kit, then sequenced with the ABI PRISM BigDye Terminator v3.1 cycle sequencing kit and analyzed on an ABI310 DNA sequencer. All DNA sequence editing, analysis and manipulation was performed by using Assembly LIGN, Clustal and MacVector version 6 or 7 formulated for a MacIntosh computer. Genbank accession Nos. are the following: case No 1 POL, EU658934; Case No 1 TER, EU658935; case No. 2 POL, EU658936; case No 2 TER, EU658937.
Results
Necropsy
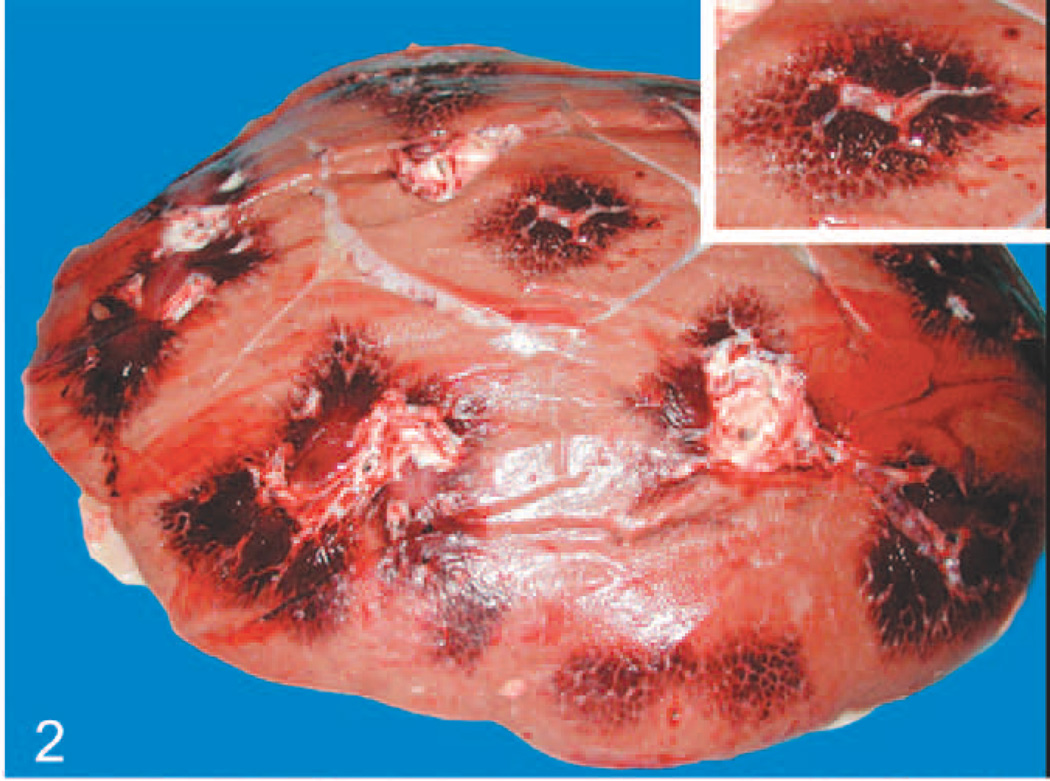
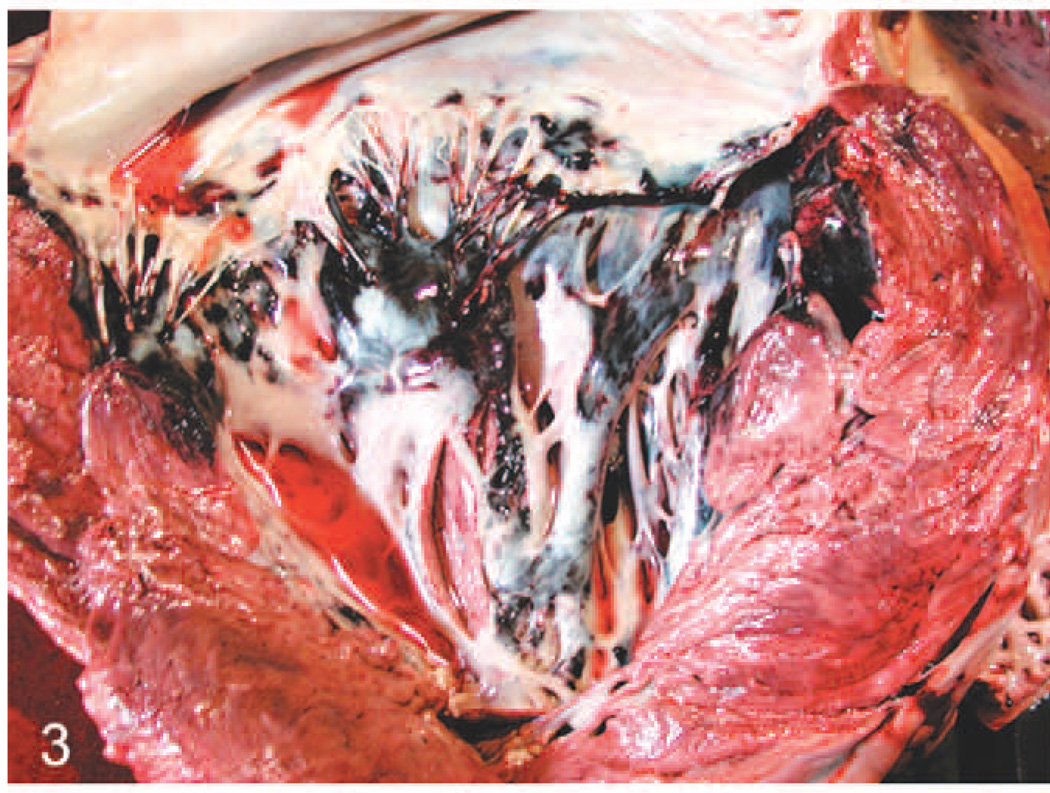
Case No. 2 had approximately 10 liters of straw colored fluid in the abdominal cavity. Marked edema was noted in the mesenteric root and omentum (Fig. 1). Petechiae were noted throughout the serosal surfaces of the gut. Marked thickening of the intestinal wall because of edema and congestion was noted throughout the alimentary tract. Foci of punctate reddening and ulceration were noted throughout the mucosa, the small intestine, and the proximal aspect of the large intestine. Some petechiae were noted on serosal surfaces of the kidney. On cut surface, the renal medullary regions of all lobes were markedly reddened (Fig. 2). The urinary bladder had petechiae on the serosal surfaces and mucosal surfaces. The heart had extensive petechiae and ecchymoses on the endocardial and serosal surfaces (Fig. 3). The lungs were diffusely and mildly reddened, edematous, and swollen. Generalized reddening and mild enlargement of all examined visceral and subcutaneous lymph nodes was also noted.
Fig. 1.
Elephant No. 2. Note marked edema in mesentery root with associated petechiae and ecchymoses.
Fig. 2.
Elephant No. 2, kidney on cut surface shows lobular reddening, especially of the medullary regions, corresponding to congestion within and hemorrhage around the peritubular capillaries. Inset: Higher magnification of medullary reddening.
Fig. 3.
Heart; elephant No. 2. Note hemorrhage in left atrioventricular valve leaflets and associated endocardium, and to a lesser extent in the myocardium of the ventricular septum and left ventricular free wall.
