Abstract
Increases in atmospheric carbon dioxide (pCO2) are projected to contribute to a 1.1–6.4°C rise in global average surface temperatures and a 0.14–0.35 reduction in the average pH of the global surface ocean by 2100. If realized, these changes are expected to have negative consequences for reef-building corals including increased frequency and severity of coral bleaching and reduced rates of calcification and reef accretion. Much less is known regarding the independent and combined effects of temperature and pCO2 on critical early life history processes such as fertilization. Here we show that increases in temperature (+3°C) and pCO2 (+400 µatm) projected for this century negatively impact fertilization success of a common Indo-Pacific coral species, Acropora tenuis. While maximum fertilization did not differ among treatments, the sperm concentration required to obtain 50% of maximum fertilization increased 6- to 8- fold with the addition of a single factor (temperature or CO2) and nearly 50- fold when both factors interact. Our results indicate that near-future changes in temperature and pCO2 narrow the range of sperm concentrations that are capable of yielding high fertilization success in A. tenuis. Increased sperm limitation, in conjunction with adult population decline, may have severe consequences for coral reproductive success. Impaired sexual reproduction will further challenge corals by inhibiting population recovery and adaptation potential.
Introduction
Atmospheric carbon dioxide (pCO2) has increased from approximately 280 ppm to 390 ppm since the start of the industrial revolution due to anthropogenic activities such as the burning of fossil fuels, cement production, and land use changes [1]. Two major consequences of this increase have been a rise in global average temperature of 0.7°C, i.e., global warming, and a decrease in the average global surface ocean pH of 0.1 units (a 30% increase in acidity), i.e., ocean acidification or OA [1]. Projections, based on Special Report of Emissions Scenarios, suggest atmospheric CO2 concentration could reach up to 560 ppm by the year 2050 and over 800 ppm by 2100. This elevation in pCO2 will likely contribute to a further increase in global average surface warming of 1.1–6.4°C and a reduction in the average global surface ocean pH of between 0.14 and 0.35 units over the 21st century [1].
For coral reefs, the most likely consequences of ocean acidification and warming, respectively, are generally regarded as reduced calcification/reef accretion [2] and increased frequency and severity of coral bleaching (a breakdown of the symbiotic relationship between corals and their endosymbiotic algae) [3]. These concerns, in combination with recent trends in reef degradation caused by local stressors (e.g., overexploitation and pollution), have led to predicted future scenarios that range from spatially heterogenous declines dominated by shifts in community composition [4], [5] to global-scale loss of coral reefs [6], [7]. Recently there has been a growing awareness that rising ocean temperatures and declining pH may affect a variety of physiological and biological processes that span multiple life history stages [8]. Reproductive and early life history stages can be particularly vulnerable to environmental stress [9], and reduced reproduction and recruitment threaten reef resilience, i.e., the ability to recover post-disturbances. In light of the recent trends in reef loss, there is an urgent need to understand how global warming and ocean acidification will affect the reproductive capacity, and the recovery potential, of reef-building corals.
While many corals reproduce asexually, sexual reproduction is important for maintaining genetic diversity, populating denuded areas, determining the community structure of coral reefs, and replenishing reefs post disturbances. Corals have two primary strategies for sexual reproduction: (1) brooding, in which sperm are released into the water column and taken in by conspecifics for internal fertilization; and (2) broadcast spawning, in which eggs and sperm are released into the environment for external fertilization [10]. The majority of documented coral species are hermaphroditic broadcast spawners: they release gamete bundles, containing both eggs and sperm, into the water column and are reliant on external fertilization and larval development. Gamete bundles generally break apart approximately 30 minutes following release, whereupon gametes mix at the water's surface. Fertilized embryos develop into planula larvae over the course of hours to days before they are capable of settling on the reef [10].
As fertilization occurs externally, coral gametes are particularly vulnerable to the conditions of the surrounding water column. Consequently, fertilization success depends on a variety of factors, including sperm density, gamete age, water temperature, oxygen-availability, salinity, nutrients, pH, and pCO2 (reviewed in [11], [12]). As the oceans continue to warm and acidify, it becomes essential to understand the repercussions these changes will have on sexual reproduction. The number of studies investigating the independent effects of temperature and pH on early life history stages of corals is growing; however, few studies have investigated the interactive effects of these combined stressors [13].
Here we report the independent and combined effects of elevated temperature and pCO2 on the fertilization success of Acropora tenuis (Dana, 1846), a common species on upper reef slopes throughout the Indo-Pacific and Red Sea. Gametes were collected from naturally spawning colonies of A. tenuis and fertilized under control and experimental conditions. Two temperatures (27°C and 30°C) and two pCO2 levels (400 µatm and 800 µatm) were targeted to represent present-day conditions and a middle-of-the-road scenario projected for the end of the century [1]. The effects of these parameters were investigated across a range of sperm concentrations (102–107 sperm ml−1), spanning concentrations considered optimal for fertilization in broadcast-spawning corals, 105–106 sperm ml−1 [14].
