Abstract
Converging evidence from neuroimaging studies and computational modelling suggests an organization of language in a dual dorsal–ventral brain network: a dorsal stream connects temporoparietal with frontal premotor regions through the superior longitudinal and arcuate fasciculus and integrates sensorimotor processing, e.g. in repetition of speech. A ventral stream connects temporal and prefrontal regions via the extreme capsule and mediates meaning, e.g. in auditory comprehension. The aim of our study was to test, in a large sample of 100 aphasic stroke patients, how well acute impairments of repetition and comprehension correlate with lesions of either the dorsal or ventral stream. We combined voxelwise lesion-behaviour mapping with the dorsal and ventral white matter fibre tracts determined by probabilistic fibre tracking in our previous study in healthy subjects. We found that repetition impairments were mainly associated with lesions located in the posterior temporoparietal region with a statistical lesion maximum in the periventricular white matter in projection of the dorsal superior longitudinal and arcuate fasciculus. In contrast, lesions associated with comprehension deficits were found more ventral-anterior in the temporoprefrontal region with a statistical lesion maximum between the insular cortex and the putamen in projection of the ventral extreme capsule. Individual lesion overlap with the dorsal fibre tract showed a significant negative correlation with repetition performance, whereas lesion overlap with the ventral fibre tract revealed a significant negative correlation with comprehension performance. To summarize, our results from patients with acute stroke lesions support the claim that language is organized along two segregated dorsal–ventral streams. Particularly, this is the first lesion study demonstrating that task performance on auditory comprehension measures requires an interaction between temporal and prefrontal brain regions via the ventral extreme capsule pathway.
Keywords: stroke, voxelwise lesion-behaviour mapping, extreme capsule, superior longitudinal fascicle, arcuate fascicle
Introduction
Current models on the functional neuroanatomy of language favour an organization along two processing streams: a ventral stream that is responsible for mapping sound onto meaning and a dorsal stream that is involved in mapping sound onto articulation (Wise, 2003; Hickok and Poeppel, 2007; Rauschecker and Scott, 2009; Weiller et al., 2011).
Using a combination of functional MRI and diffusion-tensor imaging-based probabilistic tractography (Kreher et al., 2008) in healthy subjects, Saur et al. (2008) identified white matter fibre tracts connecting activated cortical regions during repetition of pseudowords and comprehension of short sentences, two language tasks preferentially involving either the dorsal or ventral language processing stream. The authors found that temporofrontal interaction during repetition of pseudowords is subserved by the dorsal arcuate and superior longitudinal fascicle, connecting the superior temporal lobe and premotor cortices in the frontal lobe. In contrast, temporofrontal interaction during language comprehension is mediated by the ventral extreme capsule, connecting the middle temporal lobe and the ventrolateral prefrontal cortex. These results are in line with previous tractography studies (Parker et al., 2005; Anwander et al., 2007; Frey et al., 2008) demonstrating the importance of at least two distinct ventral and dorsal long-distant white matter association tracts for language processing.
Building upon this neuroanatomical evidence, Ueno et al. (2011) developed a ‘neurocomputational’ model of the dual stream language framework by combining neuroanatomical information and computational modelling. Brain lesions were simulated by reducing both function and connectivity of the network components (layers). Simulated lesions demonstrated that damage to the layers of the dorsal stream had a greater impact on repetition accuracy, whereas damage to layers of the ventral stream had a stronger impact on comprehension accuracy. Further simulations indicated that model performance of the dual stream architecture was superior compared with a single stream ‘ventral-only’ architecture (Ueno et al., 2011).
If this converging evidence from both structural and functional neuroimaging and computational modelling in favour of a dual stream segregation for language processing holds true, impairments of repetition and comprehension in patients with acute aphasia should correlate with lesions of either the dorsal or ventral stream.
To test this hypothesis, we conducted a voxelwise lesion-behaviour mapping (VLBM; Rorden et al., 2007) study in a large cohort of 100 acute stroke patients. We decided for acute stroke, as in this early phase, structure–function relationships in the brain are less biased by neuronal reorganization.
In a first step, we analysed lesion-behaviour relationships without any previous assumptions using a voxelwise whole-brain analysis correlating the patients’ repetition and comprehension impairments with the acute brain lesions. Subsequently, we measured the involvement of damage to the underlying white matter fibre tracts derived from healthy subjects. We, thus, computed the overlap of the statistical lesion maps for repetition and comprehension resulting from the VLBM procedure with the functional MRI-defined probabilistic tractography maps of dorsal and ventral white matter fibre tracts reported by Saur et al. (2008).
We hypothesized that repetition and comprehension deficits in acute aphasic patients not only depend on cortical damage but also on damage to white matter association tracts. In particular, we expected that lesions to the dorsal arcuate and superior longitudinal fascicle fibres would be related to repetition deficits, whereas lesions to the ventral extreme capsule fibres would predominantly be associated with comprehension deficits.
Materials and methods
Patients
Patients were recruited from the Stroke Unit of the Department of Neurology at the University Medical Centre of Freiburg, Germany. From October 2006 to September 2009, a total of 615 patients who presented with aphasia caused by a (thromb-) embolic stroke of the left-hemisphere were screened for inclusion in the study. Aphasia was diagnosed either with the Aachen Aphasia Bedside Test (Biniek et al., 1992) or (in cases of less severe impairment) with the Aachen Aphasia Test (Huber et al., 1984). From this cohort, 515 patients were excluded because of the following reasons: (i) age >90 years (n = 85); (ii) inability to tolerate MRI examination owing to reduced general health status (n = 138); (iii) previous infarcts (n = 156); (iv) native language other than German (n = 23); (v) any major cognitive impairment other than aphasia (n = 46); and (vi) other reasons e.g. contraindications for MRI, compliance issues or technical problems (n = 67). Finally, we included 100 native German-speaking patients (mean age 62 years, 67 male, 6 left-handed) with acute aphasia caused by ischaemic stroke (Table 1), who were examined on average 3 ± 2.6 (± SD) days after onset.
