Abstract
Conversion of soluble α-synuclein into insoluble and fibrillar inclusions is a hallmark of Parkinson’s disease and other synucleinopathies. Accumulating evidence points towards a relationship between its generation at nerve terminals and structural synaptic pathology. Little is known about the pathogenic impact of α-synuclein conversion and deposition at nigrostriatal dopaminergic synapses in transgenic mice, mainly owing to expression limitations of the α-synuclein construct. Here, we explore whether both the rat as a model and expression of the bacterial artificial chromosome construct consisting of human full-length wild-type α-synuclein could exert dopaminergic neuropathological effects. We found that the human promoter induced a pan-neuronal expression, matching the rodent α-synuclein expression pattern, however, with prominent C-terminally truncated fragments. Ageing promoted conversion of both full-length and C-terminally truncated α-synuclein species into insolube and proteinase K-resistant fibres, with strongest accumulation in the striatum, resembling biochemical changes seen in human Parkinson’s disease. Transgenic rats develop early changes in novelty-seeking, avoidance and smell before the progressive motor deficit. Importantly, the observed pathological changes were associated with severe loss of the dopaminergic integrity, thus resembling more closely the human pathology.
Keywords: Parkinson’s disease, dopamine, animal models, alpha-synuclein, synapse function
Introduction
Human α-synuclein protein has been implicated in the pathogenesis of several neurodegenerative disorders, such as Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy, which are characterized by the presence of Lewy bodies, Lewy neurites or glial inclusions, consisting of insoluble α-synuclein fibrils (Spillantini et al., 1997, 1998). These structures are typically proteinase K resistant (Miake et al., 2002; Neumann et al., 2004) and post-translationally modified by truncation, phosphorylation, oxidation, nitrosylation or ubiquitination (recently reviewed Oueslati et al., 2010). In addition to missense mutations in families with autosomal dominant Parkinson’s disease (Polymeropoulos et al., 1997; Krüger et al., 1998; Zarranz et al., 2004), genetic studies in humans and transgenic mice have indicated that an excess of the wild-type α-synuclein protein load increases susceptibility of Parkinson’s disease. Evidence for the latter was obtained from experiments showing that transcriptional upregulation owing to gene multiplications (Singleton et al., 2003; Chartier-Harlin et al., 2004), polymorphic variations in the untranslated regions (Krüger et al., 1999; Maraganore et al. 2006; Jowaed et al., 2010; Matsumoto et al., 2010) and high immunoreactivity of α-synuclein in mice (Masliah et al., 2000; Giasson et al., 2002; Lee et al., 2002; Daher et al., 2009) correlates with the risk of developing Parkinson’s disease. It also suggests that the full α-synuclein gene locus may contribute to Parkinson’s disease pathogenesis. This is further endorsed by the observation that post-transcriptional modifications such as alternative splicing of the α-synuclein messenger RNA lead to an increase in C-terminally truncated isoforms in Lewy body disorders (Beyer et al., 2004). C-terminal truncated α-synuclein species have been detected predominantly in the core of Lewy body formations (Dufty et al., 2007; Muntane et al., 2012), implying its function as a possible ‘seed’ of protein aggregation. The topographical mapping of Lewy body pathology revealed that both the limbic and motor system brain nuclei are vulnerable and may directly correlate with Parkinson’s disease symptoms (Braak et al., 2003). However, although olfactory dysfunction, anxiety and depression are frequent symptoms of early Parkinson’s disease, correlating Lewy bodies in olfactory bulb and other limbic nuclei in Braak stage 1 is low (Sengoku et al., 2008; Ubeda-Banon et al., 2010). This observation implies that in these brain regions, generation of α-synuclein intermediates before the fibrillization process might incite the underlying neuropathology. Further, growing evidence points towards alteration of synaptic plasticity of newborn neurons in olfaction (recently reviewed by Lazarini and Lledo, 2011), and depression as α-synuclein has been shown to decrease olfactory bulb neurogenesis (Winner et al., 2004; Marxreiter et al., 2009; Lelan et al., 2011), whereas treatment with antidepressants resulted in an increased neurogenesis in α-synuclein transgenic mice (Kohl et al., 2012; Ubhi et al., 2012). However, α-synuclein transgenic mice that model mesolimbic synucleinopathy often fail to mimic in parallel nigrostriatal pathology of dopaminergic neurons, which may either relate to integration effects or promoter-restricted expression (Matsuoka et al., 2001; Giasson et al., 2002; Neumann et al., 2002; Lim et al., 2011; Nuber et al., 2011; Rieker et al. 2011) or relate to strain differences, as it was suggested that rats might be more sensitive to dopamine psychomotor stress than mice (Ralph-Williams et al., 2003; Ponnusamy et al., 2005; Ralph and Caine, 2005), thus highlighting the need for novel animal models.
To overcome limitations in promoter-restricted direction of transgene expression, i.e. to address α-synuclein-induced vulnerability in all Parkinson’s disease-relevant brain regions and to create the precondition for modifications on a transcriptional and post-transcriptional level, we generated a bacterial artificial chromosome (BAC) α-synuclein transgenic rat model carrying the full-length human wild-type SNCA locus, including all introns and exons, the upstream localized regulatory promoter sequences and parts of the 3′untranslated region. Further, usage of the BAC construct will allow us to study gene dosage underlying the neuropathology of α-synuclein multiplication disorder (PARK4) in more detail.
Detailed analyses of α-synuclein expression pattern revealed a relatively strong accumulation of insoluble full-length and C-terminal truncated α-synuclein, paralleled by the presence of proteinase K resistant fibres and inclusion body formation in aged rat brain. Increase in striatal insoluble full-length and C-terminal truncated α-synuclein re-emphasized biochemical changes seen in Parkinson’s disease brain within Braak staging. Changes in α-synuclein pattern were functionally accompanied by early changes in avoidance behaviour and smell deficit and late locomotor impairments. Underlying neuropathological analyses revealed an increase in olfactory bulb neurogenesis in young animals, a strong reduction of striatal dopamine transmission associated with a severe degeneration of dopaminergic nerve terminals and astrogliosis in aged animals. Thus, our findings suggest a high vulnerability of rat dopaminergic synapses to conversion of transgenic human α-synuclein into insoluble neurotoxic conformers.
Materials and methods
Generation of BAC transgenic rats
For the generation of transgenic rats, we used a 190-kb fused AF163864 PAC/AC097478 BAC clone (Yamakado et al., 2012). It contained the entire human SNCA sequence (GenBank AF163864), with 30-kb upstream regulatory promoter sequences and a 45-kb flanking downstream region, cloned into pBACe3.6 vector as described previously (Yamakado et al., 2012). BAC-DNA was separated from the vector by NotI and FseI restriction and purified by Agarase digestion (Thermo Scientific). Subsequently, the DNA solution was concentrated with Microcon® concentrators, followed by dialysis in injection buffer (10 mM Tris pH 7.4, 0.15 mM EDTA pH 8.0, 100 mM NaCl). The size and integrity of the purified BAC DNA fragment was analysed by pulse-field gel electrophoresis. Transgenic rats were obtained by injecting the purified BAC fragment into fertilized Sprague–Dawley oocytes at a concentration of 1.5 µg/ml. Founder animals were identified by PCR using DNA isolated from ear biopsies and primers specific for human α-synuclein promoter (SynProm-F: 5′-ccgctcgagcggtaggaccgcttgttttagac-3′, SynProm-R: 5′-tccccgcggggacctctagcctgtcgtcgaat-3′); for testing integrity of the construct, founders were also genotyped for human SNCA exon 2 (exon2F: 5′-gtaaaacgacggccagtgccccgaaagttctcattcaa-3′, exon2R: 5′-ggaaacagctatgaccatgacccatcactcatgaacaagc-3′), human SNCA exon 4 (exon4F: 5′-gtaaaacgacggccagtgctaccaccctttaatctgttg-3′, exon4R: 5′-ggaaacagctatgaccatgataacacaaaacgtacacagcc-3′) and human SNCA exon 6 (exon6F: 5′-gtaaaacgacggccagtgtgtaagtggggagccatttc-3′, exon6R: 5′-ggaaacagctatgaccatgaggatggaacatctgtcagca-3′). To distinguish between homozygous and heterozygous animals, the relative number of DNA copies was estimated by quantitative real-time PCR on a LightCycler® 2.0 (Roche) using a LightCycler® FastStart DNA MasterPLUS SYBR Green I kit (Roche) and rat tail genomic DNA. Reactions were performed in 20μl of mixture containing 10 pmol of each primer, 40 ng DNA and 1 × SYBR Green Mix (Roche). Quantitative PCR was carried out in duplicates and normalized to a reference gene (β-actin; β-actin-F: 5′-agccatgtacgtagccatcca-3′; β-actin-R: 5′-tctccggagtccatcacaatg−3′). Primer sequences to detect the copy number of the α-synuclein transgene were located in the promoter sequence (SynProm-F: 5′-ccgctcgagcggtaggaccgcttgttttagac−3′; LC-SynPromR: 5′-cctctttccacgccactatc-3′). The amplification conditions were as follows: 10 min at 95°C; 45 cycles of 20 s at 95°C, 20 s at 58°C, 20 s at 72°C; melting curve: 10 s at 95°C, 20 s at 60°C; cooling: 30 s 40°C.
All rats were kept in normal light dark cycle (12 h light/12 h dark) and had free access to food and water. All procedures used followed the guidelines by international standards for the care and use of laboratory animals and were approved by the local Animal Welfare and Ethics committee of the Country Commission Tuebingen, Germany.
Sequential extraction
Expression pattern of α-Syn was examined at 3 and 16 months of age (wild-type n = 3, synuclein n = 3). Animals were anaesthetized, decapitated and dissected brains subdivided on a chilled stage. Sequential extraction of α-synuclein was performed as described previously (Tofaris et al., 2006). Tissues were homogenized in 2.5 vol of TBS + (50 mM Tris-HCl, pH 7.4, 175 mM NaCl; 5 mM EDTA, protease inhibitor cocktails) (Calbiochem, CA) and spun for 30 min at 120 000 g. The pellet was subsequently extracted in TBS + containing 1% Triton® X-100, and TBS+, 1 M sucrose and RIPA buffer (TBS+, 1% NP-40, and 0.5% sodium deoxycholate, 0.1% SDS), and each extraction step was followed by centrifugation for 20 min at 120 000 g. The detergent-insoluble pellet was solubilized in 8 M urea/5% SDS.
Western blot analyses
For western blot analyses, 25 μg of TBS, Triton® X-100 and urea of human and rat brain protein extracts and 0.5 µg recombinant α-synuclein cleaved by calpain I were run on 4–12% Bis-Tris gels (Invitrogen, Life Technologies) and electroblotted onto nitrocellulose membranes (Millipore). For improved immunodetection of α-synuclein, membranes were fixed in 0.4% paraformaldehyde for 30 min (Lee and Kamitani, 2011). After washing in PBS, membranes were blocked for 30 min in PBS and 0.2% Tween-20 (PBST) containing 5% bovine serum albumin at room temperature and subsequently incubated with human-specific antibody 15G7 (1:20) (Kahle et al., 2000) or antibody that recognizes both rodent and human α-synuclein (BD Bioscience, clone 42; syn1) (1:1000) in PBST containing 5% bovine serum albumin overnight. After washing with PBST, membranes were probed with corresponding secondary antibodies (1:5000, American Qualex), visualized with enhanced chemiluminescence (PerkinElmer) and analysed usign the VersaDoc gel imaging system (BioRad). Proteins were normalized to β-actin (1:3000), used as a loading control. Quantification of signal intensities was performed as previously described (Nuber et al., 2008).
Fibril assembly
Purified α-synuclein was fibrillized as described previously (Herrera et al., 2008). Briefly, soluble recombinant α-synuclein (1 mg/ml) was incubated in assembly buffer (20 mM Tris, 150 mM NaCl, pH 7.4, containing 0.1% NaN3) with constant agitation (200 rpm). The reaction was stopped after 72 h, and proteins diluted with PBS to a final concentration of 10 µM and stored at −20°C. Fibrillization was monitored by thioflavin S assay, and the presence of high molecular structures was verified by western blot technique.
Dot blot analysis
Formation of α-synuclein fibrils in urea extracts was confirmed by dot blot measurements (Schleicher & Schuell Minifold-I Dot-Blot System, Whatman) with the FILA-1 antibody, which specifically detects oligomeric and insoluble α-synuclein aggregates, but not the monomeric form of α-synuclein (Lindersson et al., 2004; Paleologou et al., 2009). The procedure was followed as described by the manufacturer. Briefly, 8 µg samples of the urea fraction (see sequential protein extraction) were spotted as duplicates on a polyvinylidene difluoride membrane and subsequently blocked for 30 min with 5% bovine serum albumin in PBST and incubated with FILA-1 for 1.5 h at room temperature and then treated with horseradish peroxidase-conjugated secondary antibody for 45 min. Bands were visualized with an enhanced chemiluminescence detection kit and densitometric analysis of dots representing the α-synuclein fibrils performed using ImageQuant Software (Amersham Bioscience). Membrane was re-probed with human specific antibody (15G7, 1:20) and human + rodent anti-α-synuclein (BD biosciences, 1:1000) in PBST containing 5% bovine serum albumin overnight to confirm specificity of FILA-1 towards human α-synuclein fibrils in standard and urea extracts of transgenic rats.
Proteinase K digest
In situ detection of proteinase K-resistant, α-synuclein was performed with the proteinase K–PET blot method as previously described (Neumann et al., 2004; Freichel et al., 2007). Briefly, 7 μm paraffin sections were transferred and fixed onto a nitrocellulose membrane, digested with proteinase K (50 μg/ml, 3 h at 55°C) and stained with human specific monoclonal 15G7 anti-α-synuclein (dilution 1:20) (Kahle et al., 2000). Antibody binding was detected by an alkaline phosphatase-coupled secondary anti-rat antibody and formazan reaction using nitrotetrazolium blue/5-bromo-4-chloro-3-indolyl phosphatase P-toluidine salt. For higher power photomicrographs of proteinase K-resistant structures in aged homozygous rats, free floating vibratome sections were digested with proteinase K as described previously (Taschenberger et al., 2012). Staining was performed using antibodies against human anti-α-synuclein (1:20; 15G7) and anti-β-syn (1:1000; BD Biosciences).