Histopathology
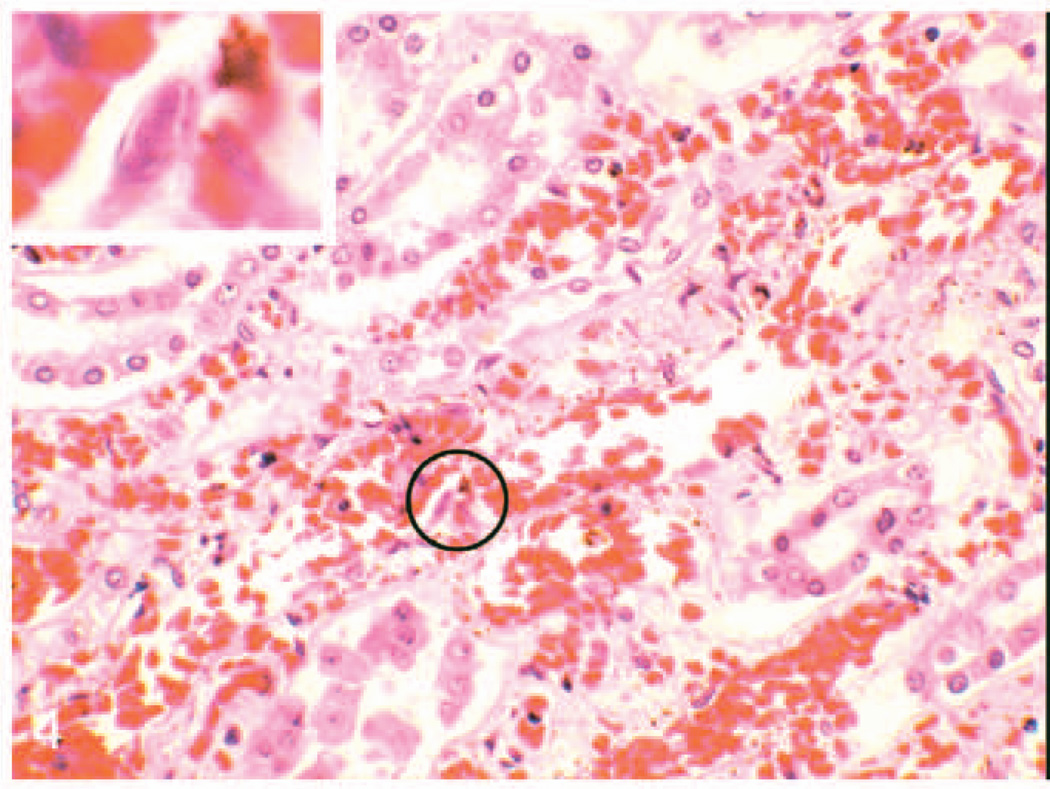
Blood vessels throughout all tissues from both elephants, especially the uvea, alimentary tract, kidney, and liver, had varying degrees of endothelial cell hypertrophy or necrosis, edema, or fibrinoid degeneration of the vessel wall, thrombosis, and perivascular edema or hemorrhage. Affected vessels included capillaries, small veins and venules, small arteries, and arterioles. Especially in the large arteries of the splenic capsule (case No. 1) and renal medulla and uvea (case No. 2), endothelial cells occasionally contained single, variably sized, eosinophilic or amphophilic smudged intranuclear inclusions (Figs. 4, 5). The small intestine and colon of case No. 2 had numerous foci of hemorrhage and necrosis that corresponded to the punctate reddened foci grossly noted, and these were associated with extensive bacterial overgrowth in the colon. Mild neutrophilic inflammation was noted multifocally in the heart adjacent to or within foci of perivascular hemorrhage and multifocally within the interstitium of the lung. Lymph nodes had mild erythrophagocytosis in the medullary sinuses. Hepatic sinusoids had slightly increased numbers of neutrophils and hypertrophied Kupffer cells that contained phagocytized cell debris. Few bile canaliculi contain bile casts, and few very small foci of clustered neutrophils were noted randomly throughout the hepatic parenchyma. “Blue bodies” were frequently seen in the hepatic sinusoids and rarely in renal peritubular capillaries of case No. 2. These structures were roughly spherical, 15–20 microns in diameter, basophilic and resembled necrotic cells (Fig. 6). Mild rhabdomyolysis was noted in skeletal muscle from the legs and tongue of case No. 2. No bacteria were seen when using Goodpasture’s and Warthin-Starry stains on multiple tissues from either elephant.
Fig. 4.
Kidney; elephant No. 2. Note congestion of the medullary peritubular capillaries associated with some mild hemorrhage and scattered interstitial cellular debris. Inset: Higher magnification of circled region showing intranuclear amphophilic inclusion in an endothelial cell. HE.
Fig. 5.
Artery in splenic capsule; elephant No. 1. Note hypertrophy of endothelial cells, Some of which contain intranuclear inclusions (arrows). Inset: Higher magnification of circled endothelial cell showing intranuclear amphophilic inclusion. HE.
Fig. 6.
Liver; elephant No. 2. Note congestion and sinusoidal leukocytosis. Sinusoids also contain blue bodies (arrow), determined by electron microscopy to be degenerative inflammatory cells. Inset: Higher magnification of 2 blue bodies from circled region. HE.
