Abstract
Among the secretory phospholipase A2s (sPLA2), sPLA2 group X (PLA2GX) has the most potent hydrolyzing activity toward phosphatidylcholine, and has recently been shown to be implicated in chronic inflammatory diseases. The aim of the present study was to investigate PLA2GX expression in colorectal cancer (CRC) and its correlation with patient clinicopathological features. The present study comprises a series of 158 patients who underwent surgical resection for primary CRC. PLA2GX expression in CRC tissues was examined by immunohistochemistry and compared with patient clinicopathological findings and survival. A total of 64% of the tumors expressed PLA2GX at high levels. Statistical analysis revealed that PLA2GX expression was inversely correlated with hematogenous metastasis (P=0.005). In the subgroup analysis, left-sided tumors with high PLA2GX expression showed an inverse correlation with lymph node metastasis (P=0.018) and hematogenous metastasis (P=0.017). Patients with high PLA2GX expression tended to have a longer disease-specific survival compared with those with low PLA2GX expression in left-sided, but not right-sided, CRC (P=0.08). In light of the present results, we suggest that PLA2GX has an inhibitory effect on the progression of CRC.
Keywords: phospholipase A2, colorectal cancer, immunohistochemistry, metastasis
Introduction
Globally, colorectal cancer (CRC) is the third most commonly diagnosed cancer in males and the second in females (1). A number of studies have demonstrated the critical involvement of cyclooxygenase (COX) in the development and progression of CRC (2,3). COX is a rate-limiting enzyme in the synthesis of bioactive prostaglandins or thromboxanes from arachidonic acid (AA), which is mainly released from membrane-bound glycerophospholipids. Phospholipase A2 (PLA2) is a key esterase that cleaves the glycerophospholipids at the sn-2 ester bond to release a fatty acid and lysophospholipid (4). Therefore, the tissue expression of PLA2 is thought to have important roles in the development of CRC.
PLA2 proteins are broadly defined into three different classes: secretory PLA2 (sPLA2), cytosolic PLA2 (cPLA2) and Ca2+-independent PLA2 (iPLA2). Approximately one-third of the PLA2s belong to the sPLA2 family, which contains typically disulfide-rich, low molecular weight enzymes with strict Ca2+ dependence and a His-Asp catalytic dyad (5). To date, 11 sPLA2s (IB, IIA, IIC, IID, IIE, IIF, III, V, X, XIIA and XIIB) have been identified in mammals. After being secreted to the extracellular space, sPLA2s act on cellular membrane-bound phospholipids in an autocrine or paracrine manner, leading to the production of various inflammatory mediators, including prostaglandins, leukotrienes and thromboxane. The other cleavage products, namely lysophospholipids, such as lysophosphatidylcholine (LPC) and lysophosphatidic acid (LPA), also have various bioactivities. Moreover, sPLA2s also act on non-cellular phospholipids, including those in microvesicles, pulmonary surfactant, lipoproteins, microbial membranes and food substances (4).
The physiological functions of the different sPLA2s have been gradually elucidated. They have been implicated in lipid digestion and obesity, activation of immune cells, asthma, atherosclerosis, acute respiratory distress syndrome and host defense against bacteria, viruses and parasites (5–8). However, differences in pathophysiological roles as well as the expression profile of each enzyme remain largely unknown. Of the sPLA2 family, sPLA2 group X (PLA2GX) has the most powerful AA-releasing activity from cell membrane-bound phospholipids, leading to eicosanoid formation (9,10). Morioka et al have shown that PLA2GX also releases AA from cultured human colon carcinoma cell lines, leading to COX-2-dependent PGE2 formation (11). The authors also showed enhanced expression of PLA2GX in adenocarcinoma cells in comparison with the normal colonic epithelia, by immunohistochemistry. PLA2GX has also been shown to stimulate the proliferation of colon cancer cells (12). From these data, the positive role of PLA2GX on colorectal carcinogenesis is speculated. In fact, previous studies have also described the expression of PLA2GX in human colon cancer tissue at the mRNA (13) and protein (14) levels. However, the precise expression and distribution patterns of PLA2GX in colonic cancer tissues remain to be characterized. In the present study, we aimed to examine the expression of PLA2GX in human CRC tissue and its possible correlation with clinical and pathological variables as well as with patient outcome.