Calpain digestion
Calpain digestion of recombinant α-synuclein was performed as previously described (Dufty et al., 2007). Briefly, 15 µg of recombinant α-synuclein was incubated with calpain I (2 U) in buffer containing 40 mM HEPES and 1 mM calcium at 37°C. To demonstrate fragment specificity to calpain cleavage, 4 mM calpeptin was added to calpain buffer and co-incubated for 60 min. To stop proteolysis, LDS sample buffer with 100 mM DTT (Invitrogen) was added and samples either heated at 70°C for 10 min and used for western blot analysis or stored at −20°C.
Immunohistochemistry
Rats were anaesthetized with an intraperitoneal injection of a mixture of xylazine (10 mg/kg)/ketamine (100 mg/kg) followed by intracardial perfusion with PBS and 4% of ice cold (w/v) paraformaldehyde in PBS (pH 7.4). The brain was dissected out of the skull and post-fixed in 4% paraformaldhyde for an additional 72 h at 4°C. Brains were either embedded in paraffin and cut into 7-µm sagittal sections for in situ analyses of proteinase K-resistant α-synuclein or were sagittally cut into 40-µm vibratome sections and immunostained as previously described (Nuber et al., 2008). After treatment with H202 (0.3% in PBS, 20 min) and blocking (10% normal serum; 0.1% Triton® X-100, 1 h), sections were incubated for 24 h at 4°C with anti-human α-synuclein (15G7; 1:50) or anti-rodent/human α-synuclein (syn1, BD Bioscience; 1:2000) in 10% normal serum. After washing with PBS, sections were incubated with the respective biotinylated secondary antibodies (Vector; 1:200 in PBS) and subsequently transferred in ABC solution (Vector; Vectastain Kit) (1:500 in PBS) for 1 h and visualized with 3,3′ diaminobenzidene. Double labelling was performed as previously described (Masliah et al., 2001). Briefly, sections were blocked in 10% normal serum and then incubated with anti-human α-synuclein (15G7; 1:500) overnight at 4°C, followed by detection with Tyramide Signal Amplification-Direct (Red) system (1:100 NEN Life Sciences). Thereafter, sections were incubated with rabbit anti-GFAP (1:500, Millipore) or rabbit tyrosine hydroxylase (1:200, Chemicon), followed by incubation with FITC-conjugated secondary antibodies (1:75 in PBS). Confocal microscopy was carried out as described with Axiovert 35 microscope (Zeiss) mounted on a MRC1024 laser scanning confocal microscope (Bio-Rad).
Electron microscopy
For ultrastructural analyses, vibratome sections were post-fixed with 2% glutaraldehyde/0.1% osmium tetroxide in 0.1 M sodium cacodylate buffer, embedded in epoxy and analysed on a Zeiss EM 10 electron microscope as described (Masliah et al., 2000).
Cell counting
The number of tyrosine hydroxylase-positive cells was estimated with systematic random sampling of approximately every 10th section to yield six to eight sections to encompass the full rostral-caudal extent of the substantia nigra. The respective region was outlined using a ×4 objective attached to an Olympus BX51 microscope. Tyrosine hydroxylase positive neurons of wild-type and BAC α-synuclein transgenic rats (3 months: n = 5; 18 months: n = 5 per group) were estimated using Stereo-Investigator 8.21.1 Software (MicroBrightField, Biosciences) as previously described (Jaffar et al., 2001; Overk et al., 2009). Total cell numbers were estimated according to the optical fractionator method according to West et al. (1991). To avoid counting of tyrosine hydroxylase-positive cells of the ventral tegmental area, we used a conservative approximation of the substantia nigra. Besides a possible shrinkage of sections, this likely led to the relative low cell numbers presented, which are, however, still in range when compared with previous studies (Sugama et al., 2003; Mantoan et al., 2005; Winner et al., 2011). Region definitions were based on the Paxinos and Watson (2005) rat brain atlas, and sections were analysed using a ×100 1.3 Plan Apo oil immersion objective. Average thickness was ∼40 µm, and a guard height of 5 µm was used for sampling brick depth of ∼20 µm. Counts were taken at predetermined intervals (x = 150, y = 150) and with a counting frame of 86 × 86 µm (7.396 μm2). For assessment of tyrosine hydroxylase and GFAP immunoreactivity levels in striatum of BAC α-synuclein transgenic rats and respective wild-type control subjects, sections were digitized to greyscale pictures and analysed for optical densities using ImageQuant 1.43 software (NIH). Data were corrected for tissue background staining by subtracting optical density values from fibre tracts within identical sections and presented as corrected optical density. All analyses were performed on a blinded basis.
Human samples
Control and Parkinson’s disease human brain samples (Supplementary Table 1) were supplied by the Parkinson’s UK Tissue Bank, funded by Parkinson’s UK, a charity registered in England and Wales (258197) and Scotland (SC037554), and analyses were carried out in accordance with the Ethics Committee guidelines. Tissue was sequentially extracted and 25 µg of TBS and urea extracts used for immunoblot studies as described earlier in the text.
High performance liquid chromatography
For estimation of olfactory bulb and striatal amine levels, 12-month-old synuclein transgenic (n = 7) and wild-type control rats (n = 5) were deeply anaesthetized with CO2, decapitated, and the striatum and olfactory bulb were dissected out on ice and homogenized in 0.5 M perchloric acid, centrifuged, filtered and stored at −80°C until analysis for monoamine content estimation (Pum et al. 2008). Samples containing 500 pg dihydroxybenzylamine as an internal standard were analysed by HPLC with electrochemical detection. The column was an ET 125/2, Nucleosil 120-5, C-18 reversed phase column (Macherey and Nagel). For detecting noradrenaline and serotonin (5-HT), the mobile phase consisted 75 mM NaH2PO4, 4 mM KCl, 20 µM EDTA, 1.5 mM SDS, 100 µl/l diethylamine, 12% methanol and 12% acetonitril adjusted to pH 6.0 using phosphoric acid. The electrochemical detector (Intro) was set at 500 mV versus an ISAAC reference electrode (Antec) at 30°C (Amato et al., 2011). For detection of dopamine, the mobile phase was composed of 99.5 mM chloroacetic acid, 0.53 mM SDS, 0.5 mM EDTA, 4 mM KCl, with 6% v/v acetonitrile and 0.8% v/v tetrahydrofuran, with the pH adjusted to 3.25 using 6 M NaOH solution. Quantification was performed by amperometric detection (Decade) with the potential set at +530 mV versus an ISAAC reference electrode (Antec) at 30°C (Jocham et al., 2007).
Study of olfactory bulb neurogenesis
To determine the impact of BAC α-synuclein expression on neurogenesis, 3-month-old rats (n = 7 per group) received daily intraperitoneal injections of bromodeoxyuridine (BrdU) (50 mg/kg; Sigma) for 5 consecutive days starting at 20 weeks of age and were analysed 4 weeks after the BrdU injections. Immunohistochemistry and double-labelling immunofluorescence with antibody against BrdU were performed as previously described (Winner et al., 2004).
Positron emission tomography of dopamine transporter binding
Neuroimaging of dopamine transporter ligand-binding capacity provides a measure to estimate dopamine terminal function and to assess striatal dopamine deficiency (recently reviewed by Brooks et al., 2010). To analyse striatal dopamine transporter function in vivo, four 16-month-old synuclein transgenic rats and three respective wild-type control rats were used with the dopamine transporter radioligand 11C-d-threo-methylphenidate PET imaging studies as described previously (Nuber et al., 2008). Briefly, rats were anaesthetized with 1.5% isoflurane (Vetland Anesthesia System) at a flow rate of 0.8 l/min oxygen. PET imaging studies were performed with an Inveon dedicated small-animal PET scanner (Siemens Preclinical Solutions), and data were acquired in list mode >3600 s and histogrammed into 16 time frames. The radiolabelling was performed in a PET tracer synthesizer for 11C methylations from GE Healthcare. The total synthesis time was 60 min, and specific activities of 75–140 GBq/μmol at the end of synthesis were obtained. The mean injected activity of 11C-d-threo-methylphenidate was 27.7 ± 1.5 MBq, which corresponds to a mean carrier of 650 ng. Data were acquired in full 3D mode, and images were reconstructed using filtered back projection with a matrix size of 256 × 256 and a zoom of two.
Behavioural assessment in automated cages
The circadian pattern of home cage activity was conducted with the LabMaster homecage apparatus (TSE Systems). Rats (wild-type: n = 12, synuclein: n = 12) were placed individually during the light phase with 200 g of sawdust in the LabMaster cage, and a grid of photocells automatically detected horizontal (x, y level; locomotor activity) and vertical (z-level; rearing, defined as a simultaneous break of light beam on both cage bottom and top level) activity in both light and dark phase (each 12 h in duration) during a 72 h testing period. All parameters were measured continuously and analysed with custom-made software. Horizontal activity was summed for 60 min intervals and presented as mean ± SEM of the sum (x + y) of recorded ambulatory activity.
Smell test
For testing olfactory discrimination in rats, only male rats were tested, as variations in oestrogen level may influence smell performance (Sorwell et al., 2008) and locomotor behaviour (Ogawa et al., 2003) in rodents. A smell task was assessed as described for Parkinson’s disease rat models by Prediger et al. (2006, 2009) with 3-month-old BAC α-synuclein transgenic rats (n = 8) and wild-type control rats (n = 6), and 16-month-old BAC α-synuclein transgenic rats (n = 9) and wild-type control rats (n = 5). This task was based on the preference of rodents for bedding impregnated with their own odour (familiar compartment) when compared with fresh sawdust (novel compartment). Briefly, each rat was placed into a testing arena (920 × 920 mm), which was divided into two equal compartments, one containing fresh sawdust and one containing 3-day-old bedding of the rat’s home cage. The time the animal spent in each compartment was recorded for a 5-min period.
Challenging beam walking
To assess motor performance, rats were tested in a challenging beam traversal modified from Fleming et al. (2004). Briefly, 3- and 12-month-old animals (n = 6 per group) were placed on a wide square beam (3 cm × 2 m) and a narrow square beam (2 cm × 2 m), which ended at the animal home cage; thus, animals were encouraged to move along the beam. After four assisted trials, animals able to traverse the entire length of the beam unassisted were tested on their latency to traverse the middle section (160 cm) of the beams with and without a mesh grid (2 cm squares) of corresponding width.
Statistical analysis
Phenomaster data, collected for 72 h periods, were evaluated with two-way ANOVA and post hoc Bonferroni as described previously (Nuber et al., 2011). Significant main effects were analysed further using one-way ANOVA followed by planned comparisons (post hoc Bonferroni) based on the expectation of genotype (software: Prism 6.0 software, GraphPad). The Kaplan–Meyer survival curve, a log-rank (Mantel–Cox) test was performed using Prism 6. Statistical analysis for HPLC, PET imaging, stereology and protein expression between genotypes was assessed by unpaired two-tailed t-tests. Significance was set at P < 0.05, and all values were presented as mean ± SEM.
Results
Overexpression of BAC α-synuclein leads to C-terminal truncation and conversion into insoluble, proteinase K resistant α-synuclein species, strongly enriched in nigrostriatal system
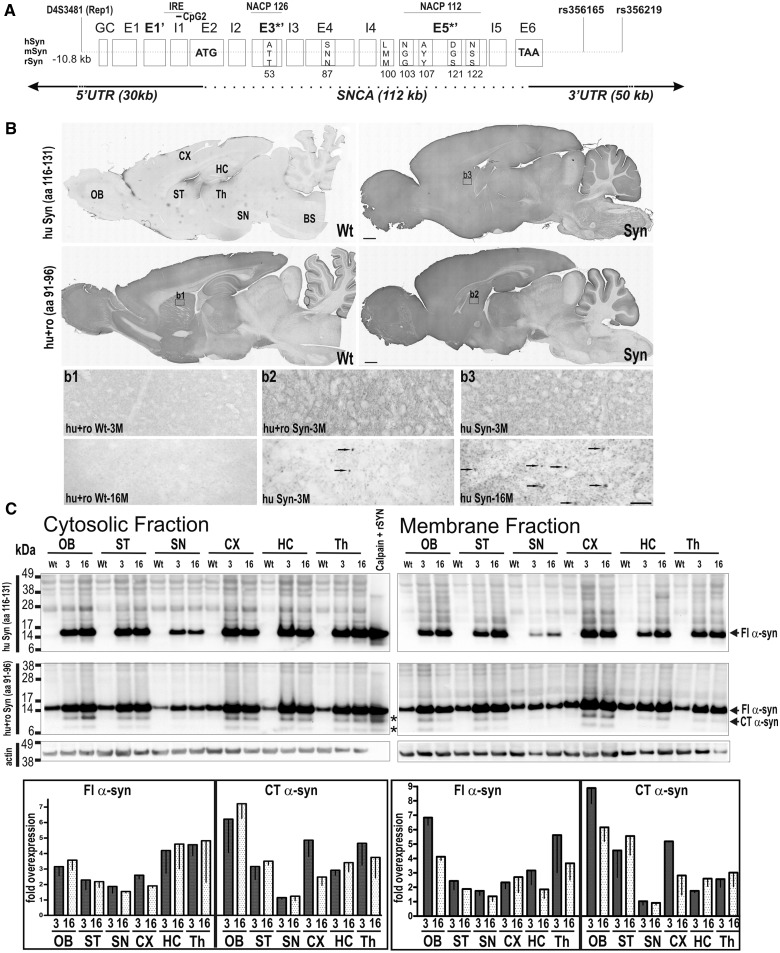
To develop a novel transgenic rat modelling characteristics of Parkinson’s disease, our strategy was to create rats overexpressing α-synuclein transgene derived from a human BAC construct, which contains the coding sequence, intronic regions and also the human regulatory promoter elements (Fig. 1A) that potentially lead to α-synuclein induced pathology in all Parkinson’s disease relevant brain regions. As the vast majority of Parkinson’s disease cases are of a sporadic nature, we used a wild-type human α-synuclein construct to inject Sprague Dawley rats. Of the 63 offspring, we identified six lines that contained all exons and introns and the flanking promoter region. One founder lacked sequences downstream of exon 4 and did not display any protein expression (data not shown). All founder rats showed germline transmission, and the resulting offspring were analysed by quantitative PCR for relative integration levels of BAC constructs. As a high α-synuclein protein load has been correlated with a pathological phenotype (Masliah et al., 2000; Giasson et al., 2002; Lee et al., 2002; Neumann et al., 2004; Daher et al., 2009; Kuo et al., 2010), we chose a founder line displaying the highest integration number of BAC DNA construct, which led to a highest transgene expression in fore- and midbrain regions and crossbred it to homozygosity (Fig. 1A). When comparing with wild-type control rats, the expression pattern of the transgenic α-synuclein expression closely resembled that of the endogenous rodent α-synuclein (Fig. 1B) but with a more dot-like distribution of the human α-synuclein in striatal nerve end terminals. With increasing age, intense α-synuclein positive deposits were detected in dilated axon terminals (Fig. 1B). To analyse the human transgenic α-synuclein expression pattern in more detail, brains of 3- and 16-month-old animals were subdivided and proteins sequentially extracted with buffers containing increasing concentrations of denaturing agents, separating them into TBS soluble (cytosolic), Triton®X (membrane) and Urea insoluble (aggregate) fractions as previously established for Parkinson’s disease models and human Parkinson’s disease as well as dementia with Lewy bodies brain (Culvenor et al., 1999; Tofaris et al., 2006; Seidel et al., 2010). Antibody for human α-synuclein (15G7; amino acid 116-131), which is specific for the C-terminus of α-synuclein protein, substantiated brain regional overexpression on immunoblots as seen by immunohistochemistry (Fig. 1B). By using an antibody specific for rodent and human α-synuclein, which binds to an epitope within the end of the NAC domain (syn1; amino acid 91–96), we additionally observed a remarkable overexpression of lower molecular weight signals in all brain regions (Fig. 1C). These fragments were not detectable with the 15G7 antibody, and thus are likely to consist of C-terminally truncated α-synuclein. Interestingly, these bands comigrated with fragments seen after partial proteolytic cleavage of recombinant α-synuclein with calpain 1, suggesting implication of calpain in intracellular protein turnover. As C-terminal truncated α-synuclein was not detected in the analysed brain regions of wild-type control rats, calpain-mediated fragmentation is likely to be specific for human α-synuclein protein. Reverse-phase chromatography was used to specify C-terminal truncated α-synuclein. Peaks were collected, and peptide masses were determined by sequencing of tryptic peptides using mass spectrometry. Overall peptides detected in the N-terminus, the NAC domain and C-terminus (to amino acid 122) of the calpain-cleaved recombinant α-synuclein were also detected in the 12 kDa slice of BAC α-synuclein rat striatum sample (Supplementary Fig. 1), further implicating calpain in proteolytic processing of human α-synuclein in transgenic rats.