Methods
Gamete collection and preparation
Gravid colonies of A. tenuis were collected from Orpheus Island, GBR (Permit G09/30237.1) and maintained in flow-through seawater in outdoor aquaria at the Australian Institute of Marine Science (AIMS), Townsville, Queensland. On evenings of predicted gamete release, colonies were placed in separate containment tanks and monitored for spawning activity. Spawning occurred on November 15, 2011 at approximately (1930 h). Upon release, gamete bundles were collected by pipette from each of five colonies and transferred to 50 ml falcon tubes for transport to the laboratory. Care was taken to avoid cross-contamination between genotypes. The volume of seawater in the tubes was adjusted to achieve 1∶4 (v/v) ratios of gametes∶seawater to ensure a concentrated stock solution (≥108 sperm ml−1). Tubes were gently agitated to assist disintegration of gamete bundles, i.e., the release of eggs and sperm. Bundles disintegrated ∼60 min after release, whereupon eggs and sperm from each colony were separated.
Sperm handling
Concentrated sperm (∼108 sperm ml−1) were removed from each tube by pipette and combined in a glass bottle to create a stock sperm solution consisting of equal parts sperm from each of the five parent colonies. The stock sperm solution was mixed by gentle inversion of the jar, and experimental sperm solutions were made by serial, ten-fold dilutions into 0.2 µm filtered treatment water (400 or 800 µatm, refer to ‘Seawater chemistry’ for details). Six dilutions (102–107 sperm ml−1) were made for each pCO2 level (400 and 800 µatm), totaling 12 sperm*CO2 solutions (102–107 sperm ml−1*400 µatm and 102–107 sperm ml−1*800 µatm); each dilution was gently inverted at least 5 times to ensure mixing. Borosilicate glass Schott bottles were used to maintain sperm viability and avoid abnormal cleavage of embryos that can occur with the use of plastics [14]. The sperm*CO2 solutions were then used to fill the replicate scintillation vials in each of the two temperature baths (see ‘Experimental design’ section). After, two 1-ml subsamples of each dilution in each sperm*CO2 combination were fixed in 10% formaldehyde for verification of sperm concentrations (ten replicate counts per subsample by hemocytometer).
Egg handling
Eggs from each parent colony were divided equally into new falcon tubes and washed 5–7 times with 0.2 µm filtered treatment water (400 or 800 µatm) to remove residual sperm. Once washed, equal volumes from each parent colony were combined to create two stock egg solutions (400 and 800 µatm) consisting of equal parts from each of the five parent colonies.
Experimental design
A balanced factorial design was used to evaluate the effect of temperature and CO2 on the fertilization curve of A. tenuis. The design consisted of 144 scintillation vials: 2 CO2 levels (400 and 800 µatm)×2 temperatures (27 and 30°C)×6 sperm concentrations (102, 103, 104, 105, 106, and 107 sperm ml−1)×6 replicates (N = 6). Fifteen ml of each sperm*CO2 combination (see ‘Sperm handling’ section) was transferred (Pipetaid, Gilson) into each of 6 replicate 20-mL glass scintillation vials for each of the four CO2*T treatments. Twelve additional vials (three per CO2*T treatment) were dedicated for no-sperm controls and filled with 20-mL of sperm-free seawater. Scintillation vials were nested within re-circulating water baths for temperature control during the experiment (±0.2°C). Temperatures within the vials were allowed to equilibrate (∼30 min.) to the treatment levels prior to the addition of eggs. Thirty microliters of eggs (∼300 eggs) were added to each vial. To avoid sperm contamination (i.e., introduction of additional sperm between treatments), the addition of eggs was conducted in order from the lowest to highest sperm concentration, and tips were changed between sperm concentrations. Vials were gently swirled, and eggs were left to fertilize and develop. Fertilization experiments were initiated within 3 h of spawning. Embryos were sub-sampled at 3 hours (∼16–32 cell stage) and fixed in 10% formaldehyde; the samples were collected in the same order in which the eggs were introduced to ensure that gametes in all treatments were allowed equal sperm-egg contact time. Subsequently, 200–300 eggs/embryos from each subsample were examined using a dissecting microscope and scored as either fertilized (showing normal cleavage patterns of cell division) or unfertilized (showing no signs of cleavage) (Fig. 1).
Figure 1. Representative images of unfertilized and fertilized Acropora tenuis embryos.
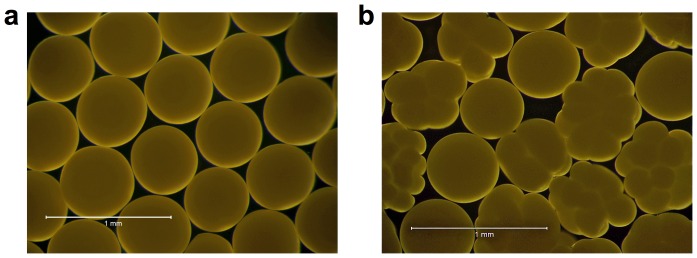
Unfertilized eggs (a) are spherical and show no signs of cleavage while fertilized embryos (b) show distinct cell division.
