Abstract
Background
The renin-angiotensin system (RAS) is present in human placental tissue and participates in regulation of maternal-fetal blood flow during pregnancy. RAS expression in placental tissue is regulated by various hormones and is altered in various disease conditions. An in vitro system is needed to further investigate regulation of the placental RAS. To this end, we studied RAS expression in the human placenta-derived cell line, CRL-7548.
Methods
CRL-7548 cells were cultured in plastic plates. Total RNA was extracted, reverse transcribed, and amplified by polymerase chain reaction (PCR) with specific primers. Angiotensin II peptide in the culture media was measured by radioimmunoassay. Renin activity was detected by radioimmunoassay measuring angiotensin I generated. Angiotensin receptor type I was detected by Western blot.
Results
Specific mRNA for angiotensin, renin, angiotensin converting enzyme, and angiotensin receptor type I was detected by real-time PCR. Renin activity was detected in the placental cell lysate, and angiotensin II peptide, the final product of the RAS system, was detected in cell culture media by radioimmunoassay. Angiotensin receptor type I was identified as a 41 kDa protein in cell lysates by Western blot.
Conclusions
These results demonstrate that all necessary components of the classic RAS are expressed in the human placental cell line CRL-7548. This cell line may prove useful as an in vitro system for studying RAS regulation in the placenta.
Keywords: Renin-angiotensin system, Placenta, Expression, Pre-eclampsia, Pregnancy
The renin-angiotensin system (RAS) plays an important role in the development and regulation of arterial pressure during a variety of physiologic and pathophysiologic conditions. In the classic RAS, angiotensin I (Ang I) is formed by the proteolytic cleavage of the N-terminus of angiotensinogen (AGT) by renin. Angiotensin converting enzyme (ACE) in turn converts the decapeptide Ang I to the octapeptide angiotensin II (Ang II). Ang II exerts the biological functions of the RAS by binding to the two major Ang II receptors: Ang II receptor type I (AT1R) and type II (AT2R). Ang II has a similar affinity for both receptors.
In addition to the presence of RAS in circulating blood, the actions of RAS can occur via generation and activity of RAS components at tissue sites. The presence of a local RAS has been demonstrated in several tissues, including human placenta.1–6 There is significant evidence to suggest that the local RAS in the placenta is involved in physiologic and pathophysiologic processes during pregnancy.7,8 Ang II is involved in the regulation of uteroplacental vascular resistance and blood flow.9 Low Ang II concentrations increase uteroplacental blood flow, whereas high concentrations of Ang II reduce blood flow.10,11 Additionally, Ang II activates plasminogen activator inhibitor-1 gene expression through AT1R and, thus, inhibits human trophoblast invasion, a process involved in the physiological union between maternal and fetal circulatory systems.12
To facilitate the research of RAS regulation in the placenta and study of the underlying mechanisms, an in vitro system, such as a placental cell line, is needed. Here we report a human placental cell line, CRL-7548, that expresses all the components of the classic RAS necessary for function.
Materials and Methods
Cell Culture
A human placenta-derived cell line, CRL-7548 (5 months gestation), was obtained from the American Type Culture Collection (Manassas, VA). The human placental cells were cultured in plates with Dulbecco’s Modified Eagle Medium (DMEM [Mediatech, Inc., Herndon, VA]) and 10% fetal bovine serum (FBS [Sigma, St. Louis, MO]) at 37° C for 2 to 3 days to reach approximately 80% to 90% confluence before experiments.
RNA Preparation
Placental cells were cultured without FBS overnight and washed with phosphate buffered saline (PBS) before collection and RNA extraction. Total RNA was extracted from cells using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA was resuspended in diethylpyrocarbonate-treated water and quantitated by spectrophotometry.
Real-time Polymerase Chain Reaction
RNA (1 μg) was reverse transcribed with random primers and Moloney murine leukemia virus reverse transcriptase in a final volume of 50 μl. A 2 μl volume of cDNA was amplified by TaqMan real-time polymerase chain reaction (PCR) using the Roche LightCycler 480 system in a final volume of 50 μl in LightCycler Probe Master solution containing 0.5 μM of each specific primer and 0.2 μM fluoresceine labeled probe for 40 cycles. Primers were designed from different exons of each gene to avoid amplifying genomic DNA. The sequences of the primers and probes used in this study are listed in Table 1.
Table 1.