Table 1.
Demographic data and language scores of all 100 patients
| Mean (SD) | Min/max | Principal component analysis |
||
|---|---|---|---|---|
| 1st component | 2nd component | |||
| Demographic data | ||||
| Age (years) | 62 (13) | 16/86 | ||
| Gender (male/female) | 67/33 | |||
| NIHSS | 8 (5) | 1/22 | ||
| Lesion volume (ml) | 50 (44) | 0.4/233 | ||
| Language scores (number of items) | ||||
| AABT | ||||
| BLIKO (n = 10) | 80 (25) | 0/100 | ||
| MUMO (n = 10) | 67 (28) | 0/100 | ||
| SIREI (n = 10)* | 61 (34) | 0/100 | 0.820 | 0.463 |
| IDENT (n = 10)** | 68 (30) | 0/100 | 0.388 | 0.770 |
| BENENN (n = 10) | 53 (36) | 0/100 | ||
| AAT | ||||
| Token test (n = 50) | 41 (35) | 0/100 | ||
| Subtest 1 (n = 10)** | 55 (42) | 0/100 | 0.320 | 0.894 |
| Subtest 2 (n = 10)** | 42 (42) | 0/100 | 0.308 | 0.882 |
| Subtest 3 (n = 10)** | 31 (39) | 0/100 | 0.286 | 0.818 |
| Subtest 4 (n = 10) | 23 (36) | 0/100 | ||
| Subtest 5 (n = 10) | 21 (33) | 0/100 | ||
| Repetition (n = 50) | 53 (36) | 0/99 | ||
| Repetition of sounds (n = 10)* | 65 (39) | 0/100 | 0.816 | 0.416 |
| Repetition of nouns (n = 10)* | 59 (39) | 0/100 | 0.893 | 0.382 |
| Repetition of loanwords (n = 10)* | 56 (39) | 0/100 | 0.927 | 0.327 |
| Repetition of compound nouns (n = 10)* | 46 (37) | 0/97 | 0.897 | 0.340 |
| Repetition of sentences (n = 10)* | 41 (36) | 0/100 | 0.869 | 0.334 |
| Writing (n = 30) | 40 (34) | 0/97 | ||
| Naming (n = 40) | 41 (35) | 0/99 | ||
| Comprehension (n = 40) | 49 (30) | 0/98 | ||
| Auditory comprehension of words (n = 10)** | 58 (30) | 0/100 | 0.460 | 0.719 |
| Auditory comprehension of sentences (n = 10)** | 48 (31) | 0/100 | 0.421 | 0.791 |
| Reading comprehension of words (n = 10) | 51 (35) | 0/100 | ||
| Reading comprehension of sentences (n = 10) | 42 (34) | 0/97 | ||
Language scores are given as percentages correct.
Subtests written in bold contributed to the principal component analysis for repetition (highlighted with *) and comprehension (highlighted with **). Factor loadings of each subtest on both principal components are given (1st and 2nd components).
AABT = Aachen Aphasia Bedside Test with the subtests; AAT = Aachen Aphasia Test; BENENN = naming of objects; BLIKO = prompts for eye and head movement; IDENT = identification of objects; MUMO = prompts of oral movement; NIHSS = National Institute of Health Stroke Scale; SIREI = repetition of automated functions.
We did not explicitly exclude haemodynamic infarcts, in which neural damage may exceed the demarcated infarct (Weiller et al., 1991), and structure–function relationship, therefore, may be difficult to be assessed. A retrospective review of the ultrasound examinations revealed high-grade stenosis (>80%) or occlusion of the left internal carotid artery in 20 of the 100 patients (14 male). However, the infarct pattern was suggestive of embolic rather than haemodynamic stroke in all of these patients (i.e. these patients showed territorial rather than watershed infarcts or infarcts in the terminal supply area of the deep perforators). In addition, transcranial ultrasound demonstrated sufficient intracranial collateralization. Therefore, these cases were not excluded from analysis.
Full written consent was obtained from all subjects. In cases of severe aphasia or paralysis of the right hand, detailed information was given to the patient’s relatives. The study was approved by the local ethics committee.
Behavioural testing
Parallel to MRI, patients were tested with the Aachen Aphasia Bedside Test and Aachen Aphasia Test (Table 1). Since we were seeking a robust and reliable behavioural measure of the patients’ acute repetition and comprehension impairments, we applied a principle component analysis to a set of 12 repetition and comprehension scores. For repetition, we included the subtest SIREI (singen, reihensprechen; translated: singing, recitation) of the Aachen Aphasia Bedside Test (as one variable) and the five-item sets of the subtest repetition (as five variables) of the Aachen Aphasia Test. SIREI tests basic speech production abilities by asking the patients to repeat automated functions like singing, counting or greeting. The Aachen Aphasia Test subtest ‘repetition’ probes repetition of sounds, nouns, loanwords, compound nouns and sentences.
For comprehension, we included the subtest IDENT (Identifizieren; translated: identifying) of the Aachen Aphasia Bedside Test (as one variable), the Token Test subtests 1–3 (as three variables) and auditory comprehension on word and sentence level (as two variables). IDENT probes basic auditory comprehension through object identification: patients are confronted with four objects (cup, fork, knife and plate) and are asked to take or point to one of the objects. The subtest auditory comprehension of the Aachen Aphasia Test assesses comprehension on both word and sentence level. This task requires the selection of a target picture out of four presented pictures of varying complexity after auditory prompting. On word level, one of the pictures represents a semantic or phonemic distractor, or, on sentence level, a semantic or syntactic distractor, another picture is thematically related and one is unrelated. The Token Test of the Aachen Aphasia Test probes both auditory comprehension as well as more general cognitive abilities: patients are asked to identify geometrical objects, varying in shape, size and colour. We did not include the Token Test subtests 4 and 5, since these two most complex subtests strongly rely on verbal working memory, a function beyond the mere auditory comprehension component of interest.
