Abstract
Many studies provide evidence relating lower human arsenic (As) methylation efficiency, represented by high % urinary monomethylarsonic acid (MMA(V)), with several arsenic-induced diseases, possibly due to the fact that MMA(V) serves as a proxy for MMA(III), the most toxic arsenic metabolite. Some epidemiological studies have suggested that indigenous Americans (AME) methylate As more efficiently, however data supporting this have been equivocal. The aim of this study was to characterize the association between AME ancestry and arsenic methylation efficiency using a panel of ancestry informative genetic markers to determine individual ancestry proportions in an admixed population (composed of two or more isolated ancestral populations) of 746 individuals environmentally exposed to arsenic in northwest Mexico. Total urinary As (TAs) mean and range were 170.4 and 2.3–1053.5 μg/L, while %AME mean and range were 72.4 and 23–100. Adjusted (gender, age, AS3MT 7388/M287T haplotypes, body mass index (BMI), and TAs) multiple regression model showed that higher AME ancestry is associated with lower %uMMA excretion in this population (p <0.01). The data also showed a significant interaction between BMI and gender indicating negative association between BMI and %uMMA, stronger in women than men (p <0.01). Moreover age and the AS3MT variants 7388 (intronic) and M287T (non-synonymous) were also significantly associated with As methylation efficiency (p = 0.01). This study highlights the importance of BMI and indigenous American ancestry in some of the observed variability in As methylation efficiency, underscoring the need to be considered in epidemiology studies, particularly those carried out in admixed populations.
Keywords: arsenic, arsenic metabolism, ancestry, admixture, body mass index, BMI, AS3MT
INTRODUCTION
Environmental exposure to inorganic arsenic (As) and its related adverse health effects in exposed populations have been the focus of intensive research during the past few decades (Sohel et al. 2009; Yoshida, et al. 2004; Smith et al. 1992; Cebrian et al. 1983). Chronic exposure to inorganic As via drinking water is associated with the development of several health problems including cancerous and non-cancerous diseases (Chen and Ahsan 2004; Yoshida et al. 2004; Tseng 2007a; Tsai et al., 1998; Yang 2006; Yang et al., 2008). Notwithstanding the major, population-level impact of duration-weighted As exposure, individual variability in susceptibility to As toxicity has been associated with human As methylation efficiency in many studies of environmentally exposed populations (Steinmaus, et al., 2006; Tseng 2007b; 2007a).
The metabolism of As creates metabolites that are readily excreted in urine and have different degrees of toxicity (Tseng 2007a; Styblo et al. 2002). Using arsenic (+3 oxidation state) methyltransferase (AS3MT) as the main catalytic enzyme, arsenite (As(III)) is first methylated to monomethylarsonic acid (MMA(V)) and then reduced to monomethylarsonous acid (MMA(III)) which then follows a second methylation and reduction steps in order to create dimethylarsinic acid (DMA(V)) and dimethylarsinous acid (DMA(III)) respectively (Thomas et al. 2004; 2007). Within the As metabolic scheme, MMA(III) was shown to be the most toxic of all As metabolites, being able to induce cellular malignant transformation in vitro (Wnek et al. 2010; Petrick et al. 2000; Styblo et al. 2000). In epidemiological studies, the % of urinary As excreted as MMA(V) (%uMMA) was used as a measure of As methylation efficiency (Hernandez et al., 2008; Steinmaus et al., 2010). Several studies showed that individuals with low As methylation efficiency, indicated by high %uMMA, are more susceptible to develop As-induced diseases compared to individuals with high methylation efficiency that excrete low %uMMA (Pu et al. 2007; Tseng et al. 2005; Chen et al. 2003). Noteworthy in this regard is the considerable inter-individual variability in As methylation efficiency frequently reported among As exposed populations (Vahter 2002; Tseng 2009, Gomez-Rubio et al. 2011).
The relevance of As methylation efficiency in the toxicity of As led to efforts to identify the potential factors affecting the inter-individual variability in this process. Population ancestry-related differences in the distribution of urinary As metabolites have been reported, as shown by the variation in %uMMA excretion between different global populations (Loffredo et al. 2003; Vahter et al. 1995; Vahter 2000). Studies specifically suggested greater As methylation efficiency in indigenous American (AME) populations (Vahter et al. 1995; Engstrom et al. 2010; Hopenhayn-Rich et al. 1996). Conversely, other results contradict the hypothesis of better As methylation efficiency among AME-derived populations. For example, it was reported that AME and European (EURO) populations possess similar As methylation profiles and disease incidences (Concha et al. 1998; Smith et al. 2000; Chung et al. 2002). One possible contributor to the inconsistent findings among these studies is the loss of information in applying categorical classification of ancestry in populations that may, in fact, exhibit genetic admixture.
Genetic admixture refers to mating between two or more previously isolated populations. At a genome-wide level, geographically and culturally isolated populations develop genetic variation over time that is unique to that particular population, and this population-specific genetic variation may be associated with, or causative of, important phenotypes such as disease risk and xenobiotic response (Seldin et al. 2008; Fejerman et al. 2008). For this reason, genetic admixture may be an important component of individual variability in many traits. In Mexico, admixture occurred within the last 500 years between indigenous Americans, Europeans and, to a lesser extent, Africans (Rubi-Castellanos et al. 2009). This history is reflected in contemporary genetic variation in Mexico, which represents a continuum of admixture of the previously isolated populations from America, Europe and Africa (Wang et al. 2008; Gonzalez Burchard et al. 2005). One of the advances made possible by the public effort to catalog human genetic variation has been the use of panels of unlinked genetic polymorphisms with large allele frequency differences between ancestral populations in order to provide quantitative estimates of genomic ancestral composition in admixed individuals (Shriver et al. 2003).
The present study in As-exposed Mexican populations generated an interest in whether genetically ascertained indigenous American ancestry might prove to be associated with As methylation efficiency, as suggested from some studies using categorical descriptors of ancestry.
