Abstract
Background
The association between methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and hepatocellular carcinoma (HCC) risk was inconsistent and underpowered. To clarify the effects of MTHFR gene polymorphisms on the risk of HCC, a meta-analysis of all available studies relating C677T and/or A1298C polymorphisms of MTHFR gene to the risk of HCC was conducted.
Methods
The authors searched PubMed, EMBASE, Cochrane Library, Web of Science, and Chinese Biomedical Literature database (CBM) for the period up to July 2012. Data were extracted by two independent authors and pooled odds ratio (OR) with 95% confidence interval (CI) was calculated. Metaregression and subgroup analyses were performed to identify the source of heterogeneity.
Results
Finally, 12 studies with 2,351 cases and 4,091 controls were included for C677T polymorphism and 6 studies with 1,333 cases and 1,878 controls were included for A1298C polymorphism. With respect to A1298C polymorphism, significantly decreased HCC risk was found in the overall population (CC vs. AA: OR = 0.660, 95%CI 0.460–0.946, P = 0.024; recessive model: OR = 0.667, 95%CI = 0.470–0.948, P = 0.024). In subgroup analyses, significantly decreased HCC risk was found in Asian population (CC vs. AA: OR = 0.647, 95%CI = 0.435–0.963; P = 0.032) and population-based studies (CC vs. AA: OR = 0.519, 95%CI = 0.327–0.823; P = 0.005). With respect to C677T polymorphism, no significant association with HCC risk was demonstrated in overall and stratified analyses.
Conclusions
We concluded that MTHFR A1298C polymorphism may play a protective role in the carcinogenesis of HCC. Further large and well-designed studies are needed to confirm this association.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related death worldwide, which is still a global health challenge [1], [2]. The mechanism of its carcinogenesis, like other cancers, still remains unclear. Folate is a form of the water-soluble vitamin B9. It is necessary for the production and maintenance of new cells and is involved in DNA methylation, DNA synthesis and DNA repair [3]. Some studies have indicated that folate deficiency could influence cancer risk [4], [5]. Methylenete trahydrofolate reductase (MTHFR) is a key enzyme for intracellular folate homeostasis and metabolism. It catalyses the irreversible conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, which is the primary circulating form of folate and provides methyl groups for the methylation of homocysteine to methionine [6]. Altered MTHFR enzyme activity has been linked to the development of cancer [7], [8], [9].
The MTHFR gene is located at chromosome 1p36.3 and is 2.2 kb in length with a total of 11 exons [10]. There are at least 247 single nucleotide polymorphisms (SNPs) in the MTHFR gene, reported in the dbSNP database (http://www.ncbi.nlm.nih.gov/snp/). However, only two common polymorphisms, C677T (rs1801133) and A1298C (rs1801131), have been extensively investigated. For the MTHFR C677T polymorphism, a C to T transition at nucleotide position 677 in exon 4 generates an alanine (Ala) to valine (Val) change at amino acid 222 (Ala222Val). This substitution lies at the binding site for the flavin adenine dinucleotide, an important cofactor for MTHFR [11]. As a result, carriers of the MTHFR 677TT genotype possess a thermolabile enzyme of reduced activity [12], which results in decreased folate concentration and increased homocysteine level in the serum [13]. Another polymorphism in MTHFR, A to C transversion at nucleotide 1298 (A1298C), results in an amino acid substitution of glutamic acid for alanine (Ala) at codon 429 (Glu429Ala), which may also induce decreased activity of MTHFR [14]. Hence, it is biologically reasonable to hypothesize a potential relationship between MTHFR polymorphisms and HCC risk.
A number of studies have been conducted to investigate the association between MTHFR C677T and/or A1298C polymorphisms and HCC risk, but the results are somewhat controversial and underpowered. With respect to C677T polymorphism, a meta-analysis by Jin et al. [15] found that MTHFR C677T polymorphism was associated with an increased HCC risk in an overdominant model, however, they only included 10 eligible studies in the meta-analysis, which make their conclusions questionable. With respect to A1298C polymorphism, to the best of our knowledge, no meta-analyses on this issue have ever appeared. To derive a more precise estimation of the relationship between MTHFR polymorphisms and HCC risk, we conducted a meta-analysis of all available case–control studies relating the C677T and/or A1298C polymorphisms of the MTHFR gene to the risk of developing HCC.
Methods
Search Strategy
This study was performed according to the proposal of Meta-analysis of Observational Studies in Epidemiology group (MOOSE) [16]. A comprehensive search strategy was conducted towards the electronic databases including PubMed, EMBASE, Cochrane Library, Web of Science, and Chinese Biomedical Literature database (CBM), using the search strategy based on combinations of the keywords “hepatocellular carcinoma or HCC” and “methylenetetrahydrofolate reductase, MTHFR, one-carbon metabolism or folate” and “polymorphism, mutation or variant”. The last search was updated on July 01, 2012. Although no language restrictions were applied initially, for the full-text review and final analysis our resources only permitted the review of articles published in English and Chinese. Reference lists of the identified articles were also examined and the literature retrieval was performed in duplication by two independent reviewers (Xue Qin and Qiliu Peng). When multiple publications reported on the same or overlapping data, we chose the most recent or largest population. When a study reported the results on different subpopulations, we treated it as separate studies in the meta-analysis.
Selection Criteria
We reviewed abstracts of all citations and retrieved studies. The following criteria were used to include published studies: (1) evaluating the association between MTHFR gene polymorphisms and HCC; (2) case-control design; (3) the papers must offer the size of the samples, distribution of alleles, genotypes or other information that can help us infer the results to estimate the odds ratio (ORs) and their 95% confidence intervals (CIs); and (4) studies published in English or Chinese language. Participants could be of any age. Studies were excluded if one of the following existed: (1) the design was based on family or sibling pairs; (2) the genotype frequency was not reported; or (3) there was insufficient information for data extraction.