Figure 1.
Human BAC transgenic α-synuclein expression matches endogenous rodent expression pattern, with additional conversion of human full-length α-synuclein (FL α-syn) into C-terminal truncated forms (CT α-syn). (A) Schematic representation of the human α-syn gene structure used for generation of BAC synuclein transgenic rats (Syn), including all introns (I1–I5) and exons (E1–E6) and additional 30-kb upstream regulatory sequences and 50-kb downstream into 3′ untranslated region. Scheme also shows genetic variants described to influence risk of Parkinson’s disease by modulating expression level (Rep1), differential binding of transcription factors (IRE = iron responsive element), alternative splicing sites (untranslated: E*, translated: E3*’, E5*’), methylation site (CpG2) or binding sites of microRNAs. Alignments are α-synuclein for human, mouse and rat (for references, see text). (B) Sagittal brain section of a homozygous rat showing α-synuclein expression in the olfactory bulb (OB), cortex (CX), striatum (ST), thalamus (Th), substantia nigra (SN), hippocampus (HC), cerebellum (CE) with lowest level in brainstem (BS), matching the endogenous α-synuclein expression pattern seen in wild-type control rats. Arrows indicate an accumulation of α-synuclein positive granules in dilated neuritic spheroids in aged homozygous rats. (C) Brains were sequentially extracted with TBS to resolve cytosolic proteins, followed by 1% Triton® X-100 to resolve membrane-associated species. Immunoblots of α-synuclein expression in subregional dissected brains substantiated expression of human α-synuclein in all brain regions in the cytosolic and membrane fraction using human α-synuclein specific antibody (15G7) with its epitope localized at the end of C-terminus (aa 116–131). Using BD antibody specific for both rodent and human transgenic α-synuclein with its epitope localized at the NAC domain (syn1; aa 91–96) detected 14-kDa full-length α-synuclein and α-synuclein species of lower kDA (∼ 8 and 12 kDa), which co-migrated with calpain1 partially digested recombinant α-synuclein (Calpain recSYN; asterisks). These fragments are not detected with C-terminal localized antibody and thus most likely consist of C-terminal truncated α-synuclein. C-terminal truncated α-synuclein was not detected in brain regions of control rats (wild-type, wt) (C) Means for independent samples (n = 3 per group) of three independent blots were quantified after normalization to β-actin and then to α-synuclein protein expression level of wild-type controls and presented as mean − SEM. Scale bars: overviews = 3 mm; upper panels = 50 µm; lower panels = 20 µm.
When compared with rodent α-synuclein of 16-month-old wild-type control rats, cytosolic human α-synuclein was overexpressed by ∼2–3-fold in all brain regions analysed, both in young and old transgenic rats (Fig. 1C). Membrane associated α-synuclein was decreased in most brain regions in aged rats, but without statistical significance. Fragmentation of soluble and membrane-associated α-synuclein was strongly specific for human α-synuclein and highest in olfactory bulb (7-fold), followed by striatum, hippocampus, cortex and thalamus (3-fold) and lowest in substantia nigra (1-fold).
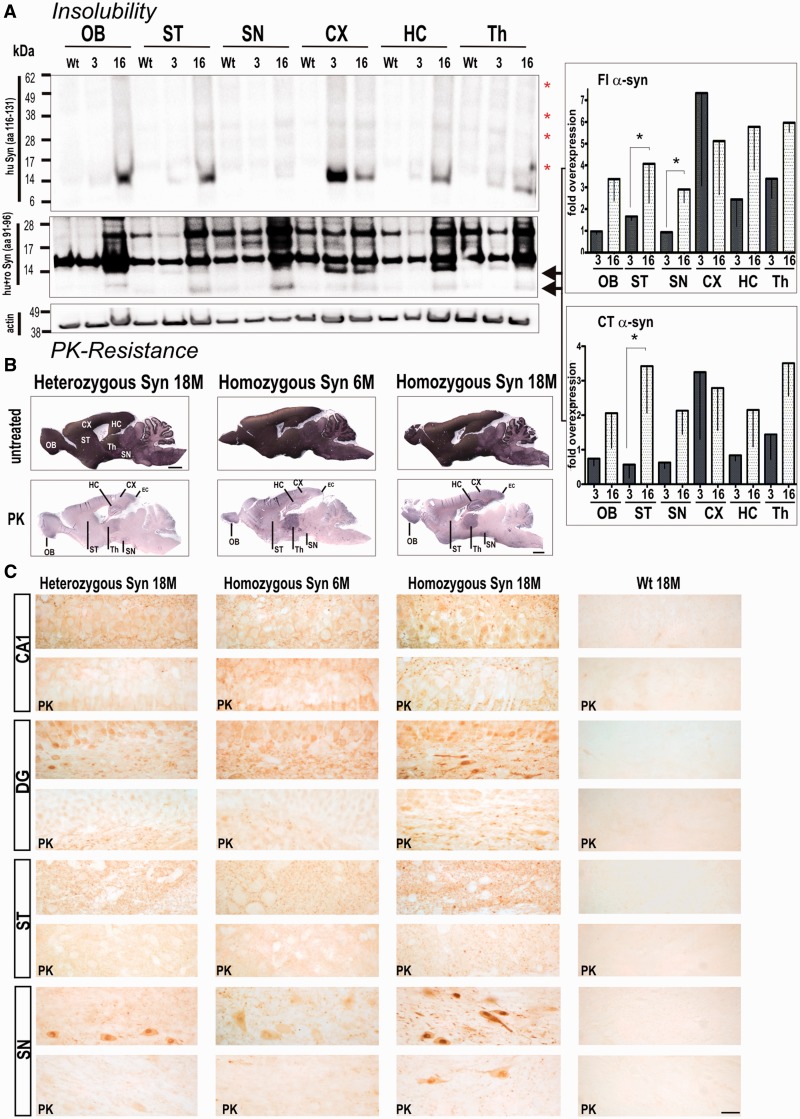
We then analysed whether overexpression of full-length and C-terminal truncated α-synuclein led to its neurotoxic conversion into insoluble aggregates. At 3 months of age, the typical smear of higher oligomeric and a prominent 14-kDa monomeric band of insoluble α-synuclein in urea-treated extracts was detected in cortex and also slightly increased in the thalamic region using an antibody against human α-synuclein protein (Fig. 2A). At 16 months, transgenic rats displayed a strong increase in insoluble monomeric and higher molecular α-synuclein in all brain regions analysed. When using an antibody against amino acid (aa) 91–96, we observed a strong signal at ∼17 kDa overlaying those of 14-kDa monomeric full-length α-synuclein (Fig. 2B). Immunoblot detection of urea fraction with an antibody specific for rodent α-synuclein did not reveal insoluble monomeric rodent α-synuclein (data not shown); thus, the detected 17-kDa signal is likely due to a non-specific cross-reaction to another protein enriched in insoluble fractions (e.g. cytoskeletal proteins). At 16 months of age, insoluble monomeric full-length α-synuclein was increased in both striatal and midbrain region, reaching statistical significance when compared with young rats (3 months synuclein transgenic: 1.7 ± 0.2; 16 months synuclein: 4.1 ± 1.8). Additionally, the insoluble C-terminal truncated α-synuclein significantly increased in striatum of 16-month-old rats (3 months synuclein: 0.6 ± 0.4; 16 months synuclein: 3.4 ± 1.4).
Figure 2.
Accumulation of proteinase K (PK) resistant and insoluble full-length (FL) α-synuclein is age- and gene-dosage dependant with insoluble full-length (Fl) α-synuclein and C-terminal truncated α-synuclein significantly enriched in striatum. (A) Brains of 3- and 16-month-old rats were sequentially extracted and the final pellet resolved in 8 M urea and 5% SDS. Analysis of α-synuclein insolubility showed a marked increased of monomeric full-length α-synuclein, and the typical high molecular smear of SDS promoted breakdown of aggregated α-synuclein species in 16-month-old transgenic rats (Syn) when using C-terminally localized 15G7 antibody (red asterisks). Consistently, detection with syn1 antibody (aa 91–96) revealed an increase in insoluble full-length α-synuclein and C-terminal truncated α-synuclein in aged animals. Syn1 antibody also detected α-synuclein unspecific bands at ∼17 and 28 kDa in both wild-type control rats and BAC synuclein transgenic rats. Band intensities were quantified, normalized to β-actin and then to control expression level and expressed as x-fold overexpression (–SEM) relative to 16-month-old wild-type control rats. Bar graphs represents means of n = 3 animals per group of three independent blots. *P < 0.05. (B) Representative images of abundant proteinase K-resistant fibres detected in several brain regions of BAC synuclein transgenic rats. Heterozygous BAC synuclein transgenic rats (18-month-old, 18 M) and 6-month-old (6 M) homozygous rats were used as additional controls to evaluate the increase of human α-synuclein positive fibres. (C) Proteinase K treatment diminished α-synuclein reactivity in both neuronal cell soma and neuropil of heterozygous and young homozygous animals, whereas proteinase K resistant α-synuclein immunoreactive granules were observed in neuropil of the CA1 and striatum and in the perinuclear region of neurons of the dentate hilus and substantia nigra. Scale bars: upper panels = 3 mm in (in situ stained overviews); lower panels = 20 µm. OB = olfactory bulb; CX = cortex; DG = dentate gyrus; HC = hippocampus; SN = substantia nigra; ST = striatum; Th = thalamus; wt = wild-type.
Biochemical data are consistent with in situ proteinase K-resistant α-synuclein profiling, showing that pathological α-synuclein fibrils formed in several brain regions of aged rats, including the thalamus, hippocampus and cortex, including the entorhinal cortex, olfactory bulb, substantia nigra and striatum. Pathological fibres were already seen in thalamus, cortex and olfactory bulb in BAC α-synuclein transgenic rats at 6 months of age (Fig. 2B), suggesting that not all protease-resistant filaments display detergent resistance. Proteinase K-resistant α-synuclein was greatly reduced in age-matched heterozygous animals (Fig. 2B), implying that both ageing and gene dosage cause the α-synuclein fibrillization process. In order to visualize cellular localization of proteinase K resistant α-synuclein aggregates in more detail, additional vibratome sections were stained with the human α-synuclein-specific antibody, digested with proteinase K as previously described (Taschenberger et al., 2012) and imaged with higher resolution light microscopy (Fig. 2C). In 18-month-old homozygous rats, proteinase K resistant granules were predominantly detected in pyramidal cell dendrites of the CA1 layer and as perinuclear aggregates in neurons of the dentate hilus and of the substantia nigra; whereas in age-matched heterozygous and young homozygous rat sections, the relatively weak α-synuclein accumulations displayed less proteinase K resistance. Additionally, proteinase K resistant structures were detected as a dot-like pattern in neuropil of striatal nerve fibres of aged homozygous rats (Fig. 2C). Minimal background staining was detected in age-matched wild-type animals (Fig. 2C), and a greatly reduced immunoreactivity was observed when digest was followed by an antibody against β-synuclein, which was used as an internal staining control (Supplementary Fig. 2).
