Abstract
Loss of the Merlin tumour suppressor causes abnormal de-differentiation and proliferation of Schwann cells and formation of schwannoma tumours in patients with neurofibromatosis type 2. Within the mature peripheral nerve the normal development, differentiation and maintenance of myelinating and non-myelinating Schwann cells is regulated by a network of transcription factors that include SOX10, OCT6 (now known as POU3F1), NFATC4 and KROX20 (also known as Egr2). We have examined for the first time how their regulation of Schwann cell development is disrupted in primary human schwannoma cells. We find that induction of both KROX20 and OCT6 is impaired, whereas enforced expression of KROX20 drives both myelin gene expression and cell cycle arrest in Merlin-null cells. Importantly, we show that human schwannoma cells have reduced expression of SOX10 protein and messenger RNA. Analysis of mouse SOX10-null Schwann cells shows they display many of the characteristics of human schwannoma cells, including increased expression of platelet derived growth factor receptor beta (PDGFRB) messenger RNA and protein, enhanced proliferation, increased focal adhesions and schwannoma-like morphology. Correspondingly, reintroduction of SOX10 into human Merlin-null cells restores the ability of these cells to induce KROX20 and myelin protein zero (MPZ), localizes NFATC4 to the nucleus, reduces cell proliferation and suppresses PDGFRB expression. Thus, we propose that loss of the SOX10 protein, which is vital for normal Schwann cell development, is also key to the pathology of Merlin-null schwannoma tumours.
Keywords: Schwann, merlin, SOX10, KROX20, schwannoma
Introduction
The genetic condition neurofibromatosis type 2 is characterized by formation of multiple tumours of the peripheral nervous system. The predominant tumours formed are vestibular schwannomas of the eighth cranial nerve (vestibulocochlear), but also include cutaneous schwannomas, meningiomas and ependymomas (Hanemann, 2008). Neurofibromatosis type 2 is caused by biallelic loss of the NF2 gene that encodes the tumour suppressor protein merlin (NF2), which is also lost in all sporadic schwannoma tumours (Rouleau et al., 1993; Trofatter et al., 1993). Research has shown that merlin acts to ensure cell–cell contact inhibition of growth and regulates protein ubiquitination in the nucleus through interaction with DCAF1 (McClatchey and Giovannini, 2005; Curto et al., 2007; Lallemand et al., 2009; Li et al., 2010). Loss of merlin in cultured Schwann cells leads to altered morphology, enhanced proliferation and over activation of multiple signalling pathways including Rac GTPase, extracellular regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and phosphatidyl inositol-3 kinase (PI-3 kinase) MAP kinase pathways (Shaw et al., 2001; Kaempchen et al., 2003; Ammoun et al., 2008). During Schwann cell development, a number of signalling pathways and transcription factors regulate axon–Schwann cell interaction, cell cycle withdrawal and differentiation of Schwann cells within peripheral nerves (Jessen and Mirsky, 2005; Pereira et al., 2012), and the impact of merlin loss on these regulators of normal Schwann cell differentiation has not yet been investigated. The zinc finger transcription factor KROX20 is essential for the differentiation of myelinating Schwann cells and regulates both cell cycle withdrawal and myelin gene expression (Topilko et al., 1994; Zorick et al., 1999; Parkinson et al., 2004). Expression of KROX20 in turn is regulated by a number of other transcription factors such as SOX10, OCT6, NFATC4 and YY1, which bind and co-operatively activate the myelinating Schwann cell enhancer of the KROX20 promoter (Ghislain and Charnay, 2006; Kao et al., 2009; He et al., 2010; Kipanyula et al., 2012). In addition to its role in myelin gene regulation, in synergy with KROX20, SOX10 functions to regulate Schwann cell proliferation and controls the dynamics of Schwann cell–axon interactions during development (Finzsch et al., 2010). SOX10 is both essential for the generation of Schwann cell precursors from the neural crest (Britsch et al., 2001) and the sequential induction of OCT6 and KROX20 during myelination (Finzsch et al., 2010). Given that the key characteristics of human schwannoma tumours are loss of cell differentiation, loss of axonal contact and increased cell proliferation, we have investigated this transcriptional network in these cells.
As a first step, we tested both the regulation and function of KROX20 in human Merlin-null schwannoma cells. We found that the induction of both KROX20 and OCT6 is impaired in these cells, whereas enforced expression of KROX20 induces cell cycle arrest and differentiation identically in human control Schwann cells and Merlin-null schwannoma cells. As SOX10 is key in the regulation of both OCT6 and KROX20, we measured the levels of SOX10 in schwannoma cells and found reduced levels of SOX10 messenger RNA and protein in all the tumours we analysed (n = 17). In keeping with a role for SOX10 in these tumours, SOX10 re-expression restores the ability of these cells to induce Kroz-20, relocalizes NFATC4 to the nucleus and drives myelin gene expression. Human schwannoma cells express raised levels of PDGFRB and consequently show increased signalling and proliferation in response to platelet derived growth factor (PDGF) (Ammoun et al., 2008). We find that re-expression of SOX10 in human schwannoma cells reduces PDGFRB expression and consequently PDGF-induced signalling and proliferation. To separately study the roles of SOX10 and merlin, we have used conditional mouse knockouts for both genes together with small interfering RNA knockdown of merlin in Schwann cells. We demonstrate that loss of merlin alone, both in vitro and in vivo, does not reduce SOX10 levels in Schwann cells. Similarly, restoration of merlin expression in human schwannoma cells does not rescue SOX10 expression. Analysis of SOX10-null mouse Schwann cells shows that loss of SOX10 alone appears to give many of the phenotypes of human schwannoma cells, namely increased levels of PDGFRB, increased cell proliferation, schwannoma-like morphology with increased focal adhesions and the failure to myelinate in vitro (Flaiz et al., 2009). These findings link, for the first time, the processes of normal cell differentiation and tumourigenesis in Schwann cells and demonstrate that loss of both merlin and SOX10 contribute to the aberrant phenotype of human schwannoma cells.
Materials and methods
Reagents
Adenoviruses expressing green fluorescent protein (GFP), KROX20/GFP, Cre recombinase, JNK-binding domain of JNK-interacting protein 1 (JIP-1) and Δ169 c-Jun have been described elsewhere (Parkinson et al., 2001, 2004, 2008). Adenoviruses expressing NF2/GFP (Xiao et al., 2005) and dominant negative Rac1 (Wojciak-Stothard et al., 2001) were kind gifts from J. Testa (Fox Chase Cancer Centre, Philadelphia, USA) and A. Ridley (King’s College London, UK), respectively. The SOX10 adenoviral construct was generated using a C-Terminal haemagglutinin (HA)-tagged human SOX10, using the AdEasy™ system (He et al., 1998) to generate an adenovirus co-expressing GFP and HA-tagged SOX10. Antibodies against OCT6 (sc-11661), NFATC4 (sc-13036), Rho GDI (sc-359), Beta2a tubulin (sc-134229) and merlin (sc-55575) were from Santa Cruz Biotechnology (Wembley, UK). Guinea pig and rabbit antibodies against SOX10 were as described (Stolt et al., 2003; Maka et al., 2005). Phospho-NFκB (Ser276) p65 antibody (#3037), phospho-CREB (Ser133, #9198) and PDGFRB (#3169) were obtained from New England Biolabs. S100β antibodies were from Dako, Ki67 antibodies from Abcam, KROX20 antibodies were from Cambridge Bioscience, phospho-ERK1/2 (V8031) antibody was from Promega and paxillin (5H11) antibody from Millipore. Antibody to periaxin was a gift from P. Brophy (University of Edinburgh, UK), and antibody to myelin protein zero (MPZ or P0) a gift from J. Archelos (Medical University of Graz, Austria). Biotinylated anti-guinea pig, anti-goat, anti-mouse and anti-rabbit were obtained from Vector laboratories. Alexa Fluor® fluorescently conjugated anti-rabbit, mouse or goat IgGs and fluorescently conjugated streptavidin were from Life Technologies (Paisley, UK). Superfrost glass slides were from VWR Merck. All reagents for small interfering RNA knockdown of merlin were obtained from Qiagen (Crawley, UK).
Preparation of Schwann cell cultures
Schwann cells were prepared from the sciatic nerve and brachial plexus of post-natal day 3 rodents as previously described (Brockes et al., 1979). Human Schwann cells were isolated from nerve donors and schwannoma cells from tumour samples of patients with neurofibromatosis type 2 (following informed consent). Neurofibromatosis type 2 diagnosis was established using criteria set down in the NIH Consensus Conference (National Institutes of Health Consensus Development Conference, 1988; Mulvihill et al., 1990). Cells were harvested and cultured as previously described (Hanemann et al., 1998; Rosenbaum et al., 1998). All experiments were performed in serum-free supplemented medium known as defined medium (Jessen et al., 1994; Parkinson et al., 2008).
Immunocytochemistry, western blotting, polymerase chain reaction and adenoviral infections
Immunostaining and counting were performed as previously described (Parkinson et al., 2001, 2003, 2004, 2008). Anti-SOX10 (both antibodies at 1:1000), anti-OCT6 (1:150), anti phospho-NFκB (Ser276) p65 (1:100), phospho-CREB (1:100), NFATC4 (1:1000) and PDGFRB (1:100) primary antibodies were incubated overnight at 4°C. Biotinylated secondary antibodies (1:200), anti-guinea pig, anti-goat and anti-rabbit, were incubated at room temperature for 45 min and the streptavidin-568 third layer (1:500) incubated for 30 min at room temperature. Confocal imaging was performed using the Zeiss meta 510 confocal microscope and the Zeiss LSM software (Zeiss). Fluorescence microscopy was performed on a Nikon Eclipse 80i. Unless otherwise stated, western blotting, PCR and adenoviral infections were performed as previously described (Parkinson et al., 2001, 2003, 2004, 2008; Kaempchen et al., 2003; Flaiz et al., 2007). For semi-quantitative PCR, the following primers were used: 18S rRNA For, CAGCCACCCGAGATTGAGCA, Rev, TAGTAGCGACGGGCGGTGTG; SOX10 For: CAGGCGAGCTGGGCAAGGTCAAG, Rev: CTGCCTGAGCCCACACCATGAAG; PDGFRB For: CGAGTTGGACCTGAACATGAC, Rev: CGCACAATCTCGATCTTTCTC; CD44 For: TGAATATAACCTGCCGCTTTG, Rev: GTCATACTGGGAGGTGTTGGA.
