Abstract
Trypanosoma cruzi, the causative agent of Chagas disease, is a multiclonal parasite with high levels of genetic diversity and broad host and geographic ranges. Molecular characterization of South American isolates of T. cruzi has demonstrated homologous recombination and nuclear hybridization, as well as the presence of 6 main genetic clusters or “discrete typing units” (DTUs). Few studies have extensively investigated such exchange events and genetic diversity in North American isolates. In the current study, we genetically characterized over 50 US isolates from wildlife reservoirs (e.g., raccoons, opossums, armadillos, skunks), domestic dogs, humans, nonhuman primates, and reduviid vectors from nine states (TX, CA, OK, SC, FL, GA, MD, LA, TN) using a multilocus sequencing method. Single nucleotide polymorphisms were identified in sequences of the mismatch-repair class 2 (MSH2) and Tc52 genes. Typing based on the two genes often paralleled genotyping by classic methodologies using mini-exon and 18S and 24Sα rRNA genes. Evidence for genetic exchange was obtained by comparing sequence phylogenies of nuclear and mitochondrial gene targets, dihydrofolate reductase-thymidylate synthase (DHFR-TS) and the cytochrome oxidase subunit II- NADH dehydrogenase subunit I region (COII-ND1), respectively. We observed genetic exchange in several US isolates as demonstrated by incongruent mitochondrial and nuclear genes phylogenies, which confirms a previous finding of a single genetic exchange event in a Florida isolate. The presence of SNPs and evidence of genetic exchange illustrates that strains from the US are genetically diverse, even though only two phylogenetic lineages have been identified in this region.
Introduction
Trypanosoma cruzi, the causative agent of Chagas disease, is a clonally proliferative parasite with a heterogeneous population [1], [2]. It is a biologically, molecularly, and biochemically diverse parasite that has been detected in over 200 mammalian species, including humans [3]. Prior to advances in molecular biology and genetics, differences in T. cruzi were based solely on growth characteristics and manifestations of disease in various hosts [4]. Today, T. cruzi is segregated into six major discrete typing units, TcI to TcVI and a scarcely described Tcbat genotype [5]–[8]. Characterizing a strain of T. cruzi into one of these six genotypes is useful in determining the evolutionary ecology of the parasite in a region, as well as, associating biological characters with disease manifestations. Previously, a predominately clonal population structure for T. cruzi was accepted [9], but with recent evidence for genetic exchange events, hybridization, and mitochondrial introgression this model has been challenged [10]–[17].
Looking at ten intergenic regions of the T. cruzi genome in well-characterized isolates, TcV and TcVI have been confirmed as direct hybrids of parental groups TcII and TcIII [18]. Machado and Ayala [10] have also illustrated genetic exchange by comparing nuclear and mitochondrial gene phylogenies and while the majority of isolates included in their study were from South and Central America, three isolates were from the US, of which one exhibited genetic exchange. Additionally, experimental evidence of genetic exchange in a laboratory system was revealed with the hybridization of clones [11]; however, such events in nature are rare [9] and the mechanism of recombination in T. cruzi is still unknown.
Because T. cruzi is such a significant cause of morbidity and mortality in Central and South America, considerable characterization work on T. cruzi has been conducted in these regions, but because human cases in the US are rare, little work has been conducted to characterize US isolates. Since 1955, six autochthonous human cases have been reported in the United States with the most recent occurring in 2006 [19]–[23]. In addition to these six cases, over 1,750 individual blood donors currently residing in the United States were positive for antibodies reactive to T. cruzi [24]. The objective of this study was to explore the molecular diversity of T. cruzi from the US. Our goals were to investigate genetic exchange in US isolates and compare sequences of several gene targets with those from South America to identify evidence of genetic diversity between these different regions. To accomplish these goals, nucleotide sequences of two nuclear genes, the mismatch-repair class 2 gene (MSH2) and the thiol-disulfide oxido-reductase Tc52 gene (Tc52), were compared with a selection of T. cruzi isolates from the United States to identify single nucleotide polymorphisms that indicate heterogeneity and potential virulence differences. To investigate potential genetic exchange, the phylogenies of a nuclear gene [dihydrofolate reductase-thymidylate synthase (DHFR-TS)] and mitochondrial gene targets [cytochrome oxidase subunit II-NADH dehydrogenase subunit I region (COII-ND1)] were compared.
Materials and Methods
Ethics Statement
Samples labeled as “human” origin and from various animals were obtained through a material transfer agreement with CDC, Pasteur Institute, and Southeastern Cooperative Wildlife Disease Study. The conditions and approvals for the archived samples are unknown to the authors. No authors came in contact with human subjects during the research. Remaining samples from animals were obtained by handling wild-trapped animals. These animals were cared for in accordance with the guidelines of the Institutional Animal Care and use Committee and under animal use protocol A2009-3-006 approved by this committee at the University of Georgia.
Isolates
T. cruzi was isolated from multiple species of free-ranging and captive wildlife, domestic animals, triatomine bug vectors, and humans who were autochthonously infected in the United States; host and origin of each isolate can be seen in Table 1. Some isolates were obtained as liquid nitrogen-stored parasites from the Centers for Disease Control and Prevention, Pasteur Institute, and the Southeastern Cooperative Wildlife Disease Study and were established in axenic LIT medium as previously described [25]. Additional isolates were obtained from wild-trapped animals in axenic LIT medium or canine macrophage-cell culture as previously described [26]. Biological clones are indicated with the prefix “clX.”
Table 1. Lineage typing of Trypanosoma cruzi isolates from the United States.