To summarize, six repetition and six comprehension scores were entered as variables into the principle component analysis. The principle component analysis was calculated with SPSS software (version 20). Principal components were extracted with a varimax rotation to maximize the sum of the variance of the loading vectors. Importantly, the number of resulting principle components was not pre-determined in the principle component analysis set-up. Nevertheless, the analysis resulted in two principle components (Table 1) from which the individual single factor loadings were used as behavioural variables in the VLBM analyses. The two principle components represent the functions of interest labelled as auditory ‘repetition’ and ‘comprehension’ as best as detectable with aphasia tests in a cohort of stroke patients with acute aphasia.
The rationale to include subtests from the Aachen Aphasia Bedside Test and Aachen Aphasia Test was to provide test material for a broad range of impairments. To further characterize each patient’s language impairment, severity [mild (I), moderate (II) and severe (III)], fluency (fluent versus non-fluent) and type of aphasia (global, Broca, anomic, Wernicke, non-classifiable, residual) as indicated by the Aachen Aphasia Test were evaluated (Fig. 1). Although the type of aphasia is usually not stable in the early phase after stroke, this information may provide a basic estimate of the patient’s impairment.
Figure 1.
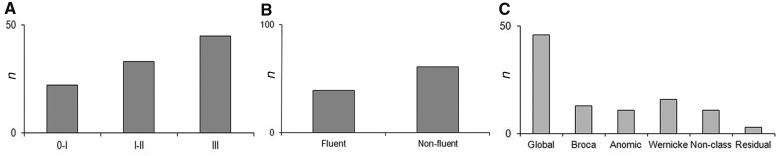
Behavioural data of 100 stroke patients with acute aphasia ∼3 days post stroke. Histograms of (A) severity [mild (I), moderate (II) and severe (III)], (B) fluency (fluent, non-fluent), and (C) type of aphasia as indicated by the Aachen Aphasia Test (non-class = non-classifiable).
Magnetic resonance imaging data acquisition
MRI data were obtained on a 3 T TIM Trio scanner (Siemens). For diffusion-weighted imaging, we used a standard sequence (23 slices, matrix = 128 × 128 pixel, voxel size = 1.8 × 1.8 × 5 mm3, repetition time = 3.1 s, echo time = 79 ms, flip angle = 90°, six diffusion-encoding gradient directions with a b-factor of 1000 s/mm2). Lesions were delineated on the averaged diffusion-weighted images. As a prerequisite for spatial normalization, a high-resolution T1 anatomical scan was obtained for each patient (160 slices, matrix = 240 × 240 pixel, voxel size = 1 × 1 × 1 mm3, repetition time = 2.2 s, echo time = 2.6 ms).
Lesion analysis
In a first step, a rough delineation of the diffusion-weighted imaged lesion was performed using a customized region of interest toolbox implemented in the SPM 8 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm8). We applied individual intensity thresholds to ensure that the resulting lesion maps best covered the diffusion-restricted tissue. Subsequently, individual lesion maps were spatially normalized using the normalization parameters obtained from the individual co-registered T1 scan. Finally, individual lesion maps were binarized and registered to the Montreal Neurological Institute (MNI) template brain (voxel size 0.5 × 0.5 × 0.5 mm3). The resulting images were manually inspected by two independent investigators and compared with the original lesions to ensure that our procedure performed correctly.
For VLBM, we used the non-parametric mapping software (Rorden et al., 2007) implemented in MRIcron (http://www.cabiatl.com/mricro/mricron/stats.html). Non-parametric testing was chosen, as behavioural data were not normally distributed (Supplementary Fig. 1). Specifically, we performed the Brunner–Munzel test, a rank test for continuous behavioural variables and binary images to identify lesioned voxels associated with repetition or comprehension deficits. The resulting maps display voxels with a significant difference in the distribution of the behavioural measure depending on whether the voxel was lesioned. To increase statistical power, only voxels affected in at least 10 patients were considered for analysis. This resulted in lesion maps for repetition and comprehension.
Finally, we computed two different logistic regression analyses. In the first one, lesion size was included to account for a potential correlation of lesion size with severity of impairment. In the second one, we included the concomitant opposite deficit (i.e. comprehension in the lesion map for repetition and vice versa) as covariates of no interest, since only few patients of our sample presented with an isolated deficit of either comprehension or repetition but rather showed some degree of impairment of both functions.
The statistical threshold was set to P = 0.01 (using a false discovery rate correction for multiple comparisons). Coordinates of lesion maxima are reported in MNI space.
Percentage of white and grey matter voxels
To determine the percentage of white and grey matter voxels, we segmented the MNI template into grey and white matter using the segmentation routine in SPM 8. The percentage of overlap of white and grey matter tissue with the statistical lesion maps for repetition and comprehension, respectively, was determined by using MRIcron software (Rorden et al., 2007).
Damage to ventral and dorsal association tracts
To quantify damage to the ventral and dorsal long-distant fibre tracts, we calculated the overlap of our lesion maps for repetition and comprehension with the probability maps of the Saur et al. (2008) study. In that work, we combined functional MRI and diffusion-tensor imaging in healthy subjects to identify the most probable white matter fibre tracts that connect activated cortical regions in repetition and comprehension. From that study, we took 32 individual, spatially normalized probability maps of eight different region-to-region connections, four defined in the repetition and comprehension experiment, respectively. The fibre tracking procedure has been described in detail previously (Saur et al., 2008; 2010).