Electron microscopy
Intranuclear inclusions in endothelial cells of splenic vessels from case No. 1 and uveal vessels of case No. 2 were examined by electron microscopy. The inclusions were associated with peripheral margination of chromatin and were composed of electron-dense granular material that gave rise to hexagonal viral particles 70–80 nm in greatest dimension. These particles were seen budding through the nuclear envelope in case No. 1 and were enveloped, and 100–120 nm in diameter when seen in the cytoplasm of infected cells from both elephants (Fig. 7). Blue bodies in the hepatic sinusoids were unidentified cells with pyknotic nuclei and degenerative cytoplasmic organelles.
Fig. 7.
Electron photomicrograph of artery from splenic capsule; elephant No 1. Note hypertrophy of endothelial cells and accumulation of electron dense granules in the cytoplasm of center cell, m = muscular tunic. Inset left: Note nonenveloped hexagonal particle in nucleus (n), budding of particle through nuclear membrane (arrow), and enveloped particles in the cytoplasm. Inset right: Intracytoplasmic enveloped hexagonal particles typical of herpesvirus, uranyl acetate and lead citrate. Bar =125 nm.
Detection of herpesvirus by PCR
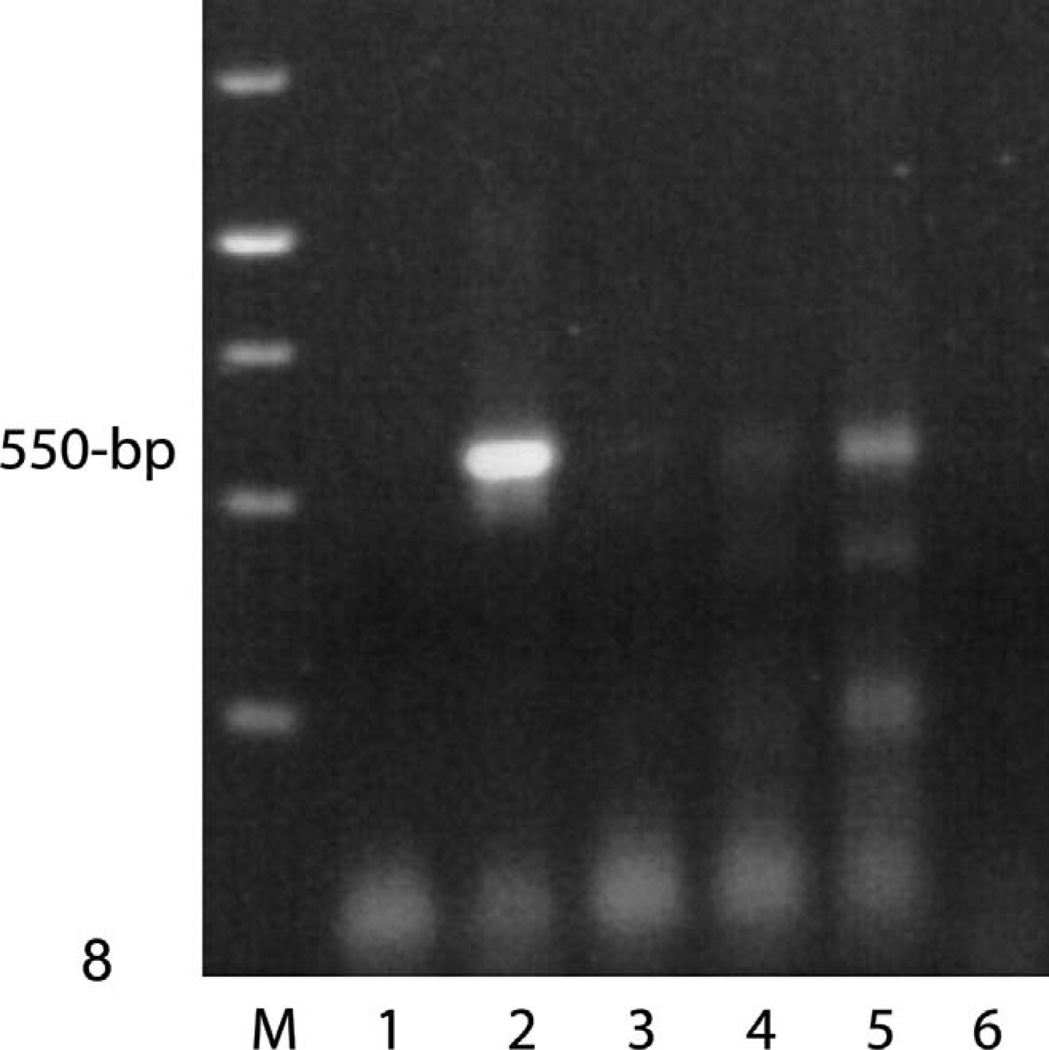
Initially, the 2 standard PCR DNA tests for the specific TER region loci of EEHV1 and EEHV2 were negative on necropsy tissue samples from case No. 1. Similarly, negative results were also obtained on 2 whole-blood samples obtained during the acute illness of case No 2. Therefore, additional PCR searches for a possible novel herpesvirus were carried out with both the redundant PAN herpesvirus TER primers7 used in the original discovery of EEHV113 and with the more recently developed PAN herpesvirus POL region Codehops primers that detect the majority of known mammalian herpesvirus DNA polymerase genes (Figs. 8, 9).15 The TER consensus primers produced secondround PCR bands of 415 bp in the heart-tissue DNA sample from case No. 1, as well as from the colon and kidney tissue DNA sample from case No. 2, but not in the whole blood or several other disease tissue samples of both. Similarly, the consensus POL primers also produced a 550-bp band from the heart-tissue sample from case No. 1 and the colon sample of case No. 2, but in no other tissue or blood samples from either case. The second-round consensus POL primers were negative on all samples.