MATERALS AND METHODS
Population description
Participants of this study were recruited between 2007 and 2009 in the area surrounding Ciudad Obregon, Sonora, Mexico. This region was selected for its previous history of moderate concentrations of arsenic in drinking water (Meza et al. 2004). Any person living in this area was considered a target for this study; inclusion criteria included using the community ground well as the main drinking water supply. Recruitment was conducted through door-to-door visits and no more than one individual per household was sampled. Participants were not excluded based on current health status, and were not medically screened for signs of As-induced diseases. From the total approached individuals 5% refused to participate, leaving a total of 808 consenting individuals (67.8% females), aged ≥ 6 years old recruited for this study. Written informed consent was obtained for all participants as approved by the Human Subjects Protection Program of the University of Arizona and the Instituto Tecnologico de Sonora (ITSON). For participants younger than 18 years of age, parental consent was obtained in addition to specific assent from the minor subject.
DNA isolation and genotyping
Buccal cells from cheek brushes were collected for DNA isolation. DNA was isolated by either the Qiagen DNeasy Blood & Tissue kit, or the Qiagen BioSprint 96 DNA Blood kit modified protocol (Gomez-Rubio et al. 2010). Polymorphisms with substantially different allele frequencies between ancestral populations, also known as ancestry informative markers (AIM), are markers widely used to quantify individual genetic ancestral proportions in admixed populations (Kosoy et al. 2009). In this study, 58 AIM were genotyped using the MassARRAY platform (Sequenom, San Diego, CA, USA) following the manufacturer’s protocol and reaction conditions (Jurinke et al. 2002; Oeth et al. 2005). Three AIM (rs723822, rs938431, and rs1471939) failed due to high rates of missing genotypes, but were successfully genotyped using Taqman (Applied Biosystems, Foster City, CA, USA) assays according to the manufacturer’s recommended protocols. Polymorphisms in the AS3MT gene, rs3740393 and rs11191439 (also known as 7388 and M287T, respectively) were genotyped using Taqman assays as previously described (Gomez-Rubio et al. 2010).
Genetic admixture analysis
The 58 AIM used in this study were selected from previous publications (Kosoy et al. 2009; Klimentidis et al. 2009). These markers were chosen because they exhibit large frequency differences among geographically separated human groups (indigenous American, European and West African). Individual West African, indigenous American, and European genetic admixture estimates were obtained by maximum likelihood estimation (Hanis et al. 1986) using the genotypes at each AIM as well as available information on the allele frequencies of these AIM in non-admixed populations (see Supplementary Material). Given allele frequencies in non-admixed populations at a locus, the probability of observing a marker genotype in each subject was computed for each locus. The logs of the individual locus probabilities at all loci were then summed. For every possible admixture proportion from 0 to 100, the probability of the observed genotype was computed. The admixture proportion that corresponded to the maximum combined probability across all loci was the one selected as the maximum likelihood estimate of ancestry for that individual. To test our hypothesis of an association between AME ancestry and As methylation efficiency, %AME ancestry (of the three tested ancestries) was used as a covariate in all models.
Quality control
All samples included in the analysis produced genotypes for at least 53 of the 58 AIM. AIM included in the analysis produced genotypes in more than 97% of the samples. Taqman genotyped SNP produced genotypes for all study subjects. Each polymorphism was repeat-genotyped in separate reactions in 10% of the samples; concordance between runs was used as a quality control measure. To provide validation of the accuracy of our AIM and analytical approach 20 samples of European origin (CEPH Utah and France) were included, as well as 19 samples of indigenous American origin (Mexico, Peru and Brazil) from the Coriell Cell repositories at the Coriell Institute for Medical Research (Camden, NJ; http://ccr.coriell.org/). Coriell sample ID and ordering information for these samples are available from the authors.
Arsenic speciation
First morning urine samples were collected for As speciation analysis. Samples were stored at − 20°C until shipment to the University of Arizona; samples were shipped at −60°C. All samples were stored at −80°C until As speciation analysis. Arsenic metabolites (As(III), As(V), MMA(V), DMA(V)) were determined in urine using high performance liquid chromatography (Agilent 1100 HPLC) with inductively couple plasma mass spectroscopy (Agilent 7500 ICP/MS) as previously described (Meza et al. 2005). Limit of detection values for As(V), As(III), MMA(V) and DMA(V) were: 0.41, 0.15, 0.12 and 0.12 μg/L respectively.
Anthropometry
Weight was measured using bathroom scales, and height was measured using stadiometers. Anthropometric measures were taken at time of urine sample collection and used to calculate current body mass index (BMI). BMI was calculated as: body weight (kg)/ (height (m))2.
Statistical analysis
A linear regression model was created in order to determine variability in arsenic methylation efficiency using %uMMA as the dependent variable, and gender, age, total urinary arsenic (TAs), BMI, AME proportion and 7388/M287T haplotypes as independent variables. Gender was included in the model using women as reference. Age, TAs, BMI and AME proportion were all included in the model as continuous variables. TAs was calculated as the sum of As(V), As(III), MMA(V) and DMA(V). TAs and %uMMA were natural log transformed in order to improve distributional characteristics and better meet linear regression assumptions. AS3MT polymorphisms were evaluated for deviation from Hardy Weinberg equilibrium using a Chi-square analysis. AS3MT polymorphisms were included in the model as haplotypes. Imputation of haplotypes and multiple linear regression analysis was performed using the HaploStats package in R (Schaid et al. 2002). This analysis evaluated subject diplotypes as the basis for quantifying the effect of each haplotype compared to the referent haplotype (in this case, haplotype 1 which was the common homozygous genotype at both polymorphic positions). Preliminary analysis of scatterplots showed a possible interaction between gender and BMI on % uMMA. To address this, an interaction term between gender and BMI was included in the regression model. Statistical significance was considered when p ≤ 0.05.
RESULTS
Of the 808 individuals recruited, 62 subjects were not included in the analysis due to missing measurements (urinary As, questionnaire, anthropometric) or due to poor DNA sample quality (> 5 failed AIM tests of the 58 test panel), resulting in 746 subjects in the analysis. Fourteen samples had As(III) below limit of detection, 50 samples had As(V) below limit of detection, while MMA(V) and DMA(V) values were uniformly above the limit of detection. Samples with As species below the limit of detection where replaced with half the detection limit. A comparison of models excluding or not excluding samples that have As metabolite below the detection level showed no difference between analyses. Characteristics of the population, stratified by gender, are shown in Table 1. Total number of women was higher than total number of men. This may have been the result of recruitment occurring during working hours in communities in which men frequently worked in local agricultural operations. In the whole population, TAs mean (range) was 170.4 μg/L (2.2–1053.5), and mean urinary As metabolites (range) of 24.3 (0.27–180.3) for InAs, 19.9 (0.26–132.7) for uMMA, and 126.2 (1.32–797.5) for uDMA. The mean age was 29 years with range between 5 and 86 years; 294 subjects were below 18 years. BMI categories were similarly distributed among the population with 21.7% of the population being underweight, 26.1% normal weight, 28.2% overweight, and 24% obese. Surprisingly, compared to men, women were of a similar height and greater body weight. This may be partially explained by the younger mean age of men in the study.