Each spatially normalized region-to-region probability map was thresholded using a value of 0.0148. This value was generated empirically from the distribution observed in a large sample of region-to-region probability maps in our previous study (Saur et al., 2008). The resulting binary maps of each region-to-region connection were summed across subjects to produce group variability maps (Newton et al., 2006). In these maps, the voxel intensity ranged from 1 to 32, indicating spatial overlap between subjects. These variability maps were again thresholded at an arbitrary value of 75%, indicating that in the surviving voxels, a connection was present in 24 of the 32 subjects. As our study focuses on ventral and dorsal fibre systems rather than region-to-region connections, we calculated ventral and dorsal composite tracts. The ventral composite tract consisted of the four region-to-region connections between the anterior and posterior middle temporal gyrus and the ventrolateral prefrontal cortex (pars orbitalis and triangularis of the inferior frontal gyrus) defined in the comprehension experiment. In contrast, the dorsal composite tract consisted of the four connections between the anterior and posterior superior temporal gyrus and the premotor cortex (pars opercularis of the inferior frontal gyrus and dorsal premotor cortex) defined in the repetition experiment.
The number of overlapping voxels between our statistical lesion maps and the ventral and dorsal composite tracts in the left-hemisphere was determined with MRIcron. Relative fibre damage was calculated by dividing the overlap volume by the total tract volume. In addition, a non-parametric regression analysis was performed between individual overlap of each patient’s ischaemic lesion with the ventral and dorsal fibre systems and each patient’s comprehension and repetition deficit, respectively. Thus, tract integrity was not tested using probabilistic tracking directly in the patient data but rather by taking the probabilistic tracking data for each fibre system from healthy subjects and overlapping this with each patient’s structural lesion.
Results
Demographic and behavioural results
Patients’ demographic and behavioural data are given in Table 1. Descriptive statistics of the different subtests of the Aachen Aphasia Bedside Test and the Aachen Aphasia Test demonstrate that all language domains were equally affected across patients. Histograms of severity, fluency and type of aphasia show that most patients presented with severe language impairment (Fig. 1A), non-fluent aphasia (Fig. 1B) and global aphasia (Fig. 1C). Notably, the variance of the different subtests reflects that the analysis encompassed both patients with no or slight impairments and patients with severe deficits in different language functions.
The principle component analysis across the 12 repetition and comprehension scores revealed two distinct components that could be assigned either to the repetition or comprehension tests based on the correlations of the factor loadings with the respective test scores (Table 1). The Bartlett’s test of sphericity indicated that our data were adequate for factor analysis. The first component explained 82–93% and the second component 72–89% of the total variance introduced by the repetition and comprehension scores, respectively.
Lesion volume showed a moderate negative correlation with the repetition (r = −0.41) and comprehension (r = −0.49) scores (Spearman rank correlation, P< 0.01), whereas factor loadings for repetition and comprehension were not correlated with each other (r = 0.012, P= 0.9).
Voxelwise lesion-behaviour mapping analysis for repetition and comprehension deficits
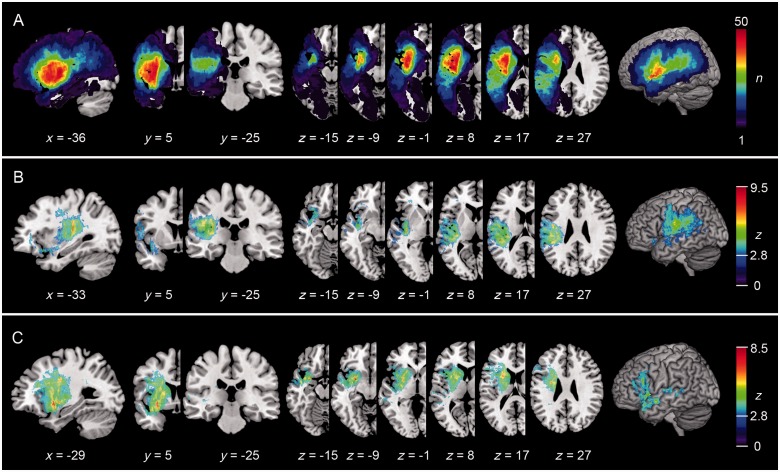
Figure 2A displays the lesion overlap based on the binary normalized lesion maps of all patients. Maximum lesion overlap was found in subcortical areas in the vascular territory of the lenticular-striatal arteries.
Figure 2.
Lesion overlap and VLBM lesion maps for repetition and comprehension. (A) Lesion overlap is displayed as summarized binary lesion maps. The colour bar in A indicates the degree of overlap of lesions [e.g. red values indicate that in 50 of 100 subjects (n) tissue was affected by stroke]. (B and C) VLBM maps based on the non-parametric Brunner–Munzel test of repetition (B) and of comprehension (C) deficits in 100 acute aphasic stroke patients. Colour bars indicate Z-scores at P < 0.01, FDR corrected. Coordinates are reported in MNI space.
Figure 2B and C shows the VLBM results of the Brunner–Munzel test. Lesions associated with repetition deficits were located more dorsal-posterior in the temporoparietal region, whereas lesions associated with comprehension deficits were found more ventral-anterior in the temporoprefrontal region.
Impairments of repetition (Fig. 2B) were associated with lesions of the superior and middle temporal gyrus, the parietal operculum, primary sensory-motor and premotor cortex, posterior insular cortex as well as large subcortical tissue. The statistically most significant voxels were found in the deep white matter of the frontal semioval centre (−36, −20, 28; z = 9.5) and the posterior temporal cortex (−30, −24, 8; z = 8.7) in projection of the arcuate and superior longitudinal fascicle fibres, respectively, and in the posterior most part of the insular cortex (−38, −12, −1; z = 7.1).