Data Extraction
Two investigators (Xue Qin and Qiliu Peng) independently extracted data from the studies included. Data extracted from eligible studies included the first author’s name, publication date, country of origin, ethnicity, genotyping method, matching criteria, source of control, HCC diagnosis, QC when genotyping, total numbers of cases and controls and genotype frequencies of cases and controls. The two investigators checked the data extraction results and reached consensus on all of the data extracted. If different results were generated, they would check the data again and have a discussion to come to an agreement. A third reviewer (Li Shan) was invited to the discussion if disagreement still existed.
Quality Score Assessment
The quality of the eligible studies was independently assessed by two investigators (Xue Qin and Qiliu Peng) according to a set of predefined criteria (Table 1), which was originally proposed by Thakkinstian et al [17]. The revised criteria cover the credibility of controls, the representativeness of cases, specimens of cases determining genotypes, Hardy-Weinberg equilibrium in controls, and total sample size (Table 1). The disagreements between two investigators were resolved by consensus. The total scores ranged from 0 (lowest) to 15 (highest), and studies with scores ≥10 were classified as high-quality studies, whereas studies with scores <10 were considered as low-quality studies.
Table 1. Scale for quality assessment.
| Criteria | Score |
| Representativeness of cases | |
| Selected from population or cancer registry | 3 |
| Selected from hospital | 2 |
| Selected from pathology archives, butwithout description | 1 |
| Not described | 0 |
| Credibility of controls | |
| Population-based | 3 |
| Blood donors or volunteers | 2 |
| Hospital-based (cancer-free patients) | 1 |
| Not described | 0 |
| Specimens of cases determining genotypes | |
| White blood cells or normal tissues | 3 |
| Tumor tissues or exfoliated cells of tissue | 0 |
| Hardy-Weinberg equilibrium in controls | |
| Hardy-Weinberg equilibrium | 3 |
| Hardy-Weinberg disequilibrium | 0 |
| Total sample size | |
| ≥1000 | 3 |
| ≥400 but <1000 | 2 |
| ≥200 but <400 | 1 |
| <200 | 0 |
Statistical Analysis
Summary odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated for each polymorphism in different comparison models, including additive genetic models, recessive genetic model, and dominant genetic model.
The Q test and I2 statistics were used to assess the statistical heterogeneity among studies [18], [19]. If the result of the Q test was PQ <0.1 or I2≥50%, indicating the presence of heterogeneity, a random-effects model (the DerSimonian and Laird method) was used to estimate the summary ORs [20]; otherwise, when the result of the Q test was PQ ≥0.1 and I2<50%, indicating the absence of heterogeneity, the fixed-effects model (the Mantel–Haenszel method) was used [21]. To explore the sources of heterogeneity among studies, we performed logistic metaregression and subgroup analyses. The following study characteristics were included as covariates in the metaregression analysis: genotyping methods (PCR-RFLP versus not PCR-RFLP), ethnicity (Caucasian population versus Asian population), quality score (high quality studies versus low quality studies), source of controls (Hospital-based versus Population-based), QC when genotyping (Yes versus no), and HCC diagnosis (pathologically or histologically confirmed versus other diagnosis criteria). Subgroup analyses were conducted by stratification of ethnicity and source of controls. Galbraith plots analysis was performed for further exploration of the heterogeneity.
Sensitivity analysis was performed by sequential omission of individual studies. For each polymorphism, publication bias was evaluated using a funnel plot and Egger’s regression asymmetry test [22]. If publication bias existed, the Duval and Tweedie non-parametric “trim and fill” method was used to adjust for it [23]. The distribution of the genotypes in the control population was tested for Hardy-Weinberg equilibrium using a goodness-of-fit Chi-square test. All analyses were performed using Stata software, version 10.0 (Stata Corp., College Station, TX). All p values were two-sided. To ensure the reliability and the accuracy of the results, two authors entered the data into the statistical software programs independently with the same results.
Results
Study Characteristics
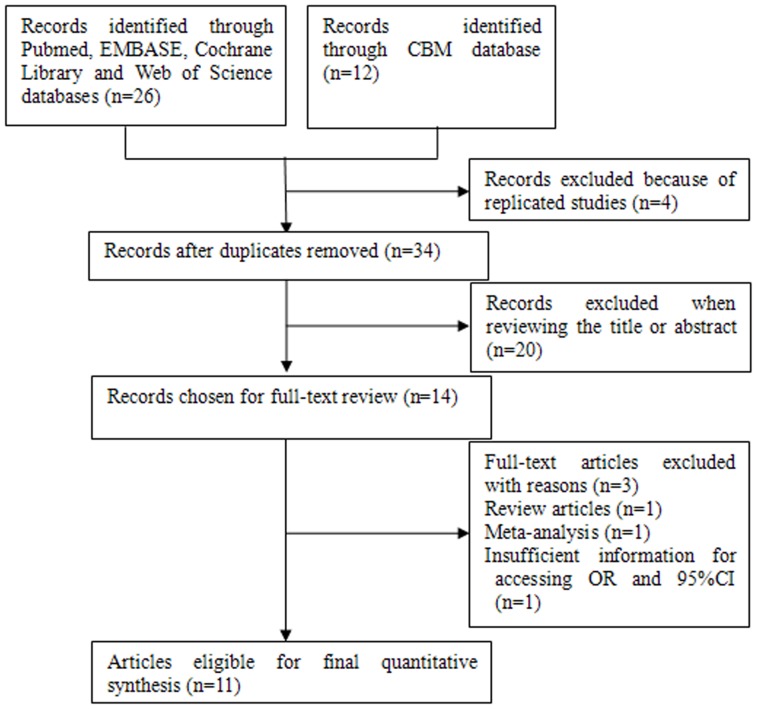
Based on the search criteria, 14 studies relevant to the role of MTHFR gene polymorphisms on HCC susceptibility were identified. Three of these articles were excluded: one of these articles was a review [24], one was a meta-analysis [15], and one did not provide allele or genotyping data [25]. Manual search of references cited in the published studies did not reveal any additional articles. As a result, a total of 11 relevant studies including 9 English articles [26]–[34] and 2 Chinese papers (one was a dissertation of postgraduate student) [35], [36] met the inclusion criteria for the meta-analysis (Figure 1). Among them, one of the eligible studies contained data on two different ethnic groups [30], and we treated it independently. Therefore, a total of 12 separate comparisons were finally included in our meta-analysis. The main characteristics of the studies were presented in Table 2. Among them, six studies evaluated the C677T variant and 6 studies evaluated the C677T and A1298C variants. Therefore, a total of 12 studies including 2,351 cases and 4,091 controls were available for the meta-analysis of C677T polymorphism and 6 studies containing 1,333 cases and 1,878 controls were included for A1298C polymorphism. The sample size in these studies varied considerably, ranging from 150 to 1,051 individuals. Of all the eligible studies, 5 were conducted in Caucasian population, and 7 were in Asians for C677T polymorphism; 5 were conducted in Asians and only one [30] was in Caucasians for A1298C polymorphism. Six studies were population–based and 6 were hospital–based studies. Only 3 articles of all eligible studies used quality control when genotyping and 4 studies in the present meta-analysis did not provide definite criteria for the HCC diagnosis. Several genotyping methods were used, including PCR-RFLP, TaqMan assay, and RT-PCR. The genotype distributions of the controls in two studies were not consistent with HWE for C677T polymorphism [27], [30] and one was not consistent with HWE for A1298C polymorphism [34].
