Sir, There is a growing body of evidence supporting the beneficial effects of idebenone in Leber’s hereditary optic neuropathy (LHON, MIM 353500), an inherited mitochondrial disease that causes rapid bilateral vision loss and lifelong legal blindness. Until recently, reports were limited to isolated case studies and small open-label case series (Mashima et al., 1992, 2000; Cortelli et al., 1997; Carelli et al., 1998, 2001; Barnils et al., 2007). However, in 2011, two articles published in Brain provided additional evidence for the therapeutic use of idebenone in LHON (Carelli et al., 2011; Klopstock et al., 2011).
In the first complete randomized, placebo-controlled, double-blind clinical trial in LHON [Rescue of Hereditary Optic Disease Outpatient Study (RHODOS), ClinicalTrials.gov identifier: NCT00747487], 85 unselected patients with LHON ≥14 years of age were randomized to receive 900 mg/day of idebenone or placebo in a 2:1 ratio for 24 weeks (Klopstock et al., 2011). In the intent-to-treat population, visual acuity data were available for 82 patients harbouring one of the three primary mitochondrial DNA mutations (m.11778G>A, m.3460G>A and m.14484T>C) and experiencing first vision loss up to 5 years before study enrolment. All visual acuity end-points in the intent-to-treat population showed a consistent trend, with idebenone protecting patients from further vision loss, in contrast to the placebo group where visual acuity continued to deteriorate. Efficacy seen on visual acuity end-points was supported by independent measures including tests of colour contrast sensitivity. Following a pre-specified analysis by mutation, exclusion of patients with the m.14484T>C mutation, which is known for its high spontaneous recovery rate, resulted in a larger treatment effect in all visual acuity end-points. This finding was in agreement with the data from a retrospective analysis of 103 patients, published in the same issue of Brain (Carelli et al., 2011). This study showed a significant difference in the incidence of visual recovery in idebenone-treated patients harbouring the m.11778G>A mitochondrial DNA mutation, known to cause ∼70% of LHON cases in Europe, and concluded that an early treatment start is recommended.
Vision loss in LHON is rapid, and in the majority of cases results in persistent lifelong visual impairment, typically rendering patients legally blind. Therefore, in light of the relatively short treatment duration of 24 weeks for RHODOS, we have now determined whether the observed treatment effects persisted after discontinuation of treatment. For this, we invited patients previously participating in RHODOS for reassessment of their visual acuity to a single visit observational follow-up study (RHODOS-OFU), which had ethical and institutional review board approval and was sponsored by Santhera Pharmaceuticals (ClinicalTrials.gov identifier: NCT01421381). According to the study protocol, the primary end-point was the change in best (logMAR) visual acuity at this study visit compared with baseline and Week 24 of RHODOS.
Sixty patients (70.6%) who participated in RHODOS were enrolled into this RHODOS–OFU study, of whom 58 patients provided visual acuity data in both studies and were included in the analysis. Of these, 39 patients had been randomized to idebenone in the course of RHODOS and 19 patients to placebo, in keeping with the 2:1 ratio of idebenone:placebo in the RHODOS study design. There were no significant differences in the demographics or molecular genetic characteristics of the RHODOS–OFU group compared with the original RHODOS cohort (Table 1), indicating that the smaller subpopulation recruited to the RHODOS–OFU study was representative of the RHODOS study population. The mean ± SD time that had elapsed between Week 24 of RHODOS and the RHODOS–OFU study visit was 30.5 ± 4.9 months (median: 30.1 months). The time since onset of vision loss at baseline of RHODOS for this subpopulation was 23 ± 17 months (median: 18 months).
Table 1.