Table 1.
Characteristics of the study population stratified by gender.
| men | women | |
|---|---|---|
| Total n (%) | 240 (32.2) | 506 (67.8) |
| Age, mean (SD) | 23.3 (19) | 31 (17.7) |
| SNP genotypes | ||
| 7388 (GG/GC/CC) | 145/80/15 | 320/162/24 |
| M287T (TT/TC/CC) | 219/21/0 | 448/57/1 |
| Weight, mean* (SD) | 54.4 (24.3) | 63.6 (21.9) |
| Height, mean^ (SD) | 153.3 (19.6) | 153.1 (12.4) |
| Body mass index, n (%) | ||
| underweight (<18.4) | 87 (36.2) | 81 (16) |
| normal (18.5–24.9) | 68 (28.3) | 135 (26.7) |
| overweight (25–29.9) | 69 (28.8) | 132 (26.1) |
| obese (>30) | 16 (6.7) | 158 (31.2) |
| AME ancestry proportion, mean (SD) | 72.8 (15.4) | 73.6 (15.5) |
| Total urinary arsenic+, mean (SD) | 223.9 (170.5) | 145 (121.2) |
| InAs+, mean (SD) | 33.9 (30.1) | 19.7 (19.8) |
| uMMA+, mean (SD) | 28.2 (23.1) | 16 (15.3) |
| uDMA+, mean (SD) | 161.7 (125.1) | 109.3 (91.9) |
| %uMMA, mean (SD) | 12.5 (3.9) | 11.1 (4.3) |
SD: standard deviation. AME: Indigenous American. Age reported in years,
Weight reported in Kg,
Height reported in centimeters,
Arsenic species reported in part per billion (ppb).
Genotype frequencies of AS3MT SNP 7388 and M287T were concordant with Hardy-Weinberg equilibrium. Repeat-genotyped samples showed a 100% concordance between runs. Fifty-eight AIM were used to determine the individual genetic ancestral proportions in this population. Maximum likelihood analysis of these markers revealed that this population was predominantly of indigenous American (73.4%) and European background (22.1%), consistent with other studies in Mexico (Martinez-Fierro et al. 2009). To provide validation for our marker set, 20 Coriell repository samples of defined European ancestry and 19 of defined indigenous American ancestry were included. Sample identity was blinded throughout the ancestry analysis process. The expected ancestry was identified in each reference sample. Mean values for these reference samples were: European samples: 94% Europe, 4% America, 2% Africa, indigenous American samples: 7% Europe, 90% America, 3% Africa. The extent of African ancestry measured in our study population (4.5%) was of the same general magnitude as that measured in the Coriell reference European (2%) and indigenous American (3%) populations, and could represent true admixture or alternatively could be a result of measurement error (Shriver et al. 2003).
Distribution of gender, age and smoking status (current/former versus never) was similar between upper and lower AME quartiles while statistically significant difference was observed for BMI; individuals in the upper AME quartile showed lower BMI than individuals in the lower AME quartile (24.6 Vs. 26.2, p=0.03).
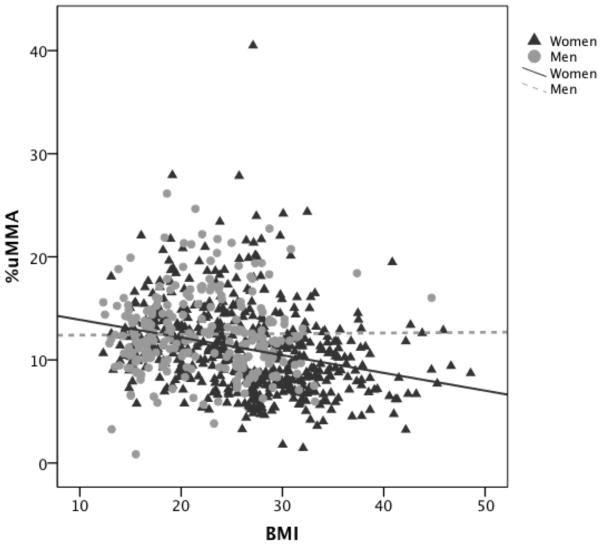
AS3MT polymorphisms 7388 and M287T have minimal linkage disequilibrium, and are thus inherited independently of one another. Analyzing two polymorphisms in the same gene in a genetic association study necessitated a haplotype-based approach as the best way to incorporate the DNA sequence at both polymorphic sites into a single variable. This was accomplished using the HaploStats software package (Schaid et al. 2002). This analytical software enabled the inclusion of the effect of the subjects’ haplotypes within a multiple linear regression model that also allowed incorporating interaction terms (Table 2). In the model, TAs was not found to be significantly associated with %uMMA. Age was positively associated with %uMMA, while AME proportion was negatively associated with %uMMA. The coefficient of the AME ancestry variable in the multivariate regression model and the same variable in a univariate model differed by only 12%, suggesting that there was not substantial confounding by the remaining variables. The effect of AS3MT genetic variation was evaluated as the haplotypes resulting from 2 SNP (7388, M287T) that have been consistently shown to be associated with %uMMA levels (Gomez-Rubio et al. 2011). Three of the 4 possible haplotypes were observed in this population. Haplotype 1 (referent, comprised of the common allele at both sites) was most commonly observed, followed by haplotype 3 (variant allele at 7388, common at M287T), with haplotype 2 (common allele at 7388, variant at M287T) as the least frequent (Table 3). Relative to individuals carrying haplotype 1, individuals with haplotype 2 have significantly higher %uMMA excretion while individuals with haplotype 3 have significantly lower %uMMA excretion. The regression model also showed a significant interaction between gender and BMI, indicating that the effect on BMI over %uMMA excretion was markedly different between men and women in this population. The negative association observed between BMI and %uMMA appeared to be stronger in women than men (Figure 2). Based on the interaction term (coefficient: 0.0179, p<0.01), intercepts and BMI coefficients were calculated separately for men and women. This demonstrated a negative trend for both genders, however the association was only statistically significant for women (Table 2). The unadjusted mean estimates of %uMMA in each of the 4 BMI categories by gender shows that mean %uMMA decreases by BMI category in women while the relationship is more complex in men (Table 4).