Model
Fertilization curves in broadcast spawners are typically bell-shaped, in which sperm availability limits fertilization success at lower sperm concentrations, and polyspermy is thought to limit fertilization success at higher sperm concentrations [15]. In our study, fertilization success reached an asymptotic maximum, and we did not observe a decline in fertilization at higher sperm concentrations. Accordingly, sigmoidal dose-response curves were fit to the data, defined as follows: % Fert = % Fertmax/(1+10LogEC50-LogSC), where % Fert is the percent fertilization at a given sperm concentration (SC), % Fertmax is the asymptotic average maximum percent fertilization, and EC50 is the ‘half maximal effective concentration’, or the sperm concentration that yields 50% of Fertmax (Fig. 2a). The model used follows the general equation for a standard, three parameter sigmoidal dose-response curve in which the bottom asymptote has been constrained to zero (0% Fert at 0 sperm ml−1) and the top asymptote, % Fertmax, has been constrained to less than or equal to 100%. Nonlinear regressions were fit separately for each CO2*T treatment using least squares residuals. For each parameter, Fertmax and LogEC50, best-fit values were compared between treatments using One-way ANOVAs. Where significant differences were detected, Tukey's Multiple Comparisons tests were conducted to determine which treatments differed from one another (Table S1).
Figure 2. Theoretical and actual fertilization curves for Acropora tenuis.
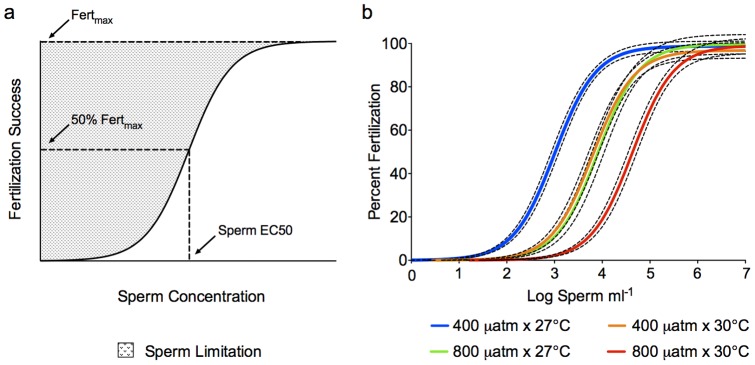
Schematic (a) showing the graphical representation of Fertmax and Sperm EC50, the sperm concentration at which 50% of Fertmax is achieved. The shading represents the region of sperm limitation resulting in less than maximal fertilization success (adapted from Marshall [15]). Fertilization curves (b) of Acropora tenuis by temperature and CO2 treatments. Dashed black lines indicate 95% confidence intervals for each curve. Parameter estimates are given in Table 3.
Respiratory alterations of CO2 treatments
To test whether the addition of sperm to seawater caused respiratory alterations of desired CO2 levels, we measured pH in each T*CO2 combination for sperm concentrations 103–107 sperm ml−1 at the start and end of the experiment.
Changes were not measured in 102 sperm ml−1 as preliminary work showed that changes in this concentration were comparable to seawater controls. So as not to disturb fertilization and/or embryonic development in the scintillation vials, separate 30 ml, screw-top glass vials, were used for pH measurements. Twenty milliliters of each sperm*CO2 combination were added to each vial (4 CO2*T treatments×5 sperm concentrations×2 replicates = 40 vials) and nested in the same water baths as the fertilization vials. Preliminary work indicated that chemical changes were highly consistent within a sperm concentration and that a sample size of 2 was sufficient to accurately document changes. pH was measured in each vial (SC*T*CO2 combination) using an Orion ROSS combination pH electrode (Thermo Scientific) calibrated at 27°C and 30°C against a seawater TRIS buffer [16] (SOP6a).
Seawater chemistry
One ambient (400 µatm) and one elevated pCO2 concentration (800 µatm) were chosen for the study based on near-future projections determined by the Intergovernmental Panel on Climate Change (IPCC) [1]. These may represent conservative pCO2 levels, as modified projections (e.g., Representative Concentration Pathway (RCP) 8.5) used in the forthcoming Assessment Report (AR5) by the IPCC indicate that atmospheric CO2 may exceed 1,000 ppm by the end of this century [17].