| Gene target | |||||||
| Host (Order) | Isolate | Origin | Lineage>>† | Tc52 | MSH2 | DHFR-TS | COII-ND1 |
| Human | TC CC | Corpus Christi, TX | I | I | I | I‡ | n.d. |
| (Primate) | CA R | California | I | I | I | I | I |
| TC California | Lake Don Pedro, TX | I | I | I | n.d. | I‡ | |
| Domestic Dog | Caesar Dog | Not known | IV | IV | IV | IV | IV |
| (Carnivora) | Dog Theis | Not known | IV | IV | IV | IV | IV |
| Griffin Dog | Hillsboro, TN | I/IV | IV/I | IV/I | IV | I | |
| OK Dog | Bartlesville, OK | IV | IV | IV | IV | IV | |
| Samantha Dog | South Carolina | IV | IV | IV | IV | IV | |
| Smokey | South Carolina | IV | IV | IV | IV | IV‡ | |
| USA Dog Y | California | IV | I | I | I | IV | |
| VA Opossum | 92101601P cl2 | Statesboro, GA | n.d. | I | I | I | IV |
| (Didelphimorphia) | 93041401P cl1 | Statesboro, GA | I | I | I | I | IV‡ |
| 93070103P cl2 | Fort Stewart, GA | I | I | I | I | IV | |
| FH4 | South Georgia | I | I | I | I | IV | |
| FL Opo 2 | Wakulla Springs, FL | I | I | I | I | IV | |
| FL Opo 3 | Wakulla Springs, FL | I | I | I | I | IV | |
| FL Opo 15 | Maclay State Park, FL | I | I | I | n.d. | IV | |
| Opossum 1970 | New Orleans, LA | I | I | I | I | I‡ | |
| USA Opossum | South Louisiana | I | I | I | I | I | |
| Raccoon | 92122102R | Statesboro, GA | IV | IV | IV | IV | IV |
| (Carnivora) | 93040701R cl2 | Statesboro, GA | IV | IV | IV | IV | IV |
| 93053103R cl3 | Harrold Preserve, GA | I | I | I | I | I | |
| 93071502R cl2 | Fort Stewart, GA | IV | IV | IV | IV | IV | |
| 93072805R cl3 | Fort Stewart, GA | IV | IV | IV | IV | IV | |
| FL Rac 13 | Maclay State Park, FL | I/IV | I | I | IV | n.d. | |
| FL Rac 15 | Wakulla Springs, FL | IV | IV | IV | IV | IV | |
| FL Rac 30 | Wakulla Springs, FL | IV | IV | IV | IV | IV | |
| FL Rac 46 | Tall Timbers, FL | IV | IV | IV | IV | n.d. | |
| FL Rac 5 | Torreya State Park, FL | IV | IV | IV | IV | n.d. | |
| FL Rac 7 | Lake Talquin, FL | IV | IV | IV | IV | IV | |
| FL Rac 9 | Torreya State Park, FL | IV | IV | IV | IV | IV | |
| GA Rac 107 | Ossabaw Island, GA | IV | IV | IV | IV | IV | |
| GA Rac 134 | Whitehall Forest, GA | IV | IV | IV | IV | IV | |
| GA Rac 143 | Athens, GA | IV | I | IV | IV | IV | |
| GA Rac 45 | Skidaway Island, GA | IV | IV | IV | IV | n.d. | |
| GA Rac 69 | Athens, GA | IV | IV | IV | IV | IV | |
| Maryland Rac | Laurel, MD | IV | IV | IV | IV | IV‡ | |
| STC 10R cl3 | St. Catherine's Island, GA | IV | IV | IV | IV | IV | |
| STC 35R | St. Catherine's Island, GA | IV | IV | IV | IV | IV | |
| TN Rac 18 | Rutherford Co., TN | IV | IV | IV | IV | IV | |
| T. sanguisuga | Florida | Gainesville, FL | I | I | I | I | n.d. |
| (Hemiptera) | Florida C1F8 | Gainesville, FL | I | I | I | I | IV |
| T. sang 5 cl1 | Bulloch Co., GA | I | IV | IV | IV | n.d. | |
| RT lemur | Nilda | St. Catherine's Island, GA | IV | IV | n.d. | IV | n.d. |
| (Primate) | Clarence | St. Catherine's Island, GA | IV | IV | IV | IV | IV‡ |
| Meg | St. Catherine's Island, GA | IV | IV | IV | n.d. | IV‡ | |
| Rh. Macaque (Primate) | Texas Theis | Not known | I | IV | IV | n.d. | IV |
| Nb Armadillo (Cingulata) | Armadillo 1973 | New Orleans, LA | I | I | I | I | I |
| GA Arm 20 | Ossabaw Island, GA | IV | IV | IV | IV | IV | |
| USA Armadillo | South Louisiana | I | I | I | I | I | |
| Str. Skunk (Carnivora) | GA Sk 1 | Ludiwici, GA | IV | IV | IV | n.d. | IV‡ |
Table abbreviations: VA opossum = Virginia Opossum; RT lemur = Ring-tailed Lemur; Rh. Macaque = Rhesus Macaque; Nb Armadillo = Nine-banded Armadillo; Str. Skunk = Striped Skunk; n.d. = not determined.
previously characterized using mini-exon, D7 divergent domain of 24 s alpha rRNA, and 18 s rRNA genetic analysis in [34].
partial sequences were analyzed.
Molecular Technique
Template was obtained for polymerase chain reactions by boiling parasites for 15 min and using the resulting supernatant for DNA extraction with the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA) following the manufacturer's protocol. PCR amplification with GoTaq Taq polymerase (Promega Corporation, Madison, WI) was completed for the four gene targets, MSH2, Tc52, DHFR-TS, and COII-ND1, following the respective previously published protocols [27], [28], [10]. DNA extraction, amplification, and product analysis were performed in separate dedicated laboratory areas. A negative water control was included in each set of extractions and PCR reactions as contamination controls. Sequencing reactions were performed at the Clemson University Genomics Institute (Clemson, SC). Sanger sequencing reactions were carried out with purified PCR product and amplicons were bidirectionally sequenced on an ABI 3100 Automated Sequencer using the provided ABI equipment software for basecalling and sequencing analysis (Applied Biosystems, Foster City, CA). In the case of COII-ND1 products, reactions that did not yield complete sequences were identified and a heminested PCR reaction was performed using primers ND1.3A and COII.2A in the primary reaction and ND1.3S and COII.A400 or COII.2A and COII.A400R in the secondary reactions [10]. Because of the size (1,473 bp, DHFR-TS; 1,226 bp, COII-ND1; 875 bp, MSH2; 1,300 bp, Tc52) and inherent properties of the amplicons, cloning attempts were unsuccessful. Therefore, multiple sequences were obtained for each isolate and polymorphisms were noted if observed in at least two sequences for an analyzed sample.
Phylogenetic Analysis and Genotyping
Contiguous sequences were assembled in Sequencher and sequences aligned by the Clustal Wallis method in Mega4. Three phylogenetic trees were created by neighbor-joining, minimum evolution and maximum parsimony methods from the alignment of each gene target with the bootstrap consensus tree being inferred from 500 replicates and the bat trypanosome T. cruzi marinkellei [593 (B3)] or T. brucei [TReu927] used as the outgroup [29]–[32]. A strict consensus tree was interpreted from the three methods based on topology with no observed bootstrap supported incongruencies. Evolutionary distances were computed using the Kimura 2-parameter method [33]. Lineage typing of each isolate was performed with whole or partial sequences of the obtained gene sequence and a BLAST search was administered on GenBank to determine sequence identity with previously genotyped T. cruzi strains. Nucleotide sequence data reported in this paper are available in the GenBank database under accession numbers: GU212870-GU212990, GU212992-GU213035.