Table 2.
Multiple regression model using natural log transformed %uMMA as the dependent variable. Intercept and BMI coefficients were calculated separately by gender based on gender × BMI interaction term, all other coefficients are the same for both genders. Adjusted R2 for the model is 0.15
| Coefficient | Standard error | p-value | ||
|---|---|---|---|---|
| Intercept | Women | 3.1022 | 0.1804 | <0.01 |
| Men | 2.7663 | 0.1446 | <0.01 | |
|
| ||||
| BMI | Women | −0.0216 | 0.0027 | <0.01 |
| Men | −0.0037 | 0.0045 | 0.39 | |
|
| ||||
| Age, yrs | 0.0025 | 0.0009 | <0.01 | |
| Total urinary arsenic (log normalized) | 0.0158 | 0.0167 | 0.34 | |
| Haplotype 2, GC (GT as reference) | 0.1141 | 0.0420 | <0.01 | |
| Haplotype 3, CT (GT as reference) | −0.0816 | 0.0222 | <0.01 | |
| Indigenous American proportion | −0.4496 | 0.0883 | <0.01 | |
Table 3.
Haplotype frequencies in the study population.
| Haplotypes | Alleles (7388, M287T) | Frequency |
|---|---|---|
| 1 | GT | 0.74 |
| 2 | GC | 0.05 |
| 3 | CT | 0.21 |
Figure 2.
Scatter plot showing %uMMA as a function of BMI in men and women in the study population. Regression lines were fit for men and women in order to illustrate the trend of the association for each sex.
Table 4.
%uMMA in the different BMI categories by gender in the study population.
| Body mass index category
|
||||
|---|---|---|---|---|
| Underweight %uMMA (95% CI) | Normal weight %uMMA (95% CI) | Overweight %uMMA (95% CI) | Obese %uMMA (95% CI) | |
| Gender | ||||
| Women | 12.36 (11.7–13) | 12.35 (11.7–13) | 10.9 (9.9–11.8) | 9.47 (8.9–10) |
| Men | 11.84 (11.2–12.5) | 14.06 (13–15.1) | 11.73 (12.6–11.5) | 11.88 (9.7–14.1) |
DISCUSSION
The As epidemiology literature has included speculation about higher As methylation efficiency in AME populations, while studies in populations from U.S and Taiwan noted %uMMA excretion values between 20 to 30% (Warner et al. 1994; Chiou et al. 1997). Vahter et al. (1995) reported an unusually low uMMA excretion, of about 2%, in a small study population of 30 indigenous Andean women from Argentina. In addition, other studies also suggested differences in As methylation efficiency specifically between AME and EURO populations. A study performed in a population located in the Atacama Desert of Northern Chile reported that individuals of indigenous Atacameno descent had statistically significantly higher As methylation efficiency (12.6 %uMMA) than those from European descent (17.2 %uMMA) (Hopenhayn-Rich et al. 1996). Similarly, a subsequent study in a population of women from the Andean region of Argentina reported a statistically significant difference in %uMMA excretion between AME and EURO women, medians of 7% and 9% respectively (Engstrom et al. 2010). These findings, however, were not supported in an Argentinean population from a village comprised of mainly Criollo individuals (descendants of Spanish immigrants). This population had similar %uMMA excretion compared to a population recruited from a village of mainly AME individuals (2.2–3.4 and 2.1–3.6 %uMMA, respectively) (Concha et al. 1998). Another study showed that the prevalence of As skin lesions in a population of indigenous Atacameno individuals from Chile was as high as that found in other As exposed populations around the world (Smith et al. 2000). Although the As methylation profile was not discussed in this paper, a subsequent study using the same population reported a mean uMMA excretion of 14%, similar to the %uMMA excretion profile of other populations globally (Chung et al. 2002; Hall et al. 2009; Huang et al. 2009). In this study we show that genetically determined AME ancestry is associated with less %uMMA excretion. Although not the main focus of our study, %uMMA in our population was strongly, negatively correlated with uDMA/uMMA ratio, and not surprisingly AME ancestry was also statistically significantly associated with uDMA/uMMA ratio.
Individuals in this northern Mexican population varied widely in AME proportion, ranging from 23 to 100% (Figure 1), highlighting the potential loss of information in applying conventional ethnic or racial categorization to admixed populations. A negative association between AME proportion and %uMMA was observed in this study represented by a −0.4469 coefficient in the multivariate regression model, which is interpreted as a 36% decrease in %uMMA for a one unit increase in AME proportion (1 unit = 100%). Stated differently, if the %uMMA of an average 0% AME subject was 20%, an approximately 12.8 %uMMA excretion would be predicted in an average 100% AME subject. The significance and underlying cause of the association between AME ancestry and As methylation efficiency reported here needs to be interpreted with care. Associations between ancestry and a phenotype of interest may be due to ancestry-skewed genetic variation at some locus (or loci) in the genome. Exemplary in this regard is the increased warfarin sensitivity observed in patients of Asian ancestry that is substantially explained by Asian variant allele frequency at the VKORC1 gene (Limdi et al. 2010). Several intronic polymorphisms in the AS3MT gene have been consistently associated with lower excretion of %uMMA in urine (Meza et al. 2005; Schlawicke Engstrom et al. 2007). Allele frequencies in some of these AS3MT genetic variants were found to be different between AME and EURO populations. SNP 7388, which is in strong linkage disequilibrium (LD) with other AS3MT SNP, was reported to have minor allele frequency of 42% in AME subjects while a minor allele frequency of 20% was observed in EURO subjects (Meza et al. 2005; Gomez-Rubio et al. 2010). By including AS3MT haplotypes (7388/M287T) in our linear regression model, it was possible to determine that the association between AME ancestry and As methylation efficiency is not likely to result from confounding by the ancestry-skewed frequency of AS3MT variants. This suggests that if the association between %uMMA and AME ancestry is genetically based, genes other than AS3MT may be involved.