Patients and methods
Patients and samples
A total of 158 consecutive patients with colorectal adenocarcinoma who underwent curative resection with lymph node dissection at the University of Tokyo Hospital (Tokyo, Japan), in the period between January 1991 and March 1994, were enrolled. There were 96 males and 62 females (mean age, 62 years; range, 38–90 years). Cases of ulcerative colitis and familial adenomatous polyposis were excluded from this study. None of the patients had received preoperative chemotherapy or radiation therapy. All pertinent clinical and histopathological data of the patients and their tumors were collected from the patients’ case records. Clinicopathological features were analyzed based on the TNM classification of malignant tumors of the Union for International Cancer Control (UICC; 7th edition). All patients had been subsequently followed up at regular clinical visits until mortality or when last seen alive, for a mean observation period of 108 months. Informed consent was obtained from all patients and the study was approved by the Ethics Committee of the Hospital of the University of Tokyo, Tokyo, Japan.
The surgically resected specimens were immediately fixed in 10% buffered formalin and the cross-sections of the entire cancerous lesion were embedded in paraffin. Conventional pathological diagnosis of the primary lesion and the dissected lymph nodes was performed on hematoxylin and eosin (H&E)-stained sections. PLA2GX expression in the cancerous lesion was examined by immunohistochemical staining, as described below.
Immunohistochemical study
Rabbit anti-sPLA2GX polyclonal antibody was generated by the immunization of rabbit with a polypeptide at The Tokyo Metropolitan Institute of Medical Science (Tokyo, Japan). The specificity and immunoreactivity of the antibody was verified by immunoblotting with sPLA2-transfected cells (15). Consecutive formalin-fixed paraffin-embedded sections (4 μm thick) were immunohistochemically stained by the streptavidin-biotin (SAB) immunoperoxidase method. For immunohistochemical staining, the sections were deparaffinized with xylene and dehydrated with 98% ethanol, placed in 0.01 M sodium citrate buffer (pH 6.0) and heated in an autoclave oven for 15 min. After washing twice in PBS, endogenous peroxidase activity was inhibited by incubation with 0.3% hydrogen peroxide in methanol for 20 min. After three washes in PBS, non-specific reactions were blocked by incubation with 10% goat serum for 30 min at room temperature. Biotinylated goat anti-rabbit immunoglobulin and SAB complex, supplied commercially [Histfine SAB-PO(R) kit, Nichirei, Tokyo, Japan] were used as the reagents in the subsequent steps. The sections were incubated with the anti-PLA2GX antibody overnight at 4°C. The color was then developed with diaminobenzidine solution. The sections were then lightly counterstained with a cocktail of Mayer’s/Lillie-Mayer’s hematoxylin and mounted. Spermatozoa were used as a positive control (16). For the negative control, the antibody was replaced with PBS.
Evaluation of immunostaining
The expression of PLA2GX in the cancerous lesion and in the surrounding normal mucosa was assessed by two observers (S.K. and M.H.) without knowledge of the corresponding clinical data. All tissue samples were assessed in a consecutive analysis to ensure maximal internal consistency. For the objective assessment of the PLA2GX expression level, it was stratified into three groups, as follows: −, not detected; +, focally positive in cancer cells; ++, diffusely positive in carcinoma cells. The consistency between the observers was 80.1% (κ test), and in the discrepant cases, a consensus was reached after a joint review. In the statistical analysis, − and + were considered to be the low expression group, and ++ was considered to be the high expression group.
Statistical analysis
The statistical significance of the differences was evaluated by χ2 test, Fisher’s exact test or a non-paired Student’s t-test, as appropriate. The disease-specific survival (DSS) rate was analyzed by the Kaplan-Meier method and the log-rank comparison test. To assess the value of PLA2GX as an independent predictor, a multivariate survival analysis was performed, using the Cox proportional hazards regression model. All statistical analyses were performed with JMP 9.0 (SAS Institute, Cary, NJ, USA). P<0.05 was considered to indicate a statistically significant result.