Seawater chemistry was manipulated via direct bubbling with carbon dioxide-enriched air into two separate aquaria. The control was bubbled with outside air. Treatment water was filtered (0.2 µm) before use in experiments, to minimize changes of pCO2 due to background, microbial respiration. Duplicate water samples of each pCO2 treatment were collected prior to the start of the fertilization experiment and poisoned with mercuric chloride (0.05% by volume) to verify distinct treatments. Samples were analyzed for total alkalinity (TA) and dissolved inorganic carbon (DIC) using a VINDTA 3C® (Versatile INstrument for the Determination of Total dissolved inorganic carbon and Alkalinity, Marianda). Accuracy was checked against certified seawater reference material (from A. Dickson, Scripps Institute of Oceanography, Batch 106). pCO2, pH (total scale), and Ωarag were computed from TA, DIC, temperature, and salinity using the CO2SYS program (from E. Lewis, Brookhaven National Laboratory); dissociation constants for carbonate and boric acid were determined as described in Albright et al. [18].
Results
Seawater chemistry
Actual chemical and physical conditions of the seawater used in fertilization assays are presented in Table 1. pCO2 levels differed slightly between temperature treatments due to the affect of temperature on the solubility of CO2.
Table 1. Physical and chemical conditions of seawater used to fill experimental vials for fertilization experiments (Mean ± 1 SD, N = 2)a.
| Treatment | T* (°C) | Salinity* | TA* (µmol kg−1) | pHT | pCO2 (µatm) | HCO3 − (µmol kg−1) | CO3 −2 (µmol kg−1) | CO2 (µmol kg−1) | TCO2* (µmol kg−1) | Ωarag |
| 400 µatm×27°C (control) | 27.0±0.2 | 36.0±0.2 | 2327±2 | 8.013±0.001 | 434±1 | 1787±2 | 219.5±0.1 | 11.63±0.02 | 2018±2 | 3.491±0.001 |
| 400 µatm×30°C | 30.0±0.2 | 36.0±0.2 | 2327±2 | 7.970±0.001 | 488±1 | 1786±2 | 220.3±0.1 | 12.19±0.02 | 2018±2 | 3.559±0.001 |
| 800 µatm×27(C | 27.0(0.2 | 36.0(0.2 | 2322(2 | 7.783(0.001 | 815(2 | 1972(2 | 142.5(0.1 | 21.83(0.03 | 2137(2 | 2.267(0.001 |
| 800((atm(30(C | 30.0(0.2 | 36.0(0.2 | 2322(2 | 7.741(0.001 | 912(2 | 1970(2 | 143.6(0.1 | 22.77(0.03 | 2137(2 | 2.320(0.001 |
Asterisks represent parameters that were directly measured; remaining parameters were calculated using CO2SYS (see ‘Methods’).
Error represents the analytical error of the TA and DIC analyses.
Fertilization model
Percent fertilization by sperm concentration and treatment is presented in Table 2, and model parameters for nonlinear regressions are presented in Table 3. Maximum fertilization (Fertmax) did not differ among treatments (F3,148 = 0.502, P = 0.681), but the fertilization curve shifted horizontally such that the sperm concentration required to achieve half of the maximum fertilization (EC50) increased with the addition of experimental factors (F3,148 = 141.7, P<0.0001) (Fig. 2b; Table 3). Fifty percent of Fertmax was attained at 1.0×103 sperm ml−1 under control conditions (400 µatm and 27°C). Independent stressors, i.e., increasing temperature by 3°C or pCO2 by 400 µatm, resulted in a six- to eight-fold increase in EC50 (6.3×103–8.2×103 sperm ml−1), with no significant difference between the two factors (Table 3; Table S1). The combined effects resulted in a five- to seven- fold increase above the single factors, and a near 50-fold increase in EC50 relative to controls (4.3×104 sperm ml−1) indicating a multiplicative interaction between temperature and CO2.
Table 2. Percent fertilization by sperm concentration and treatment (Mean ± SEM, N = 6).
| No Sperm Control | 5.21×102 sperm ml−1 | 5.51×103 sperm ml−1 | 5.82×104 sperm ml−1 | 6.15×105 sperm ml−1 | 6.49×106 sperm ml−1 | 6.86×107 sperm ml−1 | |
| 400 µatm×27°C | 0.9±0.6 | 33.9±4 | 82.96±5 | 98.3±0.5 | 97.7±0.4 | 98.6±0.4 | 98.8±0.3 |
| 800 µatm×27°C | 1.5±0.7 | 2.2±0.4 | 39.37±10 | 90.93±3 | 98.3±0.5 | 98.0±0.6 | 98.7±0.3 |
| 400 µatm×30°C | 0.0±0.0 | 19.8±4 | 42.88±5 | 83.6±2 | 96.6±0.7 | 98.1±0.5 | 98.1±0.3 |
| 800 µatm×30°C | 1.3±0.1 | 1.4±0.5 | 3.14±0.5 | 59.7±6 | 94.9±0.5 | 96.3±0.6 | 98.3±0.3 |
Table 3. Parameter estimates for nonlinear regressions of fertilization dataa.