Figure 1.
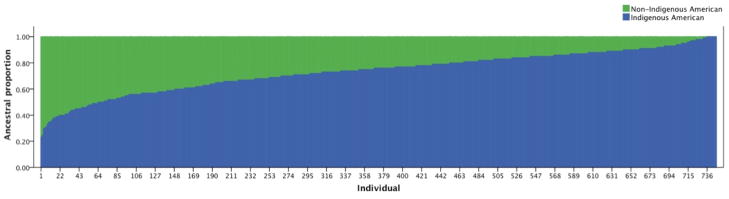
Individual ancestry proportions in the study population. Individual ancestry proportions are depicted in the Y-axis; indigenous American and non-indigenous American proportions are shown. The X-axis represents each individual in the population, sorted in increasing indigenous American ancestry proportion.
It is, however, equally important to understand that while ancestry quantification utilizes genetic testing, it simultaneously serves as a marker for a host of environmental factors that may correlate with ancestry. Cultural norms and practices, socioeconomic status, and health care availability can be different between different ancestral populations (Bhattacharyya et al. 2010; Sluyter et al. 2010). Within admixed populations differences in socioeconomic status have been reported to be associated with genetic admixture (Florez et al. 2009; Gower et al. 2003) and phenotypic characteristics such as skin color (Parra et al. 2004). Thus, one cannot exclude environmental factors, including social and cultural practices that correlate with indigenous American ancestry, as possible explanations for this association. More studies will be needed to confirm, and to dissect the effect of ancestry on arsenic metabolism.
Previously Gomez-Rubio et al. 2011 reported a statistically significant association between BMI and As methylation efficiency in three independent populations of adult women from the southwest USA and northwest Mexico, where higher BMI was associated with low %uMMA urinary excretion and high uDMA to uMMA ratio. In this study that included men and women of a broad age range (5 to 86 years old), an interaction between gender and BMI was identified for this association. In our study population, women showed a strong negative association between BMI and %uMMA while men show a less obvious relationship between the two variables (Figure 2, Table 4). After stratifying the BMI coefficients by gender, the association did not remain significant in men (Table 2). This observation, however, is not completely surprising as the distribution of BMI in this population varied by gender, with men having lower BMI than women (mean BMI of 21.8 and 26.4 respectively). In previous reports, the significance of the association between BMI and As methylation efficiency has been inconsistent from study to study. One explanation for this incongruity may be related to the lack of a representation of different BMI categories within some studies. A previous publication noted a statistically significant association between high BMI and low %uMMA in men of a European population with a median BMI of 27 (Lindberg et al. 2007). Therefore it is plausible that the lack of representation of the upper BMI scale in the men of our study population resulted in the weaker association between BMI and %uMMA in men. Future studies need to focus on a population of men that has a good representation of all BMI categories. However, it is evident that BMI plays an important role in the As methylation processes, and needs to be carefully considered in future association studies seeking to better understand the inter-individual variability in As methylation efficiency, as well as related diseases.
In this study an association was also observed between each of the AS3MT genetic variants, 7388 and M287T, and As methylation efficiency. Individuals with the minor allele in 7388 intronic polymorphism have lower %uMMA excretion, an association that has been highly replicated, though no functional explanation has been reported (Meza et al., 2005; Schlawicke Engstrom et al., 2007; Gomez-Rubio et al., 2010). Conversely, individuals with the minor allele in M287T exonic polymorphism showed significantly higher %uMMA excretion, consistent with other reports that show that change of methionine to threonine in amino acid position 287 modifies AS3MT enzymatic function altering the arsenic methylation process (Drobná et al., 2004; Valenzuela et al., 2009; Agusa et al., 2011). The multivariate regression model revealed that individuals with haplotype 2, carrying 7388 common allele and M287T minor allele, have significantly higher %uMMA excretion than individuals carrying haplotype 1 (both common alleles). Conversely, individuals with haplotype 3, carrying 7388 minor allele and M287T common allele, have significantly lower %uMMA excretion than individuals with haplotype 1 (Table 2). These findings are in agreement with prior AS3MT genetic association studies, highlighting the importance of these AS3MT genetic variants in the inter-individual variation of As methylation efficiency in human populations.
In this population, the multivariate model showed that age was positively associated with %uMMA, thus suggesting that As methylation efficiency decreases with age (Table 2). Meza et al. (2005) reported a similar association in an independently recruited population from the same region of northwest Mexico. In that study, children had statistically significantly higher As methylation efficiency, represented by a higher uDMA/uMMA, than adults. Our results are in concordance with other publications demonstrating a positive association between age and As methylation efficiency (Huang et al. 2008; Lindberg et al. 2008; Tseng et al. 2005; Agusa et al. 2009). Total gender effect cannot be evaluated from regression model due to the interaction term included, however simple %uMMA mean comparison between genders showed that in average women methylate As more efficiently than men, excreting lower proportions of uMMA, consistent with previous reports (Lindberg et al. 2008; Tseng et al. 2005; Hopenhayn-Rich et al. 1996; Huang et al. 2008). The underlying cause for the association between BMI, age, and gender with %uMMA is unknown; a possible explanation may be related to the association of these factors with AS3MT expression. Although age and gender have not been previously associated with this phenotype, changes in AS3MT expression induced by obesity in rodent models have been reported (Shockley et al., 2009).
Limitations of this study include a relative under-representation of men in the population. Variables evaluating socioeconomic, dietary or nutritional status were not recorded, limiting the ability to evaluate environmental factors that could potentially be covariate with AME ancestry. While the study had a high participation rate, no specific effort was made to randomize household selection, so we can not exclude the possibility of a selection bias. While drinking water as a source of arsenic exposure was confirmed, other potential routes of arsenic exposure were not assessed. Agriculture is a key source of employment in these communities, thus potential occupational arsenic exposure is possible. Strengths of the study included a reasonably large population size, the measurement of total urinary arsenic as a biomarker of all-source arsenic exposure, and the incorporation of genomic methodology aimed at more accurate ascertainment of ancestry.
Taken in the context of substantial evidence implicating %uMMA as an As-associated disease risk factor, our study suggests that being indigenous American, female, AS3MT 7388 variant, AS3MT M287T non-variant, and young would confer some level of risk mitigation within a homogenously exposed population. BMI may be more complex, in that increased As methylation efficiency associated with high BMI might be offset by the deleterious consequences of obesity.