Fig. 8.
PCR detection of a novel herpesvirus POL gene in the heart tissue; elephant No. 1. Gel electrophoretic separation of first-round PCR products by using Codehops primers LGH2595/2597 (550 bp). Photograph of ethidium bromide stained agarose gel. Lanes 1 to 4, undiluted DNA samples from No. 1. Lane 1, whole blood; 2, heart; 3, intestine; 4, spleen; 5, EEHV1B positive control; 6, no DNA negative control.
Fig. 9.
PCR detection of EEHV3 TER gene in all tissues; elephant Nos. 1 and 2. Gel electrophoretic separation of first-round PCR products by using primers LGH6707/6708 (320 bp). Photograph of ethidium bromide stained agarose gel. Lanes 1, 2, 9, and 10, elephant No. 1, undiluted DNA samples; 1, intestine; 2, spleen; 9, whole blood; 10, heart. Lane 3, human placental DNA negative control. Lanes 4 to 8, elephant No. 2., undiluted DNA samples; 4, whole blood; 5, colon; 6, kidney; 7, liver; 8, spleen. Elephant No. 1 intestine was also positive (not shown).
DNA sequencing and sequence analysis
DNA sequence analysis was carried out on second-round PCR products from the 3 positive consensus TER samples and on the first-round Codehops POL PCR products from both cases (Figs. 9, 10). The results revealed evidence for the presence of 2 closely related but novel highly diverged herpesvirus genomes. Both were more closely related to the previously described EEHV1 and EEHV2 viruses than to any other known herpesviruses. The 317-bp TER domain DNA sequence of No. 1 differed by 25.4% at the nucleotide level from both EEHV1 and EEHV2 but with just 4 of 104 predicted amino-acid differences. Moreover, the 499-bp DNA POL block from case No. 1 displayed 34.2% nucleotide differences from EEHV1 and 27% (44/166 amino acids and 42/167 amino acid) differences from EEHV1 and EEHV2, respectively, at the protein level. The 317-bp TER sequence from No. 2 produced the same predicted amino sequence as case No. 1 but differed at 12 nucleotides (3.8%) from case No. 1. Furthermore, the 488-bp POL region DNA sequence obtained from case No. 2 differed from those of case No. 1 by 7.3% at the nucleotide level and by 9% (15/162 amino acids) at the protein level. Therefore, both viruses were designated as A and B variants of a new herpesvirus named EEHV3 within the proboscivirus genus of the betaherpesvirinae. All other herpesvirus genomes in the Genbank database showed less than 54% and 55% amino-acid identity with the TER and POL proteins of these 2 EEHV3 genomes, respectively, with the closest being chimpanzee CMV for TER and human HHV6A for POL.
Based on these initial DNA sequences, a set of new second-generation PCR primers specific for case No. 2 in the TER locus were generated. These EEHV3 TER specific primers proved to give positive 310-bp first-round PCR bands from all 4 tested diseased tissue samples from case No. 1 and also from all 5 tested diseased tissue samples from No. 2. In both cases, the whole-blood samples gave weaker bands than the other tissues. Two separate acute disease blood samples taken 4 days apart from case No. 2 were positive, but a routine whole-blood sample taken a year earlier was negative. After sequencing, all of these bands proved to represent the same new EEHV3A or EEHV3B viruses observed with PCR products from the original consensus TER primers. All PCR product DNA sequences obtained from the 4 different positive necropsy tissue and blood samples from case No. 1 were identical to one another, as also were the 6 different positive tissue or blood samples from case No. 2.