Primer and Probe Design and Accession Number in GeneBank.
| Gene | Primers | Probe | GeneBank Accession # |
|---|---|---|---|
| ATG | (F) cca ttc tgc aca ccg ag (R) caa gac ctc agg ctt gtt aag | 6FAM-tag act ctg tgg gct ctc tct cat ccg-BBQ | NM-000029 |
| Renin | (F) tga cac tgg ttc gtc caa tg (R) agc tgg agg aat ccg aag c | 6FAM-tgc cct cct cca agt gca gcc-BBQ | NM-000537 |
| ACE | (F) caa ctt cga ctg gtg gta tct t (R) ctt cat gga act gga act gc | 6FAM-ccc act ttg atg ctg gag cta tgt ttc-BBQ | NM-000789 |
| AT1R | Purchased from Applied Biosystems Sequences unavailable | Assay ID: Hs00258938-1 Sequences unavailable | NM-000685 |
| GAPDH | (F) gaa ggt gaa ggt cgg agt c (R) gaa gay ggt gat ggg att tc | 6FAM-caa gct tcc cgt tct cag ct-BBQ | NM-002046 |
Preparation of Cell Lysate and Conditioned Media
Culture medium was changed to FBS-free DMEM overnight and cells were washed several times with PBS to avoid possible contamination with FBS, which may contain RAS proteins. Cells were harvested and sonicated twice for 20 seconds on ice in 1 ml of protein lysis buffer with a protease inhibitor cocktail (Sigma, St. Louis, MO), followed by centrifugation at 1500 rpm for 2 minutes at 4°C. The protein concentration in the supernatant was determined using the Bio-Rad Protein Assay as instructed. For detection of Ang II in culture medium from the human placental cells, cells were washed with PBS and the culture medium was replaced with FBS-free DMEM. After two days, cell culture media was collected and a protease inhibitor cocktail was added. This is referred to as conditioned media. As a control, unconditioned media was prepared the same way in the absence of cells.
Radioimmunoassay for Ang II and Renin Activity Assay
Conditioned culture medium (100 μl) was used for radioimmunoassay (RIA) of Ang II according to the manufacturer’s protocol (ALPCO Diagnostics, Buhlmann Laboratories, Switzerland). Unconditioned culture medium was used as a control. Each sample was analyzed in duplicate. Renin activity was indirectly measured by quantitation of Ang I generated by cleavage from AGT. Cell lysates were split in two, and one fraction was incubated on ice while the other was incubated at 37°C. Ang I was measured by RIA. Specific renin activity was determined by subtracting the concentration of Ang I in the tube incubated on ice from the Ang I concentration obtained after incubation at 37°C to correct for endogenous Ang I.
Western Blot Analysis
Thirty micrograms of protein from cell lysates were subjected to electrophoresis in 12% denaturing polyacrylamide gels (SDS-PAGE) and electrotransferred onto nitrocellulose membranes. The membrane was incubated with AT1R-specific primary antibodies (AT1 N-10P [Santa Cruz Biotechnology, Santa Cruz, CA]) overnight at 4°C. The membrane was incubated with a secondary antibody conjugated to horseradish peroxidase for one hour at room temperature. The film was developed using a chemiluminescent substrate (Amersham, Buckinghamshire, UK) and was exposed to X-ray film for one hour.
Results
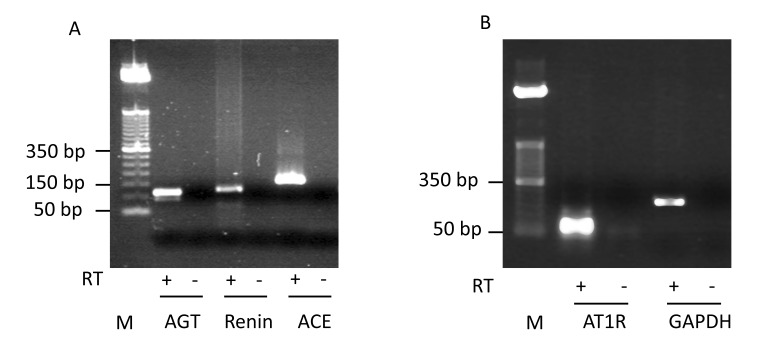
Specific mRNAs for AGT, renin, ACE and AT1R were detected in the human placental cell line by TaqMan real-time PCR analysis. PCR products for AGT, renin, ACE, AT1R and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were found to be of the predicted size on agarose gels (figure 1). The authenticity of the PCR products was confirmed by DNA sequencing (data not shown). We were unable to reliably detect AT2R mRNA suggesting that AT2R is either not expressed or expressed at a very low level in the human placental cell line.
Figure 1.