Figure 1. Flow diagram of included studies for this meta-analysis.
Table 2. Characteristics of eligible studies.
| First author (Year) | Country | Ethnicity | Sample size (case/control) | Genotyping methods | Matching criteria | Source of control | HCC diagnosis | QC when Genotyping | PI | HWE(P value) | Quality scores | |
| C677T | A1298C | |||||||||||
| Saffroy, 2004 | France | Caucasian | 148/232 | PCR-RFLP | Age, gender | PB | HC | No | C677T | 0.291 | – | 10 |
| Ventura 2005 | Italy | Caucasian | 22/128 | PCR-RFLP | Age | PB | NA | No | C677T | 0.003 | – | 6 |
| Zhu 2006 | China | Asian | 508/543 | PCR-RFLP | Gender, smoking | HB | PC and HC | Yes | C677T | 0.921 | – | 12 |
| Yang 2007 | China | Asian | 322/185 | PCR-RFLP | Gender, smoking | HB | HC | Yes | C677T, A1298C | 0.139 | 0.769 | 14 |
| Mu 2007 | China | Asian | 194/391 | PCR-RFLP | Region | PB | PC | No | C677T, A1298C | 0.235 | 0.249 | 10 |
| Yuan1 2007 | China | Asian | 247/248 | TaqMan Assay | Age, gender | PH | HC | No | C677T, A1298C | 0.033 | 0.305 | 11 |
| Yuan2 2007 | America | Caucasian | 118/209 | TaqMan Assay | Age, gender | PB | HC | No | C677T, A1298C | 0.944 | 0.666 | 10 |
| Kwak 2008 | Korea | Asian | 96/201 | PCR-RFLP | Age | HB | NA | No | C677T, A1298C | 0.234 | 0.261 | 10 |
| D’Amico 2009 | Italy | Caucasian | 94/308 | PCR-RFLP | Age, gender | HB | NA | No | C677T | 0.207 | – | 9 |
| Fabris 2009 | Italy | Caucasian | 65/381 | PCR-RFLP | Age, gender | HB | HC | No | C677T | 0.521 | – | 11 |
| Liu 2010 | China | Asian | 181/624 | TaqMan Assay | Region | HB | NA | Yes | C677T | 0.189 | – | 11 |
| Cui 2011 | China | Asian | 356/641 | RT-PCR | Age, Region | PB | PC | No | C677T, A1298C | 0.483 | 0.003 | 10 |
PI, Polymorphism(s) investigated; HC, Histologically confirmed; PC, Pathologically confirmed; NA, Not available; QC, Quality control; PB, Population–based; HB, Hospital–based; HWE, Hardy–Weinberg equilibrium in control population; PCR–RFLP, Polymerase chain reaction-restriction fragment length polymorphism; RT–PCR, Real time–polymerase chain reaction.
Meta-analysis Results
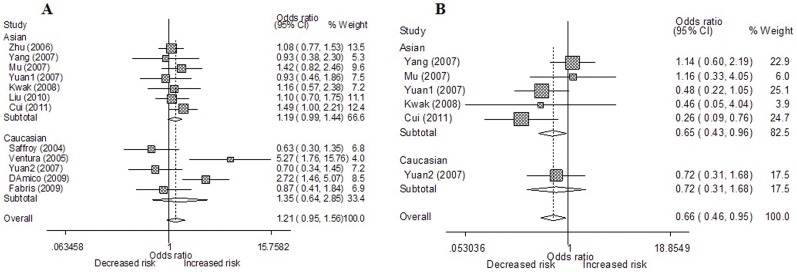
The meta-analysis suggested that the C677T polymorphism was not associated with HCC risk in all genetic models (additive models TT vs. CC and CT vs. CC, recessive model, and dominant model; Table 3) in the overall populations. Moreover, we failed to identify any significant association between the C677T polymorphism and HCC risk in all comparison models in subgroup analyses according to ethnicity and source of controls (Table 3, Figure 2A).