Demographics of patients enrolled in the RHODOS study compared with the RHODOS observational follow-up study
| Parameter | RHODOS Study |
RHODOS–OFU Study |
||||
|---|---|---|---|---|---|---|
| Idebenone | Placebo | Total | Idebenonea | Placeboa | Total | |
| Population, n (%) | 55 (64.7) | 30 (35.3) | 85 (100) | 39 (73.6)b | 19 (65.5)b | 58 (70.7)b |
| Male, n (%) | 47 (85.5) | 26 (86.7) | 73 (85.9) | 34 (87.2) | 16 (84.2) | 50 (86.2) |
| Age in years, mean ± SD [median], (range)c | 33.8 ± 14.8, | 33.6 ± 14.6 | 33.7 ± 14.6 | 34.4 ± 15.3 | 31.5 ± 14.2 | 33.4 ± 14.9 |
| [30.0] (14–63) | [28.5] (14–66) | [30.0] (14–66) | [30.0] (14–63) | [27.0] (14–66) | [28.0] (14–66) | |
| Time since onset in months, mean ± SD [median], (range)c | 22.8 ± 16.2 | 23.7 ± 16.4 | 23.1 ± 16.2 | 22 ± 16 | 25 ± 18 | 23 ± 17 |
| [17.8] (3–62) | [19.2] (2–57) | [18.2] (2–62) | [18] (3–60) | [19] (2–57) | [18] (2–60) | |
| Patients with m.11778G>A or m.3460G>A, n (%) | 44 (80.0) | 24 (80.0) | 68 (80.0) | 33 (84.6) | 17 (89.5) | 50 (86.2) |
| Onset of vision loss within 1 year, n (%)c | 19 (34.5) | 11 (36.7) | 30 (35.3) | 16 (41.0) | 6 (31.6) | 22 (37.9) |
| Patients with both eyes off-chart, n (%)d,e | 25 (47.2) | 13 (44.8) | 38 (46.3) | 18 (46.2) | 8 (42.1) | 26 (44.8) |
| Eyes off-chart, n (%)d,e | 61 (57.5) | 29 (50.0) | 90 (54.9) | 44 (56.4) | 19 (50.0) | 63 (54.3) |
| Best eye visual acuity at BL, mean ± SD [logMAR]d,e | 1.61 ± 0.64 | 1.57 ± 0.61 | 1.59 ± 0.62 | 1.56 ± 0.70 | 1.51 ± 0.64 | 1.55 ± 0.68 |
| Worst eye visual acuity at BL, mean ± SD [logMAR]d,e | 1.89 ± 0.49 | 1.79 ± 0.44 | 1.86 ± 0.47 | 1.89 ± 0.54 | 1.81 ± 0.41 | 1.86 ± 0.50 |
| Both eyes visual acuity at BL, mean ± SD [logMAR]d,e | 1.75 ± 0.58 | 1.68 ± 0.54 | 1.73 ± 0.57 | 1.72 ± 0.64 | 1.66 ± 0.55 | 1.70 ± 0.61 |
a Former treatment group in RHODOS study.
b Percentage RHODOS–OFU population relative to the corresponding group from the RHODOS intent-to-treat population.
c At RHODOS baseline.
d For RHODOS based on efficacy population, n = 82 (53 idebenone, 29 placebo).
e Off-chart defined as >logMAR 1.68 and applying logMAR 2.0/2.3/2.6 for counting fingers/hand motion/light perception.
BL = baseline.