Schwann cell differentiation and proliferation assays
For myelination assays, defined medium was supplemented with 1 mM dibutyryl cAMP for 48 h unless otherwise stated. Human Schwann and schwannoma cells were infected with adenoviruses, in defined medium without forskolin, for 24 h before cAMP stimulation in fresh defined medium. For proliferation assays in human schwannoma cells, 20 ng/ml NRG1, 100 ng/ml IGF-1 or 100 ng/ml PDGF-DD was added to fresh defined medium for 24 h or 72 h after the initial adenoviral infection. For proliferation assays in mouse Schwann cells defined medium was supplemented with 10 ng/ml PDGF-DD with or without 2 µm forskolin for 48 h.
Transgenic mice
Transgenic mouse breeding and experiments were performed following guidelines from the UK Home Office. Mice with the conditional SOX10fl/fl allele have been described (Finzsch et al., 2010), and mice with the conditional NF2fl/fl allele (Giovannini et al., 2000) were obtained from the Riken BioResource Centre (Experimental Animal Division, Japan). For in vitro analysis of null cells, mouse Schwann cells were prepared from the sciatic nerves of either Sox10fl/fl or Nf2fl/fl mouse pups and SOX10 or merlin gene expression removed in culture by infection with Cre-recombinase expressing adenovirus. Schwann cells from the same culture were infected with GFP virus and used as a control. Mice expressing Cre recombinase under the control of the P0 promoter (mTOTA P0-Cre) have been previously described (Feltri et al., 1999). For analysis of Merlin-null sciatic nerve in vivo, NF2fl/fl mice were crossed with P0-Cre mice to generate NF2fl/wt Cre+ animals that were then back-crossed to NF2fl/fl mice to generate control (NF2fl/fl Cre−) and Merlin-null (NF2fl/fl Cre+) sciatic nerve for analysis.
Small interfering RNA knockdown
Merlin was knocked down in Schwann cells using 3 nM of validated small interfering RNA oligos Rn_Nf2_2 and Rn_Nf2_3 in combination. Alternatively, 3 nM Allstars negative control was used as a scrambled control. Small interfering RNA/lipid complexes were prepared using 6 µl of HiPerfect® reagent and incubated with cell cultures for 20 h. Transfection was performed twice at 0 and 48 h, and protein expression assayed a further 24 or 48 h later.
Fluorescent immunohistochemistry
Transverse 7-µm thick cryostat sections of schwannoma and control peroneal nerve were fixed with 4% paraformaldehyde on Superfrost plus glass slides. Sections were then stained sequentially with antibodies against SOX10 (1:1000) and S100β (1:100) following the fluorescent immunocytochemistry protocol.
Scanning electron microscopy
For scanning electron microscopy, mouse Schwann cells were cultured on glass coverslips and infected with GFP or Cre expressing adenovirus as above. Forty-eight hours after addition of adenovirus, coverslips were fixed in 2.5% w/v glutaraldehyde, diluted in 0.2 M sodium cacodylate buffer, at room temperature for 2 h and then washed twice in 0.2 M sodium cacodylate buffer. Coverslips were washed in 30%, 50%, 70%, 90% and 100% absolute ethanol for 15 min each at room temperature. Samples then underwent critical point drying through CO2 in a K850 Critical Point Dryer (Emitech), and coverslips were mounted on support stubs and gold coated (nominal thickness 10 nm) in a K550 Sputter Coater (Emitech). Samples were imaged on a JSM-5600LV scanning electron microscope (JEOL Ltd.) operated at 15 kV, with an 11 mm working distance and at ×1000 or ×2000 magnification.
Statistics
For all experiments, n = 3 was used unless otherwise stated. All graphs display the arithmetic mean with error bars representing one standard error of the mean. P-values were calculated using a Student t-test and are denoted on figures as: *P < 0.05, **P < 0.01 and ***P < 0.005. For all cell differentiation and proliferation assays, ∼200 cells were counted in duplicate. In adenoviral experiments, the number of positive cells was divided by the number of GFP positive cells. For all other experiments, the number of positive cells was divided by the number of Hoechst positive cells. A minimum of 500 cells were counted for SOX10 positivity in each cryostat section.
Results
KROX20 drives myelin gene expression in Merlin-null schwannoma cells
It has been well characterized that KROX20 is the key regulator of Schwann cell myelination. Enforced expression of KROX20 in vitro is sufficient to drive increased expression of compact myelin proteins (P0 and MBP), myelin associated proteins (myelin associated glycoprotein and periaxin) and essential enzymes in myelin lipid synthesis (Nagarajan et al., 2001; Parkinson et al., 2003). KROX20 expression also downregulates expression of the myelination inhibitory transcription factors c-Jun and Sox2 (Parkinson et al., 2004, 2008; Le et al., 2005). As a first step in this analysis of Merlin-null schwannoma cells, we tested whether these events downstream of KROX20 could be normally regulated in these tumour cells. No significant basal differences in the expression of myelin proteins or negative myelin-regulators were observed in schwannoma cells. To investigate whether KROX20 could drive myelination in schwannoma cells, we infected primary human schwannoma (NF2−/−) and control Schwann (NF2+/+) cells with adenoviral constructs co-expressing KROX20 and GFP or GFP alone. After KROX20 expression, we assessed, by immunolabelling and western blotting, the induction of myelin targets and downregulation of c-Jun expression.
KROX20 was able to drive strong upregulation of the myelin protein P0 and the myelin marker periaxin in both wild-type Schwann cells and Merlin-null schwannoma cells, shown by both fluorescent immunocytochemistry and western blotting (all P< 0.02). Similarly, KROX20 was also able to downregulate the inhibitory transcription factor c-Jun in Merlin-null schwannoma cells (P< 0.001) (Fig. 1). The regulation of P0, periaxin and c-Jun by KROX-20 in human Schwann and schwannoma cells was indistinguishable from that seen in primary rat Schwann cells (data not shown). These data suggest that once expressed, KROX-20 is apparently fully able to drive the downstream myelination programme in Merlin-null schwannoma cells.
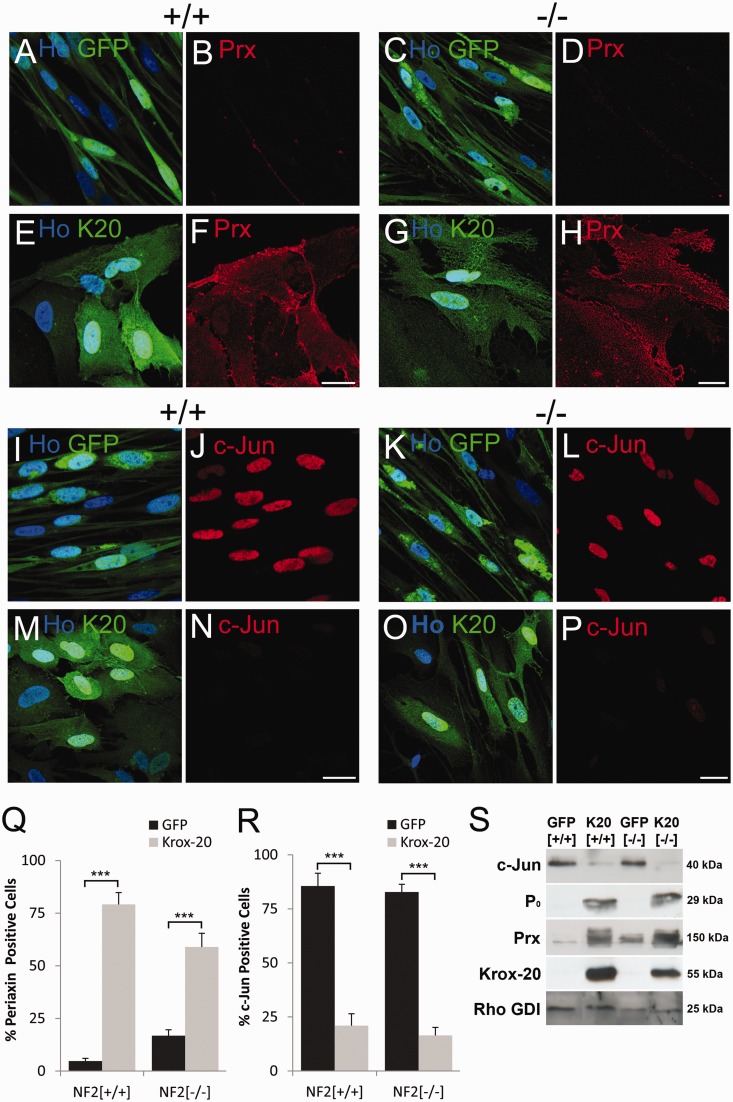
Figure 1.
Kroz-20 induces periaxin and P0 and downregulates c-Jun expression in both control and Merlin-null human Schwann cells. (A–H) Immunofluorescence of control Schwann +/+ (A, B, E and F) and Merlin-null schwannoma −/− (C, D, G and H) cells infected with control GFP (A–D) or GFP/KROX20 (E–H, K20) expressing adenoviruses showing equal induction of periaxin (Prx) protein in both control and Merlin-null cells (F and H). (I–P) Immunofluorescence of control +/+ (I, J, M and N) and Merlin-null −/− (K, L, O and P) cells infected with GFP and GFP/KROX20 (K20) expressing adenoviruses, showing down regulation of c-Jun in both control and Merlin-null cells (N and P). Scale bars = 20 µm. (Q and R) Graphs showing percentage periaxin/GFP (Q) and c-Jun/GFP (R) positive control Schwann (+/+) and schwannoma (−/−) cells following infection with GFP control and GFP/KROX20 expressing adenoviruses. (S) Western blot showing similar upregulation of periaxin and P0 protein and downregulation of c-Jun expression by KROX20 in both control Schwann (+/+) and Merlin-null schwannoma cells (−/−).