Results
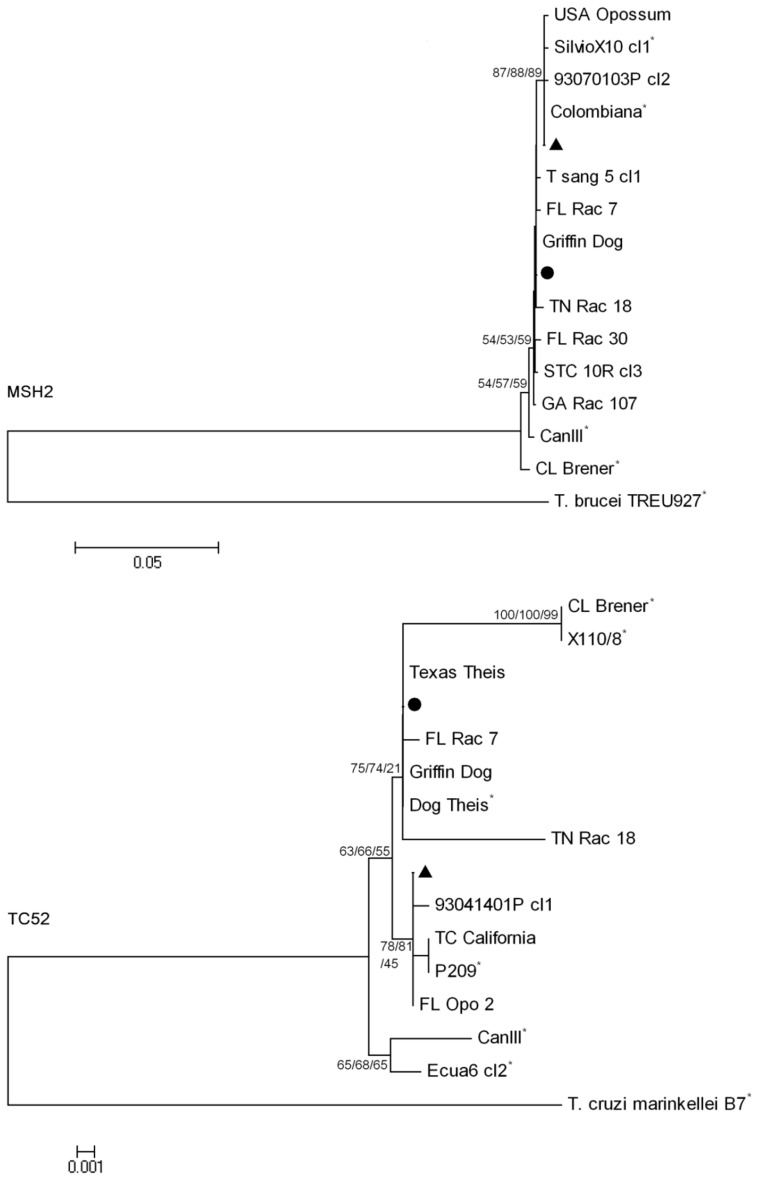
Based on MSH2, Tc52, and DHFR-TS sequences, all human (TcI), ring-tailed lemurs (TcIV), armadillo (TcI or TcIV) and skunk (TcIV) isolates were genotyped as the equivalent lineages previously determined [34]. In contrast, some isolates from domestic dogs, Virginia opossums, raccoons, a rhesus macaque, and a Triatoma sanguisuga were classified as different lineages by different gene targets. The domestic dog isolate ‘Griffin Dog,’ previously thought to be a mixed population of TcI and TcIV [34], was confirmed in this study to have multiple sequences consistent with the TcI and TcIV reference isolates. A mixed population was demonstrated with polymorphic positions identified in the MSH2 and Tc52 genes (Tables 2 and 3).
Table 2. Nucleotide sequence variations within the MSH2 gene sequence of 50 T. cruzi isolates from the United States compared to reference strains.
| Genotype/Isolate | Nucleotide Position | ||||||||||||||||||||
| 71 | 97 | 109 | 172 | 244 | 351 | 367 | 373 | 403 | 404 | 460 | 478 | 490 | 500 | 634 | 645 | 658 | 735 | 750 | 775 | 816 | |
| TcI Reference strain (Silvio X10 cl1) | C | A | G | A | C | T | A | G | G | G | A | T | C | A | T | C | A | C | A | G | A |
| TcI – 17 US sequences* | • | • | • | • | • | A | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • |
| TcI-USA Opossum | • | • | • | • | • | A | • | • | • | • | • | • | • | • | • | • | • | • | • | • | G |
| TcI-93070103P cl2 | • | • | • | • | • | A | • | • | • | • | • | Y | • | • | Y | S | • | • | T | • | • |
| Amino acid change | V→D | S→C | |||||||||||||||||||
Genotype of each isolate precedes the isolate name. Nucleotide positions correspond to sites from SilvioX10 cl1 (Genbank AY540739). Dots represent nucleotide site identical to reference strain (either Silvio X10 cl1 for TcI or CANIII cl1 for TcIV).
The following 17 US TcI sequences were identical: Human isolates (TC CC, CA R, TC California), domestic dogs (USA Dog Y), Virginia opossums (92101601P cl2, 93041401P cl2, FH4, FL Opo 2, FL Opo 3, FL Opo 15, Opossum 1970), armadillos (Armadillo 1973, USA Armadillo), triatomine bugs (Florida C1F8, Florida), and raccoons (93053103R cl3, FL Rac 13).
The following 23 US TcIV sequences were identical: Raccoons (FL Rac 9, 92122102R, 93071502R cl2, 93040701R cl1, 93072805R cl3, FL Rac 15, FL Rac 46, FL Rac 5, GA Rac 134, GA Rac 143, GA Rac 69, Maryland Rac, STC 35R), domestic dogs (Samantha Dog, Caesar Dog, Dog Theis, OK Dog, Smokey), ring-tailed lemurs (Clarence, Meg), rhesus macaque (Texas Theis), striped skunk (GA Sk 1), and armadillo (GA Arm 20).
Table 3. Nucleotide sequence variations within the Tc52 gene sequence of 51 T. cruzi isolates from the United States compared to reference strains.
| Genotype/Isolate | Nucleotide Position | |||||||||||||||||
| 85 | 91 | 121 | 148 | 151–153 | 155–156 | 159–160 | 200 | 221 | 231 | 242 | 336 | 357 | 392 | 443 | 500 | 560 | 588 | |
| TcI reference strain (P209) | C | A | T | G | ACA | GG | CT | A | A | T | T | A | C | T | G | A | G | A |
| TcI – 17 US sequences* | • | • | • | • | ••• | •• | •• | • | • | • | • | • | • | • | • | • | • | • |
| TcI-TC California | • | • | • | • | ••• | •• | •• | • | • | • | • | • | • | • | • | • | • | • |
| TcI-93041401P cl1 | • | • | C | • | ••• | •• | •• | • | • | • | • | • | • | • | • | • | • | • |
| TcI-FL Opo 2 | • | • | • | • | ••• | •• | •• | • | • | • | • | • | • | • | • | • | • | • |
| Amino acid change | V→A | L→P | ||||||||||||||||
| TcIV reference strain (CANIII cl1) | A | A | T | G | ACA | GG | CT | G | A | T | C | G | G | C | G | G | G | G |
| TcIIa- 27 US sequences† | C | • | • | • | ••• | •• | •• | A | G | G | T | • | • | • | A | • | • | A |
| TcIIa-TN Rac 18 | C | C | • | A | GGT | CA | GA | A | G | G | T | • | • | • | A | • | • | A |
| TcIIa-FL Rac 7 | C | • | • | • | ••• | •• | •• | A | G | G | T | • | • | • | A | • | A | A |
| TcI/IIa-Texas Theis | • | • | • | • | ••• | •• | •• | A | G | G | • | • | • | • | A | • | • | A |
| TcI/IIa-Griffin Dog | • | • | • | • | ••• | •• | •• | A | R | K | • | K | S | Y | R | R | • | A |
| Amino acid change | E→A | E→A | Y→W | K→Y | E→K | L→E | S→A | A→T | ||||||||||
Genotype of each isolate precedes the isolate name. Nucleotide positions correspond to sites from P209 (Genbank EF065175). Dots represent nucleotide site identical to reference strain (either P209 for TcI or CANIII cl1 for TcIV). Dashes represent missing nucleotides.