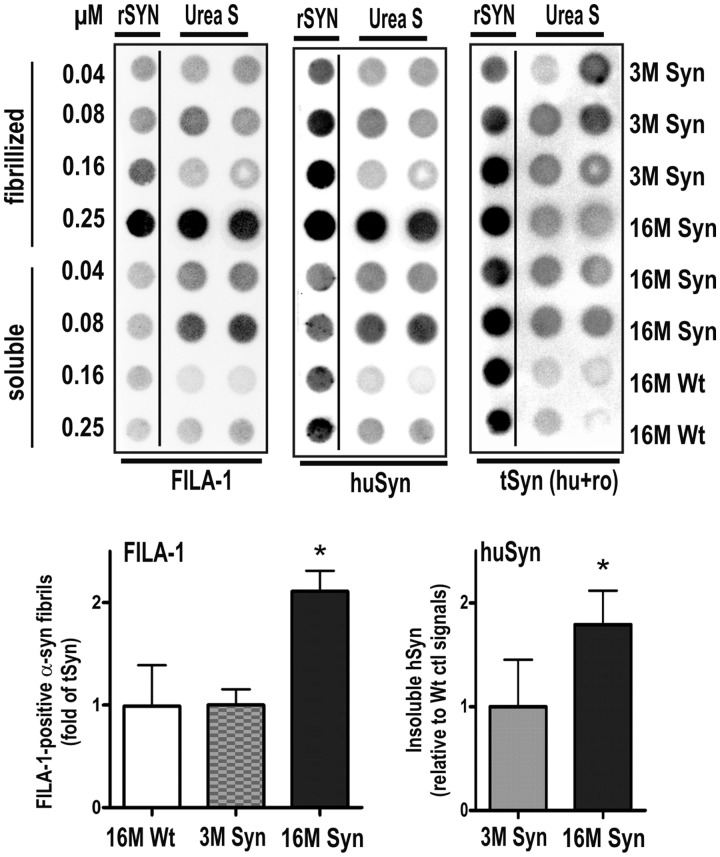
To further confirm that human BAC α-synuclein formed aggregates in transgenic rat brain, we also analysed striatum urea extracts by dot-blot assay using the FILA-1 antibody, which is specific for aggregates of misfolded α-synuclein but does not detect the soluble monomeric α-synuclein formations (Lindersson et al., 2004; Paleologou et al., 2009). Figure 3 demonstrates that there is a 1–2-fold increase in total human α-synuclein from 3 to 16 months that correlates to a similar increase in the amount of FILA-1 positive aggregates in striatal extracts of BAC α-synuclein transgenic rats. This change occurred on the background of an almost unchanged total level of α-synuclein (rat endogenous and transgenic human α-synuclein).
Figure 3.
Dot blot analysis of α-synuclein fibrils in striatal urea extracts of human BAC synuclein transgenic rats. Dot-blot analysis was performed using FILA-1 antibody specific for aggregated α-synuclein and an antibody for human α-synuclein (huSyn) to confirm its aggregation. The levels of FILA-1 signals in individual rat samples were normalized to the average total levels of α-synuclein (human + rodent α-synuclein). The levels of human α-synuclein are expressed relative to the background signals detected in wild-type control rats. Values are represented as mean + SEM. The FILA-1 antibody detected specifically fibrillized recombinant α-synuclein (rSYN) of the standard and in striatal urea extracts (Urea S) of aged transgenic (Syn) rat brain. Both FILA-1 and human α-synuclein antibodies detected an average doubling of insoluble α-synuclein aggregates at 16 month when compared with 3-month-old rats (*P < 0.05; two-tailed t-test).
Increase of insoluble full-length α-synuclein and C-terminal truncated α-synuclein in striatum resembles changes in α-synuclein pattern with increasing Braak stages of patients with Parkinson’s disease
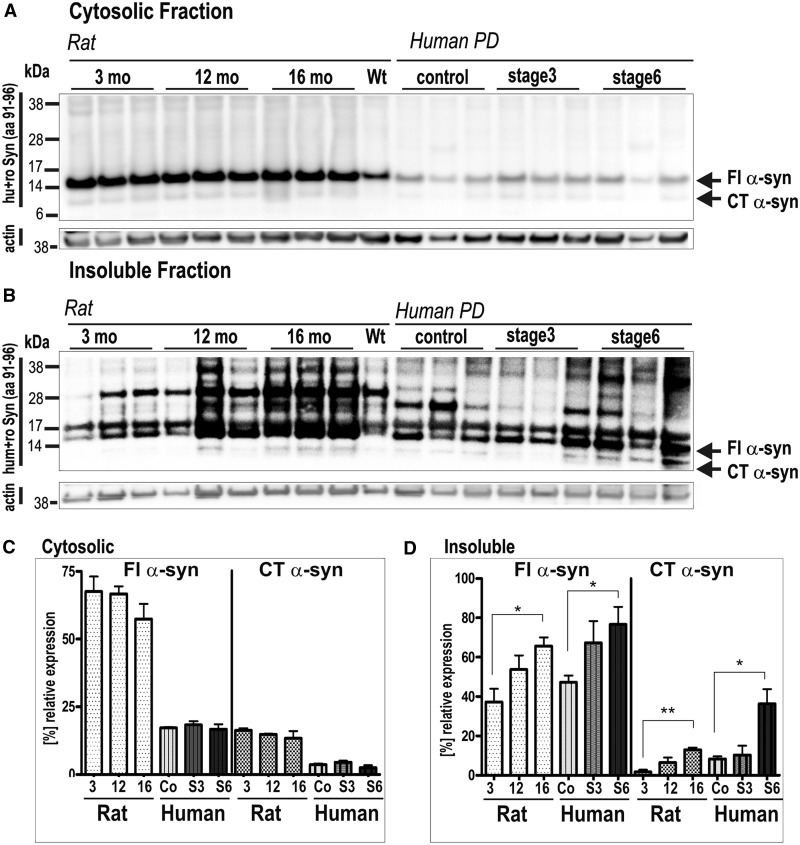
The strong accumulation of insoluble full-length and C-terminal truncated α-synuclein in striatum of aged synuclein transgenic rats prompted us to directly compare expression pattern with TBS and urea extracts of humans diagnosed with clinically and neuropathologically confirmed Parkinson’s disease of Braak stage 3 and 6 and normal aged control subjects (Fig. 4). In the TBS-soluble fraction, full-length and C-terminal truncated α-synuclein of transgenic rats resolved with equivalent migration in striatum of control subjects and patients with Parkinson’s disease. BAC α-synuclein transgenic rats displayed a 2–3-fold overexpression when compared with α-synuclein in human Parkinson’s disease brain (Fig. 4A). Neither soluble full-length α-synuclein nor soluble C-terminal truncated α-synuclein levels changed significantly within Parkinson’s disease stages or ageing of synuclein transgenic rats (Fig. 4C). Importantly, in striatum of both human and BAC α-synuclein transgenic rat, the insoluble full-length α-synuclein increased ∼2-fold with increasing Braak stage or age, respectively (synuclein: 3 months/12 months: 1.4-fold, 3 months/16 months: 1.7-fold; P = 0.03; control/stage 3: 1.5-fold, control/stage 6: 1.7-fold; P = 0.03). Additionally, the insoluble ∼12-kDa C-terminal truncated α-synuclein increased both at 16 months in rats (3 months/12 months: 3-fold; 3 months/16 months: 5.4-fold; P = 0.001) and with increasing Braak stage of human brain (control/stage 3: 1.2-fold, control/stage 6: 4-fold; P = 0.02) (Fig. 4B and D). Together, these data suggest that α-synuclein pathology in striatum of synuclein transgenic rats strongly resembles those observed at late stages in human Parkinson’s disease.
Figure 4.
Increased formations of insoluble full-length (Fl) α-synuclein and C-terminal truncated (CT) α-synuclein in striatum of human BAC synuclein transgenic rats, reflecting biochemical changes within Braak staging of Parkinson’s disease (PD) brain. (A and B) Western blots show full-length α-synuclein and C-terminal truncated α-synuclein in the cytosolic and insoluble fraction of transgenic rats at 3, 12 and 16 months (mo) of age. (C and D) Bar graph comparing the levels of α-synuclein and C-terminal truncated α-synuclein in both soluble (C) and insoluble fraction (D) of striatum of BAC synuclein transgenic rats with increasing age and of patients with increasing Braak staging (S3, S6) and healthy controls (Co) (see also Supplementary Table 1). *P < 0.05, **P < 0.01 (two-tailed t-test); wt = wild-type; S3 = stage 3; S6 = stage 6.
BAC α-synuclein expression leads to striatal dopamine depletion in transgenic rats
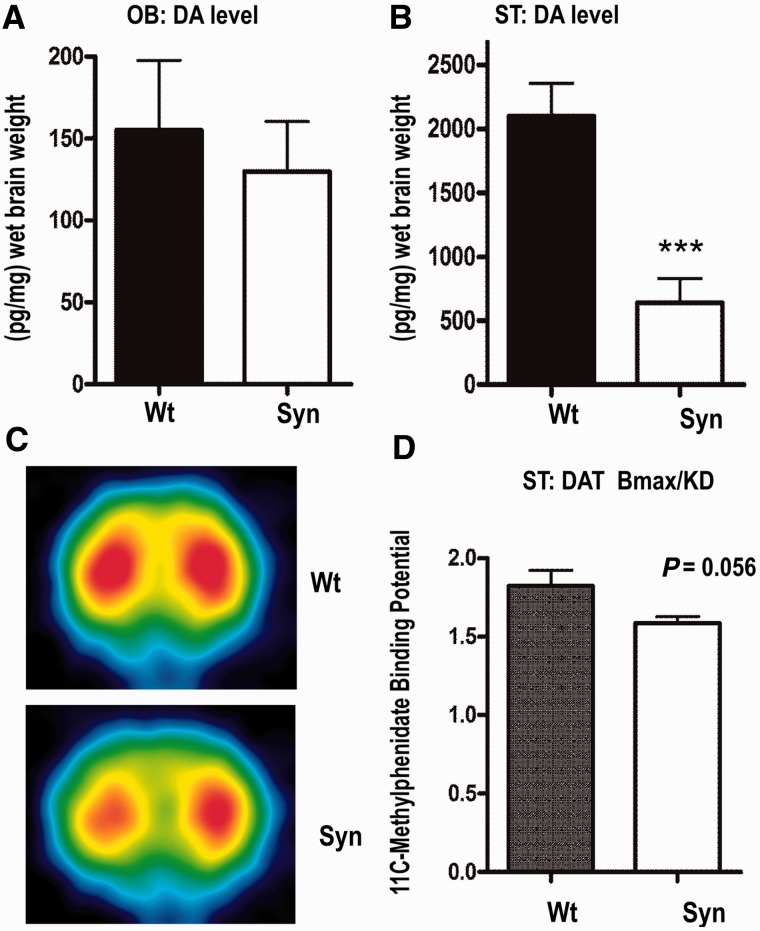
To estimate the integrity of dopaminergic nerve terminals, levels of dopamine and dopamine transporter density were evaluated using standard HPLC analysis or small animal PET, respectively (Fig. 5). We found a marked decrease of striatal dopamine (30% of wild-type level; Fig. 5B) in 12-month-old animals. In contrast, we did not detect a difference in dopamine level of the olfactory bulb (Fig. 5A). The striatal effect was specific for dopamine, as we did not find differences of noradrenalin or serotonin (Supplementary Table 2). Presynaptic dopamine dysfunction is often correlated with reduction of dopamine transporter density and its binding affinity in the striatum of human Parkinson’s disease (Antonini et al., 2001; Ribeiro et al., 2009). Thus, microPET analysis of the dopamine transporter binding potential using the specific radioligand 11C-d-threo-methylphenidate was performed to analyse dopaminergic integrity in vivo. The microPET images showed a clear-cut visualization of the striatum of transgenic rats (Fig. 5C). Subsequent calculation of striatal and cerebellar time-activity curves yielded a decrease of 13% of dopamine transporter binding potential at presynaptic terminals, bordering significance (Fig. 5D; P = 0.056).
Figure 5.
Effects of transgenic BAC synuclein expression on dopamine (DA) markers in the olfactory bulb (OB) and striatum (ST) of aged rats. (A and B) Evaluation of dopamine levels in olfactory bulb (A) and the striatum (B) revealed strong dopamine depletion in striatum of 12-month-old transgenic rats (Syn) (n = 8) when compared with wild-type (wt) controls (n = 6). (C) Example of coronal 11C-methylphenidate PET image from BAC synuclein transgenic rat and a wild-type control. (D) The average binding potential derived from time activity showed a decrease of dopamine transporter (DAT) binding in 16-month-old BAC synuclein transgenic rats (n = 4) in comparison with respective wild-type controls (n = 3).***P < 0.001 (two-tailed t-test). Error bars indicate the means + SEM.
BAC α-synuclein expression is associated with impaired novelty seeking, locomotor decline and an early smell deficit, which is paralleled with an increase of olfactory bulb neurogenesis in transgenic rats
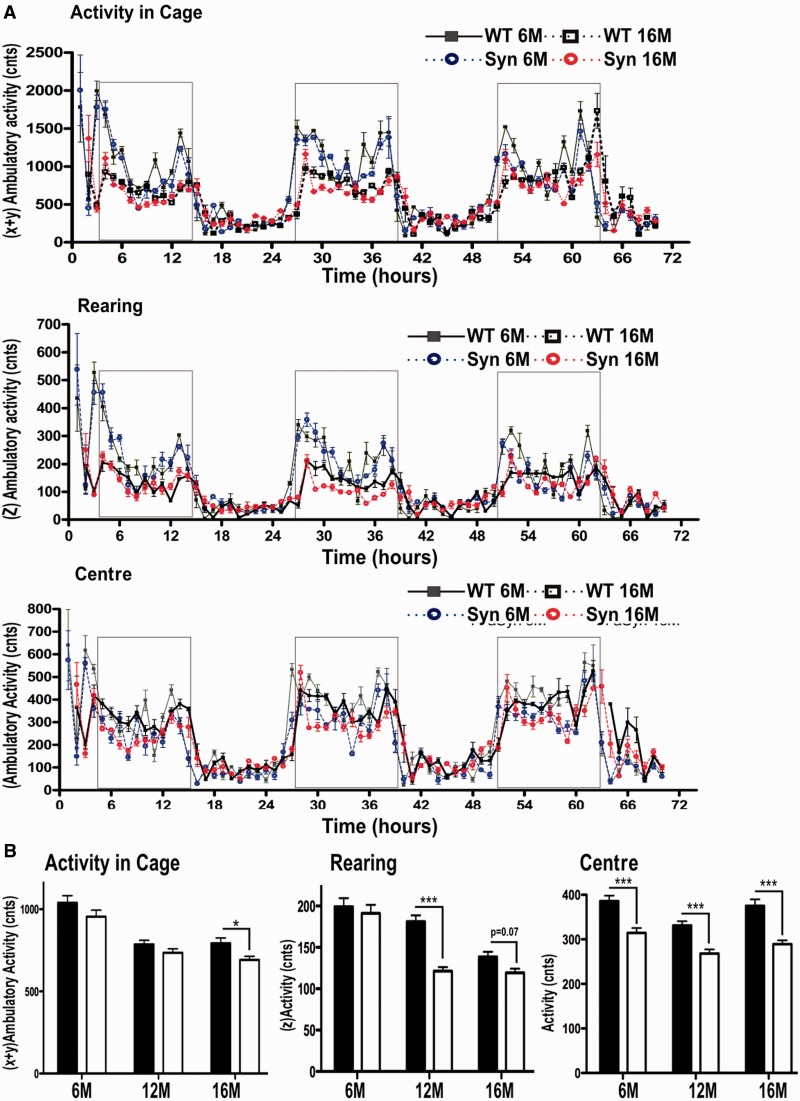
To analyse whether the age-dependent increase of insoluble and proteinase K-resistant fibrils in dopaminergic brain regions and the detected decline in dopamine function leads to a behavioural phenotype, rats were subjected for 72 h to an infrared beam break home cage system (TSE Systems) that continuously measured rodent home cage activity (Fig. 6). Previous functional studies of rat and mice suggested that striatal dopamine depletion not only reduces locomotor activity but also rearing (Salmi and Ahlenius, 2000; Summavielle et al., 2002) and anxiety-like behaviour in rat models, the latter commonly measured by avoidance of the open-spaced centre (thigmotaxis; Thiel and Schwarting, 2001; Zhang et al., 2011). Using one-way ANOVA and planned comparisons (post hoc Bonferroni) for evaluation of dark phase activities at 3, 12 or 16 months (Fig. 6A,B), we could demonstrate that BAC α-synuclein transgenic rats displayed significantly reduced dark phase ambulatory activity at 16 months (792.2 ± 32.9 wildtype; 691.8 ± 22 synuclein; P < 0.05), but not at 6 months (1039 ± 40 wild-type; 955 ± 40 synuclein) or at 12 months (786 ± 25 wild-type; 735 ± 24 synuclein). Rearing activity was already significantly decreased in 12-month-old animals (181.2 ± 8 wild-type; 121.4 ± 5 synuclein; P < 0.001) and neared significance at 16 months (139 ± 6 wild-type; 119 ± 5; P = 0.07), but no differences were detected at 6 months (199 ± 11 wild-type; 191 ± 10 synuclein). A statistically significant avoidance of cage centre was already present at 6 months (386 ± 12 wild-type, 315 ± 11 synuclein; P < 0.001) and consecutively at 12 months (332 ± 10 wild-type; 268 ± 9 synuclein; P < 0.001) and at 16 months in transgenic rats (375 ± 15 wild-type; 290 ± 9 synuclein; P < 0.001). Thus, synuclein transgenic rats show an age-dependent decrease in locomotor activity and early disturbance in novelty-seeking and avoidance behaviour, most likely related to changes in dopamine-related constitution.