KROX20 expression inhibits the proliferation of Merlin-null schwannoma cells
In addition to controlling myelin gene expression, KROX20 has been shown to regulate the proliferation of Schwann cells, inhibiting the proliferation of cells in response to mitogens such as beta-neuregulin (NRG1) (Zorick et al., 1999; Parkinson et al., 2004). Loss of merlin causes schwannoma cells to proliferate at an increased basal rate, to lose cell–cell contact dependent inhibition of cell proliferation and continue to grow in overlapping layers past confluency in vitro (Lallemand et al., 2003; Curto et al., 2007; Flaiz et al., 2007, 2008). This proliferation could be driven by either PDGF or IGF-1, which have both been shown to be mitogenic for schwannoma cells in vitro (Ammoun et al., 2008, 2012).
To test whether KROX20 effectively controls proliferation in schwannoma cells, primary human Merlin-null cells were infected with either GFP- or KROX20/GFP-expressing adenoviruses in defined medium and then treated with the Schwann cell mitogens NRG1 (10 ng/ml), PDGF-DD (100 ng/ml) or IGF-1 (100 ng/ml). Proliferation was measured by immunofluorescence using an antibody against Ki-67, which has a punctate nuclear stain in proliferating cells (Fig. 2). Basal proliferation of schwannoma cells in defined medium alone was 2.3 ± 0.5%, which was increased by NRG1, PDGF or IGF-1 addition to 8.1 ± 1.1, 8.7 ± 1.1 and 11.2 ± 2.1%, respectively, in control GFP infected schwannoma cells (Fig. 2). This proliferation was dramatically and significantly reduced to <1% with all mitogens in KROX20-expressing schwannoma cells (Fig. 2; NRG1, P< 0.001; PDGF, P< 0.001; IGF-1, P= 0.002; n = 4). KROX20 therefore effectively controls proliferation of schwannoma cells in mitogen-stimulated conditions. In conclusion, we saw that enforced KROX20 expression in Merlin-null schwannoma cells is sufficient to drive myelin gene expression, downregulate inhibitors of myelination and to inhibit the proliferation of schwannoma cells in response to a range of mitogens. These findings implied that the differentiation network downstream of KROX20 is intact in Merlin-null schwannoma cells; therefore, we next investigated the regulatory events upstream of KROX20 in these cells.
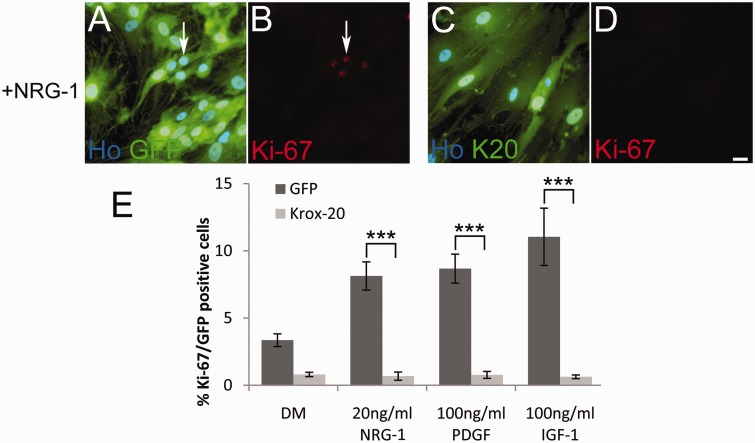
Figure 2.
KROX20 halts the proliferation of Merlin-null schwannoma cells. (A–D) Human Merlin-null schwannoma cells were infected with GFP (A and B) or GFP/KROX20 (K20) adenoviruses (C and D) for 24 h and then treated with 20 ng/ml of NRG-1 (+NRG-1) for a further 24 h. Cells were fixed and immunolabelled with antibodies against Ki-67. Arrows in A and B indicate Ki-67 positive nuclei. Scale bar = 20 µm. (E) Graph showing that KROX20 expression causes a significant decrease in schwannoma cell proliferation in response to NRG-1 (P < 0.001), PDGF (P < 0.001) or IGF-1 (P = 0.002).
Impaired induction of KROX20 and OCT6 in schwannoma cells
During Schwann cell myelination in vivo, expression of KROX20 is controlled by a complex signalling cascade involving the transcription factors OCT6, SOX10, YY1 and NFATC4, as well as through activation of the G-protein coupled receptor GPR126 (Topilko et al., 1994; Jaegle et al., 1996; Kao et al., 2009; Monk et al., 2009, 2011; Finzsch et al., 2010; He et al., 2010). This process of myelination can be mimicked in vitro by addition of cyclic AMP, which causes Schwann cell flattening and upregulation of myelin proteins (e.g. P0, myelin basic protein and periaxin), myelin lipids (e.g. O4) and myelinating transcription factors (e.g. OCT6 and KROX20) (Morgan et al., 1991; Parkinson et al., 2008). The ability of Merlin-null schwannoma cells to upregulate KROX20 in response to cyclic AMP was tested by incubating primary human Schwann and schwannoma cells in defined medium alone or in defined medium supplemented with 1 mM of the non-hydrolysable cyclic AMP analogue dibutyryl-cAMP (hereafter referred to as cAMP) for 24, 48 and 72 h and examining KROX20 induction by immunofluorescence and western blotting. Human control Schwann cells displayed no detectable KROX20 expression in basal conditions, but dramatically induced KROX20 on cAMP treatment, peaking at 34.8 ± 4.2% of nuclei positive for KROX20 at 72 h (Fig. 3 at 48 h and Supplementary Fig. 1). In contrast, Merlin-null schwannoma cells from seven independent tumours were significantly reduced in their ability to induce KROX20 protein at all time points examined (Fig. 3 and data not shown) peaking at only 9.1 ± 2.3% at 72 h, with a significantly decreased induction at 24 h (P= 0.037), 48 h (P< 0.001) and 72 h (P< 0.001). Schwannoma cells from three of these tumours displayed an absolute block in KROX20 induction, with <1% of cells KROX20 positive after any duration of cAMP treatment. This result was confirmed by western blotting at the 48 h time point in control human Schwann and schwannoma cells, again showing no apparent induction of KROX20 in Merlin-null schwannoma cells from a further two schwannoma tumours (Fig. 3). The myelinating Schwann cell marker periaxin is also induced by cAMP in Schwann cells (Parkinson et al., 2003) and we found that the induction of periaxin was also significantly blocked in Merlin-null schwannoma cells at all timepoints examined (Fig. 3, 48 h and Supplementary Fig. 1, P< 0.001, 72 h).
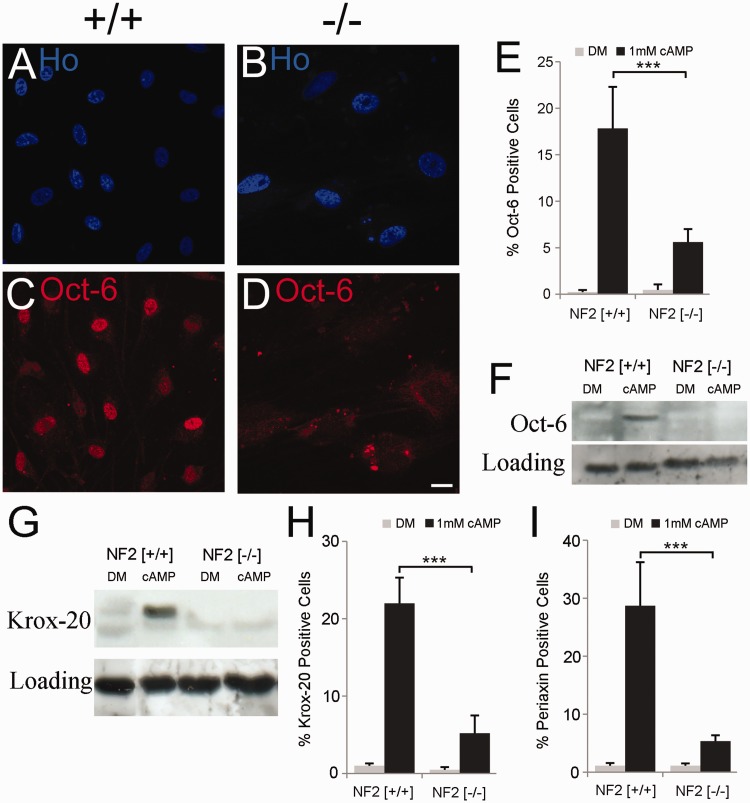
Figure 3.
Merlin-null schwannoma cells do not induce OCT6 or KROX20 in response to cyclic AMP. (A–F) Impaired induction of OCT6 in schwannoma cells. Control (NF2+/+) and Merlin-null (NF2−/−) cells were treated for 48 h with 1 mM cAMP and OCT6 levels measured by immunolabelling (A–D) and western blotting (F). Note clear induction of OCT6 in nuclei of control (+/+) cells (C), whereas no OCT6 is observed in Merlin-null (−/−) cells (D). Scale bar = 20 µm (E). Graph of percentage OCT6-positive Schwann and schwannoma cells in response to cAMP. (F and G) Western blot showing lack of OCT6 (F) and KROX20 (G) induction in response to cAMP at 48 h. (H and I) Graphs showing percentage KROX20 (H) and periaxin (I) positive control (NF2 +/+) and Merlin-null (NF2 −/−) cells after cAMP treatment (48 h). DM = defined medium control.