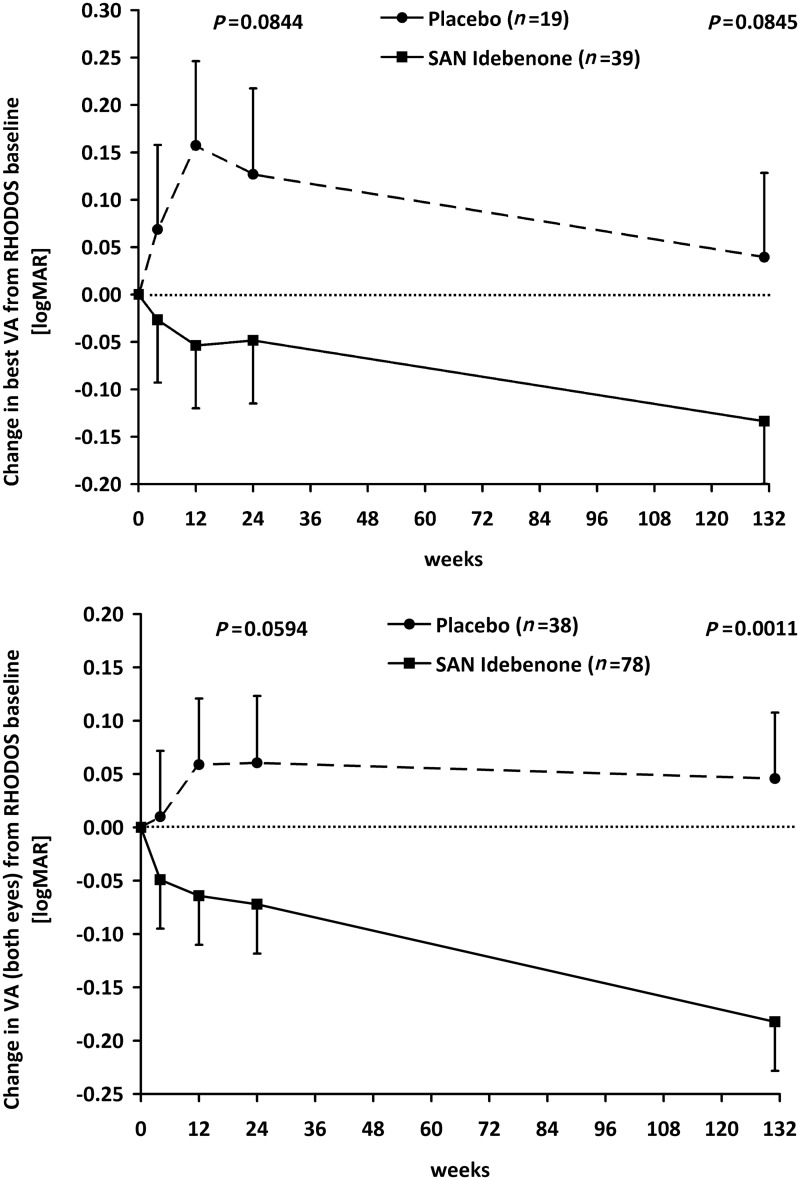
The change in best visual acuity from Week 24 of RHODOS to the follow-up visit did not correlate with the time elapsed (Spearman’s correlation coefficient = −0.045; P = 0.73) for the patients in this study. Therefore, the RHODOS–OFU visit was treated as a single categorical event in all statistical analyses, and for illustration purposes, the median time elapsed since the end of RHODOS (30 months = 131 weeks) is shown (Fig. 1). The change in best visual acuity during the RHODOS treatment period up to Week 24 was very similar in the subpopulation of this study (n = 58) when compared with the outcome of this end-point in the intent-to-treat population of RHODOS (n = 82). Specifically, the mean difference in best visual acuity estimated by the mixed model of repeated measures for patients randomized to idebenone compared with patients receiving placebo was logMAR −0.120 (six letters; P= 0.078; Fig. 2C in Klopstock et al., 2011) for the RHODOS intent-to-treat population, compared with logMAR −0.175 (eight letters; P= 0.084; Fig. 1, top panel and Table 2) for the patients in the RHODOS–OFU study. This indicates that the 58-patient subgroup is representative of the Week 24 outcome of the RHODOS intent-to-treat population, both in terms of demographics and visual acuity end-points.
Figure 1.
Change in visual acuity over time. Change in logMAR visual acuity over time for the best visual acuity (top) and visual acuity for all eyes (bottom). Data are estimated means ± SEM from mixed model of repeated measures, based on the change from baseline (in weeks) and plotted for the two treatment groups as defined in the RHODOS study. No treatment was given between Week 24 and Week 131. Worsening/improvement of visual acuity is indicated as positive/negative values in change of logMAR. A difference of logMAR 0.1 corresponds to five letters or one line on the Early Treatment Diabetic Retinopathy Study chart. The P-values are given for the difference between treatment groups. VA = visual acuity.
Table 2.