Figure 6.
Early alterations in avoidance and novelty-seeking behaviour and late motor decline in BAC synuclein transgenic rats. (A) Wild-type (WT) control rats (n = 12) and BAC synuclein transgenic rats (Syn) (n = 12) were analysed for circadian activity in familiar home environment for 72 h at 6, 12 and 16 months (for clarity, data for 12 months have been omitted in the 72 h graph; two-way ANOVA, post hoc Bonferroni). (B) Evaluation of dark phase cycles showed a decrease in ambulatory activity at 16 months (16 M), decrease in rearing (z-activity) earliest at 12 months and centre avoidance from 6 months (6 M) in synuclein transgenic rats. No differences in activity were detected for resting in light phases. Data are presented as mean + SEM *P < 0.05; **P < 0.01, ***P < 0.001 (one-way ANOVA, post hoc Bonferroni).
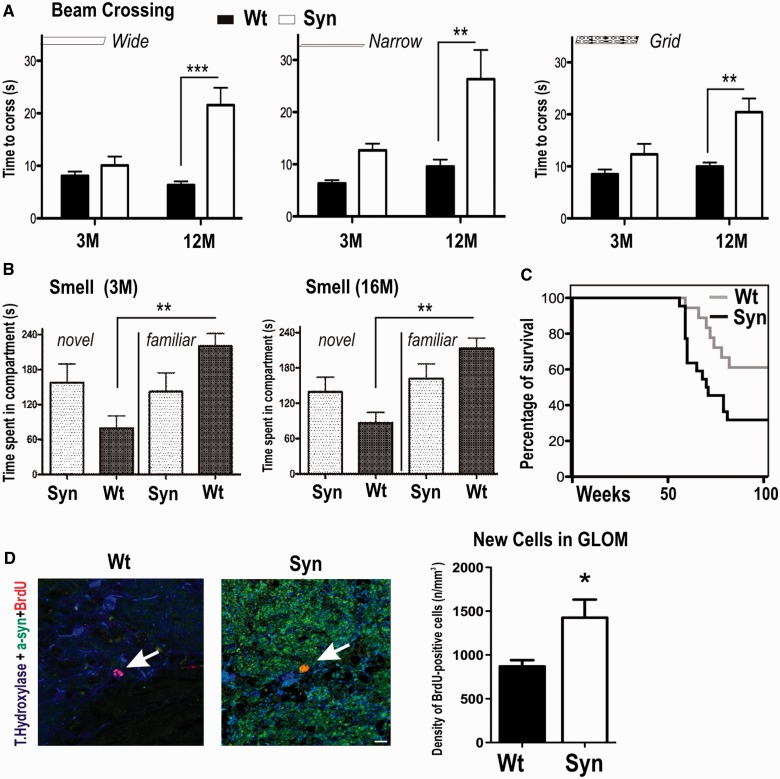
To assess whether decreased locomotor activity detected in automated cages will also lead to impairments in precise motor control, animals were tested on their ability to traverse narrow, raised wooden beams (Fig. 7A), which is known to expose locomotor impairments in 6-hydroxydopamine Parkinson’s disease rat models (Urakawa et al., 2007; Madete et al., 2011) and α-synuclein transgenic models of Parkinson’s disease (Fleming et al., 2004; Plaas et al., 2008; Sgadò et al., 2011). We used an adapted form of a challenging beam walk task; on the first test day, a wide and then a narrow wooden beam and on the second day a grid (1-cm square mesh), which challenges adjustment to a new running pattern, thus unmasking impairments that require mid- and forebrain control. At 3 months, both wild-type (8 ± 1 s) and BAC α-synuclein transgenic rats (6 ± 1 s) were able to balance on the wide beam. Traversal of the narrow beam and grid revealed subtle locomotor deficits in 3-month-old BAC α-synuclein transgenic rats, as they performed worse than control rats (narrow beam: wild-type: 6 ± 1 s; synuclein 10 ± 1 s; grid: 8.5 ± 1 s; synuclein 10 ± 1 s). In contrast, we observed an overall significantly lower performance on both beams and grid traversing in 12-month-old animals. Although wild-type rats were able to traverse the beam with only a slight increase in time when compared with 3 month-old animals, synuclein animals needed significantly more time to cross the wide beam (wild-type: 10 ± 2 s; synuclein: 22 ± 3 s; P < 0.001) and the narrow beam (wild-type: 13 ± 1 s; synuclein 26 ± 6 s; P < 0.01). This impairment of gait control also applied to performance on grid walking (wild-type: 12 ± 2 s; synuclein 20 ± 3 s; P < 0.01; two-way ANOVA, post hoc Bonferroni).
Figure 7.
Early impairment of olfactory bulb neurogenesis and odour discrimination before late impairment in precise motor control and decreased survival in BAC synuclein transgenic rats. (A) Three and 12-month-old BAC synuclein transgenic (Syn) rats were tested on their ability to transverse an elevated wooden beam or a grid. (B) Three and 16-month-old BAC synuclein transgenic males and wild-type (wt) control rats were subjected to an area containing fresh or home-cage saw dust to evaluate odour discrimination behaviour. (C) Survival curves for wild-type (n = 18) and BAC synuclein transgenic rats (n = 22) are presented. Among wild-type and BAC synuclein transgenic rats, 15 synuclein and 7 wild-type controls died with the remaining animals surviving >100 weeks, and all animals were sacrificed after 102 weeks (23 months of age). (D) Confocal image of α-synuclein, BrdU, tyrosine hydroxylase triple staining identified co-localization of human α-synuclein in BrdU-positive new dopaminergic neuron of olfactory bulb glomerular (Glom) layer BAC synuclein transgenic rats. Scale bars = 30 µm. Data are presented as mean + SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (two-way ANOVA, post hoc Bonferroni).
As the detected decrease in rearing and increase in centre avoidance may indicate functional aberrations reminiscent of early Parkinson’s disease symptoms, including anxiety and olfactory deficits, rats were also tested for their ability to discriminate odour by using a two-compartment box, containing fresh and unchanged (72 h) sawdust (Fig. 7B). This test uses the preference of rats to their own sawdust (familiar compartment) when compared with fresh sawdust (novel compartment), which has been shown to be disturbed in toxin rat models of Parkinson’s disease (Prediger et al., 2006, 2012). Most strikingly, a disruption of the olfactory discrimination was already found in young (3-month-old) BAC α-synuclein transgenic rats (novel: 158 ± 32 s; familiar: 142 ± 32 s). The pathological phenotype persisted, as it was also detected in 16-month-old rats (novel: 139 ± 25 s; familiar: 162 ± 25 s), whereas control rats were able to discriminate between both compartments and spent a significantly longer time in the familiar compartment at both tested ages (3 months: novel 79 ± 21 s; familiar 221 ± 21 s; P < 0.01; 16 months: novel 87 ± 18 s; familiar 213 ± 18 s; P = 0.001; post hoc least significance difference Bonferroni). This effect in odour discrimination seemed not to be related to locomotor deficits, as we did not detect any locomotor impairment (rearing, ambulatory activity and beam walk) in 3-month-old rats. A reduction in smell discrimination was described with an increased number of newborn neurons in the glomerular layer of the olfactory bulb of double-mutant (A30P and A53T) α-synuclein transgenic rats (Lelan et al., 2011). Thus, we quantified density of BrdU-positive cells within the olfactory bulb glomerular layer (Fig. 7D). When comparing wild-type with BAC α-synuclein transgenic rats, we found a significant increase of BrdU-positive cells (P = 0.04; two-tailed t-test), further supporting the hypothesis that a high overexpression of α-synuclein in dopaminergic neurons may lead to structural changes in the olfactory bulb contributing to smell aberrations.
While performing these tests, we noted that between the ages of 12 and 16 months, BAC α-synuclein transgenic rats developed an increased morbidity with weight loss and reduced ambulatory movement and had to be sacrificed based on animal care rules. Kaplan–Meier analysis showed a significantly reduced survival rate (P < 0.05) of BAC α-synuclein transgenic rats compared with wild-type control rats at 103 weeks of age (study end point; Fig. 7C). The median survival of BAC α-synuclein transgenic rats (70.5 weeks) correlated closely with the time point of excessive α-synuclein aggregation and progress of disease phenotype.
BAC α-synuclein expression leads to structural disruption of dopaminergic integrity
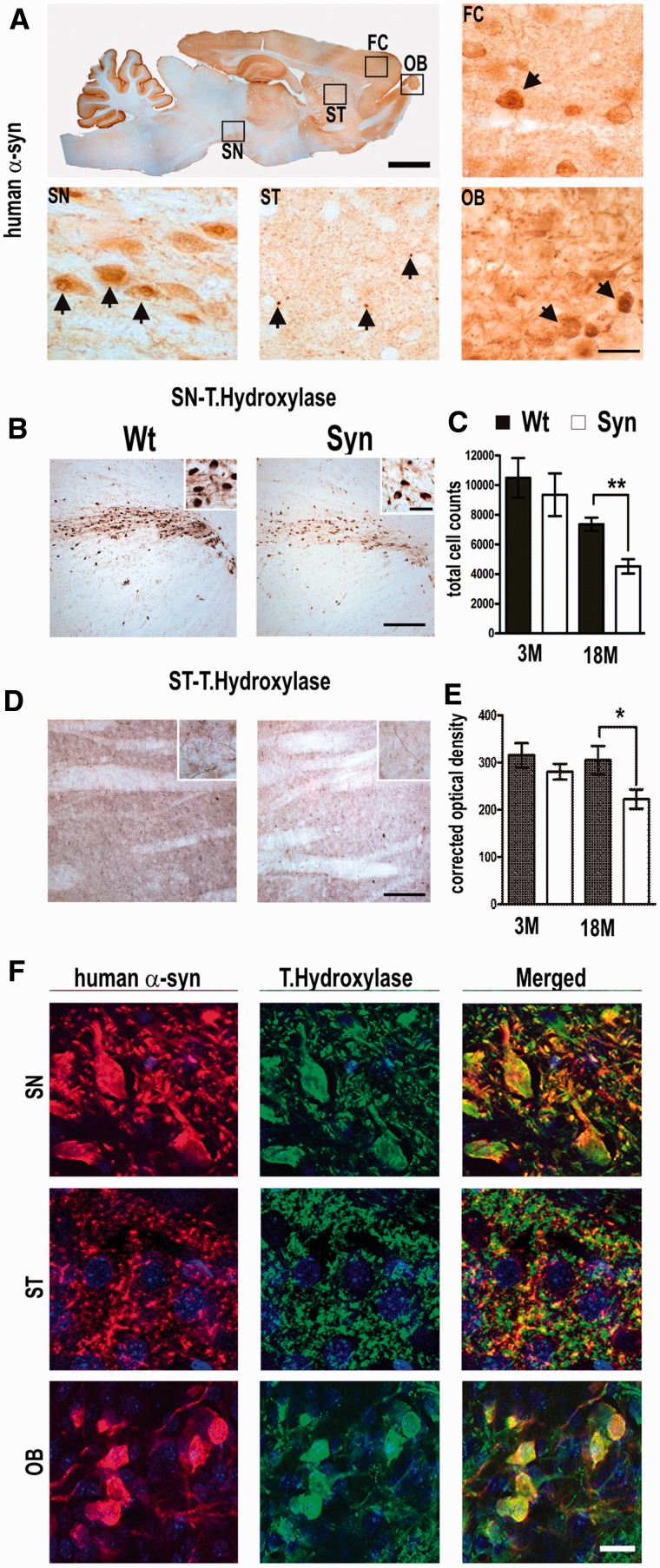
To assess whether functional deficits in smell, locomotion and avoidance behaviour, in parallel with an increase of α-synuclein insolubility and the detected decline in striatal dopamine level is caused by pathology of the dopaminergic system, we analysed dopaminergic neurons at the light microscopic and ultrastructural level. We detected a strong somatic redistribution and numerous small granular inclusions in neurons of the substantia nigra, glomerular layer of the olfactory bulb and additionally in pyramidal cells of the frontal cortex (Fig. 8A). In the striatum, we found immunoreactive spots of varying size, likely representing neuritic pathology of dopaminergic nerve fibres originating from the substantia nigra (Fig. 8A). In neurons of substantia nigra and olfactory bulb, granular inclusions were also found scattered in the perikarya. Confocal microscopy showed a substantial overlap in colocalization of α-synuclein with tyrosine hydroxylase in dopaminergic cell soma of periglomerular neurons of the olfactory bulb and importantly of nigral neurons and nigrostriatal axon terminals (Fig. 8F). As α-synuclein immunoreactivity resembled a granular dot-like pattern, likely representing pathogenic aggregation in dopaminergic cells, we estimated the number of dopaminergic neurons and dopaminergic fibres. Although we detected an age-dependent reduction of tyrosine hydroxylase-positive cells in wild-type rats as previously described (Sanchez et al., 2008), we observed an additional reduction (39 ± 6%) of total nigral dopaminergic neurons in BAC α-synuclein transgenic rats (4522 ± 486) when compared with age-matched control rats (7368 ± 450) (Fig. 8B, stereological quantification: Fig. 8C; P < 0.01). To further evaluate the integrity of the dopaminergic innervations, we quantified tyrosine hydroxylase-positive fibers in the striatum (Fig. 8D). When compared with control rats, the number of stained fibres was significantly reduced (28 ± 5%) and thus reflects a synaptic dysfunction of the nigrostriatal system (Fig. 8E; P < 0.05). We therefore stained sections with GFAP to identify astrogliosis, as it is a common cellular response surrounding axonal or synaptic pathology in brains of synuclein mice (Lee et al., 2002; Neumann et al., 2002; Cabin et al., 2005; Tofaris et al., 2006; Lim et al., 2011; Nuber et al., 2011). GFAP staining in the striatum showed that granular α-synuclein deposition was accompanied by a significant reactive astrogliosis (Supplementary Fig. 3), which may contribute to the observed dopaminergic phenotype.