Results
PLA2GX expression in human CRC
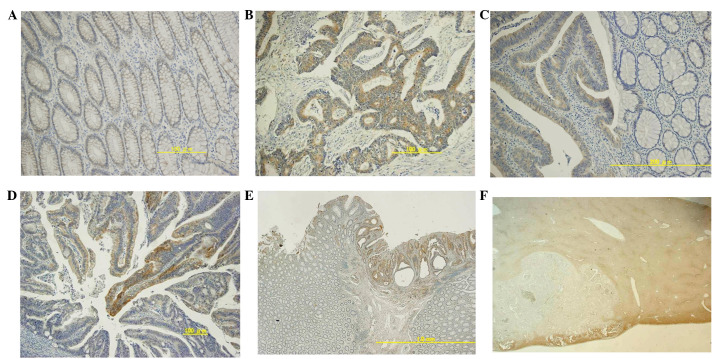
The staining patterns of PLA2GX in CRC specimens are shown in Fig. 1. In the normal colonic mucosa adjacent to the CRC, most of the colonic epithelial cells showed a weak expression of PLA2GX (Fig. 1A), although there was a variation of immunoreactivity among the cases. In the majority of the 158 tumors, PLA2GX expression was found predominantly in the cytoplasm of carcinoma cells and, compared with the normal epithelium, the staining signal was generally enhanced (Fig. 1B). The stromal tissue was not stained in any analyzed specimen. In 101 cases, PLA2GX expression was diffusely and almost equally detected in most of the cancer cells, as shown in Fig. 1B, whereas in 54 cases, the expression was observed only in focal cancer cells (Fig. 1D). In 3 cases, however, negligible staining of cancer cells was found. In addition, PLA2GX was hardly detected in the hepatic metastatic lesions, although the expression was diffuse in the primary lesion. (Fig. 1E and F). For further analyses, the tumors were divided into high expression (101 cases) and low expression (57 cases) groups.
Figure 1.
Expression pattern of PLA2GX in CRC specimens and normal counterparts. Staining in (A) the normal colonic mucosa (weak staining) ; (B) the cancer tissue (diffuse staining); (C) the boundary area between normal (right) and cancer (left); and (D) the cancer tissue (focal immunoreactivity). Representative cases of (E) primary CRC and (F) hepatic metastasis. PLA2GX, secretory phospholipase A2 group X; CRC, colorectal cancer. (A and B) Magnification, ×200. (C–E) Magnification, ×100. (F) Magnification, ×20.
Correlation between PLA2GX expression and clinicopathological features
Descriptive characteristics of the study subjects are presented in Table I. The expression of PLA2GX showed no correlation with age, gender, tumor size, tumor location, histological appearance, lymphatic invasion or venous involvement. However, the rate of hematogenous metastasis was significantly higher in the low PLA2GX expression group (10.5%) than in the high PLA2GX expression group (1.0%; P= 0.005). Similarly, there was a tendency for higher incidence of lymph node metastasis in the low PLA2GX expression group (50.9%) than in the high expression group (36.6%; P=0.081) (Table II). When the study sample was restricted to left-sided tumors (112 cases), nodal metastasis was observed in 52.4% (22/42) of the low PLA2GX expression group, which was significantly higher than that in the high expression group (30.0%; 21/70; P= 0.018). By contrast, when samples were restricted to right-sided tumors, no difference in the incidence of nodal metastasis was observed. Additionally, only border-line significance (P=0.051) was observed in the association between the PLA2GX expression and the UICC stage.
Table I.
Association of PLA2GX expression with clinical variables.