KROX20 induction may be inhibited by increased activity of the ERK1/2 and JNK1/2 MAP kinase pathways (Harrisingh et al., 2004; Ogata et al., 2004), and both pathways have been shown to be elevated in Merlin-null schwannoma cells (Ammoun et al., 2011). We therefore tested whether inhibition of either or both of these pathways would restore KROX20 induction. Experiments using a JNK1/2 inhibitor (SP600125), expression of the JNK-binding domain of JIP-1, both of which have previously been shown to inhibit JNK activity in Schwann cells (Parkinson et al., 2004), or even expression of dominant negative c-Jun (Δ169 c-Jun; Parkinson et al., 2001) did not restore KROX20 induction in schwannoma cells. Similarly, inhibition of the MEK/ERK pathway by U0126 or AZD6244, the PI3-kinase pathway using BEZ235, or even use of the ErbB2 inhibitor Lapatinib or the PDGFR inhibitors Nilotinib or Sorafenib, all of which inhibit schwannoma cell proliferation at the concentrations used, were unable to increase KROX20 induction in response to cAMP in schwannoma cells (Supplementary Fig. 2 and data not shown). Similarly, both mouse and human Merlin-null cells show increased GTP loading of Rac, and such enhanced Rac activity has been shown to block elongation of schwannoma cells along dorsal root ganglion axons in co-culture assays, an early step in myelination (Nakai et al., 2006). Inhibition of Rac activity by adenovirally mediated expression of dominant negative N17-Rac also failed to rescue KROX20 expression in schwannoma cells in response to cAMP (data not shown).
Next, we tested whether there was normal induction of the POU domain transcription factor OCT6 in Merlin-null schwannoma cells. Forty-eight hours after addition of cAMP to control human Schwann cells, we observed induction of nuclear OCT6 by immunocytochemistry and western blotting, which was significantly (P= 0.003) blocked in Merlin-null schwannoma cells (Fig. 3).
Phosphorylation and activation of NFκB and CREB following cAMP treatment are thought to be important for the induction of OCT6 and KROX20, respectively (Nickols et al., 2003; Arthur-Farraj et al., 2011). Using phospho-specific antibodies for the p65 subunit of NFκB (Ser276) and CREB (Ser133), we measured the response to cAMP in control and Merlin-null schwannoma cells. Analysis by immunocytochemistry with these antibodies after cAMP addition showed an identical amplitude and duration of activation (i.e. phosphorylation) of NFκB and CREB in control Schwann and Merlin-null schwannoma cells (Supplementary Fig. 3). Additionally, the zinc finger transcription factor YY1 has also been shown to regulate KROX20 expression during myelination. Analysis by immunolabelling showed clear nuclear YY1 expression in both control Schwann and Merlin-null schwannoma cells (data not shown). These experiments identify the regulation of both OCT6 and KROX20 by cyclic AMP as abnormal in Merlin-null schwannoma cells.
SOX10 levels are reduced in Merlin-null schwannoma cells in vitro and in vivo
The high mobility group transcription factor SOX10 is first expressed in the neural crest and is required for Schwann cell specification and subsequent myelination in the peripheral nervous system (Britsch et al., 2001; Finzsch et al., 2010). The control of both KROX20 and OCT6 have been shown to be dependent on SOX10 expression (Ghislain and Charnay 2006; Reiprich et al., 2010; Jagalur et al., 2011); therefore, we next measured SOX10 levels in vitro in control and Merlin-null schwannoma cells and in vivo by analysis of cryostat sections of control human nerve and schwannoma tumour samples. Figure 4 shows the results of immunocytochemistry and western blotting of human Schwann and schwannoma cells. In vitro, control Schwann cells displayed a uniform intense nuclear expression in all cells. In contrast, both schwannoma cells in vitro and sections of schwannoma tumours showed a consistent decreased expression in all cells or a heterogeneous staining pattern with some cells expressing SOX10 and some containing no detectable SOX10 (n = 8; Fig. 4). These findings were confirmed with two separate SOX10 antibodies. In total, we analysed cultured cells from eight schwannoma tumours and cryostat sections from a further six independent tumours and observed a significant reduction of SOX10 in all samples analysed. Counts of SOX10-positive nuclei in control nerve and schwannoma sections showed overall a significant (60.3%, P< 0.001) decrease in the percentage of SOX10-positive nuclei (Supplementary Fig. 4). Western blotting of cell extracts from three further independent schwannoma cell cultures compared with three control human primary Schwann cell extracts also showed a variable, but consistent, reduction in SOX10 expression in Merlin-null schwannoma cells. Thus, in total, we have analysed 17 human schwannoma samples, all of which showed a reduction in SOX10 protein levels. Similarly, we observed a reduction of SOX10 messenger RNA levels in schwannoma cells compared with control Schwann cells (Fig. 4).
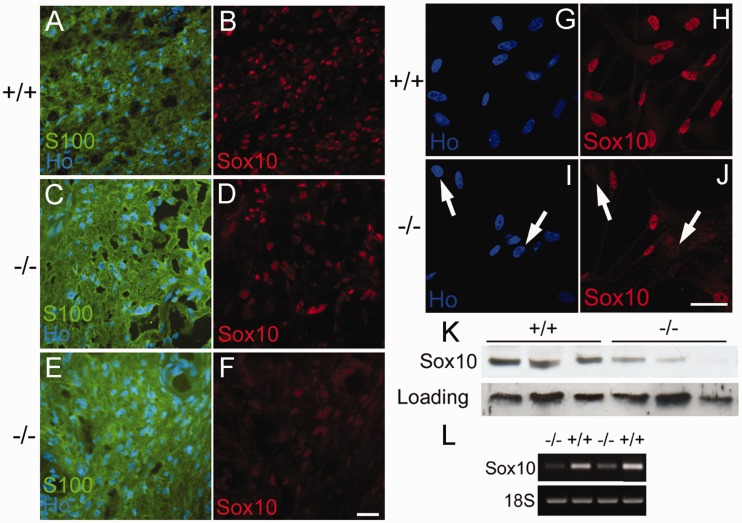
Figure 4.
Expression of SOX10 protein and messenger RNA is reduced in Merlin-null human schwannoma cells. (A–F) Cryostat sections of control human peroneal nerve (+/+, A and B) and human schwannoma tumours (−/−, C–F) stained with SOX10 antibody and the Schwann cell marker S100β (S100). Nuclei are counterstained with Hoechst dye (Ho). Almost all nuclei in control nerve (A and B) are SOX10 positive. For the tumour shown in panels C and D, a mosaic pattern is observed with many cells being SOX10 negative. For the tumour shown in panels E and F, almost no SOX10 stain is observed. Scale bar = 20 µm. (G–J) Immunolabelling of dissociated control Schwann (+/+, G and H) and Merlin-null schwannoma (−/−, I and J) cells in culture with SOX10 antibody. Note mosaic pattern in SOX10 stain in panel J; arrows show SOX10-negative cells in I and J. Scale bar = 20 µm. (K) Western blot analysis of three independent human control (+/+) and Merlin-null (−/−) schwannoma tumour samples. Note reduction of SOX10 expression in all tumour samples. (L) Semi-quantitative PCR showing reduction of SOX10 messenger RNA in two independent Merlin-null tumours (−/−) compared with normal Schwann cell controls (+/+).
Re-expression of SOX10 in human Merlin-null schwannoma cells rescues KROX20 and P0 induction
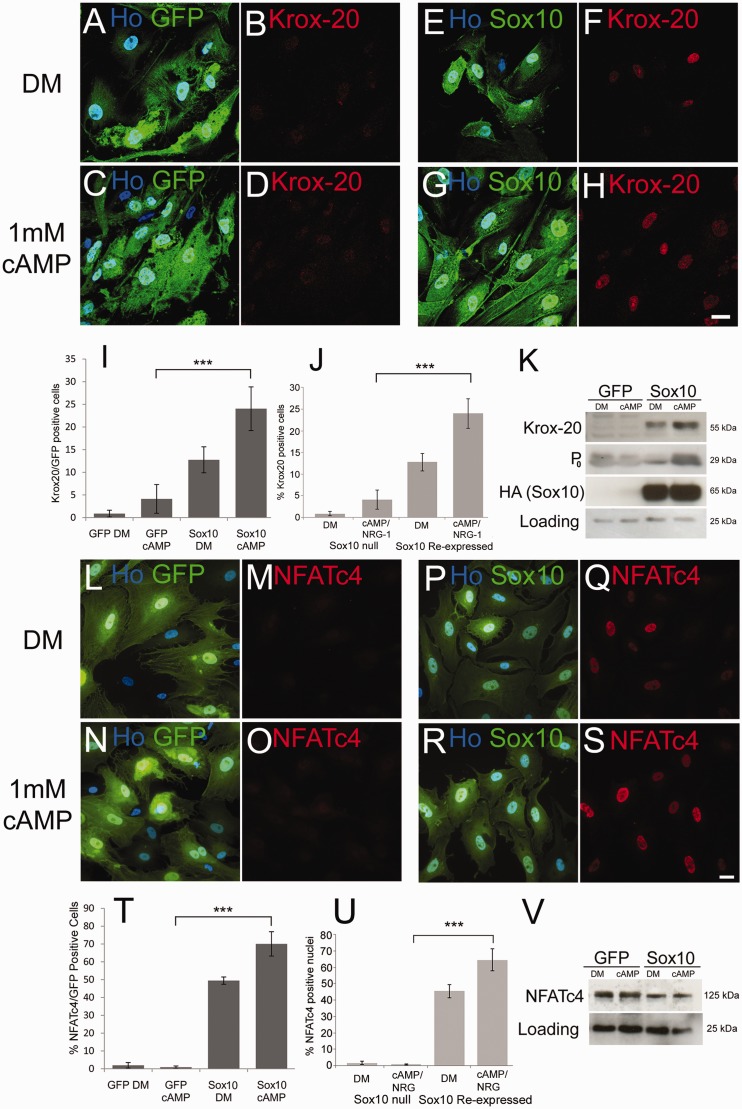
Having identified a consistent reduction in SOX10 levels in schwannoma cells as a possible cause of decreased KROX20 and OCT6, we measured the effects of haemagluttinin-tagged SOX10 re-expression in human schwannoma cells. Immunolabelling and western blotting of schwannoma cells with either haemagluttinin or SOX10 antibodies showed strong nuclear expression of SOX10 in cells following adenoviral infection (Fig. 5 and data not shown). Next, we tested whether reintroduction of SOX10 into schwannoma cells would allow induction of Krox-20 in response to cAMP. SOX10 expression was sufficient, in the absence of cAMP, to induce KROX20 in a small percentage of schwannoma cells (P= 0.001 versus controls), which was greatly enhanced by the addition of cAMP (P< 0.001 versus controls). In addition, we also observed the induction of P0 protein in these cells (Fig. 5).