Table 3. Meta-analysis of the MTHFR gene polymorphisms on HCC risk.
| Comparison | Population | No. of studies | Test of association | Mode | Test of heterogeneity | ||||
| OR | 95% CI | P Value | χ2 | PQ Value | I2 | ||||
| C677T | |||||||||
| TT vs. CC | Overall | 12 | 1.213 | 0.946–1.555 | 0.128 | R | 22.04 | 0.024 | 50.1 |
| Caucasian | 5 | 1.351 | 0.640–2.853 | 0.430 | R | 19.08 | 0.001 | 79.0 | |
| Asian | 7 | 1.192 | 0.988–1.439 | 0.067 | F | 2.82 | 0.831 | 0.0 | |
| PB | 6 | 1.211 | 0.777–1.889 | 0.398 | R | 13.87 | 0.016 | 64.0 | |
| HB | 6 | 1.190 | 0.956–1.482 | 0.119 | F | 8.12 | 0.150 | 38.4 | |
| CT vs. CC | Overall | 12 | 1.001 | 0.884–1.134 | 0.984 | F | 11.34 | 0.415 | 3.0 |
| Caucasian | 5 | 0.896 | 0.701–1.146 | 0.383 | F | 2.48 | 0.649 | 0.0 | |
| Asian | 7 | 1.040 | 0.900–1.202 | 0.593 | F | 7.85 | 0.250 | 23.5 | |
| PB | 6 | 1.081 | 0.902–1.295 | 0.399 | F | 7.65 | 0.177 | 34.6 | |
| HB | 6 | 0.934 | 0.787–1.109 | 0.436 | F | 2.43 | 0.787 | 0.0 | |
| TT vs. CT+CC | Overall | 12 | 1.194 | 0.979–1.457 | 0.080 | R | 19.01 | 0.061 | 42.1 |
| Caucasian | 5 | 1.406 | 0.740–2.672 | 0.298 | R | 16.88 | 0.002 | 76.3 | |
| Asian | 7 | 1.151 | 0.987–1.343 | 0.073 | F | 1.20 | 0.977 | 0.0 | |
| PB | 6 | 1.133 | 0.792–1.621 | 0.493 | R | 11.58 | 0.041 | 56.8 | |
| HB | 6 | 1.252 | 0.981–1.598 | 0.070 | F | 7.16 | 0.209 | 30.2 | |
| TT+CT vs. CC | Overall | 12 | 1.058 | 0.905–1.237 | 0.481 | R | 18.16 | 0.078 | 39.4 |
| Caucasian | 5 | 1.048 | 0.712–1.542 | 0.812 | R | 10.47 | 0.033 | 61.8 | |
| Asian | 7 | 1.072 | 0.935–1.228 | 0.319 | F | 7.34 | 0.290 | 18.3 | |
| PB | 6 | 1.111 | 0.837–1.475 | 0.467 | R | 12.54 | 0.028 | 60.1 | |
| HB | 6 | 1.004 | 0.856–1.179 | 0.960 | F | 5.01 | 0.415 | 0.2 | |
| A1298C | |||||||||
| CC vs. AA | Overall | 6 | 0.660 | 0.460–0.946 | 0.024 | F | 7.19 | 0.207 | 30.5 |
| Caucasian | 1 | 0.720 | 0.309–1.677 | 0.446 | – | – | – | – | |
| Asian | 5 | 0.647 | 0.435–0.963 | 0.032 | F | 7.20 | 0.126 | 44.4 | |
| PB | 4 | 0.519 | 0.327–0.823 | 0.005 | F | 3.78 | 0.286 | 20.7 | |
| HB | 2 | 1.045 | 0.567–1.927 | 0.888 | F | 0.62 | 0.432 | 0.0 | |
| AC vs. AA | Overall | 6 | 1.055 | 0.900–1.236 | 0.510 | F | 3.44 | 0.633 | 0.0 |
| Caucasian | 1 | 0.828 | 0.513–1.336 | 0.440 | – | – | – | – | |
| Asian | 5 | 1.087 | 0.919–1.286 | 0.331 | F | 2.33 | 0.676 | 0.0 | |
| PB | 4 | 1.033 | 0.860–1.241 | 0.725 | F | 1.15 | 0.756 | 0.0 | |
| HB | 2 | 1.121 | 0.817–1.538 | 0.480 | F | 2.08 | 0.149 | 32.1 | |
| CC vs. AC+AA | Overall | 6 | 0.667 | 0.470–0.948 | 0.024 | F | 8.13 | 0.149 | 38.5 |
| Caucasian | 1 | 0.780 | 0.343–1.774 | 0.554 | – | – | – | – | |
| Asian | 5 | 0.627 | 0.330–1.192 | 0.154 | R | 8.07 | 0.089 | 50.4 | |
| PB | 4 | 0.522 | 0.332–0.821 | 0.005 | F | 4.34 | 0.227 | 30.8 | |
| HB | 2 | 1.054 | 0.583–1.903 | 0.863 | F | 0.82 | 0.365 | 0.0 | |
| CC+AC vs. AA | Overall | 6 | 0.995 | 0.855–1.159 | 0.953 | F | 2.73 | 0.742 | 0.0 |
| Caucasian | 1 | 0.808 | 0.513–1.270 | 0.355 | – | – | – | – | |
| Asian | 5 | 1.023 | 0.870–1.202 | 0.788 | F | 1.80 | 0.772 | 0.0 | |
| PB | 4 | 0.958 | 0.803–1.143 | 0.634 | F | 0.67 | 0.881 | 0.0 | |
| HB | 2 | 1.113 | 0.823–1.506 | 0.485 | F | 1.33 | 0.249 | 24.8 | |
OR, odds ratio; CI, confidence intervals; R, random effects model; F, fixed effects model; PB, Population–based; HB, Hospital–based.
Figure 2. Forest plots of MTHFR gene polymorphisms and HCC risk. A.
Forest plots of MTHFR C677T polymorphism and HCC risk in subgroup analysis by ethnicity using a random-effect model (contrast TT vs. CC); B Forest plots of MTHFR A1298C polymorphism and HCC risk in subgroup analysis by ethnicity using a fixed-effect model (CC vs. AA).