| 400 µatm×27°C | 800 µatm×27°C | 400 µatm×30°C | 800 µatm×30°C | |
| Best-fit values | ||||
| EC50 | 1.0×103 | 8.2×103 | 6.3×103 | 4.3×104 |
| %Fertmax | 99 | 100 | 97 | 99 |
| 95% CI | ||||
| EC50 | 8.3×102–1.2×103 | 5.9×103–1.1×104 | 4.8×103–8.2×103 | 3.4×104–5.4×104 |
| %Fertmax | 96–100 | 95–100 | 93–100 | 96–100 |
| Degrees freedom | 37 | 37 | 37 | 37 |
| R2 | 0.97 | 0.95 | 0.95 | 0.98 |
%Fertmax did not differ significantly between treatments. However, EC50 values significantly differed between all treatments except 400 µatm×30°C and 800 µatm×27°C. See Table S1 for results of Tukey's Multiple Comparisons.
EC50 is the ‘half maximal effective concentration’, or the sperm concentration that yields 50% of the %Fertmax; %Fertmax is the asymptotic average maximum percent fertilization.
Respiratory alterations of CO2 treatments
We monitored pH at the beginning and end of our experiment to determine if respiratory activity by sperm altered our target pCO2/pH levels. The largest changes occurred in the ∼90 minutes immediately following the addition of sperm to the treatment seawater, prior to the start of the experiment. The change in pH, i.e., deviation from starting value, increased with increasing sperm concentration (Fig. 3) with a near 0.6 unit pH change in the most concentrated sperm solutions (107 sperm ml−1). pH continued to decline during the subsequent ∼180 minutes of the experiment, however, these changes were small relative to those that occurred prior to the experiment (Table S2). Despite the rapid changes in pH at higher sperm concentrations (106 and 107 sperm ml−1), high levels of fertilization (≥95%) were achieved (Table 2).
Figure 3. Respiratory alterations of target pH levels by sperm concentration and treatment (Mean ± 1 SD).
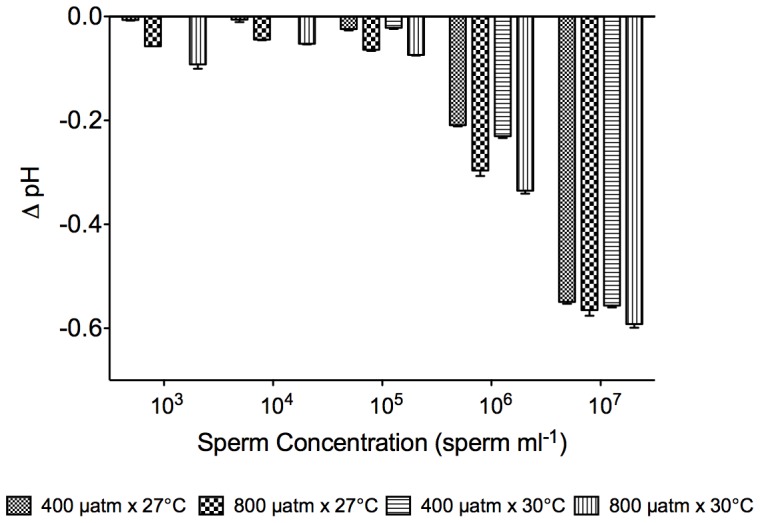
Despite the rapid changes in pH at higher sperm concentrations (106 and 107 sperm ml−1), high levels of fertilization (≥95%) were achieved. See text for details.
Discussion
By fitting fertilization curves for each temperature*CO2 treatment, we show that increases in temperature (+3°C) and pCO2 (+400 µatm), within the ranges projected for this century, negatively impact fertilization success of A. tenuis. Maximum fertilization (Fertmax) did not differ among treatments, but the fertilization curve shifted horizontally such that the sperm concentration required to achieve half of the maximum fertilization (EC50) increased with the addition of experimental factors. These results indicate that sperm concentrations that yield high fertilization success under present-day conditions are likely to be limiting as the oceans continue to warm and acidify. The nature of the effect, a shift in the fertilization curve to higher sperm concentrations without a reduction in %Fertmax, suggests that both temperature and CO2 influence fertilization through effect(s) on sperm viability or sperm-egg interactions [12], [15]. Decreased sperm motility in response to CO2-induced acidification was recently demonstrated in Acropora digitifera [19], [20] and may provide a mechanistic basis for the results observed here.