A growing body of data points to the importance of understanding ancestry-related differences in human epidemiology. As the world continues to become a smaller place, accurate estimates of ancestral composition will become an essential component of many epidemiological studies. The intriguing association between ancestry and human As metabolism illustrates the potential for human genomics to stimulate more refined scientific questions in future epidemiological studies of human As exposure and associated adverse health effects.
Acknowledgments
The authors wish to acknowledge Michael Kopplin for performing the arsenic speciation analyses. P.G-R. was supported by a fellowship from the Mexican National Council for Science and Technology (CONACyT) under the UA-CONACyT partnership. This study was supported by the NIEHS Superfund Basic Research Program (ES04940) and a NIEHS Center Grant (ES006694).
References
- Agusa T, Kunito T, Minh TB, Kim Trang PT, Iwata H, Viet PH, Tanabe S. Relationship of urinary arsenic metabolites to intake estimates in residents of the Red River Delta, Vietnam. Environ Pollut. 2009;157:396–403. doi: 10.1016/j.envpol.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Agusa T, Fujihara J, Takeshita H, Iwata H. Individual variations in inorganic arsenic metabolism associated with AS3MT genetic polymorphisms. Int J Mol Sci. 2011;12:2351–2382. doi: 10.3390/ijms12042351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N, Shapiro NL, Vakharia KT. Influence of race and ethnicity on access to care among children with frequent ear infections. Otolaryngol Head Neck Surg. 2010;143:691–696. doi: 10.1016/j.otohns.2010.06.911. [DOI] [PubMed] [Google Scholar]
- Cebrian ME, Albores A, Aguilar M, Blakely E. Chronic arsenic poisoning in the north of Mexico. Human Toxicol. 1983;2:121–133. doi: 10.1177/096032718300200110. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ahsan H. Cancer burden from arsenic in drinking water in Bangladesh. Am J Public Health. 2004;94:741–744. doi: 10.2105/ajph.94.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Su HJ, Hsueh YM, Smith TJ, Ryan LM, Lee MS, Chao SC, Lee JY, Christiani DC. Arsenic methylation and skin cancer risk in southwestern Taiwan. J Occup Environ Med. 2003;45:241–248. doi: 10.1097/01.jom.0000058336.05741.e8. [DOI] [PubMed] [Google Scholar]
- Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997;386:197–207. doi: 10.1016/s1383-5742(97)00005-7. [DOI] [PubMed] [Google Scholar]
- Chung JS, Kalman DA, Moore LE, Kosnett MJ, Arroyo AP, Beeris M, Mazumder DN, Hernandez AL, Smith AH. Family correlations of arsenic methylation patterns in children and parents exposed to high concentrations of arsenic in drinking water. Environ Health Persp. 2002;110:729–733. doi: 10.1289/ehp.02110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, Nermell B, Vahter MV. Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina. Environ Health Persp. 1998;106:355–359. doi: 10.1289/ehp.98106355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobná Z, Waters SB, Walton FS, LeCluyse EL, Thomas DJ, Stýblo M. Interindividual variation in the metabolism of arsenic in cultured primary human hepatocytes. Toxicol Appl Pharmacol. 2004;201:166–177. doi: 10.1016/j.taap.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Engstrom KS, Vahter M, Lindh C, Teichert F, Singh R, Concha G, Nermell B, Farmer PB, Stromberg U, Broberg K. Low 8-oxo-7,8-dihydro-2′-deoxyguanosine levels and influence of genetic background in an Andean population exposed to high levels of arsenic. Mutat Res. 2010;683:98–105. doi: 10.1016/j.mrfmmm.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Fejerman L, John EM, Huntsman S, Beckman K, Choudhry S, Perez-Stable E, Burchard EG, Ziv E. Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res. 2008;68:9723–9728. doi: 10.1158/0008-5472.CAN-08-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez JC, Price AL, Campbell D, Riba L, Parra MV, Yu F, Duque C, Saxena R, Gallego N, Tello-Ruiz M, Franco L, Rodriguez-Torres M, Villegas A, Bedoya G, Aguilar-Salinas CA, Tusie-Luna MT, Ruiz-Linares A, Reich D. Strong association of socioeconomic status with genetic ancestry in Latinos: implications for admixture studies of type 2 diabetes. Diabetologia. 2009;52:1528–1536. doi: 10.1007/s00125-009-1412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Meza-Montenegro MM, Cantu-Soto E, Klimecki WT. 22 Genetic association between intronic variants in AS3MT and arsenic methylation efficiency is focused on a large linkage disequilibrium cluster in chromosome 10. J Appl Toxicol. 2010;30:260–270. doi: 10.1002/jat.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O’Rourke MK, Chen Z, Cantu-Soto E, Meza-Montenegro MM, Billheimer D, Lu Z, Klimecki WT. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol. 2011;252:176–182. doi: 10.1016/j.taap.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-Stable EJ, Ziv E, Risch N. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95:2161–2168. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- Hall MN, Liu X, Slavkovich V, Ilievski V, Mi Z, Alam S, Factor-Litvak P, Ahsan H, Graziano JH, Gamble MV. Influence of cobalamin on arsenic metabolism in Bangladesh. Environ Health Persp. 2009;117:1724–1729. doi: 10.1289/ehp.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Phys Anthropol. 1986;70:433–441. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- Heck JE, Gamble MV, Chen Y, Graziano JH, Slavkovich V, Parvez F, Baron JA, Howe GR, Ahsan H. Consumption of folate-related nutrients and metabolism of arsenic in Bangladesh. Am J Clin Nutr. 2007;85:1367–1374. doi: 10.1093/ajcn/85.5.1367. [DOI] [PubMed] [Google Scholar]