The following 17 US TcI sequences were identical: Human isolates (TC CC, CA R), domestic dogs (USA Dog Y), Virginia opossums (USA Opossum, Opossum 1970, 92101601P cl2, FH4, 93070103P cl2, FL Opo 3, FL Opo 15), armadillos (Armadillo 1973, USA Armadillo), triatomine bugs (Florida C1F8, Florida), and raccoons (GA Rac 143, 93053103R cl3, FL Rac 13).
The following 26 US TcIV sequences were identical: Raccoons (STC 10R cl3, FL Rac 9, 92122102R, 93071502R cl2, 93040701R cl1, 93072805R cl3, FL Rac 15, FL Rac 46, FL Rac 5, FL Rac 30, GA Rac 134, GA Rac 69, GA Rac 107, Maryland Rac, STC 35R), domestic dogs (Samantha Dog, Caesar Dog, Dog Theis, OK Dog, Smokey), ring-tailed lemurs (Clarence, Meg, Nilda), striped skunk (GA Sk 1), triatomine bug (T sang5 cl1), and armadillo (GA Arm 20).
Single nucleotide polymorphisms (SNPs) were observed in the MSH2 and Tc52 genes of the analyzed sequences compared to TcI and TcIV reference strains from South America (Tables 2 and 3). The majority of sequences for the MSH2 (19TcI and 24 TcIV) and Tc52 (18 TcI and 27 TcIV) genes were identical among the US isolates (Tables 2 and 3). For the MSH2 gene, only one or two nucleotides distinguished US TcI isolates from the reference strain (Silvio X10 cl1) while four to six nucleotides distinguished US TcIV from the reference strain (CANIII cl1) (Table 2). For the Tc52 gene, three to four nucleotide substitutions distinguished the US TcI isolates from the reference strain (P209) with one exception; a human isolate from a California patient (Tc California) was identical to the South American reference strain (Table 3). Numerous SNPs [13]–[22] distinguished the Tc52 sequences of US and reference TcIV strains (CANIII cl1). Overall at least four SNPs were identified in these two genes that could be used to separate US isolates of TcI and TcIV from the two South American reference strains (Tables 2 and 3). Several nucleotide changes resulted in amino acid changes (Tables 2 and 3). The phylogenies of the two gene targets show the clustering of isolates with their respective genotype, including those that exhibited unique SNPs (Figure 1).
Figure 1. Evolutionary relationships among mismatch-repair class 2 gene (MSH2) and the thiol-disulfide oxido-reductase Tc52 gene (Tc52) from 50 and 51 Trypanosoma cruzi isolates, respectively.
Three phylogenetic trees were created by neighbor-joining (NJ), minimum evolution (ME), and maximum parsimony (MP) methods from the alignment of each gene target and a consensus tree was interpreted. Numbers at the branches are bootstrap values >50% (500 replicates) for the same nodes of the NJ, ME, MP trees. Evolutionary distances were computed using the Kimura 2-parameter method [29]. ▴ = the 17 US TcI isolates that were identical; • = the 24 or 27 US TcIIa isolates that were identical. * = reference strains: SilvioX10 cl1, Colombiana, P209 (TcI); X110/8 (TcIII); CANIII cl1, Dog Theis, Ecua6 (TcIV); CL Brener (TcVI).
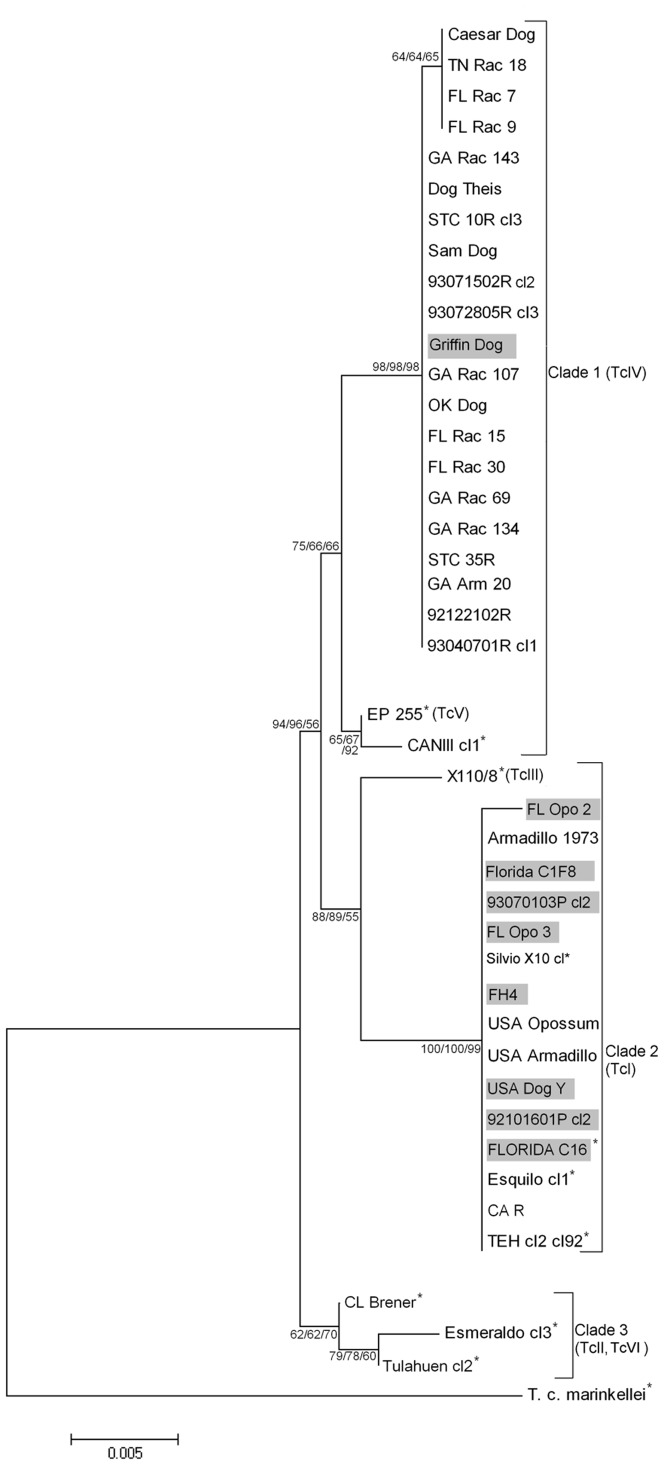
Phylogenetic analysis of the nuclear gene region, DHFR-TS, supported the findings of the Tc52 and MSH2 gene analyses and resulted in a tree that had a similar topology to a previous study [10] with three major clades (Figure 2). Little diversity was present between US TcIV isolates, but all US TcIV sequences branched separately from the reference South American TcIV sequence. Similar to sequence analysis results for the DHRF-TS gene of various TcI isolates [10], limited differences were noted within the US isolates as no separation of TcI sequences was present (Figure 2). TcIV isolates had 99% sequence identity to the CANIII cl1 reference strains with 7 SNPs; TcI isolates had 99% sequence identity to the Silvio X10 cl1 reference strain with 4 SNPs.