Figure 5.
Re-introduction of SOX10 into Merlin-null human schwannoma cells rescues induction of KROX20, P0 and NFATC4. (A–H) Merlin-null cells were infected with GFP (A–D) and GFP/SOX10 (E–H) expressing adenoviruses and either maintained in defined medium alone (A, B, E and F) or defined medium plus 1 mM cAMP (C, D, G and H). Note that SOX10 expression alone is sufficient to drive KROX20 expression in a small number of cells (F, I and K), which is further enhanced by cAMP treatment (H, I and K). Scale bar = 20 µm. (I) Percentage KROX20-positive cells in GFP- and SOX10-infected schwannoma cells. (J) Graph showing that SOX10-null mouse Schwann cells do not induce KROX20 in response to cAMP+NRG-1, but re-expression of SOX10 restores KROX20 induction. (K) Western blot showing regulation of KROX20 and P0 by SOX10 in Merlin-null cells. Probing with HA antibody detects adenovirally mediated SOX10 expression. (L–S) Re-expression of SOX10 in Merlin-null cells restores nuclear localization of NFATC4. Merlin-null cells were infected with GFP- (L–O) and GFP/SOX10- (P–S) expressing adenoviruses and either maintained in defined medium alone (L, M, P and Q) or defined medium plus 1 mM cAMP (N, O, R and S). Scale bar = 20 µm. (T) Percentage NFATC4-positive cells in GFP- and GFP/SOX10-infected schwannoma cells. (U) Graph showing that SOX10-null mouse Schwann cells do not induce nuclear localization of NFATC4 in response to cAMP+NRG-1, but re-expression of SOX10 restores NFATC4 relocalization. (V) Western blot showing expression of NFATC4 in GFP and GFP/SOX10 infected schwannoma cells. DM = defined medium.
SOX10 re-expression causes nuclear localization of NFATC4 in schwannoma cells
Signalling through the calcineurin/NFAT pathway has also recently been shown to be required for KROX20 induction and myelination (Kao et al., 2009; Kipanyula et al., 2012). Although Schwann cells express NFATC1–C4 (Kipanyula et al., 2012), only NFATC4 has been shown to bind to the myelinating Schwann cell enhancer of the KROX20 gene and drive KROX20 expression in Schwann cells (Kao et al., 2009). We therefore examined the levels and localization of NFATC4 in schwannoma cells infected with control GFP and SOX10/GFP expressing adenoviruses with or without cAMP treatment. This analysis showed that, in contrast to control GFP virus-infected cells, SOX10 reintroduction causes nuclear localization of NFATC4 that is further boosted upon cAMP treatment (P< 0.001). Western blotting demonstrated that this nuclear localisation occurred without any SOX10 driven increase in NFATC4 protein expression (Fig. 5). In addition to this, expression of SOX10 also seems to cause a shift in the molecular weight of NFATC4, which is consistent with its potential dephosphorylation, and nuclear localization, in response to SOX10 expression (Kao et al., 2009).
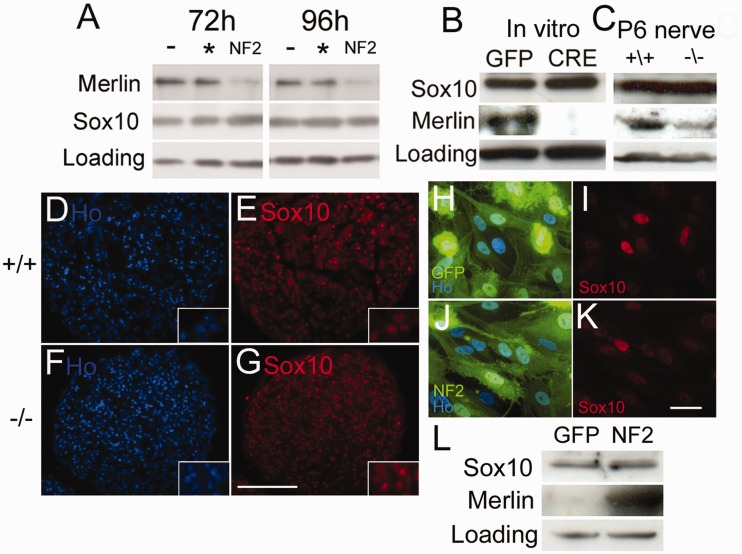
Loss of SOX10 expression in schwannoma cells is independent of Merlin loss
Having observed the clear loss of SOX10 in schwannoma cells and the effects of SOX10 re-expression in these cells, we next tested whether SOX10 expression is directly controlled by merlin in Schwann cells. To do this, we used a number of in vitro and in vivo approaches. Firstly, using small interfering RNA, we silenced merlin expression in rat Schwann cells and measured SOX10 expression by western blot and immunocytochemistry. No effect on SOX10 levels was seen at either 72 or 96 h time points after transfection, despite almost complete silencing of merlin (Fig. 6). Next, we analysed Merlin-null Schwann cells, either in vitro by infection of NF2fl/fl Schwann cells (Giovannini et al., 2000) with adenovirus expressing Cre recombinase, or in vivo by analysis of Merlin-null sciatic nerve prepared by breeding NF2fl/fl mice with P0-Cre expressing mice to specifically ablate merlin expression in Schwann cells of the nerve (Feltri et al., 1999). Using both paradigms, we did not observe a reduction of SOX10 protein expression in Merlin-null Schwann cells, either by western blot or staining of nerve cryosections (Fig. 6), demonstrating that loss of merlin does not directly lead to lower SOX10 levels. Finally, we performed experiments to reintroduce merlin into human schwannoma cells and measure levels of SOX10. Adenovirally mediated re-expression of merlin did not increase SOX10 levels in cells after 48 h as measured by either immunocytochemistry or western blot in human schwannoma cells (Fig. 6).
Figure 6.
Regulation of SOX10 expression in Schwann cells is independent of merlin. (A) Western blot of rat Schwann cells transfected with control scrambled (asterisk) or NF2 small interfering RNA (NF2) at 72 h and 96 h after transfection; untransfected cells (−). Note that knockdown of merlin at both 72 and 96 h time points, but no decrease in SOX10 protein expression. (B) Western blot of NF2fl/fl mouse Schwann cells infected with control GFP- or Cre-expressing adenoviruses, 72 h after infection. (C) Western blot of control (NF2fl/fl Cre−) and Merlin-null (NF2fl/fl Cre+) post-natal Day 6 (P6) mouse sciatic nerve, showing no reduction of SOX10 protein levels. (D–G) Cryostat sections from control (+/+; D and E) and Merlin-null (−/−; F and G) post-natal Day 6 mouse sciatic nerve shows no difference in nuclear SOX10 stain (E and G); nuclei are counterstained with Hoechst dye (Ho; D and F). Scale bar = 100 µm. Insets in panels D–G show higher magnification views of cells, confirming that SOX10 is localized to the nuclei of cells. (H–L) Re-introduction of merlin into Merlin-null human schwannoma cells does not restore expression of SOX10. Human schwannoma cells were infected with either GFP control- (H, I) or GFP/Merlin- (J and K) expressing adenoviruses and immunolabelled with SOX10 antibodies 48 h after infection (I and K). Scale bar = 20 µm. (L) Western blot showing human schwannoma cells 48 h after infection with control GFP or merlin (NF2) expressing adenoviruses. Merlin re-introduction into Merlin-null human schwannoma cells does not increase SOX10 expression.