For the A1298C polymorphism, significant decreased HCC risk was found in additive model CC vs. AA (OR = 0.660, 95%CI 0.460–0.946, P = 0.024; I2 = 30.5 and PQ = 0.207 for heterogeneity; Figure 2B) and recessive model CC vs. AC+AA (OR = 0.667, 95%CI = 0.470–0.948, P = 0.024; I2 = 38.5 and PQ = 0.149 for heterogeneity) in the overall populations. Subgroup analysis stratified by source of controls showed that the A1298C polymorphism was associated with a significantly decreased HCC risk among population-based studies for additive model CC vs. AA (OR = 0.519, 95%CI = 0.327–0.823, P = 0.005; I2 = 20.7 and PQ = 0.286 heterogeneity) and recessive model CC vs. AC+AA (OR = 0.522, 95%CI = 0.332–0.821, P = 0.005; I2 = 30.8 and PQ = 0.227 for heterogeneity). When stratified by ethnicity, significant decreased HCC risk was also found in Asians in additive model CC vs. AA (OR = 0.647, 95%CI = 0.435–0.963, P = 0.032; I2 = 44.4 and PQ = 0.126 for heterogeneity) but not in recessive model CC vs. AC+AA (OR = 0.627, 95%CI = 0.330–1.192, P = 0.154; I2 = 50.4 and PQ = 0.089 for heterogeneity). Interestingly, when we excluded the study by Yang et al [36]. which was shown as an outlier in our Galbraith plots analysis, the summary OR of recessive model CC vs. AC+AA in Asian population reached significance (OR = 0.441, 95%CI: 0.259–0.750, P = 0.003; PQ = 0.342 and I2 = 10.2 for heterogeneity).
Heterogeneity Analysis
For the C677T polymorphism, the I2 values of heterogeneity were greater than 50% and the PQ values were lower than 0.10 in additive model TT vs. CC, recessive model TT vs. CT+CC, and dominant model TT+CT vs. CC in the overall populations, which indicated statistically significant heterogeneity among studies. To explore the sources of heterogeneity, we performed metaregression and subgroup analyses. Metaregression analysis of data showed that the ethnicity and source of controls were the major sources which contributed to heterogeneity. The ethnicity and source of controls were both positively associated with the ORs in additive model TT vs. CC (regression coefficient = 0.588, 95%CI: 0.072–1.104, p = 0.026 for ethnicity and regression coefficient = 1.510, 95%CI: 0.634–2.385, p = 0.001 for source of controls, respectively), recessive model TT vs. CT+CC (regression coefficient = 0.439, 95%CI: 0.124–0.802, p = 0.041 for ethnicity and regression coefficient = 1.231, 95%CI: 0.459–2.004, p = 0.002 for source of controls, respectively), and dominant model TT+CT vs. CC (regression coefficient = 0.482, 95%CI: 0.135–0.826, p = 0.006 for ethnicity and regression coefficient = 0.917, 95%CI: 0.265–1.569, p = 0.006 for source of controls, respectively). The Genotyping methods, HCC diagnosis, QC when genotyping, and Quality scores were not effect modifiers. Subsequently, we performed subgroup analyses stratified by ethnicity and source of controls. However, heterogeneity still existed among Caucasians and population-based studies in all the above three genetic comparison models (table 3).
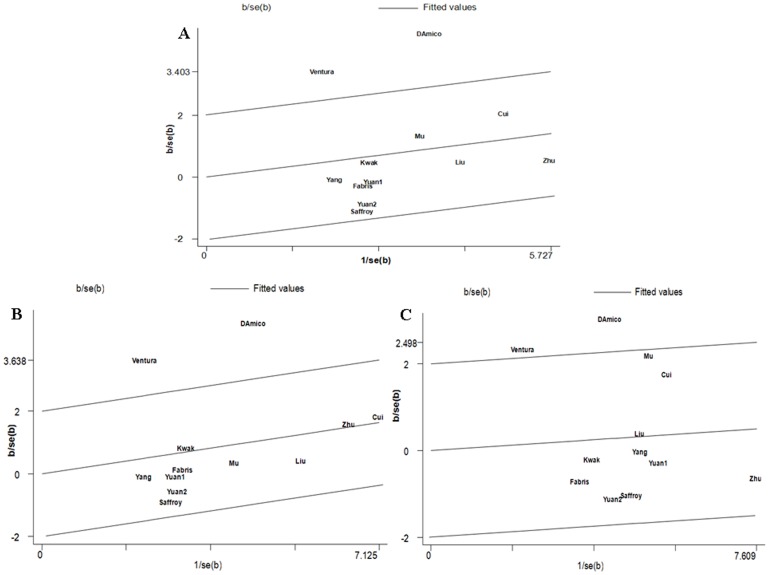
To further investigate the heterogeneity, we performed Galbraith plots analysis to identify the outliers which might contribute to the heterogeneity. Our results showed that Ventura et al. [27] and D’Amico et al. [32] were outliers in additive model TT vs. CC, recessive model TT vs. CT+CC, and dominant model TT+CT vs. CC model for C677T polymorphism (Figure 3). All I2 values decreased obviously and PQ values were greater than 0.10 after excluding the studies of Ventura et al. [27] and D’Amico et al. [32] in all genetic comparison models in the overall populations (additive model TT vs. CC: PQ = 0.596, I2 = 0.0; recessive model TT vs. CT+CC: PQ = 0.915, I2 = 0.0; dominant model TT+CT vs. CC: PQ = 0.251, I2 = 20.9 ), Caucasians (additive model TT vs. CC: PQ = 0.831, I2 = 0.0; recessive model TT vs. CT+CC: PQ = 0.740, I2 = 0.0; dominant model TT+CT vs. CC: PQ = 0.986, I2 = 0.0), and population-based studies (additive model TT vs. CC: PQ = 0.418, I2 = 41.0; recessive model TT vs. CT+CC: PQ = 0.520, I2 = 0.0; dominant model TT+CT vs. CC: PQ = 0.149, I2 = 38.2). The significance of the summary ORs for the C677T polymorphism in different comparison models in the overall population and subgroup analyses were not influenced by omitting the two studies.
Figure 3. Galbraith plots of C677T polymorphism and HCC risk in different contrast models.
A The studies of Ventura et al., D’Amico et al. were outliers in the contrast TT vs. CC. B The studies of Ventura et al., D’Amico et al. were outliers in the recessive model TT vs. CT+CC. C The studies of Ventura et al., D’Amico et al. were outliers in the dominant model TT+CT vs. CC.