- Hernandez A, Xamena N, Sekaran C, Tokunaga H, Sampayo-Reyes A, Quinteros D, Creus A, Marcos R. High arsenic metabolis efficiency in AS3MT287Thr allele carriers. Pharmacogenet Genomics. 2008;18:349–355. doi: 10.1097/FPC.0b013e3282f7f46b. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Biggs ML, Smith AH, Kalman DA, Moore LE. Methylation study of a population environmentally exposed to arsenic in drinking water. Environ Health Persp. 1996;104:620–628. doi: 10.1289/ehp.96104620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh YM, Huang YL, Huang CC, Wu WL, Chen HM, Yang MH, Lue LC, Chen CJ. Urinary levels of inorganic and organic arsenic metabolites among residents in an arsenicasis-hyperendemic area in Taiwan. J Toxicol Environ Health A. 1998;54:431–444. doi: 10.1080/009841098158728. [DOI] [PubMed] [Google Scholar]
- Huang YK, Huang YL, Hsueh YM, Yang MH, Wu MM, Chen SY, Hsu LI, Chen CJ. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: a twelve-year follow-up study. Cancer Causes Control. 2008;19:829–839. doi: 10.1007/s10552-008-9146-5. [DOI] [PubMed] [Google Scholar]
- Huang YL, Hsueh YM, Huang YK, Yip PK, Yang MH, Chen CJ. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407:2608–2614. doi: 10.1016/j.scitotenv.2008.12.061. [DOI] [PubMed] [Google Scholar]
- Jurinke C, van den Boom D, Cantor CR, Koster H. The use of MassARRAY technology for high throughput genotyping. Adv Biochem Eng Biotechnol. 2002;77:57–74. doi: 10.1007/3-540-45713-5_4. [DOI] [PubMed] [Google Scholar]
- Klimentidis YC, Miller GF, Shriver MD. Genetic admixture, self-reported ethnicity, self-estimated admixture, and skin pigmentation among Hispanics and Native Americans. Am J Phys Anthropol. 2009;138:375–383. doi: 10.1002/ajpa.20945. [DOI] [PubMed] [Google Scholar]
- Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, De La Vega FM, Seldin MF. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Human Mutat. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limdi NA, Wadelius M, Cavallari L, Eriksson N, Crawford DC, Lee MT, Chen CH, Motsinger-Reif A, Sagreiya H, Liu N, Wu AH, Gage BF, Jorgensen A, Pirmohamed M, Shin JG, Suarez-Kurtz G, Kimmel SE, Johnson JA, Klein Te, Wagner MJ. Warfarin pharmacogenetics: a single VKORC1 polymorphism is predictive of dose across 3 racial groups. Blood. 2010;115:3827–3834. doi: 10.1182/blood-2009-12-255992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg AL, Ekstrom EC, Nermell B, Rahman M, Lonnerdal B, Persson LA, Vahter M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106:110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, Rudnai P, Leonardi G, Fletcher T, Vahter M. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Persp. 2007;115:1081–1086. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo CA, Aposhian HV, Cebrian ME, Yamauchi H, Silbergeld EK. Variability in human metabolism of arsenic. Environ Res. 2003;92:85–91. doi: 10.1016/s0013-9351(02)00081-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Fierro ML, Beuten J, Leach RJ, Parra EJ, Cruz-Lopez M, Rangel-Villalobos H, Riego-Ruiz LR, Ortiz-Lopez R, Martinez-Rodriguez HG, Rojas-Martinez A. Ancestry informative markers and admixture proportions in northeastern Mexico. J Human Genet. 2009;54:504–509. doi: 10.1038/jhg.2009.65. [DOI] [PubMed] [Google Scholar]
- Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valle, Sonora, Mexico. Environ Res. 2004;96:119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Meza MM, Yu L, Rodriguez YY, Guild M, Thompson D, Gandolfi AJ, Klimecki WT. Developmentally restricted genetic determinants of human arsenic metabolism: association between urinary methylated arsenic and CYT19 polymorphisms in children. Environ Health Persp. 2005;113:775–781. doi: 10.1289/ehp.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeth P, Beaulieu M, Park C, Kosman D, del Mistro G, Van den Boom D, Jurinke C. SEQUENOM aplication note. 2005. [Google Scholar]
- Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet. 2004;36:S54–S60. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Vasken Aposhian H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol. 2000;163:203–207. doi: 10.1006/taap.1999.8872. [DOI] [PubMed] [Google Scholar]
- Pu YS, Yang SM, Huang YK, Chung CJ, Huang SK, Chiu AW, Yang MH, Chen CJ, Hsueh YM. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol Appl Pharmacol. 2007;218:99–106. doi: 10.1016/j.taap.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Rubi-Castellanos R, Martinez-Cortes G, Munoz-Valle JF, Gonzalez-Martin A, Cerda-Flores RM, Anaya-Palafox M, Rangel-Villalobos H. Pre-Hispanic Mesoamerican demography approximates the present-day ancestry of Mestizos throughout the territory of Mexico. Am J Phys Anthropol. 2009;139:284–294. doi: 10.1002/ajpa.20980. [DOI] [PubMed] [Google Scholar]
- Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Human Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlawicke Engstrom K, Broberg K, Concha G, Nermell B, Warholm M, Vahter M. Genetic polymorphisms influencing arsenic metabolism: evidence from Argentina. Environ Health Persp. 2007;115:599–605. doi: 10.1289/ehp.9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin MF, Qi L, Scherbarth HR, Tian C, Ransom M, Silva G, Belmont JW, Gamron S, Allievi A, Palatnik SA, Saurit V, Paira S, Graf C, Guilleron C, Catoggio LJ, Prigione C, Berbotto GA, Garcia MA, Perandones CE, Truedsson L, Abderrahim H, Battagliotti CG, Pons-Estel BA, Alarcon-Riquelme ME. Amerindian ancestry in Argentina is associated with increased risk for systemic lupus erythematosus. Genes Immun. 2008;9:389–393. doi: 10.1038/gene.2008.25. [DOI] [PubMed] [Google Scholar]
- Shockley KR, Witmer D, Burgess-Herbert SL, Paigen B, Churchill GA. Effects of atherogenic diet on hepatic gene expression across mouse strains. Physiol Genomics. 2009;39:172–182. doi: 10.1152/physiolgenomics.90350.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shriver MD, Parra EJ, Dios S, Bonilla C, Norton H, Jovel C, Pfaff C, Jones C, Massac A, Cameron N, Baron A, Jackson T, Argyropoulos G, Jin L, Hoggart CJ, McKeigue PM, Kittles RA. Skin pigmentation, biogeographical ancestry and admixture mapping. Human Genet. 2003;112:387–399. doi: 10.1007/s00439-002-0896-y. [DOI] [PubMed] [Google Scholar]