| PLA2GX
|
||||
|---|---|---|---|---|
| Factor | n | High expression (n=101) | Low expression (n=57) | P-value |
| Age (years), mean ± SD | 158 | 62.4±11.1 | 63.6±10.3 | 0.481 |
| Gender, n (%) | ||||
| Male | 96 | 41 (40.6) | 21 (36.8) | 0.642 |
| Female | 62 | 60 (59.4) | 36 (63.2) | |
| Size of tumor (mm), mean ± SD | 158 | 46.5±24.1 | 47.3±17.7 | 0.824 |
| T stagea, n (%) | ||||
| T1/T2 | 36 | 27 (26.7) | 9 (15.8) | 0.108 |
| T3/T4 | 122 | 74 (73.3) | 48 (84.2) | |
| Histological type, n (%) | ||||
| Well, mod differentiated | 152 | 96 (95.0) | 56 (98.2) | 0.285 |
| Muc, por differentiated | 6 | 5 (5.0) | 1 (1.8) | |
| Lymphatic invasion, n (%) | ||||
| Absent | 118 | 76 (75.2) | 42 (73.7) | 0.829 |
| Present | 40 | 25 (24.8) | 15 (26.3) | |
| Lymph node metastasis, n (%) | ||||
| Absent | 92 | 64 (63.4) | 28 (49.1) | 0.081 |
| Present | 66 | 37 (36.6) | 29 (50.9) | |
| Venous involvement, n (%) | ||||
| Absent | 75 | 50 (49.5) | 25 (43.9) | 0.495 |
| Present | 83 | 51 (50.5) | 32 (56.1) | |
| Location of the tumor, n (%) | ||||
| Colon | 117 | 78 (77.2) | 39 (68.4) | 0.229 |
| Rectum | 41 | 23 (22.8) | 18 (31.6) | |
| Right side | 46 | 31 (30.7) | 15 (26.3) | 0.559 |
| Left side | 112 | 70 (69.3) | 42 (73.7) | |
| UICC stage, n (%) | ||||
| I/II | 91 | 64 (63.4) | 27 (47.4) | 0.051 |
| III/IV | 67 | 37 (36.6) | 30 (52.6) | |
| Hematogenous metastasis, n (%) | ||||
| Absent | 151 | 100 (99.0) | 51 (89.5) | 0.005 |
| Present | 7 | 1 (1.0) | 6 (10.5) | |
UICC, Union for International Cancer Control;
TMN classification of malignant tumors, 7th edition according to UICC. PLA2GX, secretory phospholipase A2 group X; muc, mod, mucinous; moderately; por, poorly.
Table II.
Association of PLA2GX expression in the left side of the colon with clinical variables.
| PLA2GX, n (%)
|
||||
|---|---|---|---|---|
| Factor | n | High expression (n=70) | Low expression (n=42) | P-value |
| Lymphatic invasion | ||||
| Absent | 85 | 54 (77.1) | 31 (73.8) | 0.691 |
| Present | 27 | 16 (22.9) | 11 (26.2) | |
| Lymph node metastasis | ||||
| Absent | 69 | 49 (70.0) | 20 (47.6) | 0.018 |
| Present | 43 | 21 (30.0) | 22 (52.4) | |
| Venous involvement | ||||
| Absent | 54 | 35 (50.0) | 19 (45.2) | 0.625 |
| Present | 58 | 35 (50.0) | 23 (54.8) | |
| Hematogenous metastasis | ||||
| Absent | 105 | 69 (98.6) | 36 (85.7) | 0.017 |
| Present | 7 | 1 (1.4) | 6 (14.3) | |
PLA2GX, secretory phospholipase A2 group X.
Overall survival and DSS analysis of CRC with regard to PLA2GX expression
Next, we examined the correlation between PLA2GX expression and the outcome of patients, by the Kaplan-Meier analysis and the log-rank test. As shown in Fig. 2A, the high PLA2GX expression group tended to have a longer DSS, although the difference did not reach statistical significance (P=0.14). This trend was pronounced in left-sided CRC (P=0.08) and was observed in right-sided CRC (Fig. 2B).
Figure 2.
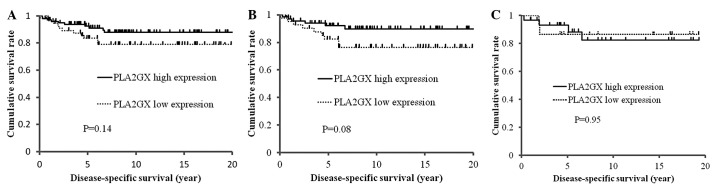
Survival outcomes of the patients grouped according to the expression of PLA2GX. Kaplan-Meier estimates of disease-specific survival of (A) all CRC patients, and those of (B) left- and (C) right-sided colon cancer. PLA2GX, phospholipase A2 group X.