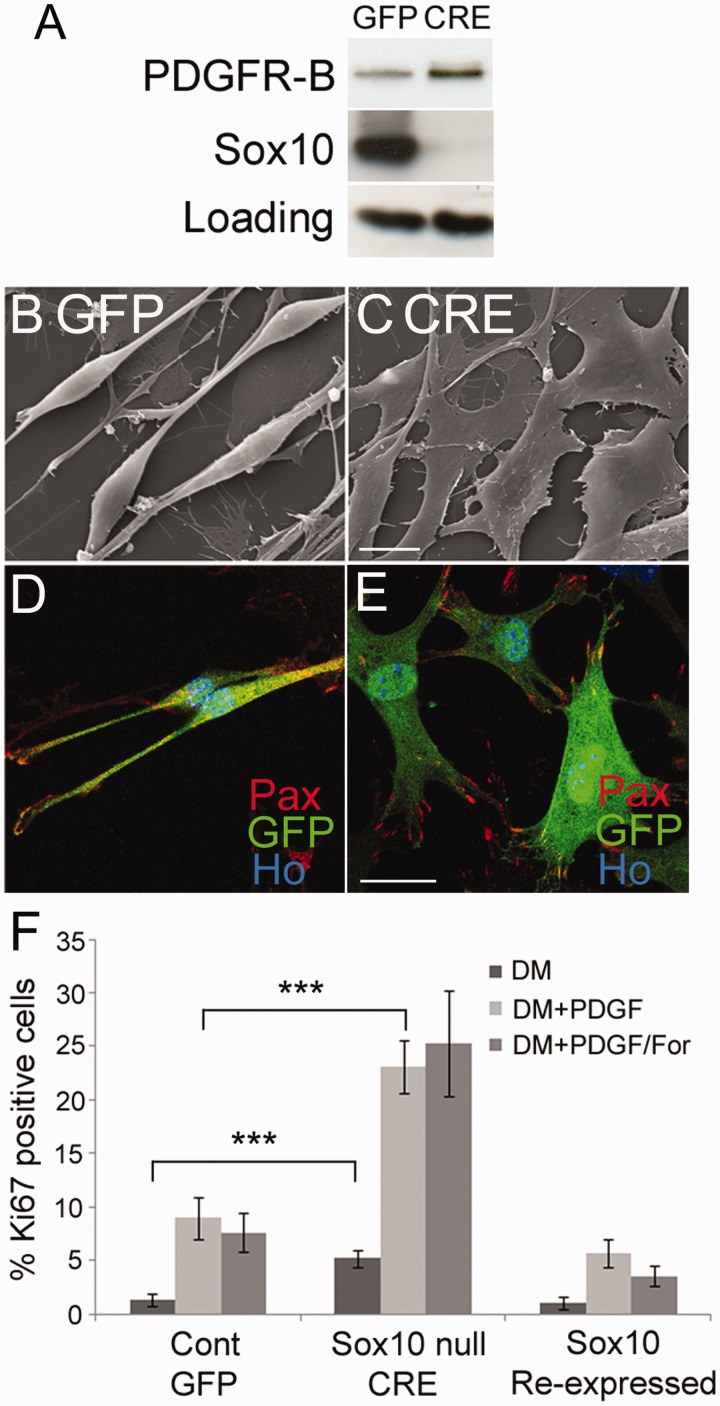
Loss of SOX10 alone results in phenotypes typical of human schwannoma cells
Loss of SOX10 in vivo leads to abnormalities in Schwann cell–axon interactions, a complete block in myelination and increased post-natal proliferation of Schwann cells in peripheral nerves (Finzsch et al., 2010; Bremer et al., 2011). To characterize changes in Schwann cells that are SOX10-dependent, we next prepared, in vitro, SOX10-null Schwann cells by treatment of SOX10fl/fl cells with Cre-recombinase expressing adenovirus. In vitro SOX10-null cells show a flattened non-bipolar morphology and increased focal adhesions as measured by paxillin immunolabelling (Fig. 7), both phenotypes of human schwannoma cells (Rosenbaum et al., 1998; Flaiz et al., 2009). Similar to human schwannoma cells, SOX10-null cells show significantly increased (P= 0.03) adhesion in a cell adhesion assay (Utermark et al., 2003) compared with control GFP virus infected cells (Supplementary Fig. 5). To confirm our idea that loss of SOX10 in Merlin-null human schwannoma cells causes reduced KROX20 induction, we performed in vitro myelination assays as described (Arthur-Farraj et al., 2011) with control and Sox10-null mouse Schwann cells. SOX10-null Schwann cells displayed minimal NFATC4 nuclear localization and KROX20 induction that was significantly increased by SOX10 reintroduction (P= 0.003, NFATC4; P< 0.001, KROX20) (Fig. 5). Similarly, in line with the in vivo evidence (Finzsch et al., 2010), SOX10-null mouse Schwann cells fail to induce OCT6 in response to cAMP plus neuregulin (data not shown).
Figure 7.
Loss of SOX10 alone in mouse Schwann cells results in phenotypes typical of human schwannoma cells. (A) Western blot of SOX10fl/fl mouse Schwann cells infected with GFP- or Cre-expressing adenoviruses, showing increased expression of PDGFRB in SOX10-null mouse cells. (B and C) Scanning electron Micrsocopy showing flattening of SOX10-null cells (C) in comparison with control cells (B). Scale bar = 10 µm. (D and E) Immunolabelling of control (D) and SOX10 null (E) cells with paxillin antibody (Pax) to reveal increased numbers of focal adhesions in SOX10 null cells (E). Scale bar = 20 µm. (F) Graph showing significantly (P < 0.001) increased proliferation of SOX10-null mouse Schwann cells in response to 10 ng/ml of PDGF or PDGF plus 2 µM of forskolin (PDGF/For). Reintroduction of SOX10 into SOX10-null cells once more decreases PDGF-induced proliferation.
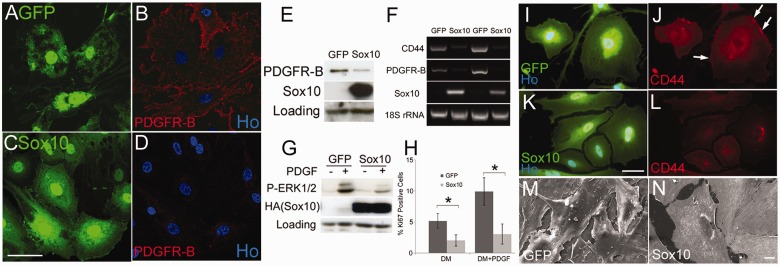
In addition, human Merlin-null schwannoma cells show a range of phenotypes, but two of the key changes in these cells are increased expression of PDGFRB, causing increased proliferation of cells, and the CD44 cell surface glycoprotein, which is involved in cell adhesion and migration (Morrison et al., 2001; Hanemann et al., 2006; Bai et al., 2007; Ammoun et al., 2008). To study whether the regulation of PDGFRB and CD44 in Schwann cells is SOX10-dependent, we analysed their expression in control and SOX10-null mouse Schwann cells and the effects of SOX10 reintroduction in Merlin-null human schwannoma cells. SOX10-null cells show higher expression levels of PDGFRB compared with controls cells and also show significantly increased basal (P= 0.002) and PDGF-induced (P< 0.001) rates of proliferation (Fig. 7).
In agreement with this, reintroduction of SOX10 into human schwannoma cells greatly reduces expression of PDGFRB and CD44 messenger RNA and protein. SOX10 re-expression also significantly reduces basal proliferation (P= 0.03) and both PDGF stimulated proliferation (P= 0.01) and MAP kinase signalling in these cells (Fig. 8).
Figure 8.
Re-introduction of SOX10 reduces PDGFRB and CD44 levels, reduces PDGF-induced signalling and prevents cell overgrowth in human Merlin-null schwannoma cells. (A–D) GFP-control- (A and B) and GFP/SOX10- (C and D) infected human schwannoma cells were immunolabelled with PDGFRB antibodies. SOX10 causes clear downregulation of membrane staining for PDGFRB. Scale bar = 20 µm. (E) Western blot showing that adenovirally mediated re-expression of SOX10 lowers PDGFRB protein levels in schwannoma cells. (F) Semi-quantitative PCR- of duplicate complementary DNA samples prepared from two independent human schwannoma cell cultures infected with control GFP or GFP/SOX10 adenoviruses. Note that downregulation of CD44 and PDGFRB messenger RNAs following SOX10 re-expression. (G) SOX10 reexpression in human schwannoma cells reduces PDGF-induced ERK1/2 MAP kinase activation. Human schwannoma cells infected with either control GFP or GFP/SOX10 adenoviruses were treated with PDGF-DD for 15 min. Blotting with HA antibody shows exogenously re-expressed SOX10 protein. (H) Re-introduction of SOX10 reduces both basal and PDGF-induced proliferation of human Merlin-null schwannoma cells at 72 h. (I–L) Immunolabelling of control GFP-infected (I and J) human schwannoma cells shows clear membrane-associated staining of CD44 (arrows in J), which is lost on SOX10 re-expression (K and L). Scale bar = 20 µm. (M and N) Scanning electron microscopy of GFP- (M) and GFP/SOX10- (N) infected human schwannoma cells. Scale bar = 10 µm. Note that control cells frequently overgrow one another, upon SOX10 re-expression the cells appear to respect cell–cell boundaries.
Schwannoma cells re-expressing SOX10, when viewed by fluorescence microscopy, showed a much more regular cell shape with fewer filopodia and appearing to grow over each other less than GFP control cells. Scanning electron microscopy of GFP- and GFP/SOX10-infected schwannoma showed that SOX10 infected cells do not overlap as frequently as control cells, displaying greater respect for the cell membrane of neighbouring cells (Fig. 8). The small GTPase Rac is a well-established regulator of the actin cytoskeleton and cell morphology, including the formation of lamellopodia. Enhanced Rac activity is a key feature of Merlin-null murine and human schwannoma cells (Shaw et al., 2001; Kaempchen et al., 2003). Rac activation was measured in control GFP- and SOX10/GFP-infected human schwannoma cells using a Rac G-LISA kit that found SOX10 re-expression caused a small but significant decrease (0.86-fold of control levels, P= 0.031) in GTP bound Rac (Supplementary Fig. 6).
Discussion
Roles for the merlin tumour suppressor have been characterized in many cell types and interaction of merlin with many other proteins regulates a diverse number of cell functions ranging from signalling at the cell membrane to controlling protein degradation in the nucleus (McClatchey and Giovannini 2005; Ammoun et al., 2008; McClatchey and Fehon 2009; Li et al., 2010; Cooper et al., 2011). The hallmark feature of neurofibromatosis type 2 is the occurrence of Merlin-null vestibular schwannomas, often bilaterally, and currently there is no effective clinical treatment for these tumours (Hanemann, 2008). Whereas a lot is now known about the transcription factors and signalling that control normal Schwann cell–axon interaction, cell cycle exit and differentiation, little is known about how merlin loss impacts on these processes and leads to tumour development. We have performed a number of experiments to examine the process of differentiation and cell cycle exit in human Merlin-null schwannoma cells. The zinc finger transcription factor KROX20 is a key regulator of Schwann cell differentiation, regulating both cell cycle exit and the gene expression that drives myelination (Topilko et al., 1994; Zorick et al., 1999; Parkinson et al., 2004, 2008). The normal control by KROX20 of cell cycle exit and myelin gene induction, using enforced KROX20 expression, is unchanged in Merlin-null schwannoma cells, but the ability of these cells to induce endogenous markers of differentiation, such as KROX20 and OCT6, in response to a myelination cue is markedly reduced, implying a Merlin-dependent loss of correct signalling upstream of KROX20. The mouse SC4 immortalized Merlin-null schwannoma cell line has been widely used in understanding the biology of merlin (Morrison et al., 2007; Hennigan et al., 2012). Interestingly, in SC4 cells, we did not observe a significant increase in either periaxin expression or downregulation of c-Jun when KROX20 was expressed (data not shown). We presume that the additional genetic events in the immortalization of these cells preclude their normal differentiation in response to KROX20 in contrast to primary human Schwann or schwannoma cells.