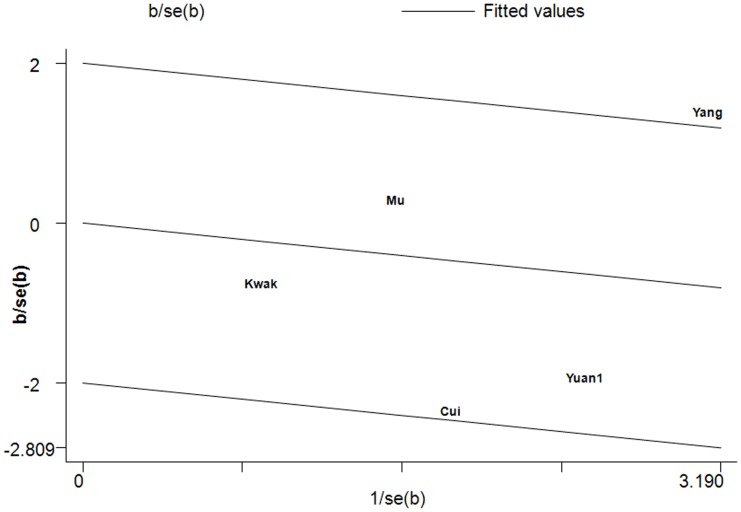
For the A1298C polymorphism, there was no statistical significant heterogeneity in all comparison models in the overall populations. Subgroup analysis by ethnicity and source of controls also indicated no significant heterogeneity in all comparison models except the recessive model CC vs. AC+AA in Asians (PQ = 0.089, I2 = 50.4; Table 3). Galbraith plots analysis showed that the study Yang et al. [36] was the outlier (Figure 4). The I2 value decreased lower than 50% and PQ values were greater than 0.10 after excluding the study of Yang et al. (PQ = 0.342, I2 = 10.2). Interestingly, the summary OR of recessive model CC vs. AC+AA in Asians reached significance after omitting this study (OR = 0.441, 95%CI: 0.259–0.750, P = 0.003).
Figure 4. Galbraith plots of A1298C polymorphism and HCC risk in Asians, The study of Yang et al. was the outlier in recessive model CC vs. AC+AA.
Sensitivity Analysis
A single study involved in the meta-analysis was deleted each time to reflect the influence of the individual data-set to the pooled ORs, and the corresponding pooled ORs were not materially altered (data not shown), indicating that our results were statistically robust. Although the genotype distribution in two studies of C677T polymorphism [27], [30] and one study of A1298C polymorphism [34] was not in accordance with HWE, the corresponding pooled ORs were not qualitatively altered with or without including these studies.
Publication Bias
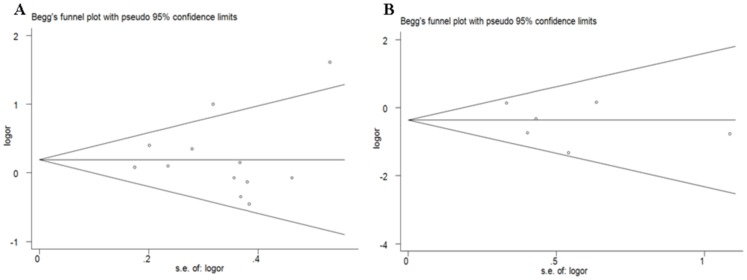
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of literatures in all comparison models. The shape of the funnel plot did not reveal any evidence of obvious asymmetry (Figure 5). Then, the Egger’s test was used to provide statistical evidence of funnel plot symmetry. The results still did not suggest any evidence of publication bias in C677T (P = 0.900 for TT vs. CC; P = 0.804 for CT vs. CC; P = 0.834 for recessive model TT vs. CT+CC; and P = 0.365 for dominant model TT+CT vs. CC) and A1298C (P = 0.508 for CC vs. AA; P = 0.717 for AC vs. CC; P = 0.458 for recessive model CC vs. AC+AA; and P = 0.409 for dominant model AC+CC vs. AA) polymorphisms.
Figure 5. Funnel plot analysis to detect publication bias.
Each point represents a separate study for the indicated association. A Funnel plot for contrast TT vs. CC of C677T polymorphism in overall analysis; B Funnel plot for allele contrast CC vs. AA of A1298C polymorphism in overall analysis.
Discussion
The folate metabolism pathway plays an important role in DNA synthesis and DNA methylation which is directed by purine and pyrimidine synthesis; folate deficiency causes uracil misincorporation into DNA with subsequent chromosome breaks [37]. MTHFR is a key enzyme in the folate metabolism pathway [38]. Two common variations in the MTHFR gene, C677T and A1298C, were associated with reduced MTHFR activity. It was reported that homozygotes (TT) and heterozygotes (CT) for C677T have, respectively, 30% and 65% of the enzyme activity compared with those whose genotype is homozygotes (CC), whereas homozygotes (CC) for A1298C have only 60% of the normal enzyme activity [12], [39]. Decreased MTHFR activity may lead to an alteration of normal intracellular distribution of folate substrates [40], and result in tumor susceptibility. This hypothesis was confirmed by our meta-analysis.
Our meta-analysis results showed that individuals with the 1298CC genotype had a reduced risk of HCC compared to those with the 1298AA genotype, especially among the Asian population. However, no association was detected among the Caucasian population. In addition, our data also showed a decreased HCC risk under the recessive genetic model (CC vs. AC+AA) in the overall populations. When we excluded the study of Yang et al. [36] which was shown as an outlier in Galbraith plots analysis, a statistically significant decreased HCC risk was also found in Asian population but not in Caucasians under the recessive genetic model. Actually, it might not be uncommon for the same polymorphism play different roles in cancer susceptibility among different ethnic populations. In Caucasians, the differences in genetic backgrounds and the environment they lived in may influence the association between the MTHFR A1298C polymorphism and the risk for HCC. In addition, the limited number of studies also makes the results from subgroup analysis by ethnicity less reliable. Thus, our results should be interpreted with caution.
In the subgroup analysis based on source of controls, significantly decreased HCC risk was found in MTHFR 1298CC genotype carriers in the population-based studies but not in hospital-based studies. This reason may be that the hospital-based studies have a high risk of producing unreliable results because hospital-based controls may not always be truly representative of the general population. Therefore, a methodologically preferable design, such as using a proper and representative population-based study, is crucial to avoid selection bias.