Figure 2. Evolutionary relationships among dihydrofolate reductase-thymidylate synthase (DHFR-TS) from 43 Trypanosoma cruzi isolates.
Three phylogenetic trees were created by neighbor-joining (NJ), minimum evolution (ME), and maximum parsimony (MP) methods from the alignment of each gene target and a consensus tree was interpreted. Numbers at the branches are bootstrap values >50% (500 replicates) for the same nodes of the NJ, ME, MP trees. Evolutionary distances were computed using the Kimura 2-parameter method [29]. The nine isolates with positions incongruent to the mitochondrial phylogenies (Fig. 3) are highlighted. * = reference strains. Sequences clustered in 3 clades: Clade 1 includes TcIV T. cruzi isolates from the US and reference TcIV and TcV S. America strains. T. cruzi isolates of TcI lineage from the US and reference strains clustered in Clade 2, while Clade 3 consists on TcII and TcVI S. American reference strains.
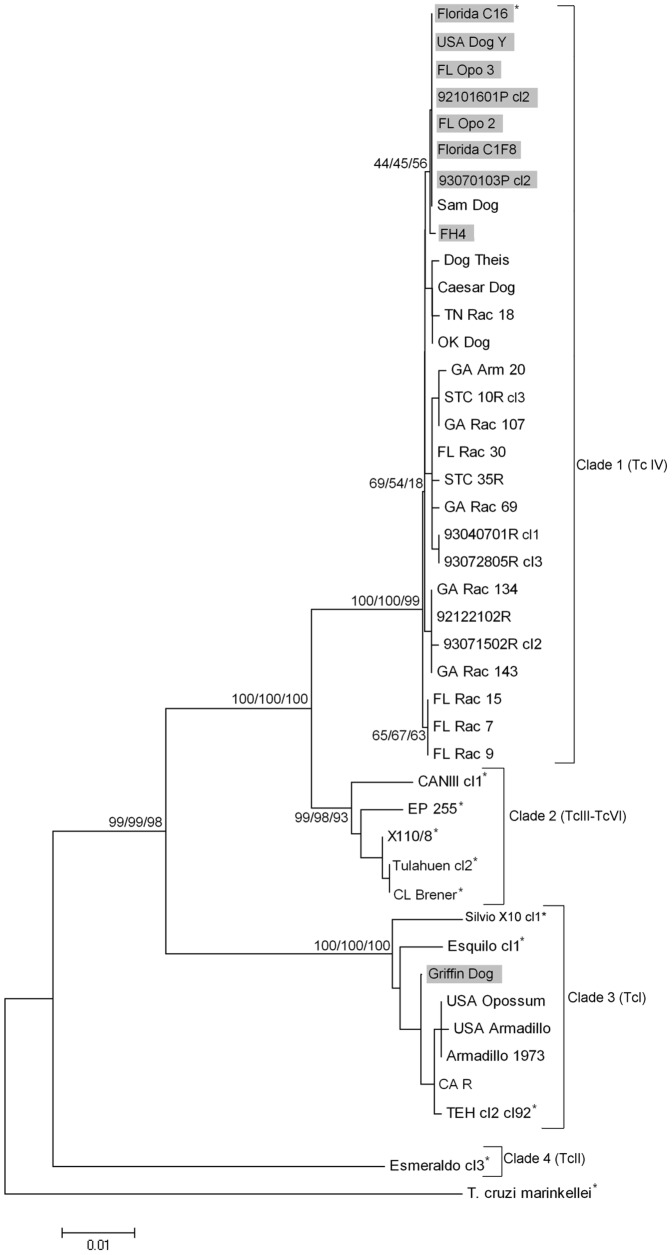
The phylogeny for the COII-ND1 mitochondrial region showed greater divergence with four clades containing additional divisions (Figure 3). Eight isolates that were classified as TcI by analysis of various nuclear genes (e.g., 18S, mini-exon, 24S alpha, MSH2, Tc52, DHFR-TS) were classified as TcIV by phylogenetic analysis of COII-NDI sequences. A single TcIV isolate, Griffin Dog, had an incongruent phylogenetic position. These nine isolates are highlighted in Figures 2 and 3.
Figure 3. Evolutionary relationships among cytochrome oxidase subunit II- NADH dehydrogenase subunit I region (COII-ND1) from 43 Trypanosoma cruzi isolates.
Three phylogenetic trees were created by neighbor-joining (NJ), minimum evolution (ME), and maximum parsimony (MP) methods from the alignment of each gene target and a consensus tree was interpreted. Numbers at the branches are bootstrap values >50% (500 replicates) for the same nodes of the NJ, ME, MP trees. Evolutionary distances were computed using the Kimura 2-parameter method [29]. The nine isolates with positions incongruent to the nuclear phylogenies (Fig. 2) are highlighted. * = reference strains. Sequences clustered in 4 distinct clades. Clade 1 contains exclusively US origin TcIV strains of T. cruzi. Reference TcIII-TcIV strains of T. cruzi clustered in Clade 2. TcI T. cruzi from the US and S. American reference strains clustered in Clade 3, while a separation of a TcII reference strain results in the fourth clade.
SNPs were not observed among the COII-ND1 US TcIV isolate sequences, but they only had 96% sequence identity to the CANIII cl1 reference strain with 36 SNPs (data not shown). Among the COII-ND1 US TcI isolate sequences, 88 SNPs were identified so that two TcI alleles were identified (data not shown). One allele corresponds to TcI strains proposed to exhibit evidence of genetic exchange based on the mitochondrial and nuclear incongruent phylogenies seen in Figures 2 and 3, while the other consists of TcI strains that do not show evidence of such events. Compared with the TcI reference strain, those that did exhibit exchange events had 92% sequence similarity to the Silvio X10 cl1 reference strain, with 85 SNPs; sequences without genetic exchange had 98% sequence identity to Silvio X10 cl1, with only 19 SNPs.