Visual acuity outcomes
| Visual acuity analysis | Estimated Differencea ± SEM (95% CI)[difference in letters], P-value |
|||
|---|---|---|---|---|
| BLb to Week 24b | BL to OFUc | Week 24 to OFU | ||
| Change in best visual acuity | ||||
| Efficacy population (n = 58: idebenone n = 39, placebo n = 19) | −0.175 ± 0.101 (−0.375 to 0.024), [+8 letters], P = 0.0844 | −0.173 ± 0.100 (−0.370 to 0.024), [+8 letters], P = 0.0845 | 0.002 ± 0.098 (−0.190 to 0.195), [0 letters], P = 0.9819 | |
| Patients with m.11778G>A or m.3460G>A (n = 50: idebenone n = 33, placebo n = 17) | −0.192 ± 0.111 (−0.411 to 0.027), [+9 letters], P = 0.0855 | −0.216 ± 0.109 (−0.432 to 0.000), [+10 letters], P = 0.0499 | −0.024 ± 0.105 (−0.231 to 0.183), [+1 letter], P = 0.8173 | |
| Change in visual acuity of individual eyes | ||||
| Efficacy population (n = 116: idebenone n = 78, placebo n = 38) | −0.133 ± 0.070 (−0.271 to 0.005) [+6 letters], P = 0.0594 | −0.228 ± 0.069 (−0.364 to −0.092) [+11 letters], P = 0.0011 | −0.096 ± 0.068 (−0.229 to 0.038) [+4 letters], P = 0.1604 | |
| Patients with m.11778G>A or m.3460G>A (n = 100: idebenone n = 66, placebo n = 34) | −0.146 ± 0.077 (−0.297 to 0.005) [+7 letters], P = 0.0573 | −0.283 ± 0.076 (−0.432 to −0.134) [+14 letters], P = 0.0002 | −0.137 ± 0.072 (−0.279 to 0.006) [+6 letters], P = 0.0599 | |
a Data are estimated mean difference [logMAR] ± SEM (95% confidence interval) between idebenone and placebo group calculated from mixed model of repeated measures. Difference in favour of idebenone is indicated by negative logMAR values and positive letter differences indicate improvement in visual acuity.
b Baseline (BL) and Week 24 in RHODOS.
c OFU = RHODOS–OFU visit (median time since Week 24 of RHODOS: 30 months).
Between Week 24 of RHODOS and the RHODOS–OFU visit 30 months later, best visual acuity followed parallel trajectories resulting in a non-significant trend towards improvement (logMAR −0.08; four letters; Fig. 1, top panel) in each of the treatment groups, with no difference between groups (logMAR 0.002; P= 0.982; Table 2). Interestingly, this improvement in best visual acuity between the end of RHODOS and the RHODOS–OFU visit was confined primarily to patients with short disease history irrespective of treatment group (disease onset at RHODOS baseline ≤1 year, idebenone: logMAR −0.155, placebo: −0.198; disease onset >1 year: idebenone: −0.038, placebo: −0.054). The observed improvement in best visual acuity after discontinuation of the RHODOS treatment period may, at least in part, result from patients learning to use peripheral (extrafoveal) vision. The parallel trajectories after the end of RHODOS led to a mean difference of logMAR −0.173 (eight letters improvement, P= 0.085) in best visual acuity between treatment groups for the entire period from baseline of RHODOS to the RHODOS–OFU visit, which is comparable with the difference observed at Week 24 (logMAR −0.175, see previous text). Thus, the treatment effect observed after 24 weeks of idebenone was maintained long after therapy was terminated. The difference between treatment groups was larger in an analysis for patients carrying either the m.11778G>A or m.3460G>A mutation (i.e. excluding patients with the m.14484T>C mutation). Here, the mean difference between idebenone and placebo-treated patients between the baseline of RHODOS and the RHODOS–OFU visit was logMAR −0.216 (10 letters, P= 0.0499).
For the analysis of best recovery of visual acuity, the primary end-point of the RHODOS study, there was a logMAR −0.147 (seven letters, P= 0.004) improvement between Week 24 of RHODOS and RHODOS–OFU in the idebenone group compared with a logMAR −0.054 (two letters, P= 0.459) improvement in the placebo group. The mean difference in best recovery of visual acuity between treatment groups for the entire period from baseline of RHODOS to the RHODOS–OFU visit was logMAR −0.158 (seven letters, P= 0.086).