Discussion
We presented evidence here that expands the syndrome of elephant endotheliotropic disease to include 2 new cases, with lesions in major internal organs associated with the presence of endothelial intranuclear inclusion bodies consistent with herpesvirus infection. By PCR sequencing analysis, the DNA genomes of the causative viruses in both of these fatal cases proved to be closely related variants of a novel herpesvirus within the proboscivirus genus that we have named EEHV3. The closest known relatives to these new viruses are the previously described EEHV1 and EEHV2 viruses, but EEHV3 is nearly twice as far diverged from both as they are from each other.
The short clinical course in both elephants, and the ascites and widespread visceral edema and petechiae seen in case No. 2 reflect the vascular damage previously described for fatal EEHV infections in elephants.12,13 The most significant gross morphologic difference was the presence of renal medullary hemorrhage described for case No. 2. The endothelial damage and presence of rare to occasional intranuclear herpetic inclusions in these elephants also were similar to those of previously described EEHV infection. The major histologic differences for the present cases were the tropism for larger veins and arteries in various tissues, as well as the previously reported involvement of capillaries. The more frequent occurrence of inclusions in the renal tissues differed from previous reports of EEHV1 and EEHV2. These findings indicate a less selective organ tropism, and this may confer a higher degree of virulence for EEHV3. Our report also describes retinal damage that was not described in previous reports of EEHV infection. This may reflect a broader tissue tropism for EEHV3, but it is also possible that eyes had not been routinely examined histologically in previous cases. Although retinal examination was not performed on case No. 2 before death, the retinal vascular damage and associated changes are lesions that perhaps can be detected by antemortem ophthalmic examination, thus providing additional clinical support for infection.
Including the recently reported description of 4 elephant gamma herpesviruses by Wellehan et al,17 these 2 variants of EEHV3 now represent the seventh species of herpesvirus found among either Asian or African elephants. Infections with gamma herpesviruses appear to be ubiquitous and largely subclinical within captive elephants, but this is probably not the case for the probosciviruses. Although the probosciviruses have evidently coevolved with their hosts throughout elephantid evolution and would also be expected to persist in a long-term latent state in animals that survive infection, it appears that unknown factors may predispose infected elephants to develop fatal manifestations of infection.
Previous attempts to isolate and culture EEHV1 from blood and necropsy tissue have been unsuccessful even in primary elephant placental endothelial cells (Richman, PhD thesis, 2003), and this, has also, so far, been the case for EEHV3. Although African elephants appear to be the natural hosts of EEHV1, the natural hosts for EEHV3 are not known. Attempts to obtain additional genomic DNA sequence data for more robust phylogenetic analysis of EEHV3, as well as to detect possible additional cases, and to deduce the natural source host species of these viruses are in progress.
Acknowledgements
The authors thank the following contributors to this study: the keepers, curators, and healthcare staff of the Woodland Park Zoo and Dr. Mitch Finnegan of The Oregon Zoo for assistance with the necropsy; Dr. Julia Hilliard, Jason Martin (Georgia State University), Dr. Noha Abou-Madi, Mary Beth Matychak, and Dr Julia Flaminio (Cornell University) for their attempts to culture these viruses; Dr. Scott Terrell for assistance with SSP data retrieval; Dr. Jim Wellehan for review of the manuscript; Histology Consulting Service, Everson, WA, for slide preparation; and Jamie Kinion, Northwest ZooPath, for pathology data retrieval. Studies at the National Zoological Park were supported by funding from Ringling Bros. and Barnum and Bailey Center for Elephant Conservation and the National Zoo. Laboratory studies at Johns Hopkins University were supported by NIH NIAID Research Grant R01 AI24576 to G.S.H. Additional funding was provided from the Woodland Park Zoo and Northwest ZooPath.