Discussion
In the current investigation, genetic diversity was demonstrated among T. cruzi isolates from the United States. T. cruzi strains are currently categorized into six major lineages, TcI to TcVI and a Tcbat genotype [5]–[8]. All six major genotypes have been characterized from South American isolates from various host species [35]. Contrastingly, strains from Mexico and Central America (Guatemala) have been characterized as TcI (both) and TcIV (Guatemala only), with a clear predominance of TcI isolates [36]–[39]. Isolates from the United States, have also been characterized only as TcI or TcIV [34], [40], [41]. Further confirming the paucity of genotypes in North America, in the current study, sequences of additional gene targets had sequence identity only to either TcI or TcIV. Regardless of gene target, TcIV isolates were clearly distinguished from the South American TcIV reference strain which provides additional evidence for considerable divergence within this lineage [10], [42]–[44].
To investigate genetic diversity among US T. cruzi isolates, the sequences of two nuclear genes, Tc52 and MSH2, were analyzed to identify SNPs. Tc52 is a single-copy gene constitutively-expressed in all developmental stages of T. cruzi and is implemented in the immune response to T. cruzi infection, where it suppresses T-cell proliferation by scavenging cysteine and glutathione (GSH) [45], [46]. Similar to previous findings [28], [47], [42], numerous SNPs were found in the sequences of 50 isolates analyzed in this study. Of the 47 SNPs identified, 17 resulted in amino acid changes, several of which have been previously linked to GSH binding [28]. Additional research is needed to determine if there is an association of these SNPs with biological differences (e.g., virulence) due to changes in GSH binding efficacy or between isolates from the US and those from South America.
Polymorphisms were also identified in MSH2, a homologue of the mutS gene of other eukaryotes [48]. The MSH2 protein is a part of the mismatch repair machinery that binds base-base mismatches and excises and repairs them. In T. cruzi, MSH2 is also a single copy gene that is constitutively-expressed in all life stages of the parasite [48]. In the current investigation, we identified 21 SNPs of the MSH2 gene, including several that could distinguish between TcI and TcIV strains. Previous findings suggested that SNPs in TcII lineage had decreased mismatch-repair ability compared to TcI strains [27]. In our study, the majority of genetic variability was noted in the TcIV isolates. Interestingly, TcIV isolates from the US tend to be less virulent to laboratory mice and to date, no human infections with this genotype have been reported in North America [49]; TcIV strains have been isolated from primates, prosimians, and domestic dogs [34], [50], [51]. Previously, different T. cruzi MSH2 phenotypes have exhibited different levels of susceptibility to cisplatin and oxidative damage [48], [52]. The ability or inability to withstand such external pressures from DNA damaging compounds was associated with genetic variability and subsequent strain differences [48], [52]. One may speculate, then, that the sequence differences within and between lineages observed in the current study may result in phenotypic differences affecting drug susceptibility. In addition to identifying sequence differences in these US isolates, phylogenies were constructed for DHFR-TS (nuclear) and COII-ND1 (mitochondrial) to elucidate genealogical relationships among isolates and illustrate evidence for genetic exchange. The nuclear phylogeny of DHFR-TS exhibited three major clades. Isolates of TcI from the US clustered with S. American isolates, illustrating the limited genetic variability of the lineage reported in previous studies with this gene [10], [39]. Although genetic variability among TcI isolates was minimal for nuclear gene targets in this study, considerable biological differences between isolates have been previously noted [53]–[55]. Other studies have differentiated TcI isolates using the microsatellite analysis, comparative genome hybridization, and mini-exon and cytochrome b genes, sometimes suggesting the subdivision of the lineage [13], [14], [56]–[59]. Division between N. and S. American TcIV strains may be evidence of the independent evolution of N. American TcIV strains from its ancestral S. American strains, as is supported by the results of this and previous studies [43], [60].
The phylogeny of COII-ND1 demonstrated greater genetic diversity with additional clustering occurring within the four clades present. As with the nuclear phylogeny, TcIV strains from the US diverged from TcIV strains of S. America. Additional clusters within the US TcIV clade indicate additional genetic diversity within the group; however, several of these subclades had low bootstrap support. The clade representing TcI strains contained both US isolates from this study and South American isolates, which is consistent with the nuclear DHFR-TS phylogeny. As previously suggested, the clustering of all TcI sequences may be due to a single origin of these strains [39], [61]. It is also possible that TcI represents a more recent introduction or spread into North America compared with TcIV, which may have been separate from the South American strains for a significant period of time which would allow divergence.
The most compelling finding from the COII-ND1 phylogeny is the clustering of several TcI strains (classified based on several nuclear genes) within the US TcIV clade. Incongruencies between nuclear and mitochondrial phylogenies have been previously reported with S. American isolates and a single US isolate and is interpreted as evidence of rare genetic exchange events in the T. cruzi population [10], [62]. These findings in addition to in vitro demonstration of genetic recombination and previous multilocus sequence typing studies illustrate that genetic exchange does occur, albeit rarely [10], [11], [43], [62]. In the current study, several TcI sequences represent isolates that may have undergone genetic exchange in comparison to few TcIV sequences. This suggests that TcI isolates may be more susceptible or likely to have recombination, possibly as a result of more rapid evolution in this lineage [61]. While these phylogenies can be associated with genetic exchange, the role of such events in driving the evolution of the species has not been explored [61]. In the current study, we identified several isolates with evidence of genetic exchange. While only two (TcI and TcIV) of the six genealogical lineages have been detected circulating in mammal populations in the US, the presence of SNPs and evidence of genetic exchange suggest that parasite populations in the US are genetically diverse.
Acknowledgments
The authors thank E. Brown, B. Wilcox and B. Hanson (SCWDS), and D. Kavanaugh (USDA, Wildlife Services) for field assistance.
Funding Statement
This study was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grant R15 AI067304. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Miles MA, Souza A, Povoa M, Shaw JJ, Lainson E, et al. (1978) Isozymic heterogeneity of Trypanosoma cruzi in the first autochthonous patients with Chagas' disease in Amazonian Brazil. Nature 272: 819–821. [DOI] [PubMed] [Google Scholar]
- 2.Miles MA (1979) Transmission cycles and the heterogeneity of Trypanosoma cruzi in Amazonian forest. In: WHR Lumsden, DA Evans (eds), Biology of Kinetoplastida, Vol. 2, London, New York, San Francisco: Academic Press. p. 117–196.
- 3. Barretto MP, Ribeiro RD (1979) Reservatorios silvestres do Trypanosoma cruzi . Rev Inst Adolfo Lutz 39: 25–26 [in Portugese]. [Google Scholar]
- 4.Hoare CA (1972) The trypanosomes of mammals. Oxford, UK: Blackwell Scientific. p. 753.