Lesions associated with comprehension deficits (Fig. 2C) involved the superior and middle temporal gyrus, the inferior frontal gyrus, the insular cortex and large parts of subcortical tissue including subinsular and periventricular white matter and the basal ganglia. The statistically most significant voxels were located in the ventral most part of the anterior insular cortex (insular apex; −33, 17, −17; z = 8.5) and the deep white matter between the insular cortex and the putamen in projection to the ventral extreme capsule fibres (−26, 7, −15; z = 7.3).
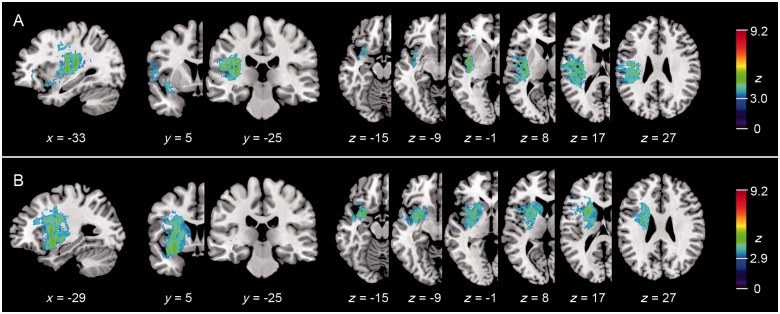
Figure 3 shows the results of the logistic regression analysis. Because factor loadings for repetition and comprehension were not correlated with each other, lesion maps look similar to those of the Brunner–Munzel test. Although overall z-values were lower, lesion maxima were almost identical to those in the Brunner–Munzel tests. This latter analysis was conducted because only few patients presented with a comprehension deficit without any repetition impairment and vice versa. Using lesion volume as a covariate of no interest, no significant voxels were found for both repetition and comprehension (not displayed). Note that a disadvantage of this method is that it has low statistical power when the anatomical structures relevant for a specific function correlate with lesion size (Karnath et al., 2004). Nevertheless, the additional analyses underline the robustness of our results demonstrating a spatial dissociation of the subcortical lesion maxima for repetition and comprehension.
Figure 3.
Lesion maps (n = 100) of the logistic regressions for repetition with the covariate comprehension (A) and for comprehension with the covariate repetition (B). Colour bars indicate Z-scores at P < 0.01, FDR corrected.
Damage of grey versus white matter
For the VLBM lesion map of repetition, we found an overlap of 62% with the grey matter portion and of 50% with the white matter portion of the segmented MNI brain. Comparably, the lesion map for comprehension overlapped to 62% with the grey and to 59% with the white matter segment. Note that some tissue at the border, between white and grey matter, was assigned to both tissue classes. Although this shows that both grey and white matter were affected to a similar degree, lesion maxima were located subcortically.
Damage to ventral and dorsal fibre tracts
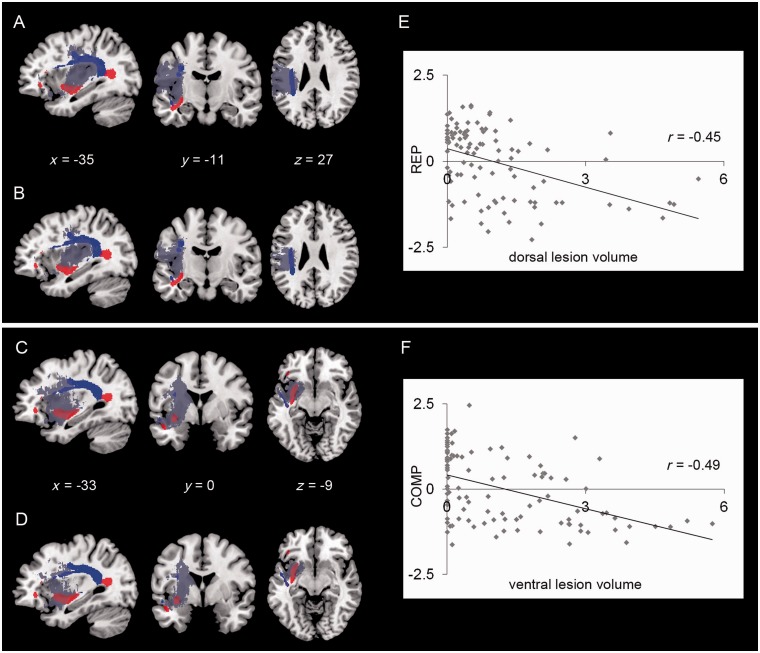
An overlap of the statistical lesion map for repetition with the ventral and dorsal association tracts showed that 51% of the dorsal fibre system and 14% of the ventral fibre system were affected (Fig. 4A). In contrast, the statistical lesion map for comprehension included 51% of the ventral fibre system but only 22% of the dorsal fibre system (Fig. 4C). This dissociation was also present when we co-varied out the concomitant opposite deficit in the logistic regression analysis. Here, we found that the statistical lesion maps for repetition overlapped to 27% with the dorsal and to 5% with the ventral fibre system (Fig. 4B). In contrast, the statistical lesion map for comprehension overlapped to 39% with the ventral and to 10% with the dorsal fibre system (Fig. 4D).
Figure 4.
Overlap of the dorsal (in blue) and the ventral (in red) long-distant association tracts from Saur et al. (2008), with the statistical lesion maps (grey) for repetition (A and B) and comprehension (C and D) derived from either the Brunner–Munzel test (A and C) or the logistic regression analysis (B and D). Scatterplots display the correlation of individual repetition (REP) performance and dorsal lesion volume (E) and comprehension (COMP) performance and ventral lesion volume (F); behavioural variables represent the individual principle component analysis factor loadings (Z-scores). Lesion volume is given in millilitres.