Detection of AGT, renin, ACE and AT1R mRNA expression by real-time PCR. Amplification products of (panel A) AGT (128 bp), renin (121 bp), ACE (164 bp), and (panel B) AT1R (80 bp) and GAPDH (225 bp) obtained from placental cell total RNA were visualized on ethidium bromide stained 2% agarose gels. Reverse transcriptase (RT) was present (+) or absent (-) in the step of reverse transcription. No PCR products were seen in samples when RT was omitted (-). A 50 bp DNA ladder was used as a size marker (M). GAPDH refers to the internal control gene, glyceraldehyde 3-phosphate dehyrodrogenase.
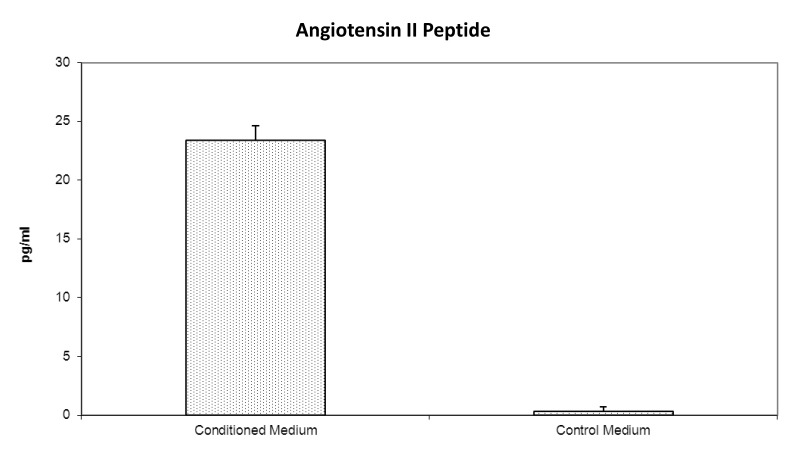
Since Ang II is the final biologically active product of the RAS and exerts its biological effects by binding to the Ang II receptors, we focused on two key proteins in the RAS, namely Ang II and AT1R. The Ang II peptide was detected in the conditioned medium by RIA, but not in the control culture medium (figure 2). AT1R was detected as a 41 kDa protein in the placental cell lysate by Western blot analysis using an AT1R-specific antibody (figure 3). Renin activity (0.01–0.04 ng A1/ml/hour) was detected in the placental cell lysate via measurement of angiotensin I by RIA. ACE activity was not detected in several attempts using a spectrophotometric assay with substrate N-[3-(2-furyl)acryloyl]-L-phenylalanyl-L-glycyl-L-glycine.
Figure 2.
Secretion of immunoreactive Ang II into culture medium by human placental cells. Conditioned media (n=6) was collected from plates with cultured placental cells. Controls are unconditioned media (n=6) from plates without cultured cells. Values are picograms immunoreactive Ang II per milliliter of culture medium.
Figure 3.
Representative Western blot experiment showing the expression of the AT1R in human placental cells. Lanes 1 and 2 are placental cell lysates from separate preparations. Approximately 30 μg of lysate proteins were loaded on each lane. A single band of 41 kDa protein was detected using an AT1R-specific antibody.
Discussion
In addition to the classical view of the systemic RAS, studies have shown that the components of the RAS are synthesized locally in many tissues, including the placenta.1–6 AGT has been detected in human placenta.13,14 While some AGT may be taken up from the maternal or fetal circulation, studies using PCR amplification have demonstrated AGT mRNA in the human placenta indicating local expression.15,16 Several studies have demonstrated in vitro secretion of prorenin from explants of the human uteroplacental unit,17,18 and renin mRNA in the placenta has been demonstrated by PCR amplification.15,19 In the human placenta, the amount of ACE mRNA increases over the course of pregnancy, but decreases near term; whereas ACE activity increases during the course of pregnancy.20 AT1R is the predominant form of Ang II receptor in the human placenta21,22 with AT2R accounting for only 0% to10%.23–26 Both AT1R protein and AT1R mRNA levels increase during pregnancy and reach the highest levels at term.27 Thus, the placental tissues contain all the necessary components for a functional RAS. The results of our study highlight the existence of a functional RAS in a human placental cell line, which may be useful for in vitro studies. We found evidence of mRNAs for all necessary components of the RAS in the human placental cell line CRL-7548. Additionally, we demonstrated secretion of Ang II peptide and the presence of its target receptor AT1R in this placental cell line, indicating that the RAS was both present and functional.