References
- 1.Burkhardt S, Hentschke J, Weiler H, Ehlers B, Ochs A, Walter J, Wittstatt U, Goltenboth R. Elephant herpes virus—a problem for breeding and housing of elephants. Berl Munch Tierarztl Wochenschr. 1999;112:174–179. (German) [PubMed] [Google Scholar]
- 2.Ehlers B, Burkhardt S, Goltz M, Ochs A, Weiler H, Hentschke J. Genetic and ultrastructural characterization of a European isolate of the fatal endotheliotropic elephant herpesvirus. J Gen Virol. 2001;82:475–482. doi: 10.1099/0022-1317-82-3-475. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers B, Dural G, Marschall M, Schregel V, Goltz M, Hentschke J. Endotheliotropic elephant herpesvirus, the first betaherpesvirus with a thymidine kinase gene. J Gen Virol. 2006;87:2781–2789. doi: 10.1099/vir.0.81977-0. [DOI] [PubMed] [Google Scholar]
- 4.Fickel J, Liechfeldt D, Richman LK, Streich WJ, Hildebrandt TB, Pitra C. Comparison of glycoprotein B (gB) variants of the elephant endotheliotropic herpesvirus (EEHV) isolated from Asian elephants (Elephas maximus) Vet Microbiol. 2003;91:11–21. doi: 10.1016/s0378-1135(02)00264-x. [DOI] [PubMed] [Google Scholar]
- 5.Fickel J, Liechfeldt D, Reinsch F, Goritz F, Hildebrandt TB. Investigations on the occurrence of herpes virus infections in Asian elephants (Elaphas maximus) Adv Ethol. 2000;35:133. [Google Scholar]
- 6.Fickel J, Richman LK, Montali R, Schaftenaar W, Goritz F, Hildebrandt TB, Pitra C. A variant of endotheliotropic herpesvirus in Asian elephans (Elephas maximus) in European zoos. Vet Microbiol. 2001;82:103–109. doi: 10.1016/s0378-1135(01)00363-7. [DOI] [PubMed] [Google Scholar]
- 7.Hargis A, Ginn P, Mansell J, Garber R. Ulcerative facial and nasal dermatitis and stomatitis in cats associated with feline herpesvirus 1. Vet Derm. 1999;10:267–274. doi: 10.1046/j.1365-3164.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 8.Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137A. [Google Scholar]
- 9.McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;7:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Reid CE, Hildebrandt TB, Marx N, Hunt M, Thy N, Reynes JM, Schaftenaar W, Fickel J. Endotheliotropic elephant herpesvirus (EEHV) infection, the first PCR-confirmed fatal case in Asia. Vet Quarterly. 2006;28:61–64. doi: 10.1080/01652176.2006.9695209. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richman LK, Montali RJ, Cambre RC, Schmitt D, Hardy D, Hidlebrandt T, Bengis RG, Hamzeh FM, Shahkolahi A, Hayward GS. Clinical and pathologic findings of a newly recognized disease of elephants caused by endotheliotropic herpesviruses. J Wildlf Dis. 2000a;36:1–12. doi: 10.7589/0090-3558-36.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Richman LK, Montali RJ, Garber RL, Kennedy MA, Lehnhardt J, Hildebrandt T, Schmitt D, Hardy D, Alcendor DJ, Hayward GS. Novel endotheliotropic herpesviruses fatal for Asian and African elephants. Science. 1999;283:1171–1176. doi: 10.1126/science.283.5405.1171. [DOI] [PubMed] [Google Scholar]
- 14.Richman LK, Montali RJ, Hayward GS. Review of a newly recognized disease of elephants caused by endotheliotrophic herpesviruses. Zoo Biol. 2000b;19:383–392. doi: 10.7589/0090-3558-36.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Rose TM. CODEHOP-mediated PCR—a powerful technique for the identification and characterization of viral genomes. Virol J. 2005;2:20. doi: 10.1186/1743-422X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russell LD, Burquet S. Ultrastructure of Leydig cells as revealed by secondary tissue treatment with a ferrocyanide:osmium mixture. Tissue Cell. 1977;9:751–766. doi: 10.1016/0040-8166(77)90040-4. [DOI] [PubMed] [Google Scholar]
- 17.Wellehan JF, Johnson AJ, Childress AL, Harr KE, Isaza R. Six novel gammaherpesviruses of Afrotheria provide insight into the early divergence of the Gammaherpesvirinae. Vet Microbiol. 2008;127:249–257. doi: 10.1016/j.vetmic.2007.08.024. [DOI] [PubMed] [Google Scholar]