With respect to C677T polymorphism, 12 studies were found in our meta-analysis. Contrary to the previous findings made by Jin et al. [15], our results showed that MTHFR C677T polymorphism was not associated with HCC risk not only in the overall population but also in the subgroup analyses stratified by ethnicity and source of controls. This is most probably because of the relatively small sample size of the previous meta-analysis. The meta-analysis of Jin et al. included only 10 studies for evaluating the association between MTHFR C677T polymorphism and HCC risk and may have insufficient statistical power to detect a true effect or may have generated a fluctuated risk estimate. Therefore, a meta-analysis with relatively larger sample size (including original studies as many as possible) is crucial to avoid selection bias in such genotype association studies.
Heterogeneity analysis of C677T polymorphism suggested significant heterogeneity in additive model TT vs. CC, recessive model TT vs. CT+CC, and dominant model TT+CT vs. CC in the overall populations. To explore the sources of heterogeneity, we performed metaregression and subgroup analyses. Metaregression analysis of data showed that the ethnicity and source of controls but not Genotyping methods, HCC diagnosis, QC when genotyping, and Quality scores might substantially influence the initial heterogeneity. Subgroup analyses by ethnicity and source of controls indicated that heterogeneity still existed in Caucasians and population-based studies in all the above three genetic comparison models. To further investigate the heterogeneity, Galbraith plots analysis was performed to identify the outliers which might contribute most to the heterogeneity. Our results showed that the studies of Ventura et al. [27] and D’Amico et al. [32] were outliers of the above three genetic comparison models (Figure 3). All I2 values decreased lower than 50% and PQ values were greater than 0.10 after excluding the studies of Ventura et al. [27] and D’Amico et al. [32] in all genetic comparison models in the overall populations, Caucasians and the population-based studies. In addition, the summary ORs for the C677T polymorphism in different comparison models in the overall population and subgroup analyses were not material change by omitting the two studies, indicating that our results were robust and reliable. The results indicated that the two studies might be the major source of the heterogeneity for the C677T polymorphism.
Significant heterogeneity was found in the recessive model CC vs. AC+AA (I2 = 50.4%, PQ = 0.089) for the A1298C polymorphism in Asian populations. Galbraith plots analysis showed that the study Yang et al. [36] was the outlier (Figure 4). The I2 value decreased lower than 50% and PQ values were greater than 0.10 after excluding this study (PQ = 0.342, I2 = 10.2). Interestingly, the pooled OR of recessive model in Asians reached significance after omitting this study (OR = 0.441, 95%CI: 0.259–0.750, P = 0.003). The results indicated that the study of Yang et al. [36] was the main source of heterogeneity for the A1298C polymorphism.
This meta-analysis should be interpreted with caution at the present time because of some limitations. First, the overall outcomes were based on individual unadjusted ORs, whereas a more precise evaluation should be adjusted by potentially suspected factors, including age, gender, smoking status, and environmental factors. In some studies, individuals who were unmatched by age and gender later developed HCC within the age range in the control group. The results would hence underestimate the OR association with the genotype. Second, the controls were not uniformly defined. Although most of the controls were selected mainly from healthy populations, some had benign disease such as liver cirrhosis, HBsAg positive subjects and so on. Therefore, non-differential misclassification bias was possible because these studies may have included the control groups who have different risks of developing HCC. Third, the number of studies included in this study for A1298C polymorphism was relatively small and there was only one study in the Caucasian group [29], which leaded to low statistical power. Forth, bias may result from the fact that unpublished data, as well as papers published in languages other than English and Chinese, were not included. Fifth, all of the studies included in the meta-analysis were performed in Asian and Caucasian populations; further studies are needed in other ethnic groups in order to capture the full range of possible ethnic differences in MTHFR polymorphisms.
In summary, the present meta-analyses did not support a prominent association between MTHFR C677T polymorphism and HCC risk. The A1298C polymorphism might be associated with decreased HCC risk in Asian populations based on current published studies. However, it is necessary to conduct large sample studies using standardized unbiased genotyping methods, homogeneous HCC patients and well matched controls. Moreover, gene–gene and gene–environment interactions should also be considered in the analysis. Such studies taking these factors into account may eventually lead to better, comprehensive understanding of the association between the MTHFR polymorphisms and HCC risk.
Supporting Information
Flow diagram of included studies for this meta-analysis.
(TIF)
Checklist.