Discussion
Due to their functional diversity, PLA2 enzymes have been implicated in various biological processes, including arthritis, asthma, defense against microbes, digestion, atherosclerosis and cancer (17). PLA2GX is known as the most potent sPLA2 capable of hydrolyzing phosphatidylcholine and acting extra-cellularly on cellular membranes and noncellular phospholipid substrates (5,9–11). It has been confirmed that PLA2GX, as well as other sPLA2s, including GIIA, GIII and GXIIA, are highly expressed in CRC tissue (13,14,18). However, no information is available concerning the correlation between PLA2GX expression in cancer and clinicopathological features. Thus, in the present study, we aimed to investigate the expression of PLA2GX in patients with CRC at various stages.
In our series, the majority of the carcinoma cells in primary CRC showed enhanced cytoplasmic expression of PLA2GX as compared with normal colonic epithelia, which is consistent with previous results (11,13,14). However, in 64% of the 158 cases, PLA2GX expression was diffusely detected, while in certain cases PLA2GX was only partially expressed and in a few cases, its expression was negative. In these cases, a significant inverse correlation was found between PLA2GX expression and hematogenous metastasis and, although without significance, also with nodal metastasis. As a consequence, patients with tumors with high PLA2GX expression had a better outcome than those with low expression. To the best of our knowledge, this is the first study to show the inverse correlation of the expression of PLA2GX with the outcome of CRC patients, and the possibility that this enzyme has suppressive effects on tumor metastasis in CRC is suggested.
Increased expression of sPLA2 has been demonstrated in numerous types of cancer, including breast (19,20), pancreatic (21), prostate (22,23), liver (24), gastric (25,26) and colorectal (14,27) cancer. Of these, group IIA PLA2 (PLA2GIIA) is one of the isoforms most commonly investigated in cancer tissue. Kashiwagi et al reported that the high expression of PLA2GIIA was correlated with longer survival in pancreatic cancer (28). More recently, Xing et al have shown the same tendency in gastric cancer, and suggested that PLA2GIIA may negatively affect the meta-static potential of gastric cancer cells (26). In fact, Ganesan et al have demonstrated that the silencing of the PLA2GIIA gene enhances the invasive activity of tumor cells, whereas enforced expression inhibited invasion (29). These results are consistent with our finding on PLA2GX, suggesting a possibility that PLA2GX, as well as PLA2GIIA, may play negative roles in the metastatic potential of gastrointestinal cancer. However, Buhmeida et al have shown a negative association of the expression of PLA2GIIA with the prognosis of stage II CRC (27). Also, Graff et al have shown that PLA2GIIA expression increases with the progression of prostate cancer in an androgen-independent manner (30). These controversial results suggest that the role of PLA2GIIA is dependent on the interaction of cancer cells with the microenvironment in different tissues.
Surrel et al have reported that, in vitro, the addition of PLA2GX stimulates the proliferative ability of colon cancer cells by producing various lipid mediators (12). However, in their study, the proliferation of the cells was examined in serum-free media, and the effect was not completely abrogated by COX and lipooxygenase inhibitors, suggesting the involvement of other lipid mediators. The released AA itself induces the apoptosis of diverse cells, including human colon cancer cells, via an intracellular ceramide-mediated pathway (31). Ceramide acts as a second messenger in the activation process of the cellular apoptotic machinery (31). Diets rich in unsatu-rated fatty acids such as AA are associated with a decreased incidence of colon cancer (32). On the bases of these facts, the negative effect of PLA2GX may be attributed to the increased levels of AA produced and the subsequent increased susceptibility to apoptosis.
Although there is no significant difference between PLA2GX expression and the tumor location, the rate of lymph node metastasis was found to be significantly lower and the DSS better in left-sided CRC than in right-sided. This finding suggests the possibility that CRCs developing in the two different colonic sites may have different biological behaviors. It is known that right- and left-sided colonic tumors have different molecular profiles, with microsatellite instability and methylator phenotypes being prevalent in right-sided tumors and chromosomal instability being predominant in left-sided tumors, resulting in different biological features (33,34). It is possible that these molecular profiles are intimately associated with the different lymphatic metastatic potentials. Indeed, the PLA2GX gene expression level was reported to be significantly higher in the normal mucosa of the left side of the colon than in that of the right side (13).