- 5. Brisse S, Barnabé C, Tibayrenc M (2000) Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int J Parasitol 30: 35–44. [DOI] [PubMed] [Google Scholar]
- 6. Brisse S, Verhoef J, Tibayrenc M (2001) Characterization of large and small subunit rRNA and mini-exon genes further support the distinction of six Trypanosoma cruzi lineages. Int J Parasitol 31: 1218–1226. [DOI] [PubMed] [Google Scholar]
- 7. Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, et al. (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104: 1051–1054. [DOI] [PubMed] [Google Scholar]
- 8. Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo A, et al. (2012) The revised Trypanosoma cruzi subspecific nomenclature: Rationale, epidemiologic relevance and research applications. Infect Gen Evol 12: 240–253. [DOI] [PubMed] [Google Scholar]
- 9. Tibayrenc M, Ayala FJ (2002) The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol 18: 405–410.10. [DOI] [PubMed] [Google Scholar]
- 10. Machado CA, Ayala FJ (2001) Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi . PNAS 98: 7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, et al. (2003) Mechanism of genetic exchange in American trypanosomes. Nature 421: 936–939. [DOI] [PubMed] [Google Scholar]
- 12. Sturm NR, Campbell DA (2010) Alternative lifestyles: the population structure of Trypanosoma cruzi . Acta Trop 115: 35–43. [DOI] [PubMed] [Google Scholar]
- 13. Minning TA, Weatherly DB, Flibotte S, Tarleton RL (2011) Widespread, focal copy number variations (CNV) and whole chromosome aneuploidies in Trypanosoma cruzi strains revealed by array comparative genomic hybridization. BMC Genomics 12: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrera C, Bargues MD, Fajardo A, Montilla M, Triana O, et al. (2007) Identifying four Trypanosoma cruzi I isolates haplotypes from different geographic regions in Colombia. Infect. Gen Evol 7: 535–539. [DOI] [PubMed] [Google Scholar]
- 15. Barnabe C, Breniere SF (2012) Scarce events of mitochondrial introgression in Trypanosoma cruzi: new case with a Bolivian strain. Infect Gen Evol 12: 1879–1883. [DOI] [PubMed] [Google Scholar]
- 16. Ferreira R, Briones MRS (2012) Phylogenetic evidence based on Trypanosoma cruzi nuclear gene sequences and information entropy suggest that inter-strain intragenic recombination is a basic mechanism underlying the allele diversity of hybrid strains. Infect Gen Evol 12: 1064–1071. [DOI] [PubMed] [Google Scholar]
- 17. Ramirez JD, Guhl F, Messenger LA, Lewis MD, Montilla M, et al. (2012) Contemporary cryptic sexuality in Trypanosoma cruzi . Molec Ecol 21: 4216–4226. [DOI] [PubMed] [Google Scholar]
- 18. Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA (2003) Evidence for multiple hybrid groups in Trypanosoma cruzi . Int J Parasitol 33: 269–279. [DOI] [PubMed] [Google Scholar]
- 19. Woody NC, Woody HB (1955) American trypanosomiasis (Chagas' disease); first indigenous case in the United States. JAMA 159: 676–677. [DOI] [PubMed] [Google Scholar]
- 20. Schiffler RJ, Mansur GP, Navin TR, Limpakarnjanarat K (1984) Indigenous Chagas' disease (American trypanosomiasis) in California. JAMA 251: 2983–2984. [PubMed] [Google Scholar]
- 21. Ochs DE, Hnilica VS, Moser DR, Smith JH, Kirchhoff LV (1996) Postmortem diagnosis of autochthonous acute chagasic myocarditis by polymerase chain reaction amplification of a species-specific DNA sequence of Trypanosoma cruzi . Am J Trop Med Hyg 54: 526–529. [DOI] [PubMed] [Google Scholar]
- 22. Herwaldt BL, Grijalva MJ, Newsome AL, McGhee CR, Powell MR, et al. (2000) Use of polymerase chain reaction to diagnose the fifth reported US case of autochthonous transmission of Trypanosoma cruzi, in Tennessee. J Infect Dis 181: 395–399.20. [DOI] [PubMed] [Google Scholar]
- 23. Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, et al. (2007) Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 13: 605–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AABB: AABB Chagas' Biovigilance Network website. Available: www.aabb.org/Content/Programs_and_Services/Data_Center/Chagas/. Accessed 2013 Jan 3.
- 25. Castellani O, Ribeiro LV, Fernandes JF (1967) Differentiation of Trypanosoma cruzi in culture. J Protozool 14: 447–451. [DOI] [PubMed] [Google Scholar]
- 26. Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, et al. (2010) Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector-borne Zoonot Dis 10: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Augusto-Pinto L, Teixeira SMR, Pena SDJ, Machado CR (2003) Single-nucleotide polymorphisms of the Trypanosoma cruzi MSH2 gene support the existence of three phylogenetic lineages presenting differences in mismatch-repair efficiency. Genetics 164: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oury B, Tarrieu F, Monte-Alegre A, Ouaissi A (2005) Trypanosoma cruzi: Sequence polymorphism of the gene encoding the Tc52 immunoregulatory-released factor in relation to the phylogenetic diversity of the species. Exp Parasitol 111 198–206. [DOI] [PubMed] [Google Scholar]
- 29. Saitou N, Nei M (1987) The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406–425. [DOI] [PubMed] [Google Scholar]
- 30.Eck RV, Dayhoff MO (1966) Atlas of protein sequence and structure. Silver Springs, MD: National Biomedical Research Foundation.