The effects of CO2 and temperature on fertilization were dependent on the sperm concentration. Maximum differences between treatments were observed within the range of 102–104 sperm ml−1, whereas all treatments achieved ≥95% fertilization success at sperm concentrations of 105–107 sperm ml−1. Moreover, despite rapid changes in pH at higher sperm concentrations (106 and 107 sperm ml−1), high levels of fertilization (≥95%) were achieved, further demonstrating that while acidification inhibits fertilization success at non-saturating sperm concentrations, optimal sperm concentrations are capable of resulting in high fertilization success. These results are consistent with ecotoxicological studies on other marine invertebrates [12], [15] and support the concept that the effect of an environmental toxicant on fertilization success is dependent on the sperm concentration, with lower, non-saturating sperm concentrations typically showing the highest sensitivity. This is thought to occur because the addition of a toxicant typically acts to reduce the number of sperm-egg interactions, either by killing sperm or reducing sperm-egg contact efficiency [15]. Importantly, at ‘optimal’ sperm concentrations, enough interactions occur that this effect is not detected. Unfortunately, many recent studies have evaluated the effect of environmental stressors on fertilization using a single sperm concentration that has previously been identified as optimal, severely limiting our ability to make useful predictions about the likely impact of these stressors on sexual reproduction [13]. The dependence of treatment effects on the sperm concentration suggests that the susceptibility of fertilization success to warming and acidification may vary among coral populations; populations that are likely to be sperm-limited (e.g., low density populations and/or populations that experience high wind/wave activity) may be more likely to experience declines in fertilization, while those that currently experience optimal concentrations may be more resistant.
Currently, robust estimates of in situ sperm concentrations and fertilization rates are lacking for corals. Available information indicates that while optimal sperm concentrations are obtainable for some species under certain conditions [21], sperm concentrations and fertilization rates are highly variable in both space and time [14], [21] and are generally assumed to be limiting for most sessile, broadcast-spawning marine invertebrates [22]. Because sperm concentration depends on a number of factors including population density, time after spawning, and wind and water turbulence, identifying concentrations that are ecologically relevant is complex. Despite these challenges, there is an urgent need to characterize naturally-occurring fertilization conditions if we are to better gauge the threat that global warming and ocean acidification pose to sexual reproduction of corals.
The effects of CO2 and temperature on fertilization of A. tenuis are similar to the independent effects of these parameters observed in other studies [18], [23]. Albright et al. [18] showed that fertilization of a Caribbean congener, Acropora palmata, was negatively affected by elevated pCO2 (673 and 998 µatm); similar to this study, the effect of pCO2 on fertilization success was dependent on the sperm concentration and was exacerbated at lower, non-saturating concentrations. Acropora millepora experienced reduced fertilization and a higher frequency of embryonic abnormalities at 32°C (+4°C), and fertilization ceased altogether at 34°C (+6°C) [23]. However, in the same study, three other coral species, Favites abdita, Favites chinensis and Mycedium elephantotus were fertilized and developed normally at temperatures up to 32°C (+5°C), indicating that the susceptibility to climate change may differ among coral taxa. More research is needed to determine the generality of the response observed in this study; multi-stressor experiments should be emphasized as species may respond differently to dual stressors. Identifying species-specific differences in the susceptibility of fertilization and recruitment to global warming and ocean acidification may implicate future changes in community structure.
While only a handful of studies have investigated the effects of pCO2 and temperature on coral fertilization, the effects of these factors on fertilization of other spawning marine invertebrates has been the subject of many recent investigations, though results are equivocal [9], [13], [24]. For example, Havenhand et al. [25] reported acidification-induced reductions in fertilization for the sea urchin Heliocidaris erythrogramma; however, Byrne et al. [26], [27], [28] observed no effect in the same species. Similarly, Havenhand et al. [29] and Parker et al. [30] report contrasting results for fertilization success of the bivalve species, Crassostrea gigas, exposed to acidified seawater. It is not yet clear whether discrepancies between studies stem from species-specific differences or variations in the methodologies employed (reviewed in [24]). Consequently, there is an increasing awareness of the need for standardized methodologies to facilitate comparisons between studies.
Interpretation of laboratory results is further confounded by our inability to accurately replicate conditions in an organism's natural environment. For example, scintillation vials limit water movement, which may affect fertilization dynamics (although the use of multiple sperm concentrations partially accounts for diffusive effects of the open ocean); also, experimental set-up time necessitates a delay in gamete contact, which may or may not occur in nature. This time component, and the associated gamete aging, may affect the susceptibility of gametes to experimental stress. Despite these limitations, results of fertilization experiments provide valuable insight regarding the potential consequences of a warming and acidifying ocean on sexual reproduction of spawning marine organisms.
Recent investigations indicate that elevated temperatures and pCO2 levels also impact coral reproduction and recruitment through effects on post-fertilization processes (e.g., larval development, survival, settlement, metamorphosis, and early post-settlement growth). Temperatures exceeding 30°C have negative effects on the development and survival of some coral species [31], [32], [33]. Effects of temperature on larval settlement are more complex, as thermal stress has been shown to both increase [34] and decrease [34], [35] settlement. Elevated pCO2 does not appear to affect embryonic development [36], but larval respiration [37], [38] may be affected. pCO2 also affects larval settlement, though it appears to do so indirectly through impacts on the epilithic algal community [38], [39]; under future ocean acidification scenarios, certain species of crustose coralline algae (e.g., Titanoderma prototypum), that promote larval metamorphosis and facilitate survival [11] appear to lose out to less desirable taxa [39]. Acidification also reduces post-settlement growth and calcification [13], [18], [40], [41] impairing the ability of new recruits to effectively compete for benthic area. Given the range of life history stages and processes that appear negatively impacted by global warming and ocean acidification, carryover effects, and/or compounding effects on successive life history stages are likely.