The transcriptional control of OCT6 and KROX20 have been well characterized in Schwann cells, and both rely on the high mobility group transcription factor SOX10 for their expression in developing Schwann cells (Ghislain and Charnay 2006; Finzsch et al., 2010; Reiprich et al., 2010; Bremer et al., 2011). Correspondingly, we have found reduced levels of SOX10 messenger RNA and protein in all the human schwannoma tumours we have examined, and in further confirmation of the role of SOX10, re-expression of SOX10 in human schwannoma cells reverts many of the changes seen in these cells back to a normal phenotype, critically including restoring the capacity to express myelin proteins in vitro and suppressing basal and PDGF-induced proliferation.
To separate the effects of merlin and SOX10 loss in Schwann cells, we have used small interfering RNA and mice with conditional alleles of these genes, and, both in vitro and in vivo, we see that loss of merlin has no apparent direct effect on SOX10 expression in Schwann cells. Analysis of the role of SOX10 in Schwann cells in vivo has shown that it is required for normal Schwann cell–axon interaction, cell cycle withdrawal, expression of both OCT6 and KROX20 and the normal induction and maintenance of myelination in peripheral nerves. In addition, loss of SOX10 leads to an increase in other cell types within the nerve, such as endothelial cells and pericytes and an apparent decrease in S100-expressing Schwann cells (Finzsch et al., 2010; Bremer et al., 2011), although we, in agreement with other studies (Rosenbaum et al., 1998; Kanaan et al., 2008), find no decrease in S100 staining in the schwannoma tumours we have analysed; in accordance with this, we find no increase in S100β messenger RNA levels when SOX10 is re-expressed in schwannoma cells (data not shown).
Our examination of SOX10-null mouse Schwann cells in vitro shows that loss of SOX10 alone replicates phenotypic changes associated with human schwannoma cells, such as increased numbers of focal adhesions, increased proliferation and increased expression of and signalling through the PDGF receptor beta (PDGFRB). From these data, we would propose a model by which loss of Merlin and SOX10 contribute to the phenotype of human schwannoma cells. For the example of the PDGFRB, loss of Merlin in cells only causes a modest (1.3-fold) increase in PDGFRB messenger RNA levels but is involved in recycling of the receptor protein at the cell surface (Lallemand et al., 2009), whereas our data show that SOX10 appears to transcriptionally repress the PDGFRB gene; thus, the combined effects of both merlin and SOX10 loss will lead to the large increases seen in PDGFRB protein levels in human schwannoma cells. Similarly, for CD44 messenger RNA, which encodes the hyaluronate receptor that transmits proliferative signalling in Merlin-null cells (Morrison et al., 2001; Bai et al., 2007), SOX10 transcriptionally represses CD44 messenger RNA levels when reintroduced into human schwannoma cells.
The mechanism by which SOX10 expression is lost in human schwannomas is unclear; the simplest explanation would be that loss of merlin leads to changes in cell signalling that repress SOX10 transcription, but our in vitro and in vivo analysis show this to not be the case. Analysis of both Merlin-null knockout cells and Merlin re-expression experiments in human schwannoma cells shows that the acute loss of Merlin has no effect on levels of SOX10. What regulates SOX10 in Schwann cells is presently unclear, but an upstream enhancer, known as U3, contributes to SOX10 expression and can be activated by a number of transcription factors such as SOX10, SOX9, AP2α (TFAP2A) and FOXD3 in vitro (Wahlbuhl et al., 2012). A role for activated Notch has also been shown in repressing SOX10 expression in Schwann cells (Li et al., 2004), and Merlin loss in Drosophila leads to increased Notch signalling (Maitra et al., 2006), but inhibition of Notch signalling by the γ-secretase inhibitor N-[N-(3, 5-difluorophenacetyl)-lalanyl]-S-phenylglycine t-butyl ester; 25 µM of the γ-secretase SOX10 expression in schwannoma cells (data not shown).
The key to the loss of SOX10 in human schwannomas, we propose, may be the chromosomal location of SOX10 and Merlin on chromosome 22. Remarkably, the two genes are close to one another on the long arm of chromosome 22 (merlin 22q12.2, SOX10 22q13.1). The second ‘hit’ and loss of merlin function in neurofibromatosis type 2 commonly involves loss of heterozygosity or a large deletion of chromosome 22, and our meta-analysis of cytogenetic studies on schwannoma tumours indicates that the region encoding SOX10 would be lost in >50% of these tumours (Bruder et al., 1999; Antinheimo et al., 2000; Mantripragada et al., 2003; Koutsimpelas et al., 2011), although this is probably an underestimate as the chromosomal marker resolution of several of these studies is not particularly good. SOX10 has been shown to bind and activate its own promoter (Wahlbuhl et al., 2012); therefore, it is possible that the loss of one allele could lead to the transcriptional silencing of the remaining intact allele in Schwann cells and the corresponding fall in both SOX10 messenger RNA and protein observed in schwannoma tumours. Another alternative, which has been proposed, given the differences observed between patients with neurofibromatosis type 2 and the phenotype of mice with Merlin-null Schwann cells, is that there is a third ‘hit’ at another locus in addition to the loss of merlin (Woods et al., 2003). Such an additional genetic change could directly or indirectly affect SOX10 expression and contribute to the phenotype we observe in human schwannoma cells. The production and characterization of a conditional SOX10 allele, and the separation of merlin and SOX10 on different mouse chromosomes (chromosomes 11 and 15, respectively) would now allow the testing of the effects of SOX10 hemizygosity or complete loss on the biology of Merlin-null cells and may lead, perhaps, to the development of a mouse model that more closely matches the human disease.
In conclusion, we have identified consistent loss of SOX10 in human schwannoma cells, and our results identify that this loss strongly contributes to the phenotype of this tumour cell type. These results, for the first time, provide a direct link between the processes involved in normal Schwann cell differentiation and homeostasis in the peripheral nerve and the development and pathology of schwannoma tumours. Further characterization of the relative contributions of merlin and SOX10 to the biology of schwannoma cells may give important insights into how to treat these clinically important tumours.
Funding
This work was funded by a Wellcome Trust. Wellcome Trust project grant (awarded to D.B.P.), the Northcott Devon Medical Foundation and the South West Regional Development Agency for a PhD studentship (awarded to R.D.S.D).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors wish to thank Dr Roy Moate, Peter Bond and Glenn Harper from the University of Plymouth electron microscopy centre for excellent technical assistance with electron microscopy experiments.
Glossary
Abbreviations
- AMP
adenosine monophosphate
- GFP
green fluorescent protein
- P0
myelin protein zero
- PDGF
platelet derived growth factor
- MAPK
mitogen activated protein kinase
- MBP
myelin basic protein
References
- Ammoun S, Flaiz C, Ristic N, Schuldt J, Hanemann CO. Dissecting and targeting the growth factor-dependent and growth factor-independent extracellular signal-regulated kinase pathway in human schwannoma. Cancer Res. 2008;68:5236–45. doi: 10.1158/0008-5472.CAN-07-5849. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Schmid MC, Ristic N, Zhou L, Hilton D, Ercolano E, et al. The role of insulin-like growth factors signaling in Merlin-deficient human schwannomas. Glia. 2012;60:1721–33. doi: 10.1002/glia.22391. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Schmid MC, Zhou L, Ristic N, Ercolano E, Hilton DA, et al. Insulin-like growth factor-binding protein-1 (IGFBP-1) regulates human schwannoma proliferation, adhesion and survival. Oncogene. 2011;31:1710–22. doi: 10.1038/onc.2011.357. [DOI] [PubMed] [Google Scholar]
- Antinheimo J, Sallinen SL, Sallinen P, Haapasalo H, Helin H, Horelli-Kuitunen N, et al. Genetic aberrations in sporadic and neurofibromatosis 2 (NF2)-associated schwannomas studied by comparative genomic hybridization (CGH) Acta Neurochir (Wien) 2000;142:1099–104; discussion 1104–5. doi: 10.1007/s007010070036. [DOI] [PubMed] [Google Scholar]
- Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, et al. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–33. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Liu YJ, Wang H, Xu Y, Stamenkovic I, Yu Q. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–50. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- Bremer M, Frob F, Kichko T, Reeh P, Tamm ER, Suter U, et al. SOX10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–32. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. The transcription factor SOX10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes JP, Fields P, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–18. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Bruder CE, Ichimura K, Tingby O, Hirakawa K, Komatsuzaki A, Tamura A, et al. A group of schwannomas with interstitial deletions on 22q located outside the NF2 locus shows no detectable mutations in the NF2 gene. Hum Genet. 1999;104:418–24. doi: 10.1007/s004390050978. [DOI] [PubMed] [Google Scholar]
- Cooper J, Li W, You L, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 functions upstream of the nuclear E3 ubiquitin ligase CRL4DCAF1 to Suppress Oncogenic Gene Expression. Sci Signal. 2011;4:pt6. doi: 10.1126/scisignal.2002314. [DOI] [PubMed] [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltri ML, D'Antonio M, Previtali S, Fasolini M, Messing A, Wrabetz L. P0-Cre transgenic mice for inactivation of adhesion molecules in Schwann cells. Ann N Y Acad Sci. 1999;883:116–23. [PubMed] [Google Scholar]
- Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, et al. SOX10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–12. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaiz C, Ammoun S, Biebl A, Hanemann CO. Altered adhesive structures and their relation to RhoGTPase activation in Merlin-deficient Schwannoma. Brain Pathol. 2009;19:27–38. doi: 10.1111/j.1750-3639.2008.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaiz C, Kaempchen K, Matthies C, Hanemann CO. Actin-Rich protrusions and nonlocalized GTPase activation in Merlin-deficient schwannomas. J Neuropathol Exp Neurol. 2007;66:608–16. doi: 10.1097/nen.0b013e318093e555. doi: 10.1097/nen.0b013e318093e555. [DOI] [PubMed] [Google Scholar]