In conclusion, our study showed an inverse association between the reduced expression of PLA2GX and the increased metastatic potential of human CRC, especially in the left-sided tumors. Our data suggest that PLA2GX may have a protective role against the invasive ability of CRC, and the reduced ability to produce PLA2GX may result in the acquisition of a clinically more malignant phenotype. Thus, PLA2GX expression may be a potential clinical biomarker for the prediction of the invasive ability of human CRC. To date, several inhibitors of PLA2s have been developed as anticancer drugs (35). However, since PLA2s, in certain situations, may also have a suppressive effect on tumor progression, they may be two-edged swords, and thus their indication should be carefully considered.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodrigues S, Bruyneel E, Rodrigue CM, Shahin E, Gespach C. Cyclooxygenase 2 and carcinogenesis. Bull Cancer. 2004;91(Suppl 2):S61–S76. (In French). [PubMed] [Google Scholar]
- 4.Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem. 2011;150:233–255. doi: 10.1093/jb/mvr088. [DOI] [PubMed] [Google Scholar]
- 5.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 6.Touqui L, Wu YZ. Interaction of secreted phospholipase A2 and pulmonary surfactant and its pathophysiological relevance in acute respiratory distress syndrome. Acta Pharmacol Sin. 2003;24:1292–1296. [PubMed] [Google Scholar]
- 7.Triggiani M, Granata F, Frattini A, Marone G. Activation of human inflammatory cells by secreted phospholipases A2. Biochim Biophys Acta. 2006;1761:1289–1300. doi: 10.1016/j.bbalip.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Nevalainen TJ, Graham GG, Scott KF. Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Hanasaki K, Ono T, Saiga A, et al. Purified group X secretory phospholipase A(2) induced prominent release of arachidonic acid from human myeloid leukemia cells. J Biol Chem. 1999;274:34203–34211. doi: 10.1074/jbc.274.48.34203. [DOI] [PubMed] [Google Scholar]
- 10.Murakami M, Kambe T, Shimbara S, et al. Different functional aspects of the group II subfamily (Types IIA and V) and type X secretory phospholipase A(2)s in regulating arachidonic acid release and prostaglandin generation. Implications of cyclooxygenase-2 induction and phospholipid scramblase-mediated cellular membrane perturbation. J Biol Chem. 1999;274:31435–31444. doi: 10.1074/jbc.274.44.31435. [DOI] [PubMed] [Google Scholar]
- 11.Morioka Y, Ikeda M, Saiga A, et al. Potential role of group X secretory phospholipase A(2) in cyclooxygenase-2-dependent PGE(2) formation during colon tumorigenesis. FEBS Lett. 2000;487:262–266. doi: 10.1016/s0014-5793(00)02350-4. [DOI] [PubMed] [Google Scholar]
- 12.Surrel F, Jemel I, Boilard E, et al. Group X phospholipase A2 stimulates the proliferation of colon cancer cells by producing various lipid mediators. Mol Pharmacol. 2009;76:778–790. doi: 10.1124/mol.108.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mounier CM, Wendum D, Greenspan E, Fléjou JF, Rosenberg DW, Lambeau G. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br J Cancer. 2008;98:587–595. doi: 10.1038/sj.bjc.6604184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tribler L, Jensen LT, Jørgensen K, et al. Increased expression and activity of group IIA and X secretory phospholipase A2 in peritumoral versus central colon carcinoma tissue. Anticancer Res. 2007;27:3179–3185. [PubMed] [Google Scholar]