Figure 8.
BAC synuclein overexpression in dopaminergic brain regions leads to aggregation and reduction of dopaminergic markers. (A) Sagittal sections stained for human α-synuclein of a representative 18-month-old BAC synuclein transgenic rat. Boxed areas indicate areas shown in lower panels. Immunoreactivity with relative high somatic redistribution in periglomerular cells of the olfactory bulb (OB), and frontal cortex (FC) (arrows). Staining also showed numerous α-synuclein positive puncta, and perinuclear aggregates of varying size, strongly accumulating in dystrophic nerve fibres of the striatum (ST) (arrows) and cell soma of nigral cells (SN). (B) Representative figure displaying tyrosine hydroxylase immunoreactivity in wild-type (wt) and BAC synuclein transgenic rats; a strong reduction of tyrosine hydroxylase immunoreactivity was observed in the dopaminergic cells of substantia nigra (B) and also in the fibres of the striatum of BAC synuclein transgenic rats (D) when compared with wild-type rats. Quantitative analysis obtained using either Stereoinvestigator or ImageJ software (NIH) of tyrosine hydroxylase immunoreactivity showed reduction in dopaminergic neurons (C) and nerve fibres (E), suggesting strong affection of dopaminergic integrity. (F) Representative confocal immunofluorescence images of α-synuclein of periglomerular cells of olfactory bulb, nigral neurons of substantia nigra and striatum nerve fibres. Nuclei were stained with DAPI. Co-staining demonstrated a granular aggregation pattern of α-synuclein (arrows) in tyrosine hydroxylase positive dopaminergic neuronal somata of olfactory and nigral neurons and in dopaminergic nerve fibres of striatum. *P < 0.05; **P < 0.01 (two-tailed t-test). Error bars indicate the ±SEM. Scale bars: A = 3 mm (upper panel) and 30 µm (lower panels), B and D = 60 µm and 20 µm (insets), F = 10 µm.
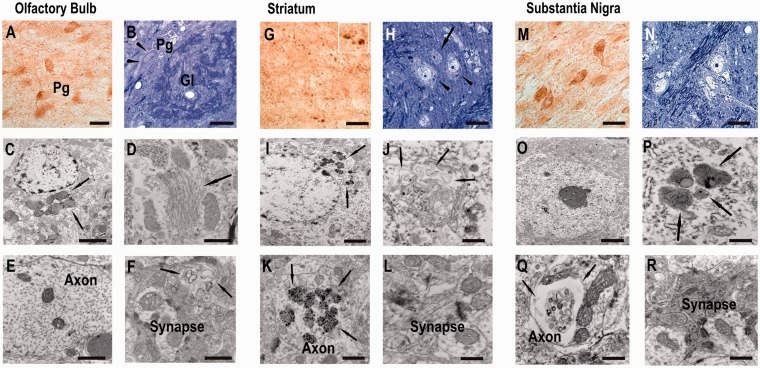
Previous studies showed a high propensity of insoluble and C-terminal truncated α-synuclein to assemble into pathogenic fibrils and nucleate fibrillization process (Giasson et al., 2001; Murray et al., 2003; Mishizen-Eberz et al., 2005). We next analysed the ultrastructure of dot-like α-synuclein immunoreactivity by transmission electron microscopy in aged rats (Fig. 9).
Figure 9.
Ultrastructural analyses revealed axonal and synaptic pathology and dark-cell degeneration in BAC synuclein transgenic rats. Semi-thin sections showed numerous α-synuclein immunopositive periglomerular (Pg), dopaminergic neurons in (A) glomerular layer (Gl) of olfactory bulb and (M) substantia nigra pars compacta surrounded by nerve fibres, presenting dot-like immunoreactive deposits as also prominently detected in numerous dilated spheroids of the striatum (G). Adjacent toluidine blue-counterstained semi-thin sections displayed shrunken dark degenerated neurons as depicted in B (arrow head) and H (arrow), filled with cytoplasmic dark blue granular deposits (arrowheads in B and H). Higher magnification revealed characteristic features of dark cell neurodegeneration with a condensed cytoplasm (C, I and O), which were found to harbour accumulated lysosomes, lipid droplets, dark organelles (arrows C, I and P) and swollen endoplasmatic reticulum (D and J). Single dilated unmyelinated nerve fibres in the glomerular core and dorsal striatum and substantia nigra showed detachment of dark axoplasm containing numerous electron dense inclusions (E; arrows in C, E, K and Q) and electron dense synaptic terminal with accumulated empty vesicles (L and R, arrows in F). Scale bars: A, G, M = 15 µm; B, H, N = 10 µm; C, I, O = 3 µm; D, J, P, F, L, R = 1 µm; E, K, Q = 5 µm. Pg = •••.
Human α-synuclein staining of olfactory bulb semi-thin sections substantiated immunoreactive dot-like structures within the glomerular core surrounded by positively stained juxtaglomerular cells (Fig. 9A). According to their localization and size, these neurons were dopaminergic periglomerular cells. Higher magnification revealed numerous small inclusion body formations in the somatodendritic compartment (Fig. 9A and B). Electron microscopy analysis of this area showed that several of the periglomerular cells were filled up with condensed organelles with a varying degree of degeneration and increased accumulation of lysosomes (Fig. 9C). These degenerated neurons showed multilaminar structures consisting of swollen endoplasmic reticulum (Fig. 9D). Interestingly, degeneration was also occasionally seen in surrounding fibres, displaying axonal dilatation (Fig. 9E) and a strong increase of empty vesicles at synapses (Fig. 9F).
Stained semithin sections of striatum showed abnormal α-synuclein immunopositive granules in neuropil as displayed in the higher magnification inset and in cell soma of medium spiny neurons (Fig. 9G). Toluidin-blue staining further detected pyknotic cells scattered between unaffected neurons (Fig. 9H). These degenerated neurons presented various accumulations of dense-core bodies (Fig. 9I), swelling of the endomembrane system and numerous multilaminar and multivesicular structures (Fig. 9J). Degenerating axons harboured electron-dense deposits (Fig. 9K) as also seen occasionally at synaptic contacts with dendritic spines (Fig. 9L).
Midbrain dopaminergic neurons showed somatodendritic and nuclear redistribution of immunoreactivity surrounded by immunopositive fibres (Fig. 9M), consisting of myelinated and unmyelinated neuritic processes (Fig. 9N). Neurons occasionally displayed slightly collapsed nuclear envelope surrounded by darkened cytoplasmic structures (Fig. 9O). Higher magnification identified further accumulation of electron dense lysosomal structures and lipid-like droplets (Fig. 9P) and axonal pathology resembling those seen in striatum (Fig. 9Q). Here also, electron-dense material accumulated at synaptic contacts (Fig. 9R).
These results suggest that overexpression of human BAC α-synuclein led to pathological alterations at synapses and axons of dopaminergic neurons in olfactory bulb and in the nigrostriatal system.
Discussion
Several α-synuclein transgenic mice (recently reviewed by Magen and Chesselet, 2010) and rats, expressing transgene (Lelan et al., 2011) or viral injected α-synuclein mainly in dopaminergic regions (Kirik et al., 2002; Lo Bianco et al., 2004; Yamada et al., 2004; Chung et al., 2009) have been generated to provide the opportunity to elucidate mechanisms underlying human synucleinopathies. The overexpression level of α-synuclein protein was correlated to its toxic gain-of-function, as comparative studies indicated that an increased total copy number of transgene induced an early phenotype (Daher et al., 2009), when comparing homozygous with heterozygous animals (Neumann et al., 2002; Gomez-Isla et al., 2003; Daher et al., 2009) or the phenotype of rats with high and low viral α-synuclein titre, accordingly (Koprich et al., 2011). In human Parkinson’s disease, the triplication of the SNCA locus (Singleton et al., 2003) correlates with a higher level of α-synuclein RNA and protein (Farrer et al., 2004; Miller et al., 2004) and might be associated with an early-onset and a rapid progressive dopaminergic phenotype (Sekine et al., 2010). In animal mouse brain, the detected high levels of α-synuclein led to conversion into insolubility and/or small inclusion formations in vivo (Masliah et al., 2000; Giasson et al., 2002; Kahle et al., 2001; Neumann et al., 2002; Gomez-Isla et al., 2003; Lim et al., 2010). By demonstrating strong and age-related increase of insoluble full-length α-synuclein in dopaminergic synapse-rich striatum of BAC α-synuclein transgenic rats comparable with different severity of human Parkinson’s disease, we provide evidence that the conversion and deposition of α-synuclein is related to pathology of nerve terminals in transgenic animals in vivo and might be concomitant with the observed increase in astrogliosis (Supplementary Fig. 3). Additionally, we detected a strong increase in insoluble C-terminal truncated α-synuclein in the striatum within aged synuclein transgenic rats reflecting biochemical data gained of striatum of patients with Parkinson’s disease with increasing Braak stages (Fig. 4). Previous studies have demonstrated that insoluble C-terminal truncated α-synuclein mediates the pathogenic conversion of additional soluble α-synuclein into pathogenic fibrils (Mishizen-Eberz et al., 2005); its accumulation in the core of Lewy bodies (Dufty et al., 2007; Muntane et al., 2012) may further hint to its role as a seed in the formation of α-synuclein aggregates. However, although we detected a relatively high load of C-terminal truncated α-synuclein in the striatum, it was not selectively detected before the increase of insoluble full-length α-synuclein in 12-month-old rats when compared with 16-month-old rats. Although the underlying mechanisms leading to generation and increase of C-terminal truncated α-synuclein require further investigation, it is apparent that its selective presence in transgenic animals, its synergistic increase with full-length α-synuclein in the insoluble fraction of the nigrostriatal system and its presence in late stage Parkinson’s disease brain indicate its role in disease pathogenesis. Small α-synuclein deposits are known to accumulate at pathogenetically alterated axons and/or synapses (Lee et al., 2002; Steidl et al., 2003; Masliah et al., 2005; Nuber et al., 2008; Lim et al., 2011); thus, it may be suggested that the detected endoplasmic reticulum pathology, increase of lysosomal structures in combination with the presence of empty vesicles, indicates vulnerability of synapses owing to malfunctioning of sorted protein synthesis. Our biochemical, structural and behavioural data further indicate that the nigrostriatal dopaminergic system of synuclein transgenic rats is strongly affected, which would be in contrast to several other pan-neuronal-expressing synuclein transgenic mouse models, showing neither gross synucleinopathy nor differences in dopaminergic innervations (Giasson et al., 2002; Lee et al., 2002; Rockenstein et al., 2002; Gomez-Isla et al., 2003). Additionally, dopaminergic neuropathology of tyrosine hydroxylase-directed expression of human α-synuclein in transgenic mice was more related either owing to embryonic alterations (Wakamatsu et al., 2008), artificial constructs, e.g. C-terminal truncation on α-synuclein null background (Tofaris et al., 2006), double-mutations (Riechfield et al., 2002) or additional toxic triggers of the dopamine pathway (Thiruchelvam et al., 2004).
In contrast, α-synuclein overexpression in dopaminergic cells induced by injection of viral vectors in vivo or in cell culture point to its function as a mediator of dopamine synthesis, storage and release (Murphy et al., 2000; Lotharius et al., 2002; Baptista et al., 2003; Larsen et al., 2006). By using several approaches to determine the impact of α-synucleinopathy on dopaminergic integrity in our BAC α-synuclein transgenic rats, we showed that reduction of dopamine levels ∼70% exceeded by far the extent of loss of dopaminergic innervations and cell numbers. A recent study by Lundblad et al. (2012) defined distinct stages of impairments that correspond to a progressive Parkinson’s disease phenotype by usage of an adeno-associated virus α-synuclein rat model. Authors reported that a 2–3-fold overexpression of human wild-type α-synuclein led to a marked (50%) reduction of dopamine uptake 10 days after injection, followed by a 70–80% reduction in dopamine release at 3 weeks. Importantly, these changes were observed, without major cell loss or gross behavioural abnormalities as reported previously (Kirik et al., 2002). Dopamine transporter immunoreactivity was maintained in the surviving dopamine innervations at all tested time points, suggesting an impaired function rather than an actual loss of the dopamine transporter protein. Indeed, post-mortem data of patients with Parkinson’s disease indicate that the loss of dopamine in the caudate nucleus at onset of motor symptoms ranges between 70 and 80%, whereas dopaminergic cells in the substantia nigra display only a moderate reduction of ∼30% (Riederer and Wuketich, 1976; Fearnley and Lees, 1991; Ma et al., 1997; Cheng et al., 2010).