What has been and will be the cumulative toll of climate change and ocean acidification on coral recruitment? Many of the dominant framework-building corals in the Caribbean (broadcast-spawning species – Acropora palmata, A. cervicornis, Montastrea spp.) have been observed to suffer from reproductive and/or recruitment failure [42]. Lack of historical baselines limits our understanding of whether recruitment of these species declined or has always been low. Furthermore, it remains unknown if, or to what extent, changing temperatures and CO2 levels have contributed to this failure, though it has been speculated that they are already impacting juvenile coral growth in the Caribbean [43]. As coral populations decline worldwide, successful reproduction and recruitment will become more challenging. The results of this study suggest that, irrespective of changes in current population structure, sexual reproduction of corals will be compromised as warming and acidification narrow the window for successful fertilization. Future work should investigate the presence of species-specific adaptations that may infer resistance to declines in sexual reproduction. Developing strategies to manage for reproductive and recruitment success may prove a necessary step towards promoting the sustainability, persistence, and recovery of coral populations.
Supporting Information
Tukey's Multiple Comparisons of LogEC50 values by treatment.
(DOCX)
Respiratory alterations of target pH values by treatment and sperm concentration (N = 2).
(DOCX)
Acknowledgments
We are grateful to A. Negri for discussion that improved the design of this study and to A. Negri and S. Uthicke for access to their experimental seawater system. A. Negri, D. Abrego, and E. Puill-Stephan were responsible for the collection and maintenance of coral colonies prior to spawning. Collection of corals was conducted under GBRMPA permit G09/30237.1. A. Negri, S. Uthicke, and J. Lough provided constructive feedback that greatly improved this manuscript.
Funding Statement
This project was supported by an Australian Research Council fellowship awarded to RA and by the Australian Institute of Marine Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC (2007) The Physical Science Basis: Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press.
- 2.Andersson AJ, Mackenzie FT, Gattuso JP (2011) Effects of ocean acidification on benthic processes, organisms, and ecosystems. In: Gattuso JP, Hansson L, editors. Ocean Acidification. Oxford University Press. pp. 122–153.
- 3. Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80: 435–471. [Google Scholar]
- 4. Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333: 418–422. [DOI] [PubMed] [Google Scholar]
- 5. Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, et al. (2012) Assembly rules of reef corals are flexible along a steep climatic gradient. Curr Biol 22: 736–741. [DOI] [PubMed] [Google Scholar]
- 6. Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- 7. Silverman J, Lazar B, Cao L, Caldeira K, Erez J (2009) Coral reefs may start dissolving when atmospheric CO2 doubles. Geophys Res Lett 36 doi: 10.1029/2008GL036282. [Google Scholar]
- 8.Gattuso JP, Hansson L (2011) Ocean Acidification. Oxford University Press. 326 p.
- 9. Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol: An Ann Rev 49: 1–42. [Google Scholar]
- 10.Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N, editors. Coral Reefs: An Ecosystem in Transition. Springer pp. 59–85.
- 11. Ritson-Williams R, Arnold SN, Fogarty ND, Steneck RS, Vermeij MJA, et al. (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38: 437–457. [Google Scholar]
- 12. Reuter KE, Lotterhos KE, Crim RN, Thompson CA, Harley CD (2011) Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus . Glob Change Biol 17: 163–171. [Google Scholar]
- 13. Albright R (2011) Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J Mar Biol 2011: Art. ID 473615, 14 pp. [Google Scholar]
- 14. Oliver J, Babcock R (1992) Aspects of the fertilization ecology of broadcast spawning corals: Sperm dilution effects and in situ measurements of fertilization. Biol Bull 183: 409–417. [DOI] [PubMed] [Google Scholar]
- 15. Marshall DJ (2006) Reliably estimating the effect of toxicants on fertilization success in marine broadcast spawners. Mar Poll Bull 52: 734–738. [DOI] [PubMed] [Google Scholar]
- 16.Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PICES Special Publication 3. North Pacific Marine Science Organization.