- 31. Rzhetsky A, Nei M (1992) A simple method for estimating and testing minimum evolution trees. Mol Biol Evol 9: 945–967. [Google Scholar]
- 32. Felsenstein J (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 33. Kimura M (1980) A simple for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16: 111–120. [DOI] [PubMed] [Google Scholar]
- 34. Roellig DM, Brown EL, Barnabé C, Tibayrenc M, Steurer FJ, et al. (2008) Molecular typing of Trypanosoma cruzi isolates, United States. Emerg Infect Dis 14: 1123–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yeo M, Acosta N, Llewellyn M, Sánchez H, Adamson S, et al. (2005) Origins of Chagas disease: Didelphis species are natural hosts of Trypanosoma cruzi I and armadillo hosts of Trypanosoma cruzi II, including hybrids. Int J Parasitol 35: 225–233. [DOI] [PubMed] [Google Scholar]
- 36. Espinoza B, Vera-Cruz JM, González H, Ortega E, Hernández R (1998) Genotype and virulence correlation within Mexican stocks of Trypanosoma cruzi isolated from patients. Acta Trop 70: 63–72. [DOI] [PubMed] [Google Scholar]
- 37. Bosseno M-F, Barnabé C, Gastélum EM, Kasten FL, Ramsey J, et al. (2002) Predominance of Trypnanosoma cruzi lineage I in Mexico. J Clin Microbiol 40: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sánchez-Guillén M, Barnabé C, Tibayrenc M, Zavala-Castro J, Totolhua J, et al. (2006) Trypanosoma cruzi strains isolated from human, vector, and animal reservoir in the same endemic region in Mexico and tryped as T. cruzi I, discrete typing unit 1 exhibit considerable biological diversity. Mem Inst Oswaldo Cruz 101: 585–590. [DOI] [PubMed] [Google Scholar]
- 39. Iwagami M, Higo H, Miura S, Yanagi T, Tada I, et al. (2007) Molecular phylogeny of Trypanosoma cruzi from Central America (Guatemala) and a comparison with South American strains. Parasitol Res 102: 129–134. [DOI] [PubMed] [Google Scholar]
- 40. Clark CG, Pung OJ (1994) Host specificity of ribosomal DNA variation in sylvatic Trypanosoma cruzi from North America. Mol Biochem Parasitol 66: 175–179. [DOI] [PubMed] [Google Scholar]
- 41. Barnabé C, Yaeger R, Pung O, Tibayrenc M (2001) Trypanosoma cruzi: A considerable phylogenetic divergence indicates that the agent of Chagas disease is indigenous to the native fauna of the United States. Exp Parasitol 99: 73–79. [DOI] [PubMed] [Google Scholar]
- 42. Westenberger SJ, Barnabé C, Campbell DA, Sturm NR (2005) Two hybridization events define the population structure of Trypanosoma cruzi . Genetics 171: 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yeo M, Mauricio IL, Messenger LA, Lewis MD, Llewellyn MS, et al. (2011) Multilocus sequence typing (MLST) for lineage assignment and high resolution diversity studies in Trypanosoma cruzi . PLoS Negl Trop Dis 5: e1049 Doi:10.1371/journal.pntd.0001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marcili A, Valente VC, Valente SA, Junqueira ACV, da Silva FM, et al. (2009) Trypanosoma cruzi in Brazilian Amazonia: Lineages TCI and TCIIa in wild primates, Rhodnius spp. and in humans with Chagas associated with oral transmission. Int J Parasitol 39: 615–623. [DOI] [PubMed] [Google Scholar]
- 45. Ouaissi MA, Dubremetz JF, Schöneck R, Fernandez-Gomex R, Gomez-Corvera R, et al. (1995) Trypanosoma cruzi: A 52-kDa protein sharing sequence homology with glutathione S-transferase is localized in parasite organelles morphologically resembling reservosomes. Exp Parasitol 81: 453–461. [DOI] [PubMed] [Google Scholar]
- 46. Ouaissi A, Guevara-Espinoza A, Chabé F, Gomez-Corvera R, Taibi A (1995) A novel and basic mechanisms of immunosuppression in Chagas' disease: Trypanosoma cruzi releases in vitro and in vivo a protein which induces T cell unresponsiveness through specific interation with cysteine and glutathione. Immunol Lett 48: 221–224. [DOI] [PubMed] [Google Scholar]
- 47. Mathieu-Daudé F, Bosseno M, Garzon E, Leliévre J, Sereno D, et al. (2007) Sequence diversity and differential expression of Tc52 immuno-regulatory protein in Trypanosoma cruzi: potential implications in the biological variability of strains. Parasitol Res 101: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 48. Augusto-Pinto L, Bartholomeu DC, Teixeira SMR, Pena SDJ, Machado CR (2001) Molecular cloning and characterization of the DNA mismatch repair gene class 2 from the Trypanosoma cruzi . Gene 272: 323–333. [DOI] [PubMed] [Google Scholar]
- 49. Roellig DM, Yabsley MJ (2010) Infectivity, pathogenicity, and virulence of Trypanosoma cruzi from sylvatic animals and vectors, and domestic dogs from the United Staets in ICR strain mice and SD strain rats. Am J Trop Med Hyg 83: 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pung OJ, Spratt J, Clark CG, Norton TM, Carter J (1998) Trypanosoma cruzi infection of free-ranging lion-tailed macaques (Macaca silenus) and ring-tailed lemurs (Lemur catta) on St. Catherine's Island, Georgia, USA. J Zoo Wildl Med 29: 25–30. [PubMed] [Google Scholar]
- 51. Hall CA, Polizzi C, Yabsley MJ, Norton TM (2007) Trypanosoma cruzi prevalence and epidemiologic trends in lemurs on St. Catherine's Island, Georgia. J Parasitol 93: 93–96. [DOI] [PubMed] [Google Scholar]
- 52. Campos PC, Silva VG, Furtado C, Machado-Silva A, Darocha WD, et al. (2011) Trypanosoma cruzi MSH2: Functional analyses on different parasite strains provide evidences for a role on the oxidative stress response. Mol Biochem Parasitol 176: 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Barrera-Pérez MA, Rodríguez-Félix ME, Guzmán-Marín E, Zavala-Velázquez J, Dumontiel E (2001) Bioligical behavior of three strains of Trypanosoma cruzi from Yucatan, Mexico. Rev Biomed 12: 224–230. [Google Scholar]
- 54. Bértoli M, Andó MH, de Ornelas Toledo MJ, de Araújo SM, Gomes ML (2006) Infectivity for mice of Trypanosoma cruzi I and II strains from different hosts. Parasitol Res 99: 7–13. [DOI] [PubMed] [Google Scholar]
- 55. Lisboa CV, Pinho AP, Monteiro RF, Jansen AM (2006) Trypanosoma cruzi (kinetoplastida trypanosomatidae): Biological heterogeneity in the isolates derived from wild hosts. Exp Parasitol 116: 150–155. [DOI] [PubMed] [Google Scholar]
- 56. O'Connor O, Bosseno M, Barnabé C, Douzery EJP, Breniére SF (2007) Genetic clustering of Trypanosoma cruzi I lineage evidenced by intergenic miniexon gene sequencing. Infect Gen Evol 7: 587–593. [DOI] [PubMed] [Google Scholar]
- 57. Spotorno AE, Córdova L, Solari A (2008) Differentiation of Trypanosoma cruzi I subgroups through characterization of cytochrome b gene sequences. Infect Gen Evol 8 898–900. [DOI] [PubMed] [Google Scholar]
- 58. Ocaña-Mayorga S, Llewllyn MS, Costales JA, Miles MA, Grijalva MJ (2011) Sex, subdivision, and domestic dispersal of Trypanosoma cruzi lineage I in Southern Ecuador. PLoS Negl Trop Dis 4: e915 Doi:10.1371/journal.pntd.0000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramirez JD, Duque MC, Montilla M, Cucunuba ZM, Guhl F (2012) Multilocus PCR-RFLP profiling in Trypanosoma cruzi I highlights and intraspecific genetic variation pattern. Infect Gen Evol 12: 1743–1750. [DOI] [PubMed] [Google Scholar]
- 60. Zumaya-Estrada F, Messenger LA, Lopez-Ordonez T, Lewis MD, Flores-Lopez CA, et al. (2012) North American import? Charting the origins of an enigmatic Trypanosoma cruzi domestic genotype. Parasites & Vectors 5: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Subileau M, Barnabé C, Douzery EJP, Diosque P, Tibayrenc M (2009) Trypanosoma cruzi: New insights on ecophylogeny and hybridization by multigene sequencing of three nuclear and one maxicircle gene. Exp Parasitol 122: 328–337. [DOI] [PubMed] [Google Scholar]
- 62. Brisse S, Henriksson J, Barnabé C, Douzery EJP, Berkvens D, et al. (2003) Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect Gen Evol 2: 173–183. [DOI] [PubMed] [Google Scholar]