Significant negative relationships were found in the regression analyses between individual damage to dorsal association tracts (i.e. ‘dorsal lesion volume’) and repetition performance (Spearman rank correlation coefficient r = −0.45, P< 0.01, Fig. 4E) and for damage to ventral association tracts (i.e. ‘ventral lesion volume’) and comprehension performance (Spearman rank correlation coefficient r = −0.49, P< 0.01, Fig. 4F). Since small lesion overlap affecting the whole-tract diameter might have a greater impact on the impairment compared with a larger overlap along the tract, it is reasonable that this correlation is only moderate.
Discussion
In this study, we used VLBM in a large sample of aphasic stroke patients to identify brain structures associated with acute impairments of repetition and comprehension. We found a spatial dissociation of the lesion maps: comprehension deficits were associated with temporoprefrontal brain damage, whereas repetition impairments were correlated with damage to the temporoparietal region. Moreover, within these clusters, lesion maxima were found predominantly in subcortical tissue with a significant correlation of repetition deficits with damage to the dorsal superior longitudinal and arcuate fascicle pathway and comprehension impairments with damage to the ventral extreme capsule pathway. Thus, our study provides lesion evidence for the implementation of language in a dual dorsal–ventral brain network. Besides these implications for understanding language processing in the healthy brain, our results provide important insights into the pathophysiological mechanisms causing acute aphasia in patients with stroke.
From a cognitive linguistic point of view, repetition and comprehension subsume a number of distinct operations. Although we cannot isolate these operations based on the tests we used, we will discuss our results in the context of the cognitive processes that are involved when performing the different aphasia subtests.
The comprehension score consisted of tests that confronted the patient with a set of competing items from which the target item had to be selected after verbal prompting. This task mainly includes the lexical-semantic analysis of the verbal command or question and a subsequent target selection. As demonstrated by a number of functional imaging studies (for a meta-analysis see Vigneau et al., 2006), lexical-semantic knowledge is stored in the temporal lobe. This region serves as a lexical interface, linking phonological and semantic information in a sound-to-meaning interface network (Hickok and Poeppel, 2007). Most studies indicate a left-dominant organization of this network with some differences in the computations carried out in both hemispheres (Hickok and Poeppel, 2007). Executive semantic processing (e.g. selection among alternatives) involves the ventrolateral prefrontal cortex (Thompson-Schill et al., 1997; Whitney et al., 2011) and requires a strong functional temporofrontal interaction. Our lesion map for comprehension revealed involvement of the ventrolateral prefrontal cortex as well as the (anterior) temporal lobe (Fig. 2C). Anatomical connectivity between these regions is mediated by the ventral extreme capsule fibres, which connect the temporal cortex with the ventrolateral prefrontal cortex through a direct pathway running between the insular cortex and the putamen (Schmahmann et al., 2007; Frey et al., 2008; Makris and Pandya, 2009). This corresponds to the location of the statistical peak in our lesion map for comprehension (Fig. 2C). Although our lesion study lacks the spatial resolution to distinguish the external from the extreme capsule (being just separated by a thin layer of grey matter, the claustrum); tracing studies in monkeys (Petrides and Pandya, 2007; Schmahmann et al., 2007) suggest that this temporoprefrontal connection is mediated via the extreme capsule, whereas the external capsule belongs to corticostriatal projection systems (see also Saur et al., 2009). Further evidence for the functional relevance of a ventral temporoprefrontal pathway comes from intraoperative electrical stimulation studies, demonstrating that stimulation of this pathway provokes semantic paraphasias (Duffau et al., 2005). While the selection of a target from a set of competing alternatives represents a meta-linguistic task, natural language comprehension requires the online evaluation of novel language input, for instance, whether we agree on something or not, or whether something is true or not (Hagoort et al., 2004). We suggest that in language comprehension, the temporoprefrontal interaction through the extreme capsule fibres is essential for this instant evaluation of language input.
The repetition score included tasks of overt repetition of verbally presented items that differed in their processing demands on verbal short-term memory and articulatory complexity. Computationally, repetition of speech requires the transformation of auditory input into articulatory motor output that is implemented through a close interaction between temporoparietal regions and the premotor cortex (Warren et al., 2005). It was recently argued that this sensorimotor integration process includes an internal feedback-control loop that allows the auditory system to define the target of the speech output (Hickok, 2012). Previous studies have identified a region in the Sylvian fissure at the parietotemporal boundary (area Spt) as the key region that supports sensory-motor integration for speech (Hickok et al., 2003; Warren et al., 2005; Tourville et al., 2008; Hickok, 2009; Baldo et al., 2012). This fits well with our lesion map for repetition, including extensive damage to the temporoparietal junction as well as premotor regions in the frontal cortex (Fig. 2B). Long-distant interaction between these regions is enabled by the arcuate and superior longitudinal fascicle fibres (Petrides and Pandya, 1988; Schmahmann et al., 2007; Frey et al., 2008), the location of the statistical maximum in our lesion map for repetition. This converges with the results of a recent VLBM study by Fridriksson et al. (2010), who investigated speech repetition in a sample of 45 patients with acute stroke. That study reported a comparable location for repetition deficits with a lesion maximum in the white matter underlying the left supramarginal gyrus. Functionally, this auditory-motor integration stream is especially important for the processing of novel stimuli, e.g. during language acquisition, the development of a new vocabulary or sensory guidance of the production of infrequent or phonologically complex word forms. Likewise, it has been suggested that verbal short-term memory is embedded in the same auditory-motor integration network (Shallice and Warrington, 1977; Hickok, 2009).
In a recent study on the structural connectivity of different insular subregions, Cloutman et al. (2012) argued that the posterior insula, among others, is associated with the translation of heard phonetic sound sequences into vocal tract motor patterns, whereas the anterior insula is rather engaged in mapping information onto conceptual representations. This converges well with our findings of an anterior–posterior dissociation of the insular region within our lesion maps, with the anterior insula being solely included in the lesion map for comprehension, whereas lesions to the posterior insula and the underlying white matter revealed a strong association with repetition impairments (Fig. 2B and C).