- Flaiz C, Utermark T, Parkinson DB, Poetsch A, Hanemann CO. Impaired intercellular adhesion and immature adherens junctions in Merlin-deficient human primary schwannoma cells. Glia. 2008;56:506–15. doi: 10.1002/glia.20629. [DOI] [PubMed] [Google Scholar]
- Ghislain J, Charnay P. Control of myelination in Schwann cells: a KROX20 cis-regulatory element integrates OCT6, Brn2 and SOX10 activities. EMBO Rep. 2006;7:52–8. doi: 10.1038/sj.embor.7400573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14:1617–30. [PMC free article] [PubMed] [Google Scholar]
- Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131(Pt 3):606–15. doi: 10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- Hanemann CO, Bartelt-Kirbach B, Diebold R, Kampchen K, Langmesser S, Utermark T. Differential gene expression between human schwannoma and control schwann cells. Neuropathol Appl Neurobiol. 2006;32:605–14. doi: 10.1111/j.1365-2990.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- Hanemann CO, Claudia R, Sandra K, Susanne W, Florian S, Hans Werner M. Improved culture methods to expand schwann cells with altered growth behaviour from CMT1A patients. Glia. 1998;23:89–98. doi: 10.1002/(sici)1098-1136(199806)23:2<89::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–71. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–14. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Kim JY, Dupree J, Tewari A, Melendez-Vasquez C, Svaren J, et al. Yy1 as a molecular link between neuregulin and transcriptional modulation of peripheral myelination. Nat Neurosci. 2010;13:1472–80. doi: 10.1038/nn.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigan RF, Moon CA, Parysek LM, Monk KR, Morfini G, Berth S, et al. The NF2 tumor suppressor regulates microtubule-based vesicle trafficking via a novel Rac, MLK and p38(SAPK) pathway. Oncogene. 2012 doi: 10.1038/onc.2012.135. Apr 23. doi: 10.1038/onc.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, et al. The POU factor OCT-6 and Schwann cell differentiation. Science. 1996;273:507–10. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jagalur NB, Ghazvini M, Mandemakers W, Driegen S, Maas A, Jones EA, et al. Functional dissection of the oct6 schwann cell enhancer reveals an essential role for dimeric sox10 binding. J Neurosci. 2011;31:8585–94. doi: 10.1523/JNEUROSCI.0659-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent AR, Hashimoto Y, et al. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–27. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–82. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet. 2003;12:1211–21. doi: 10.1093/hmg/ddg146. [DOI] [PubMed] [Google Scholar]
- Kanaan HA, Gardner PA, Yeaney G, Prevedello DM, Monaco EA, 3rd, Murdoch G, et al. Expanded endoscopic endonasal resection of an olfactory schwannoma. J Neurosurg Pediatr. 2008;2:261–5. doi: 10.3171/PED.2008.2.10.261. [DOI] [PubMed] [Google Scholar]
- Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, et al. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323:651–4. doi: 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipanyula MJ, Woodhoo A, Rahman M, Payne D, Jessen KR, Mirsky R. Calcineurin-nuclear factor of activated t cells regulation of KROX-20 expression in Schwann cells requires elevation of intracellular cyclic AMP. J Neurosci Res. 2012;91:105–15. doi: 10.1002/jnr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsimpelas D, Felmeden U, Mann WJ, Brieger J. Analysis of cytogenetic aberrations in sporadic vestibular schwannoma by comparative genomic hybridization. J Neurooncol. 2011;103:437–43. doi: 10.1007/s11060-010-0412-5. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D, Manent J, Couvelard A, Watilliaux A, Siena M, Chareyre F, et al. Merlin regulates transmembrane receptor accumulation and signaling at the plasma membrane in primary mouse Schwann cells and in human schwannomas. Oncogene. 2009;28:854–65. doi: 10.1038/onc.2008.427. [DOI] [PubMed] [Google Scholar]
- Le N, Nagarajan R, Wang JY, Araki T, Schmidt RE, Milbrandt J. Analysis of congenital hypomyelinating Egr2Lo/Lo nerves identifies SOX2 as an inhibitor of Schwann cell differentiation and myelination. Proc Natl Acad Sci USA. 2005;102:2596–601. doi: 10.1073/pnas.0407836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–90. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rao PK, Wen R, Song Y, Muir D, Wallace P, et al. Notch and Schwann cell transformation. Oncogene. 2004;23:1146–52. doi: 10.1038/sj.onc.1207068. [DOI] [PubMed] [Google Scholar]
- Maitra S, Kulikauskas RM, Gavilan H, Fehon RG. The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol. 2006;16:702–9. doi: 10.1016/j.cub.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Maka M, Stolt CC, Wegner M. Identification of SOX8 as a modifier gene in a mouse model of Hirschsprung disease reveals underlying molecular defect. Dev Biol. 2005;277:155–69. doi: 10.1016/j.ydbio.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Mantripragada KK, Buckley PG, Benetkiewicz M, De Bustos C, Hirvela C, Jarbo C, et al. High-resolution profiling of an 11 Mb segment of human chromosome 22 in sporadic schwannoma using array-CGH. Int J Oncol. 2003;22:615–22. [PubMed] [Google Scholar]
- McClatchey AI, Fehon RG. Merlin and the ERM proteins—regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol. 2009;19:198–206. doi: 10.1016/j.tcb.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Giovannini M. Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev. 2005;19:2265–77. doi: 10.1101/gad.1335605. [DOI] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–5. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–80. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991;112:457–67. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sherman LS, Legg J, Banine F, Isacke C, Haipek CA, et al. The NF2 tumor suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 2001;15:968–80. doi: 10.1101/gad.189601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res. 2007;67:520–7. doi: 10.1158/0008-5472.CAN-06-1608. [DOI] [PubMed] [Google Scholar]
- Mulvihill JJ, Parry DM, Sherman JL, Pikus A, Kaiser-Kupfer MI, Eldridge R. Neurofibromatosis 1 (Recklinghausen Disease) and Neurofibromatosis 2 (Bilateral Acoustic Neurofibromatosis) An Update. Ann Intern Med. 1990;113:39–52. doi: 10.7326/0003-4819-113-1-39. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30:355–68. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Zheng Y, MacCollin M, Ratner N. Temporal control of Rac in Schwann cell-axon interaction is disrupted in NF2-mutant schwannoma cells. J Neurosci. 2006;26:3390–5. doi: 10.1523/JNEUROSCI.4865-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Conference. Neurofibromatosis: conference statement. Arch Neurol. 1988;45:575–8. [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD. Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat Neurosci. 2003;6:161–7. doi: 10.1038/nn995. [DOI] [PubMed] [Google Scholar]
- Ogata T, Iijima S, Hoshikawa S, Miura T, Yamamoto S, Oda H, et al. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J Neurosci. 2004;24:6724–32. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Arthur-Farraj P, Noon LA, Woodhoo A, Lloyd AC, et al. c-Jun is a negative regulator of myelination. J Cell Biol. 2008;181:625–37. doi: 10.1083/jcb.200803013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Bhaskaran A, Droggiti A, Dickinson S, D'Antonio M, Mirsky R, et al. KROX-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death. J Cell Biol. 2004;164:385–94. doi: 10.1083/jcb.200307132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson DB, Dickinson S, Bhaskaran A, Kinsella MT, Brophy PJ, Sherman DL, et al. Regulation of the myelin gene periaxin provides evidence for KROX-20-independent myelin-related signalling in Schwann cells. Mol Cell Neurosci. 2003;23:13–27. doi: 10.1016/s1044-7431(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Parkinson DB, Dong Z, Bunting H, Whitfield J, Meier C, Marie H, et al. Transforming growth factor beta (TGFbeta) mediates schwann cell death in vitro and in vivo: examination of c-jun activation, interactions with survival signals, and the relationship of tgfbeta -mediated death to schwann cell differentiation. J Neurosci. 2001;21:8572–85. doi: 10.1523/JNEUROSCI.21-21-08572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Lebrun-Julien F, Suter U. Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci. 2012;35:123–34. doi: 10.1016/j.tins.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Reiprich S, Kriesch J, Schreiner S, Wegner M. Activation of KROX20 gene expression by SOX10 in myelinating Schwann cells. J Neurochem. 2010;112:744–54. doi: 10.1111/j.1471-4159.2009.06498.x. [DOI] [PubMed] [Google Scholar]
- Rosenbaum C, Kluwe L, Mautner VF, Friedrich RE, Muller HW, Hanemann CO. Isolation and characterization of Schwann cells from neurofibromatosis type 2 patients. Neurobiol Dis. 1998;5:55–64. doi: 10.1006/nbdi.1998.0179. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. The SOX9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. 2003;17:1677–89. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, et al. KROX-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–9. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- Utermark T, Kaempchen K, Hanemann CO. Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol. 2003;13:352–63. doi: 10.1111/j.1750-3639.2003.tb00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbuhl M, Reiprich S, Vogl MR, Bosl MR, Wegner M. Transcription factor SOX10 orchestrates activity of a neural crest-specific enhancer in the vicinity of its gene. Nucleic Acids Res. 2012;40:88–101. doi: 10.1093/nar/gkr734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(Pt 7):1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Woods R, Friedman JM, Evans DG, Baser ME, Joe H. Exploring the “two-hit hypothesis” in NF2: tests of two-hit and three-hit models of vestibular schwannoma development. Genet Epidemiol. 2003;24:265–72. doi: 10.1002/gepi.10238. [DOI] [PubMed] [Google Scholar]
- Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, et al. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–94. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Brown A, Gridley T, Lemke G. KROX-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126:1397–406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.