In line with the previously described findings, when considering the change in visual acuity of all eyes (treated as independent), the idebenone group continued to improve between Week 24 of RHODOS and the RHODOS–OFU visit (mean change logMAR −0.110, five letters; P= 0.005). However, the trajectory in visual acuity for patients in the placebo group did not change from Week 24 of RHODOS (Fig. 1, bottom panel). This implies that in the placebo group, the improvement in best visual acuity might be offset by further deterioration of the worse affected eye. The resulting difference between treatment groups for the mean change in visual acuity of individual eyes from baseline of RHODOS to the RHODOS–OFU study visit was highly significant, logMAR −0.228 (11 letters; P= 0.0011; Table 2), in favour of the idebenone group for patients carrying one of the three primary mitochondrial DNA mutations and logMAR −0.283 (14 letters, P= 0.0002) for patients carrying either the m.11778G>A or m.3460G>A mutation.
Responder analysis in the RHODOS trial previously showed that for patients with ‘off-chart’ visual acuity in both eyes at baseline, none of the 13 patients in the placebo group but 7 out of 25 patients in the idebenone group had improved to reading at least a full line at Week 24 (P= 0.07) (Klopstock et al., 2011). This was maintained for all five patients who participated in the RHODOS–OFU study, which again indicates the long-term persistence of the idebenone treatment effect. For the entire observation period, i.e. between baseline of RHODOS and the RHODOS–OFU visit, there were 50% (9 of 18) of patients with ‘off-chart’ visual acuity in the idebenone group and 25% (2 of 8) in the placebo group who improved to ‘on-chart’ visual acuity (P= 0.39). Counting eyes instead of patients for the same period, there were 18 of 44 (40.9%) eyes in the idebenone and 2 of 19 (10.5%) eyes in the placebo group that improved to ‘on-chart’ vision (P= 0.02).
We also performed a reviewer-requested alternative analysis of summary statistics. This included the change in average logMAR from baseline at Weeks 4, 12 and 24, the change from baseline to the follow-up visit described in this study and the change from Weeks 4, 12 and 24 to the follow-up visit. The results of this analysis were consistent with the mixed model of repeated measures analysis carried out in the original RHODOS study and the pre-specified analysis for the follow-on study.
In summary, the results from the single-visit RHODOS–OFU study demonstrated that the beneficial effect from 6 months of treatment with idebenone during RHODOS persisted despite discontinuation of therapy for a median time of 30 months. The underlying mechanism for this may be explained by the natural history and pathophysiology of LHON. Patients harbouring LHON mitochondrial DNA mutations usually remain asymptomatic until early adult life, when an ill-defined trigger precipitates acute loss of visual acuity. Reaching a nadir typically few months after disease onset, visual acuity rarely changes thereafter. Given what is known about the mode of action of idebenone (Haefeli et al., 2011), it is conceivable that the drug preserves or re-establishes retinal ganglion cell function during the acute phase, and thus protects from irreversible retinal ganglion cell loss. This is in keeping with the established hypothesis that, in the acute stage, the decrease in visual acuity in LHON is caused by respiratory chain dysfunction with viable, but inactive, retinal ganglion cells (Howell, 1998). Consequently, the therapeutic potential of idebenone therapy is likely to have the highest impact if therapy is initiated early in the disease at a time when retinal ganglion cell loss is still minimal, as suggested by the data of Carelli et al. (2011).
Mashima et al. (2000) and Carelli et al. (2011) both observed a mean time to recovery of ∼17 months while patients were continuously kept on treatment. The results of these studies indicate that prolonged treatment could result in a marked recovery of vision even in patients with established disease and severe visual acuity loss, an observation that was also supported by the recent anecdotal findings of Sabet-Peyman et al. (2012), albeit in one patient. A treatment period >6 months might thus offer additional therapeutic benefit.