- 15.Degousee N, Ghomashchi F, Stefanski E, et al. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J Biol Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- 16.Masuda S, Murakami M, Matsumoto S, et al. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim Biophys Acta. 2004;1686:61–76. doi: 10.1016/j.bbalip.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A2 research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Murakami M, Masuda S, Shimbara S, Ishikawa Y, Ishii T, Kudo I. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A(2) J Biol Chem. 2005;280:24987–24998. doi: 10.1074/jbc.M502088200. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita S, Yamashita J, Sakamoto K, et al. Increased expression of membrane-associated phospholipase A2 shows malignant potential of human breast cancer cells. Cancer. 1993;71:3058–3064. doi: 10.1002/1097-0142(19930515)71:10<3058::aid-cncr2820711028>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita S, Yamashita J, Ogawa M. Overexpression of group II phospholipase A2 in human breast cancer tissues is closely associated with their malignant potency. Br J Cancer. 1994;69:1166–1170. doi: 10.1038/bjc.1994.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyohara H, Egami H, Kako H, et al. Immunohistochemical localization of group II phospholipase A2 in human pancreatic carcinomas. Int J Pancreatol. 1993;13:49–57. doi: 10.1007/BF02795199. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Neubauer BL, Graff JR, et al. Expression of group IIA secretory phospholipase A2 is elevated in prostatic intraepithelial neoplasia and adenocarcinoma. Am J Pathol. 2002;160:667–671. doi: 10.1016/S0002-9440(10)64886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Q, Patel M, Scott KF, Graham GG, Russell PJ, Sved P. Oncogenic action of phospholipase A2 in prostate cancer. Cancer Lett. 2006;240:9–16. doi: 10.1016/j.canlet.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Ying Z, Tojo H, Komatsubara T, et al. Enhanced expression of group II phospholipase A2 in human hepatocellular carcinoma. Biochim Biophys Acta. 1994;1226:201–205. doi: 10.1016/0925-4439(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 25.Leung SY, Chen X, Chu KM, et al. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc Natl Acad Sci USA. 2002;99:16203–16208. doi: 10.1073/pnas.212646299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing XF, Li H, Zhong XY, et al. Phospholipase A2 group IIA expression correlates with prolonged survival in gastric cancer. Histopathology. 2011;59:198–206. doi: 10.1111/j.1365-2559.2011.03913.x. [DOI] [PubMed] [Google Scholar]
- 27.Buhmeida A, Bendardaf R, Hilska M, et al. PLA2 (group IIA phospholipase A2) as a prognostic determinant in stage II colorectal carcinoma. Ann Oncol. 2009;20:1230–1235. doi: 10.1093/annonc/mdn783. [DOI] [PubMed] [Google Scholar]
- 28.Kashiwagi M, Friess H, Uhl W, et al. Group II and IV phospholipase A(2) are produced in human pancreatic cancer cells and influence prognosis. Gut. 1999;45:605–612. doi: 10.1136/gut.45.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ganesan K, Ivanova T, Wu Y, et al. Inhibition of gastric cancer invasion and metastasis by PLA2G2A, a novel beta-catenin/TCF target gene. Cancer Res. 2008;68:4277–4286. doi: 10.1158/0008-5472.CAN-07-6517. [DOI] [PubMed] [Google Scholar]
- 30.Graff JR, Konicek BW, Deddens JA, et al. Expression of group IIa secretory phospholipase A2 increases with prostate tumor grade. Clin Cancer Res. 2001;7:3857–3861. [PubMed] [Google Scholar]
- 31.Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998;95:681–686. doi: 10.1073/pnas.95.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy BS. Chemoprevention of colon cancer by dietary fatty acids. Cancer Metastasis Rev. 1994;13:285–302. doi: 10.1007/BF00666099. [DOI] [PubMed] [Google Scholar]
- 33.Bendardaf R, Lamlum H, Ristämaki R, Korkeila E, Syrjänen K, Pyrhönen S. Mismatch repair status is a predictive factor of tumour response to 5-fluorouracil and irinotecan chemotherapy in patients with advanced colorectal cancer. Tumour Biol. 2007;28:212–220. doi: 10.1159/000107417. [DOI] [PubMed] [Google Scholar]
- 34.Bendardaf R, Lamlum H, Ristamäki R, Korkeila E, Syrjänen K, Pyrhönen S. Thymidylate synthase and microsatellite instability in colorectal cancer: implications for disease free survival, treatment response and survival with metastases. Acta Oncol. 2008;47:1046–1053. doi: 10.1080/02841860701678753. [DOI] [PubMed] [Google Scholar]
- 35.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem Pharmacol. 2007;74:949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]