- Sluyter JD, Schaaf D, Metcalf PA, Scragg RK. Dietary intakes of Pacific, Maori, Asian and European adolescents: the Auckland High School Heart Survey. Aust N Z J Public Health. 2010;34:32–37. doi: 10.1111/j.1753-6405.2010.00470.x. [DOI] [PubMed] [Google Scholar]
- Smith AH, Arroyo AP, Mazumder DN, Kosnett MJ, Hernandez AL, Beeris M, Smith MM, Moore LE. Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Persp. 2000;108:617–620. doi: 10.1289/ehp.00108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, Wood R, Kosnett MJ, Smith MT. Cancer risks from arsenic in drinking water. Environ Health Persp. 1992;97:259–267. doi: 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel N, Persson LA, Rahman M, Streatfield PK, Yunus M, Ekstrom EC, Vahter M. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology. 2009;20:824–830. doi: 10.1097/EDE.0b013e3181bb56ec. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Bates MN, Yuan Y, Kalman D, Atallah R, Rey OA, Biggs ML, Hopenhayn C, Moore LE, Hoang BK, Smith AH. Arsenic methylation and bladder cancer risk in case-control studies in Argentina and United States. J Occup Environ Med. 2006;48:478–488. doi: 10.1097/01.jom.0000200982.28276.70. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Moore LE, Shipp M, Kalman D, Rey OA, Biggs ML, Hopenhayn C, Bates MN, Zheng S, Wiencke JK, Smith AH. Genetic polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and metabolism of arsenic. J Toxicol Environ Health A. 2007;70:159–170. doi: 10.1080/15287390600755240. [DOI] [PubMed] [Google Scholar]
- Steinmaus C, Yuan Y, Kalman D, Rey OA, Skibola CF, Dauphine D, Basu A, Porter KE, Hubbard A, Bates MN, Smith MT, Smith AH. Individual differences in arsenic metabolism and lung cancer in a case-control study in Cordoba, Argentina. Toxicol Appl Pharmacol. 2010;247:138–145. doi: 10.1016/j.taap.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Del Razo LM, Vega L, Germolec DR, LeCluyse EL, Hamilton GA, Reed W, Wang C, Cullen WR, Thomas DJ. Comparative toxicity of trivalent and pentavalent inorganic and methylated arsenicals in rat and human cells. Arch Toxicol. 2000;74:289–299. doi: 10.1007/s002040000134. [DOI] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: a research update. Environ Health Persp. 2002;110(Suppl 5):767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Li J, Waters SB, Xing W, Adair BM, Drobna Z, Devesa V, Styblo M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med (Maywood) 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Waters SB, Styblo M. Elucidating the pathway for arsenic methylation. Toxicol Appl Pharmacol. 2004;198:319–326. doi: 10.1016/j.taap.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Tsai SM, Wang TN, Ko YC. Cancer mortality trends in a blackfoot disease endemic community of Taiwan following water sourde replacement. J Toxicol Environ Health A. 1998;55:389–404. doi: 10.1080/009841098158322. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Arsenic methylation, urinary arsenic metabolites and human diseases: current perspective. J Environ Sci Health C Environ Carcinogen Ecotoxicol Rev. 2007a;25:1–22. doi: 10.1080/10590500701201695. [DOI] [PubMed] [Google Scholar]
- Tseng CH. Metabolism of inorganic arsenic and non-cancerous health hazards associated with chronic exposure in humans. J Environ Biol. 2007b;28:349–357. [PubMed] [Google Scholar]
- Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235:338–350. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Tseng CH, Huang YK, Huang YL, Chung CJ, Yang MH, Chen CJ, Hsueh YM. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206:299–308. doi: 10.1016/j.taap.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Vahter M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol Lett. 2000;112–113:209–217. doi: 10.1016/s0378-4274(99)00271-4. [DOI] [PubMed] [Google Scholar]
- Vahter M. Mechanisms of arsenic biotransformation. Toxicology. 2002;181–182:211–217. doi: 10.1016/s0300-483x(02)00285-8. [DOI] [PubMed] [Google Scholar]
- Vahter M, Concha G, Nermell B, Nilsson R, Dulout F, Natarajan AT. A unique metabolism of inorganic arsenic in native Andean women. Eur J Pharmacol. 1995;293:455–462. doi: 10.1016/0926-6917(95)90066-7. [DOI] [PubMed] [Google Scholar]
- Valenzuela OL, Drobná Z, Hernández-Castellanos E, Sánchez-Peña LC, García-Vargas GG, Borja-Aburto VH, Stýblo M, Del Razo LM. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol Appl Pharmacol. 2009;239:200–207. doi: 10.1016/j.taap.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Ray N, Rojas W, Parra MV, Bedoya G, Gallo C, Poletti G, Mazzotti G, Hill K, Hurtado AM, Camrena B, Nicolini H, Klitz W, Barrantes R, Molina JA, Freimer NB, Bortolini MC, Salzano FM, Petzl-Erler ML, Tsuneto LT, Dipierri JE, Alfaro EL, Bailliet G, Bianchi NO, Llop E, Rothhammer F, Excoffier L, Ruiz-Linares A. Geographic patterns of genome admixture in Latin American Mestizos. PLoS Genet. 2008;4:e1000037. doi: 10.1371/journal.pgen.1000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner ML, Moore LE, Smith MT, Kalman DA, Fanning E, Smith AH. Increased micronuclei in exfoliated bladder cells of individuals who chronically ingest arsenic-contaminated water in Nevada. Cancer Epidemiol Biomarkers Prev. 1994;3:583–590. [PubMed] [Google Scholar]
- Wnek SM, Jensen TJ, Severson PL, Futscher BW, Gandolfi AJ. Monomethylarsonous acid produces irreversible events resulting in malignant transformation of a human bladder cell line following 12 weeks of low-level exposure. Toxicol Sci. 2010;116:44–57. doi: 10.1093/toxsci/kfq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY. Does arsenic exposure increase the risk of development of peripheral vascular diseases in humans? J Toxicol Environ Health A. 2006;69:1797–1804. doi: 10.1080/15287390600630237. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chang CC, Chiu HF. Does arsenic exposure increase the risk for prostate cancer? J Toxicol Environ Health A. 2008;71:1559–1563. doi: 10.1080/15287390802392065. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]