When comparing the overlap estimations of the Brunner–Munzel lesion maps with the ventral and dorsal fibre tracts, however, we found remarkable damage to both tracts in repetition and comprehension impairments (Fig. 4A and C). This reflects the clinical situation in acute stroke, where one usually observes variable combinations of symptoms rather than isolated deficits of a specific language function. In addition, impairments influence each other, i.e. impaired comprehension affects the repetition performance and vice versa. Thus, a strong interaction between both streams must be assumed (Weiller et al., 2011). This is in line with the neurocomputational study of Ueno et al. (2011), which demonstrated that both streams contribute to some extent to each language task arguing for a ‘graded division of labour’. Especially with regard to word repetition, the model makes use of both the ventral and dorsal stream to boost repetition performance of words.
Nevertheless, the use of logistic regression analyses enabled us to co-vary out the contribution of the concomitant opposite deficit and experimentally disentangle both functions. In the resulting lesion maps, the spatial dissociation remained preserved (Fig. 3A and B). Again, the lesion map for comprehension overlapped to a greater degree with the ventral extreme capsule/external capsule fibres, whereas the lesion map for repetition overlapped to a greater degree with the dorsal arcuate and superior longitudinal fascicle fibres (Fig. 4B and D). A regression analysis of individual lesion overlap with either the ventral or dorsal fibre systems and individual comprehension or repetition impairment further stressed the specific functional contribution of both pathways (Fig. 4E and F).
In addition to demonstrating the functional relevance of the ventral and dorsal pathways for auditory comprehension and repetition, our findings also have implications for understanding the pathophysiology of acute aphasia after ischaemic stroke. The subcortical lesion maxima demonstrate that disconnection represents an important pathophysiological mechanism provoking acute comprehension and repetition impairments. Long-distance association fibres represent strategic network components, which in case of their disruption, may cause a dysfunction in functionally dependent, remote cortical brain regions (i.e. diaschisis; Monakow, 1885; Price et al., 2001). Disconnection might provoke a global network breakdown causing the clinical syndrome of acute, global aphasia. Indeed, a substantial number of patients in our study presented with severe and/or global aphasia (Fig. 1). This does not imply that acute aphasia represents a pure disconnection syndrome. Rather, our lesion maps demonstrate that besides damage to cortical temporofrontal key regions, white matter damage critically contributes to the acute language impairment. Despite global network dysfunction in acute aphasia, the high statistical power within subcortical regions resulting from large subcortical lesion overlap allows us to demonstrate specific structure–function relationships for white matter fibre tracts.
The large subcortical lesion overlap in acute stroke can be attributed to the vascular architecture of the middle cerebral artery (Phan et al., 2005). In contrast to the greater heterogeneity of the cortical lesions owing to the highly variable leptomeningeal blood supply, occlusion of the lenticulostriate arteries has been shown to produce a rather uniform type of infarction (Weiller et al., 1990). As subcortical aphasia often recovers well, this lesion type might contribute less to lesion studies performed in chronic aphasia (Dronkers, 1996; Bates et al., 2003; Schwartz et al., 2009). Consequently, we argue that the contribution of white matter fibre damage to language impairments might have been underestimated in previous chronic VLBM studies.
It should be borne in mind that subcortical lesions can be accompanied by cortical hypoperfusion, which, in turn, might contribute to the deficit in acute stroke (Weiller et al., 1993; Hillis et al., 2002; Saur et al., 2006a). Both cortical hypoperfusion as well as the previously described diaschisis might lead to a potential ‘overestimation’ of the structure–function relationship in acute lesion studies. That is, the function that is associated with the subcortical lesion might effectively be a cortical function.
On the other hand, an important advantage of investigating acute ischaemic lesions with VLBM is the absence of any confounding neural long-term reorganization that might substantially change the neural representation of language functions (Weiller et al., 1995; Crinion and Price, 2005; Saur et al., 2006b; Leff et al., 2009; Ochfeld et al., 2010). In chronic VLBM studies, the putative function of the lesioned area might be significantly underestimated, as other brain regions might have taken over (part of) the function of the lesioned area. Thus, chronic lesion studies reveal the location of the persistent impairment. These fundamental differences between acute and chronic lesion studies might also resolve some discrepancies in the lesion patterns between our study and previous chronic lesion studies which, for instance, located auditory comprehension deficits more posteriorly (Bates et al., 2003; Dronkers et al., 2004). It seems likely that patients with frontal lesions and/or frontal disconnection show better recovery from comprehension impairments than those with temporal lesions.
In future VLBM studies, longitudinal study designs should aim at examining both acute and chronic lesion effects in patients with aphasia to unveil changes in structure–function relationships over time. Clinically, it would be of great interest to relate the acute infarction to the (residual) language deficits in the chronic stage to detect patterns of brain damage associated with ‘good’ versus ‘bad’ recovery as previously demonstrated for neglect after a right-hemisphere lesion (Karnath et al., 2011).
To summarize, our VLBM analyses allowed us to identify brain regions involved in acute repetition and comprehension impairments. The subcortical lesion maxima provide evidence for the importance of the dorsal superior longitudinal and arcuate fascicle fibres for repetition and the ventral extreme capsule fibres for comprehension, and thus stress the differential contribution of each pathway. Two clinical syndromes of classical aphasiology, conduction aphasia and transcortical sensory aphasia best reflect this ‘division of labour’ (Ueno et al., 2011). Conduction aphasia is characterized by outstanding deficits in repetition, whereas comprehension remains largely preserved. In contrast, transcortical sensory aphasia is characterized by impaired comprehension, with largely preserved repetition abilities. Our subcortical lesion maxima suggest that in both syndromes, damage to either the dorsal or ventral white matter fibre tracts (i.e. disconnection) contributes to the core symptoms of these syndromes.