Funding
P.F.C. is a Wellcome Trust Senior Fellow in Clinical Science and an NIHR Senior Investigator who receives funding from the Medical Research Council (UK) through the MRC Translational Neuromuscular Centre, the Wellcome Trust Centre for Mitochondrial Research, the UK NIHR Biomedical Research Centre for Ageing and Age-related Disease and Biomedical Research Unit award to the Newcastle upon Tyne Foundation Hospitals NHS Trust. P.Y-.W-.M. is an MRC (UK) Clinician Scientist. Recruiting of patients was supported by use of patient databases; the German network for mitochondrial disorders (mitoNET, 01GM0862), which is funded by the German Ministry of Education and Research (BMBF, Bonn, Germany), and the UK Mitochondrial Disease Cohort, which is funded by the Medical Research Council (UK). T.K. is the coordinator of mitoNET, and also receives other funding from the German Ministry of Education and Research and from the Deutsche Forschungsgemeinschaft (DFG).
Acknowledgements
The authors thank J. Al-Tamami for excellent work as a study nurse and G. Rudolph for support in patient investigations. They also thank Mika Leinonen (4Pharma, Sweden, statistical advisor) for discussion and performing statistical analysis.
References
- Barnils N, Mesa E, Munoz S, Ferrer-Artola A, Arruga J. Response to idebenone and multivitamin therapy in Leber's hereditary optic neuropathy. Arch Soc Esp Oftalmol. 2007;82:377–80. doi: 10.4321/s0365-66912007000600012. [DOI] [PubMed] [Google Scholar]
- Carelli V, Barboni P, Zacchini A, Mancini R, Monari L, Cevoli S, et al. Leber's hereditary optic neuropathy (LHON) with 14484/ND6 mutation in a North African patient. J Neurol Sci. 1998;160:183–8. doi: 10.1016/s0022-510x(98)00239-1. [DOI] [PubMed] [Google Scholar]
- Carelli V, La Morgia C, Valentino ML, Rizzo G, Carbonelli M, De Negri AM, et al. Idebenone treatment in Leber's hereditary optic neuropathy. Brain. 2011;134:e188. doi: 10.1093/brain/awr180. [DOI] [PubMed] [Google Scholar]
- Carelli V, Valentino ML, Liguori R, Meletti S, Vetrugno R, Provini F, et al. Leber's hereditary optic neuropathy (LHON/11778) with myoclonus: report of two cases. J Neurol Neurosurg Psychiatry. 2001;71:813–6. doi: 10.1136/jnnp.71.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortelli P, Montagna P, Pierangeli G, Lodi R, Barboni P, Liguori R, et al. Clinical and brain bioenergetics improvement with idebenone in a patient with Leber's hereditary optic neuropathy: a clinical and 31P-MRS study. J Neurol Sci. 1997;148:25–31. doi: 10.1016/s0022-510x(96)00311-5. [DOI] [PubMed] [Google Scholar]
- Haefeli RH, Erb M, Gemperli AC, Robay D, Courdier Fruh I, Anklin C, et al. NQO1-dependent redox cycling of idebenone: effects on cellular redox potential and energy levels. PLoS One. 2011;6:e17963. doi: 10.1371/journal.pone.0017963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N. Leber hereditary optic neuropathy: respiratory chain dysfunction and degeneration of the optic nerve. Vision Res. 1998;38:1495–504. doi: 10.1016/s0042-6989(97)00444-6. [DOI] [PubMed] [Google Scholar]
- Klopstock T, Yu-Wai-Man P, Dimitriadis K, Rouleau J, Heck S, Bailie M, et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134:2677–86. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima Y, Hiida Y, Oguchi Y. Remission of Leber's hereditary optic neuropathy with idebenone. Lancet. 1992;340:368–9. doi: 10.1016/0140-6736(92)91442-b. [DOI] [PubMed] [Google Scholar]
- Mashima Y, Kigasawa K, Wakakura M, Oguchi Y. Do idebenone and vitamin therapy shorten the time to achieve visual recovery in Leber hereditary optic neuropathy? J Neuroophthalmol. 2000;20:166–70. doi: 10.1097/00041327-200020030-00006. [DOI] [PubMed] [Google Scholar]
- Sabet-Peyman EJ, Khaderi KR, Sadun AA. Is leber hereditary optic neuropathy treatable? Encouraging results with idebenone in both prospective and retrospective trials and an illustrative case. J Neuroophthalmol. 2012;32:54–7. doi: 10.1097/WNO.0b013e318241da45. [DOI] [PubMed] [Google Scholar]