Although we observed a strong reduction of striatal dopamine levels and numbers of tyrosine hydroxylase-positive stained cells and fibres in our BAC α-synuclein transgenic rats, striatal dopamine transporter uptake was not markedly reduced. Possible explanations for the observed discrepancy might be as follows: (i) higher dopamine transporter levels are directly associated with lower dopamine turnover and synaptic dopamine concentrations, and therefore dopamine transporter availability may underlie compensatory mechanisms to maintain dopamine levels as seen in patients with Parkinson’s disease (Lee et al., 2000; Sossi et al., 2007) and 6-hydroxydopamine treated rats (Finkelstein et al., 2000; Stanic et al., 2003); (ii) by using brain lysates for high-performance liquid chromatography analyses, we measured total dopamine levels, including extracellular dopamine and vesicular dopamine, and thus intrinsic factors may further contribute to the reduction of specific dopamine markers, as α-synuclein has been described to directly inhibit tyrosine hydroxylase activity and dopamine synthesis (Perez et al., 2002) and to further modify dopamine transporter availability at the plasma membrane as a normative function (Wersinger et al., 2003, 2005); or (iii) longitudinal PET studies detected a significantly different dopamine transporter tracer binding between the anterior and posterior putamen owing to different stages of dopamine denervation in striatal subregions of human patients with Parkinson’s disease (Nandhagopal et al., 2009, 2011). Such a gradient may also occur in BAC α-synuclein transgenic rats and escape imaging owing to limitations in spatial resolution of small animal PET scanners.
Recently, in BAC α-synuclein transgenic mice harbouring the identical construct as used in our rats, hyperlocomotion was observed with an increase of dopamine transporter intensity and a preserved tyrosine hydroxylase and neurotransmitter content, without manifesting with α-synuclein aggregation in striatal synaptosomes (Yamakado et al., 2012). In contrast, in BAC α-synuclein transgenic rats, dopaminergic neurons developed α-synuclein aggregation, axonal dystrophy and displayed reduced dopamine level (Figs 4, 5 and 9). Rats seem to be more vulnerable to α-synucleinopathy (Kirik et al., 2002; Lo Bianco et al., 2004; Recchia et al., 2008), which may relate to strain differences in dopaminergic signalling. This was evidenced by experiments showing that rats are more sensitive to D2 receptor agonist stimulation than commonly used C57Bl6 mice, which may be owing to a higher receptor density (Ralph and Caine, 2005). Additionally, high dopamine concentrations stimulate D2 receptor-related intracellular signalling cascades, increasing calcium currents (Trantham-Davidson et al., 2004), which may lead to an increase of calpain-mediated cleavage of α-synuclein, explaining its co-migration with calpain-cleaved recombinant α-synuclein on immunoblots (Fig. 1, Supplementary Fig. 1). However, as the viral incorporation into adult neurons in adeno-associated virus-α-synuclein transgenic rats leads to a time- and site-specific expression, these rats are limited to model progressive changes in Parkinson’s disease brain. In BAC α-synuclein transgenic rats, we detected behavioural differences reminiscent of progressive Parkinson’s disease, with an early alteration of olfaction already seen in 3-month-old animals. Deficits in odour discrimination have been reported with an increase of newborn neurons in the glomerular layer of the olfactory bulb in rats expressing double-mutant (A30P and A53T) α-synuclein under control of the tyrosine hydroxylase promoter (Lelan et al., 2011). We consistently observed an increase of new neurons and an impaired sense of smell in our transgenic rats; it might be speculated that an increase of growth factors, such as BDNF, production of which has been shown to be increased by dopamine receptor-associated intracellular calcium activation in rat striatal neurons (Hasbi et al., 2009) may promote proliferation and thus density of BrdU-positive neurons in the glomerular core layer of our transgenic rats. Detection of early avoidance behaviour may point towards implication of limbic brain areas critically associated with emotional behaviour, such as the hippocampus and the frontal cortex (Smith et al., 1995; Calabrese et al., 2009) demonstrating affection of additional brain regions, which requires further investigation. In contrast to Lelan et al. (2011), we additionally detected pathology of the dopaminergic nerve terminals accompanied by motor impairments already starting with subtle deficits at 3 months of age, getting more prominent by 12 months of age. Thus, our data suggest that a high expression level and usage of the BAC construct generated an advanced model to study the relationship of pathogenetically converted α-synuclein in neurological decline.
In summary, we generated a BAC α-synuclein transgenic rat developing misfolding and modification of human α-synuclein and a behavioural phenotype recapitulating some changes seen in early and late Parkinson’s disease stages. Its severe impact on dopaminergic nerve terminal degeneration is concomitant with accumulating evidence correlating α-synuclein aggregation to synaptic pathology in disease progression of Parkinson’s disease and related disorders, and will open up research for understanding the precise role of α-synuclein in dopamine synaptic function and for discovering novel therapeutical strategies.
Funding
European Community (Ratstream, EU FP6-037846) and MEFOPA (241791) (to O.R.), and in part by fortune (F.15.13141) (to S.N.). S.N. is a fellow of the German Parkinson’s Society. NIH grants (AG 18440, AG 022074) (to E.M.); Interdisciplinary Centre for Clinical Research (IZKF) at the FAU Erlangen-Nuernberg (Germany), the Bavarian State Ministry of Sciences, Research and the Arts, ForNeuroCell (Germany), the German Ministry for Education and Science (BMBF grant 01GN0979) and the Albert Raps Foundation (Kulmbach, Germany) (to J.W.). Human brain samples were supplied by the Parkinson's UK Tissue Bank, funded by Parkinson's UK, a charity registered in England and Wales (258197) and Scotland (SC037554).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are grateful to T. Appl, H. Nguyen and J. Ehrisman for set-up of the LabMaster System. They thank M. Ghassemian for MassSpec analysis (Biomolecular/Proteomics Mass Spectrometry Facility; Department of Chemistry and Biochemistry, UCSD, USA) and C. Wurst and M. Münch for excellent technical assistance and P. Bauer, P. Kahle, L. Mucke and S. Roy for fruitful discussions.
Glossary
Abbreviations
- BAC
bacterial artificial chromosome
- BrdU
bromodeoxyuridine
- DAB
diaminobenzidine
- DTT
dithiothreitol
- GFAP
glial fibrillary acidic protein
- HPLC
high pressure liquid chromatography
- LDS
lithium dodecyl sulfate
- NAC
non-amyloid component
- 6-OHDA
6-hydroxydopamine
- PCR
polymerase chain reaction
- RIPA
radio-immunoprecipitation assay
- SDS
sodium dodecyl sulfate
- SNCA
alpha-synuclein gene
- TBS
Tris-buffered saline
- TX
triton X-100
References
- Amato D, Natesan S, Yavich L, Kapur S, Müller CP. Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. Int J Neuropsychopharmacol. 2011;14:1327–39. doi: 10.1017/S1461145711000010. [DOI] [PubMed] [Google Scholar]
- Antonini A, Moresco RM, Gobbo C, De Notaris R, Panzacchi A, Barone P, et al. The status of dopamine nerve terminals in Parkinson's disease and essential tremor: a PET study with the tracer [11-C]FE-CIT. Neurol Sci. 2001;22:47–8. doi: 10.1007/s100720170040. [DOI] [PubMed] [Google Scholar]
- Baptista MJ, O'Farrell C, Daya S, Ahmad R, Miller DW, Hardy J, Farrer MJ, Cookson MR. Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J Neurochem. 2003;85:957–68. doi: 10.1046/j.1471-4159.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- Beyer K, Lao JI, Carrato C, Mate JL, López D, Ferrer I, et al. Differential expression of alpha-synuclein isoforms in dementia with Lewy bodies. Neuropathol Appl Neurobiol. 2004;30:601–7. doi: 10.1111/j.1365-2990.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brooks DJ. Imaging dopamine transporters in Parkinson's disease. Biomark Med. 2010;4:651–60. doi: 10.2217/bmm.10.86. [DOI] [PubMed] [Google Scholar]
- Cabin DE, Gispert-Sanchez S, Murphy D, Auburger G, Myers RR, Nussbaum RL. Exacerbated synucleinopathy in mice expressing A53T SNCA on a Snca null background. Neurobiol Aging. 2005;26:25–35. doi: 10.1016/j.neurobiolaging.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Racagni G, Riva MA. Neuronal plasticity: a link between stress and mood disorders. Psychoneuroendocrinology. 2009;34:S208–16. doi: 10.1016/j.psyneuen.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364:1167–9. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715–25. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–73. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor JG, McLean CA, Cutt S, Campbell BC, Maher F, Jäkälä P, et al. Non-Abeta component of Alzheimer's disease amyloid (NAC) revisited. NAC and alpha-synuclein are not associated with Abeta amyloid. Am J Pathol. 1999;155:1173–81. doi: 10.1016/s0002-9440(10)65220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher JP, Ying M, Banerjee R, McDonald RS, Hahn MD, Yang L, et al. Conditional transgenic mice expressing C-terminally truncated human alpha-synuclein (alphaSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol Neurodegener. 2009;4:34. doi: 10.1186/1750-1326-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufty BM, Warner LR, Hou ST, Jiang SX, Gomez-Isla T, Leenhouts KM, et al. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol. 2007;170:1725–38. doi: 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, et al. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–9. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114:2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK. Axonal sprouting following lesions of the rat substantia nigra. Neuroscience. 2000;97:99–112. doi: 10.1016/s0306-4522(00)00009-9. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, et al. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24:9434–40. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel C, Neumann M, Ballard T, Müller V, Woolley M, Ozmen L, et al. Age-dependent cognitive decline and amygdala pathology in alpha-synuclein transgenic mice. Neurobiol Aging. 2007;28:1421–35. doi: 10.1016/j.neurobiolaging.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–33. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Murray IV, Trojanowski JQ, Lee VM. A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–6. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Irizarry MC, Mariash A, Cheung B, Soto O, Schrump S, et al. Motor dysfunction and gliosis with preserved dopaminergic markers in human alpha-synuclein A30P transgenic mice. Neurobiol Aging. 2003;24:245–58. doi: 10.1016/s0197-4580(02)00091-x. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Fan T, Alijaniaram M, Nguyen T, Perreault ML, O'Dowd BF, et al. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc Natl Acad Sci USA. 2009;106:21377–82. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera FE, Chesi A, Paleologou KE, Schmid A, Munoz A, Vendruscolo M, et al. Inhibition of alpha-synuclein fibrillization by dopamine is mediated by interactions with five C-terminal residues and with E83 in the NAC region. PLoS One. 2008;3:e3394. doi: 10.1371/journal.pone.0003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffar S, Counts SE, Ma SY, Dadko E, Gordon MN, Morgan D, et al. Neuropathology of mice carrying mutant APP(swe) and/or PS1(M146L) transgenes: alterations in the p75(NTR) cholinergic basal forebrain septohippocampal pathway. Exp Neurol. 2001;170:227–43. doi: 10.1006/exnr.2001.7710. [DOI] [PubMed] [Google Scholar]
- Jocham G, Lauber AC, Müller CP, Huston JP, De Souza Silva MA. Neurokinin 3 receptor activation potentiates the psychomotor and nucleus accumbens dopamine response to cocaine, but not its place conditioning effects. Eur J Neurosci. 2007;25:2457–72. doi: 10.1111/j.1460-9568.2007.05491.x. [DOI] [PubMed] [Google Scholar]
- Jowaed A, Schmitt I, Kaut O, Wüllner U. Methylation regulates alpha-synuclein expression and is decreased in Parkinson's disease patients' brains. J Neurosci. 2010;30:6355–9. doi: 10.1523/JNEUROSCI.6119-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Muller V, Jacobsen H, Schindzielorz A, et al. Subcellular localization of wild-type and Parkinson's disease-associated mutant alpha-synuclein in human and transgenic mouse brain. J Neurosci. 2000;20:6365–73. doi: 10.1523/JNEUROSCI.20-17-06365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle PJ, Neumann M, Ozmen L, Müller V, Odoy S, Okamoto N, et al. Selective insolubility of alpha-synuclein in human Lewy body diseases is recapitulated in a transgenic mouse model. Am J Pathol. 2001;159:2215–25. doi: 10.1016/s0002-9440(10)63072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–91. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl Z, Winner B, Ubhi K, Rockenstein E, Mante M, Münch M, et al. Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci. 2012;35:10–19. doi: 10.1111/j.1460-9568.2011.07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprich JB, Johnston TH, Huot P, Reyes MG, Espinosa M, Brotchie JM. Progressive neurodegeneration or endogenous compensation in an animal model of Parkinson's disease produced by decreasing doses of alpha-synuclein. PLoS One. 2011;6:e17698. doi: 10.1371/journal.pone.0017698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel N, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–8. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Krüger R, Vieira-Saecker AM, Kuhn W, Berg D, Müller T, Kühnl N, et al. Increased susceptibility to sporadic Parkinson's disease by a certain combined alpha-synuclein/apolipoprotein E genotype. Ann Neurol. 1999;45:611–7. doi: 10.1002/1531-8249(199905)45:5<611::aid-ana9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19:1633–50. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–22. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Lledo PM. Is adult neurogenesis essential for olfaction? Trends Neurosci. 2011;34:20–30. doi: 10.1016/j.tins.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lee BR, Kamitani T. Improved immunodetection of endogenous α-synuclein. PLoS One. 2011;6:e23939. doi: 10.1371/journal.pone.0023939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease. Ann Neurol. 2000;47:493–503. [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, et al. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 –> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci USA. 2002;99:8968–73. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelan F, Boyer C, Thinard R, Rémy S, Usal C, Tesson L, et al. effects of human alpha-synuclein A53T-A30P mutations on SVZ and local olfactory bulb cell proliferation in a Transgenic rat model of Parkinson disease. Parkinsons Dis. 2011:987084. doi: 10.4061/2011/987084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, et al. α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci. 2011;31:10076–87. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Kehm VM, Li C, Trojanowski JQ, Lee VM. Forebrain overexpression of alpha-synuclein leads to early postnatal hippocampal neuron loss and synaptic disruption. Exp Neurol. 2010;221:86–97. doi: 10.1016/j.expneurol.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindersson E, Beedholm R, Højrup P, Moos T, Gai W, Hendil KB, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–34. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci USA. 2004;101:17510–5. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Barg S, Wiekop P, Lundberg C, Raymon HK, Brundin P. Effect of mutant alpha-synuclein on dopamine homeostasis in a new human mesencephalic cell line. J Biol Chem. 2002;277:38884–94. doi: 10.1074/jbc.M205518200. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Björklund A. Impaired neurotransmission caused by overexpression of α-synuclein in nigral dopamine neurons. Proc Natl Acad Sci USA. 2012;109:3213–9. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Röyttä M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J Neurol Sci. 1997;151:83–7. doi: 10.1016/s0022-510x(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Madete JK, Klein A, Dunnett SB, Holt CA. Three-dimensional motion analysis of postural adjustments during over-ground locomotion in a rat model of Parkinson's disease. Behav Brain Res. 2011;220:119–25. doi: 10.1016/j.bbr.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Magen I, Chesselet MF. Genetic mouse models of Parkinson's disease. The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- Mantoan L, Stefanova N, Egger KE, Jellinger KA, Poewe W, Wenning GK. Failure of caspase inhibition in the double-lesion rat model of striatonigral degeneration (multiple system atrophy) Acta Neuropathol. 109:191–7. doi: 10.1007/s00401-004-0931-2. [DOI] [PubMed] [Google Scholar]