- 17. Van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, et al. (2011) The representative concentration pathways: an overview. Climatic Change 109: 5–31. [Google Scholar]
- 18. Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata . Proc Nat Acad Sci USA 107: 20400–20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morita M, Suwa R, Iguchi A, Nakamura M, Shimada K, et al. (2009) Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote 18: 103–107. [DOI] [PubMed] [Google Scholar]
- 20. Nakamura M, Morita M (2012) Sperm motility of the scleractinian coral Acropora digitifera under preindustrial, current, and predicted ocean acidification regimes. Aquatic Biol 15: 299–302. [Google Scholar]
- 21. Omori M, Fukami H, Kobathata H, Masayuki H (2001) Significant drop of fertilization of Acropora corals in 1999: an after-effect of heavy coral bleaching? Limnol Oceanogr 46: 704–706. [Google Scholar]
- 22. Levitan DR, Peterson C (1995) Sperm limitation in the sea. Trends Ecol Evol 10: 228–231. [DOI] [PubMed] [Google Scholar]
- 23. Negri AP, Marshall PA, Heyward AJ (2007) Differing effects of thermal stress on coral fertilization and early embryogenesis. Coral Reefs 26: 759–763. [Google Scholar]
- 24. Ross PM, Parker L, O'Connor WA, Bailey EA (2011) The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 3: 1005–1030. [Google Scholar]
- 25. Havenhand JN, Buttler FR, Thorndyke MC, Williamson JE (2008) Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr Biol 18: 651–652. [DOI] [PubMed] [Google Scholar]
- 26. Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Dworjanyn SA, et al. (2009) Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Proc R Soc B Biol Sci 276: 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn SA, Davis AR (2010a) Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar Environ Res 69: 234–239. [DOI] [PubMed] [Google Scholar]
- 28. Byrne M, Soars NA, Ho MA, Wong E, McElroy D, et al. (2010b) Fertilization in a suite of coastal marine invertebrates from SE Australia is robust to near-future ocean warming and acidification. Mar Biol 157: 2061–2069. [Google Scholar]
- 29. Havenhand JN, Schlegel P (2009) Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas . Biogeosci 6: 3009–3015. [Google Scholar]
- 30. Parker LM, Ross PM, O'Connor WA (2010) Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Mar Biol 157: 2435–2452. [Google Scholar]
- 31.Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z, editor. Ecosystems of the world: coral reefs. Elsevier. pp.133–206.
- 32. Bassim KM, Sammarco PW (2003) Effects of temperature and ammonium on larval development and survivorship in a scleractinian coral Diploria strigosa . Mar Biol 142: 241–252. [Google Scholar]
- 33. Heyward AJ, Negri AP (2010) Plasticity of larval pre-competency in response to temperature: observations on multiple broadcast spawning coral species. Coral Reefs 29: 631–636. [Google Scholar]
- 34. Nozawa Y, Harrison P (2007) Effects of elevated temperature on larval settlement and post-settlement survival in scleractinian corals, Acropora solitaryensis and Favites chinensis . Mar Biol 152: 1181–1185. [Google Scholar]
- 35. Randall CJ, Szmant AM (2009) Elevated temperature affects development, survivorship, and settlement of the elkhorn coral, Acropora palmata (Lamarck 1816). Biol Bull 217: 269–282. [DOI] [PubMed] [Google Scholar]
- 36. Medina-Rosas P, Szmant AM, Whitehead RF (2012) CO2 enrichment and reduced seawater pH had no effect on the embryonic development of Acropora palmata (Anthozoa, Scleractinia). Inv Rep Dev http://dx.doi.org/10.1080/07924259.2012.704407 [Google Scholar]
- 37. Nakamura M, Ohki S, Suzuki A, Sakai K (2011) Coral larvae under ocean acidification: survival, metabolism, and metamorphosis. PLoS ONE 6: e14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Albright R, Langdon C (2011) Ocean acidification impacts multiple early life history processes of the Caribbean coral Porites astreoides . Global Change Biol 17: 2478–2487. [Google Scholar]
- 39. Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ (2012) Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol Lett 15: 338–346. [DOI] [PubMed] [Google Scholar]
- 40. Cohen AL, McCorkle DC, de Putron S, Gaetani GA, Rose KA (2009) Morphological and compositional changes in the skeletons of new coral recruits reared in acidified seawater: Insights into the biomineralization response to ocean acidification. Geochem Geophys Geosyst 10: Q07005 doi:10.1029/2009GC002411. [Google Scholar]
- 41. Anlauf H, D'Croz L, O'Dea A (2011) A corrosive concoction: The combined effects of ocean warming and acidification on the early growth of a stony coral are multiplicative. J Exp Mar Biol Ecol 397: 13–20. [Google Scholar]
- 42. Hughes TE, Tanner JE (2000) Recruitment failure, life histories, and long-term decline of Caribbean corals. Ecology 81: 2250–2263. [Google Scholar]
- 43. Edmunds PJ (2007) Evidence for a decadal-scale decline in the growth rates of juvenile scleractinian corals. Mar Ecol Prog Ser 341: 1–13. [Google Scholar]
- 44. Willis BL, Babcock RC, Harrison PL, Wallace CC (1997) Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16: S53–S65. [Google Scholar]
- 45. Heyward AJ, Babcock RC (1986) Self- and cross-fertilization in scleractinian corals. Mar Biol 90: 191–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tukey's Multiple Comparisons of LogEC50 values by treatment.
(DOCX)
Respiratory alterations of target pH values by treatment and sperm concentration (N = 2).
(DOCX)