Funding
Bundesministerium für Bildung und Forschung [BMBF-research collaboration ‘Mechanisms of brain reorganization in the language network’ (01GW0661)], Deutsche Forschungsgemeinschaft (DFG, WE1352/14-1 to C.W. and HA 6314/1-1 to G.H.) and the European Union FP 7 research program (PLASTICISE, 223524). D.S. is supported by a Scholar Award of the James S. McDonnell Foundation (Function, dysfunction and repair of language networks).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- VLBM
voxelwise lesion-behaviour mapping
References
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca's area. Cereb Cortex. 2007;17:816–25. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verabal short-term memory deficits in aphasia: evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26:1–17. doi: 10.1080/02687038.2011.602391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Biniek R, Huber W, Glindemann R, Willmes K, Klumm H. The Aachen Aphasia Bedside Test—criteria for validity of psychologic tests. Nervenarzt. 1992;63:473–9. [PubMed] [Google Scholar]
- Cloutman LL, Binney RJ, Drakesmith M, Parker GJ, Lambon Ralph MA. The variation of function across the human insula mirrors its patterns of structural connectivity: evidence from in vivo probabilistic tractography. Neuroimage. 2012;59:3514–21. doi: 10.1016/j.neuroimage.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–71. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–61. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of brain areas involved in language comprehension. Cognition. 2004;92:145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio-Mazoyer N, Capelle L. New insights into the anatomo-functional connectivity of the semantic system: a study using cortico-subcortical electrostimulations. Brain. 2005;128:797–810. doi: 10.1093/brain/awh423. [DOI] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–44. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, et al. Impaired speech repetition and left parietal lobe damage. J Neurosci. 2010;30:11057–61. doi: 10.1523/JNEUROSCI.1120-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–41. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15:673–82. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci. 2012;13:135–45. doi: 10.1038/nrn3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6:121–43. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002;125:1094–104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Huber W, Poeck K, Willmes K. The Aachen Aphasia Test. Adv Neurol. 1984;42:291–303. [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex. 2004;14:1164–72. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. 2011;134:903–12. doi: 10.1093/brain/awq355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher BW, Schnell S, Mader I, Il'yasov KA, Hennig J, Kiselev VG, et al. Connecting and merging fibres: pathway extraction by combining probability maps. Neuroimage. 2008;43:81–9. doi: 10.1016/j.neuroimage.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–10. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–58. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monakow v. Neue experimentelle Beiträge zur Anatomie der Schleife: vorläufige Mittheilung. Neurologisches Centralblatt. 1885;12:265–8. [Google Scholar]
- Newton JM, Ward NS, Parker GJM, Deichmann R, Alexander DC, Friston K, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas—relevance to stroke recovery. Brain. 2006;129:1844–58. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochfeld E, Newhart M, Molitoris J, Leigh R, Cloutman L, Davis C, et al. Ischemia in broca area is associated with broca aphasia more reliably in acute than in chronic stroke. Stroke. 2010;41:325–30. doi: 10.1161/STROKEAHA.109.570374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27:11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TG, Donnan GA, Wright PM, Reutens DC. A digital map of middle cerebral artery infarcts associated with middle cerebral artery trunk and branch occlusion. Stroke. 2005;36:986–91. doi: 10.1161/01.STR.0000163087.66828.e9. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ. Dynamic diaschisis: anatomically remote and context-sensitive human brain lesions. J Cogn Neurosci. 2001;13:419–29. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–24. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Saur D, Buchert R, Knab R, Weiller C, Rother J. Iomazenil-single-photon emission computed tomography reveals selective neuronal loss in magnetic resonance-defined mismatch areas. Stroke. 2006a;37:2713–9. doi: 10.1161/01.STR.0000244827.36393.8f. [DOI] [PubMed] [Google Scholar]
- Saur D, Kellmeyer P, Weiller C. Reply to Yamada: the extreme capsule is the ventral pathway for language. Proc Natl Acad Sci USA. 2009;106:E15. [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006b;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Kupper H, Kellmeyer P, et al. Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. Neuroimage. 2010;49:3187–97. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Warrington EK. Auditory-verbal short-term memory impairment and conduction aphasia. Brain Lang. 1977;4:479–91. doi: 10.1016/0093-934x(77)90040-2. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39:1429–43. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72:385–96. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Warren JE, Wise RJ, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28:636–43. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Weiller C, Bormann T, Saur D, Musso M, Rijntjes M. How the ventral pathway got lost: and what its recovery might mean. Brain Lang. 2011;118:29–39. doi: 10.1016/j.bandl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, et al. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann Neurol. 1995;37:723–32. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ringelstein EB, Reiche W, Buell U. Clinical and hemodynamic aspects of low-flow infarcts. Stroke. 1991;22:1117–23. doi: 10.1161/01.str.22.9.1117. [DOI] [PubMed] [Google Scholar]
- Weiller C, Ringelstein EB, Reiche W, Thron A, Buell U. The large striatocapsular infarct. A clinical and pathophysiological entity. Arch Neurol. 1990;47:1085–91. doi: 10.1001/archneur.1990.00530100051013. [DOI] [PubMed] [Google Scholar]
- Weiller C, Willmes K, Reiche W, Thron A, Isensee C, Buell U, et al. The case of aphasia or neglect after striatocapsular infarction. Brain. 1993;116 (Pt 6):1509–25. doi: 10.1093/brain/116.6.1509. [DOI] [PubMed] [Google Scholar]
- Whitney C, Kirk M, O'Sullivan J, Lambon Ralph MA, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb Cortex. 2011;21:1066–75. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RJ. Language systems in normal and aphasic human subjects: functional imaging studies and inferences from animal studies. Br Med Bull. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.