- Maraganore DM, de Andrade M, Elbaz A, Farrer MJ, Ioannidis JP, Krüger R, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. genetic epidemiology of Parkinson's disease (GEO-PD) consortium. JAMA. 2006;296:661–70. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- Marxreiter F, Nuber S, Kandasamy M, Klucken J, Aigner R, Burgmayer R, et al. Changes in adult olfactory bulb neurogenesis in mice expressing the A30P mutant form of alpha-synuclein. Eur J Neurosci. 2009;29:879–90. doi: 10.1111/j.1460-9568.2009.06641.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–68. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–9. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, et al. Beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci USA. 2001;98:12245–50. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto L, Takuma H, Tamaoka A, Kurisaki H, Date H, Tsuji S, et al. CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson's disease. PLoS One. 2010;5:e15522. doi: 10.1371/journal.pone.0015522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, et al. Lack of nigral pathology in transgenic mice expressing human alpha-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277:19213–19. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Miller DW, Hague SM, Clarimon J, Baptista M, Gwinn-Hardy K, Cookson MR, et al. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–8. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- Mishizen-Eberz AJ, Norris EH, Giasson BI, Hodara R, Ischiropoulos H, Lee VM, et al. Cleavage of alpha-synuclein by calpain: potential role in degradation of fibrillized and nitrated species of alpha-synuclein. Biochemistry. 2005;44:7818–29. doi: 10.1021/bi047846q. [DOI] [PubMed] [Google Scholar]
- Muntané G, Ferrer I, Martinez-Vicente M. α-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience. 2012;200:106–19. doi: 10.1016/j.neuroscience.2011.10.042. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–20. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IV, Giasson BI, Quinn SM, Koppaka V, Axelsen PH, Ischiropoulos H, et al. Role of alpha-synuclein carboxy-terminus on fibril formation in vitro. Biochemistry. 2003;42:8530–40. doi: 10.1021/bi027363r. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, Lee CS, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain. 2009;132:2970–9. doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, McKenzie J, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain. 2011;134:3290–8. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kahle PJ, Giasson BI, Ozmen L, Borroni E, Spooren W, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–39. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Müller V, Kretzschmar HA, Haass C, Kahle PJ. Regional distribution of proteinase K-resistant alpha-synuclein correlates with Lewy body disease stage. J Neuropathol Exp Neurol. 2004;63:1225–35. doi: 10.1093/jnen/63.12.1225. [DOI] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Arias-Carrión O, Koch L, Kohl Z, Schneider J, et al. Olfactory neuron-specific expression of A30P alpha-synuclein exacerbates dopamine deficiency and hyperactivity in a novel conditional model of early Parkinson's disease stages. Neurobiol Dis. 2011;44:192–204. doi: 10.1016/j.nbd.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Nuber S, Petrasch-Parwez E, Winner B, Winkler J, von Hörsten S, Schmidt T, et al. Neurodegeneration and motor dysfunction in a conditional model of Parkinson's disease. J Neurosci. 2008;28:2471–84. doi: 10.1523/JNEUROSCI.3040-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chan J, Gustafsson JA, Korach KS, Pfaff DW. Estrogen increases locomotor activity in mice through estrogen receptor alpha: specificity for the type of activity. Endocrinology. 2003;144:230–9. doi: 10.1210/en.2002-220519. [DOI] [PubMed] [Google Scholar]
- Oueslati A, Fournier M, Lashuel HA. Role of post-translational modifications in modulating the structure, function and toxicity of alpha-synuclein: implications for Parkinson's disease pathogenesis and therapies. Prog Brain Res. 2010;183:115–45. doi: 10.1016/S0079-6123(10)83007-9. [DOI] [PubMed] [Google Scholar]
- Overk CR, Kelley CM, Mufson EJ. Brainstem Alzheimer's-like pathology in the triple transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2009;35:415–25. doi: 10.1016/j.nbd.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleologou KE, Kragh CL, Mann DM, Salem SA, Al-Shami R, Allsop D, Hassan AH, et al. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–9. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaas M, Karis A, Innos J, Rebane E, Baekelandt V, Vaarmann A, et al. Alpha-synuclein A30P point-mutation generates age-dependent nigrostriatal deficiency in mice. J Physiol Pharmacol. 2008;59:205–16. [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Medeiros R, Pandolfo P, Florio JC, Takahashi RN. The risk is in the air: Intranasal administration of MPTP to rats reproducing clinical features of Parkinson's disease. Exp Neurol. 2006;202:391–403. doi: 10.1016/j.expneurol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Rial D, Medeiros R, Figueiredo CP, Doty RL, Takahashi RN. Risk is in the air: an intranasal MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) rat model of Parkinson's disease. Ann N Y Acad Sci. 2009;1170:629–36. doi: 10.1111/j.1749-6632.2009.03885.x. [DOI] [PubMed] [Google Scholar]
- Pum M, Carey RJ, Huston JP, Müller CP. Role of medial prefrontal, entorhinal, and occipital 5-HT in cocaine-induced place preference and hyperlocomotion: evidence for multiple dissociations. Psychopharmacology. 2008;201:391–403. doi: 10.1007/s00213-008-1296-3. [DOI] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–41. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startle in mice. Neuropsychopharmacology. 2003;28:108–18. doi: 10.1038/sj.npp.1300017. [DOI] [PubMed] [Google Scholar]
- Recchia A, Rota D, Debetto P, Peroni D, Guidolin D, Negro A, et al. Generation of a alpha-synuclein-based rat model of Parkinson’s disease. Neurobiol Dis. 2008;30:8–18. doi: 10.1016/j.nbd.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Thobois S, Lohmann E, du Montcel ST, Lesage S, Pelissolo A, et al. A multitracer dopaminergic PET study of young-onset parkinsonian patients with and without parkin gene mutations. J Nucl Med. 2009;50:1244–50. doi: 10.2967/jnumed.109.063529. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Thiruchelvam MJ, Cory-Slechta DA, Wuertzer C, Gainetdinov RR, Caron MG, et al. Behavioral and neurochemical effects of wild-type and mutated human alpha-synuclein in transgenic mice. Exp Neurol. 2002;175:35–48. doi: 10.1006/exnr.2002.7882. [DOI] [PubMed] [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- Rieker C, Dev KK, Lehnhoff K, Barbieri S, Ksiazek I, Kauffmann S, et al. Neuropathology in mice expressing mouse alpha-synuclein. PLoS One. 2011;6:e24834. doi: 10.1371/journal.pone.0024834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, et al. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J Neurosci Res. 2002;68:568–78. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Salmi P, Ahlenius S. Sedative effects of the dopamine D1 receptor agonist A 68930 on rat open-field behavior. Neuroreport. 2000;11:1269–72. doi: 10.1097/00001756-200004270-00025. [DOI] [PubMed] [Google Scholar]
- Sanchez HL, Silva LB, Portiansky EL, Herenu CB, Goya RG, Zuccolilli GO. Dopaminergic mesencephalic systems and behavioral performance in very old rats. Neuroscience. 2008;154:1598–606. doi: 10.1016/j.neuroscience.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Seidel K, Schöls L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, et al. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol. 2010;67:684–9. doi: 10.1002/ana.21966. Erratum in Ann Neurol 2010; 67: 841. [DOI] [PubMed] [Google Scholar]
- Sekine T, Kagaya H, Funayama M, Li Y, Yoshino H, Tomiyama H, et al. Clinical course of the first Asian family with Parkinsonism related to SNCA triplication. Mov Disord. 2010;25:2871–75. doi: 10.1002/mds.23313. [DOI] [PubMed] [Google Scholar]
- Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, et al. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;267:1072–83. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- Sgadò P, Viaggi C, Pinna A, Marrone C, Vaglini F, Pontis S, et al. Behavioral, neurochemical, and electrophysiological changes in an early spontaneous mouse model of nigrostriatal degeneration. Neurotox Res. 2011;20:170–81. doi: 10.1007/s12640-010-9232-9. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Effects of stress on neurotrophic factor expression in the rat brain. Ann N Y Acad Sci. 1995;771:234–9. doi: 10.1111/j.1749-6632.1995.tb44684.x. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Wesson DW, Baum MJ. Sexually dimorphic enhancement by estradiol of male urinary odor detection thresholds in mice. Behav Neurosci. 2008;122:788–93. doi: 10.1037/0735-7044.122.4.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V, de la Fuente-Fernández R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ. Dopamine transporter relation to dopamine turnover in Parkinson's disease: a positron emission tomography study. Ann Neurol. 2007;62:468–74. doi: 10.1002/ana.21204. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. Alpha-Synuclein in filamentous inclusions of lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–73. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stanic D, Finkelstein DI, Bourke DW, Drago J, Horne MK. Timecourse of striatal re-innervation following lesions of dopaminergic SNpc neurons of the rat. Eur J Neurosci. 2003;18:1175–88. doi: 10.1046/j.1460-9568.2003.02800.x. [DOI] [PubMed] [Google Scholar]
- Steidl JV, Gomez-Isla T, Mariash A, Ashe KH, Boland LM. Altered short-term hippocampal synaptic plasticity in mutant alpha-synuclein transgenic mice. Neuroreport. 2003;14:219–23. doi: 10.1097/00001756-200302100-00012. [DOI] [PubMed] [Google Scholar]
- Sugama S, Cho BP, Degiorgio LA, Shimizu Y, Kim SS, Kim YS, Shin DH, et al. Temporal and sequential analysis of microglia in the substantia nigra following medial forebrain bundle axotomy in rat. Neuroscience. 2003;116:925–33. doi: 10.1016/s0306-4522(02)00572-9. [DOI] [PubMed] [Google Scholar]
- Summavielle T, Magalhães A, Castro-Vale I, de Sousa L, Tavares MA. Neonatal exposure to cocaine: altered dopamine levels in the amygdala and behavioral outcomes in the developing rat. Ann N Y Acad Sci. 2002;965:515–21. [PubMed] [Google Scholar]
- Taschenberger G, Garrido M, Tereshchenko Y, Bähr M, Zweckstetter M, Kügler S. Aggregation of α Synuclein promotes progressive in vivo neurotoxicity in adult rat dopaminergic neurons. Acta Neuropathol. 2012;123:671–83. doi: 10.1007/s00401-011-0926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Schwarting RK. Dopaminergic lateralisation in the forebrain: relations to behavioural asymmetries and anxiety in male Wistar rats. Neuropsychobiology. 2001;43:192–9. doi: 10.1159/000054889. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam MJ, Powers JM, Cory-Slechta DA, Richfield EK. Risk factors for dopaminergic neuron loss in human alpha-synuclein transgenic mice. Eur J Neurosci. 2004;19:845–54. doi: 10.1111/j.0953-816x.2004.03139.x. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Garcia Reitböck P, Humby T, Lambourne SL, O'Connell M, Ghetti B, et al. Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J Neurosci. 2006;26:3942–50. doi: 10.1523/JNEUROSCI.4965-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci. 2004;24:10652–9. doi: 10.1523/JNEUROSCI.3179-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda-Bañon I, Saiz-Sanchez D, de la Rosa-Prieto C, Argandoña-Palacios L, Garcia-Muñozguren S, et al. Alpha-synucleinopathy in the human olfactory system in Parkinson's disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol. 2010;119:723–735. doi: 10.1007/s00401-010-0687-9. [DOI] [PubMed] [Google Scholar]
- Ubhi K, Inglis C, Mante M, Patrick C, Adame A, Spencer B, et al. Fluoxetine ameliorates behavioral and neuropathological deficits in a transgenic model mouse of α-synucleinopathy. Exp Neurol. 2012;234:405–16. doi: 10.1016/j.expneurol.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, et al. Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci. 2010;5(30):6236–46. doi: 10.1523/JNEUROSCI.0567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S, Hida H, Masuda T, Misumi S, Kim TS, Nishino H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience. 2007;144:920–33. doi: 10.1016/j.neuroscience.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, et al. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–85. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Prou D, Vernier P, Sidhu A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003;17:2151–3. doi: 10.1096/fj.03-0152fje. [DOI] [PubMed] [Google Scholar]
- Wersinger C, Sidhu A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry. 2005;44:13612–24. doi: 10.1021/bi050402p. [DOI] [PubMed] [Google Scholar]
- Winner B, Jappelli R, Maji SK, Desplats PA, Boyer L, Aigner S, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–9. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winner B, Lie DC, Rockenstein E, Aigner R, Aigner L, Masliah E, et al. Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol. 2004;63:1155–66. doi: 10.1093/jnen/63.11.1155. [DOI] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J Neurochem. 2004;91:451–61. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Yamakado H, Moriwaki Y, Yamasaki N, Miyakawa T, Kurisu J, Uemura K, et al. α-Synuclein BAC transgenic mice as a model for Parkinson's disease manifested decreased anxiety-like behavior and hyperlocomotion. Neurosci Res. 2012;73:173–7. doi: 10.1016/j.neures.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- Zhang X, Egeland M, Svenningsson P. Antidepressant-like properties of sarizotan in experimental Parkinsonism. Psychopharmacology (Berl) 2011;218:621–34. doi: 10.1007/s00213-011-2356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.