(DOC)
Funding Statement
The work described in this paper was supported by the National Natural Science Fundation (No. 81060199). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM (2006) The global health burden of infection-associated cancers in the year 2002. Int J Cancer 118: 3030–3044. [DOI] [PubMed] [Google Scholar]
- 3. Kamen B (1997) Folate and antifolate pharmacology. Semin Oncol 24: S18–30–S18-39. [PubMed] [Google Scholar]
- 4. Heijmans BT, Boer JM, Suchiman HE, Cornelisse CJ, Westendorp RG, et al. (2003) A common variant of the methylenetetrahydrofolate reductase gene (1p36) is associated with an increased risk of cancer. Cancer Res 63: 1249–1253. [PubMed] [Google Scholar]
- 5. Zintzaras E (2006) Methylenetetrahydrofolate reductase gene and susceptibility to breast cancer: a meta-analysis. Clin Genet 69: 327–336. [DOI] [PubMed] [Google Scholar]
- 6. Rosenblatt DS (2001) Methylenetetrahydrofolate reductase. Clin Invest Med 24: 56–59. [PubMed] [Google Scholar]
- 7. Taioli E, Garza MA, Ahn YO, Bishop DT, Bost J, et al. (2009) Meta- and pooled analyses of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and colorectal cancer: a HuGE-GSEC review. Am J Epidemiol 170: 1207–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boccia S, Hung R, Ricciardi G, Gianfagna F, Ebert MP, et al. (2008) Meta- and pooled analyses of the methylenetetrahydrofolate reductase C677T and A1298C polymorphisms and gastric cancer risk: a huge-GSEC review. Am J Epidemiol 167: 505–516. [DOI] [PubMed] [Google Scholar]
- 9. Kim YI (2005) 5,10-Methylenetetrahydrofolate reductase polymorphisms and pharmacogenetics: a new role of single nucleotide polymorphisms in the folate metabolic pathway in human health and disease. Nutr Rev 63: 398–407. [DOI] [PubMed] [Google Scholar]
- 10. Goyette P, Pai A, Milos R, Frosst P, Tran P, et al. (1998) Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome 9: 652–656. [DOI] [PubMed] [Google Scholar]
- 11. Guenther BD, Sheppard CA, Tran P, Rozen R, Matthews RG, et al. (1999) The structure and properties of methylenetetrahydrofolate reductase from Escherichia coli suggest how folate ameliorates human hyperhomocysteinemia. Nat Struct Biol 6: 359–365. [DOI] [PubMed] [Google Scholar]
- 12. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 13. Pereira AC, Schettert IT, Morandini Filho AA, Guerra-Shinohara EM, Krieger JE (2004) Methylenetetrahydrofolate reductase (MTHFR) c677t gene variant modulates the homocysteine folate correlation in a mild folate-deficient population. Clin Chim Acta 340: 99–105. [DOI] [PubMed] [Google Scholar]
- 14. van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, et al. (1998) A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet 62: 1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jin F, Qu LS, Shen XZ (2009) Association between the methylenetetrahydrofolate reductase C677T polymorphism and hepatocellular carcinoma risk: a meta-analysis. Diagn Pathol 4: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 17. Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, et al. (2005) Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162: 201–211. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 22. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: 455–463. [DOI] [PubMed] [Google Scholar]
- 24. Yu MC, Yuan JM, Lu SC (2008) Alcohol, cofactors and the genetics of hepatocellular carcinoma. J Gastroenterol Hepatol 23 Suppl 1S92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Samonakis DN, Koutroubakis IE, Sfiridaki A, Malliaraki N, Antoniou P, et al. (2004) Hypercoagulable states in patients with hepatocellular carcinoma. Dig Dis Sci 49: 854–858. [DOI] [PubMed] [Google Scholar]
- 26. Saffroy R, Pham P, Chiappini F, Gross-Goupil M, Castera L, et al. (2004) The MTHFR 677C>T polymorphism is associated with an increased risk of hepatocellular carcinoma in patients with alcoholic cirrhosis. Carcinogenesis 25: 1443–1448. [DOI] [PubMed] [Google Scholar]
- 27. Ventura P, Rosa MC, Abbati G, Marchini S, Grandone E, et al. (2005) Hyperhomocysteinaemia in chronic liver diseases: role of disease stage, vitamin status and methylenetetrahydrofolate reductase genetics. Liver Int 25: 49–56. [DOI] [PubMed] [Google Scholar]
- 28. Zhu ZZ, Cong WM, Liu SF, Xian ZH, Wu WQ (2006) [A study on the association of MTHFR C677T polymorphism with genetic susceptibility to hepatocellular carcinoma]. Zhonghua Gan Zang Bing Za Zhi 14: 196–198. [PubMed] [Google Scholar]
- 29. Mu LN, Cao W, Zhang ZF, Cai L, Jiang QW, et al. (2007) Methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms and the risk of primary hepatocellular carcinoma (HCC) in a Chinese population. Cancer Causes Control 18: 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan JM, Lu SC, Van Den Berg D, Govindarajan S, Zhang ZQ, et al. (2007) Genetic polymorphisms in the methylenetetrahydrofolate reductase and thymidylate synthase genes and risk of hepatocellular carcinoma. Hepatology 46: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwak SY, Kim UK, Cho HJ, Lee HK, Kim HJ, et al. (2008) Methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR) gene polymorphisms as risk factors for hepatocellular carcinoma in a Korean population. Anticancer Res 28: 2807–2811. [PubMed] [Google Scholar]
- 32. D’Amico M, Pasta L, Sammarco P (2009) MTHFR C677TT, PAI1 4G-4G, V Leiden Q506, and prothrombin G20210A in hepatocellular carcinoma with and without portal vein thrombosis. J Thromb Thrombolysis 28: 70–73. [DOI] [PubMed] [Google Scholar]
- 33. Fabris C, Toniutto P, Falleti E, Fontanini E, Cussigh A, et al. (2009) MTHFR C677T polymorphism and risk of HCC in patients with liver cirrhosis: role of male gender and alcohol consumption. Alcohol Clin Exp Res 33: 102–107. [DOI] [PubMed] [Google Scholar]
- 34.Cui LH, Song Y, Si H, Shen F, Shin MH, et al.. (2011) Folate metabolism-related gene polymorphisms and susceptibility to primary liver cancer in North China. Med Oncol. [DOI] [PubMed]
- 35.Liu JJ, Gao YT, Du Z, Yang B, Jing X, et al. (2010) Shi Jie Hua Ren Xiao Hua Za Zhi, 2010. Shi Jie Hua Ren Xiao Hua Za Zhi 18: 1555–1562 (article in chinese). Available: http://d.g.wanfangdata.com.cn/Periodical_hrxhzz201015007.aspx. Accessed 2012 June 10.
- 36.Yang H (2007) Genetic association between candidate genes polymorphisms and susceptibility to chronic hepatitis B, hepatocellular carcinoma and nasopharyngeal carcinoma in Chinese population (article in chinese). Available at http://d.g.wanfangdata.com.cn/Thesis_Y1201379.aspx (accessed 10 June 2012).
- 37. Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, et al. (2002) A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A 99: 5606–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Botto LD, Yang Q (2000) 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151: 862–877. [DOI] [PubMed] [Google Scholar]
- 39. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64: 169–172. [DOI] [PubMed] [Google Scholar]
- 40. Bagley PJ, Selhub J (1998) A common mutation in the methylenetetrahydrofolate reductase gene is associated with an accumulation of formylated tetrahydrofolates in red blood cells. Proc Natl Acad Sci U S A 95: 13217–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow diagram of included studies for this meta-analysis.
(TIF)
Checklist.
(DOC)