The placenta is one of the major sites of extra-renal RAS during pregnancy. The RAS in the placenta is highly regulated during pregnancy and the expression of RAS in the placenta is altered significantly in diseased conditions such as preeclampsia.28,29 Several experimental models have been used in studies of the RAS in human placenta, including human placental tissues from various gestational stages and diseased conditions, primary placental tissue culture and cell culture, and placental cancer cell lines.7,8,30 Studies on human placental tissues directly observe changes of RAS activity in various physiological and diseased conditions. In vitro systems such as primary placental tissue or cell culture offer a controlled environment to test specific cellular and molecular hypotheses of RAS regulation and contribute greatly to our understanding of the underlying mechanisms. However, the preparation of primary placental organ or cell cultures is labor intensive and fresh preparation is required for every experiment. Placental cancer cell lines provide the advantage of convenience as they can be propagated indefinitely, but may have retained very little of the original in vivo characteristics. To facilitate the study of the RAS in the placenta, a non-cancerous human placental cell line is needed as a system for in vitro study. The human placental cell line reported here, CRL-7548, expresses all necessary components of the RAS and thus provides a useful tool for in vitro studies. To the best of our knowledge, this is the first reported noncancerous, non-transfected human placental cell line that expresses all necessary components of RAS.
The wide distribution of the components of the RAS in the placental unit and the presence of Ang II receptors in the placenta as a target for Ang II suggests paracrine and autocrine RAS functions in the placenta. It was suggested that Ang II produced in the maternal uterine placental bed may have a paracrine role by acting on AT1R in adjacent placenta to vasoconstrict fetal chorionic villi vessels, thus regulating maternal-fetal oxygen exchange.31 Our finding of a human placental cell line that expresses all components of the RAS, including AT1R, further supports this notion and suggests an autocrine function.
In pregnancy, in addition to the changes of the local RAS in the placental tissue, there is increasing activity of the systemic RAS including increased circulating levels of AGT.32 The systemic RAS may affect the local placental RAS. We have previously reported the presence of an AGT receptor on the same placental cell line used in this study.33 This AGT receptor may provide a link between the systemic RAS and the local placental RAS. Binding of AGT to the AGT receptor may cause internalization of the AGT-receptor complex and the released AGT could participate in an intracellular RAS. The human placental cell line with documented internal RAS reported here could be used to study the function of the AGT receptor and the interaction between the external and internal RAS in the placenta.
No AT2R mRNA was detected in the human placental cell line in the present study. This finding is consistent with reports from other studies demonstrating that AT1R is the predominant Ang II receptor in the human placenta.21,22 Additionally, both AT1R mRNA and protein have been shown to localize to the cytotrophoblast and syncytiotrophoblast in human placental villi during early and late pregnancy.22 RT-PCR is a very sensitive method to detect mRNA; therefore, it is likely that AT2R is not expressed in the placental cell line, although we cannot completely exclude the possibility that AT2R is expressed at a very low level or under different conditions.
There are some limitations to our study. We failed to detect ACE protein activity with several attempts. While the presence of Ang II in the culture media and the presence of ACE mRNA in cells indirectly suggest ACE activity, we are not able to directly prove the presence of ACE protein in this cell line. One possible explanation is that our methodology, designed to measure ACE activity in serum, may have been inadequate for the detection of ACE activity in cell lysates. Another possibility is that Ang II could be converted from Ang I nonspecifically by non-ACE enzymes, such as chymase.
Conclusion
In conclusion, we have identified a human placental cell line (CRL-7548) that expresses all necessary components of the classic RAS. Results suggest the potential for both autocrine and paracrine function of the RAS in the placenta, and this cell line may provide a useful in vitro system for the study of RAS regulation in the placenta and the underlying mechanisms.
Acknowledgements
The authors thank Marshfield Clinic Research Foundation for research support and Rachel V. Stankowski, PhD of the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication for writing assistance in the preparation of this article.
Footnotes
Financial Disclosure: This study was funded by grants from the Marshfield Clinic Research Foundation’s Disease Specific Funds and the Marshfield Clinic’s Physician Research Funds.
References
- 1.Hagemann A, Nielsen AH, Poulsen K. The uteroplacental renin-angiotensin system: a review. Exp Clin Endocrinol 1994;102:252–261 [DOI] [PubMed] [Google Scholar]
- 2.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol 1998;19:232–252 [DOI] [PubMed] [Google Scholar]
- 3.Bader M, Peters J, Baltatu O, Muller DN, Luft FC, Ganten D. Tissue renin-angiotensin systems: new insights from experimental animal models in hypertension research. J Mol Med (Berl) 2001;79:76–102 [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie SM, Fraser R, Connell JM, Davies E. Local renin-angiotensin systems and their interactions with extra-adrenal corticosteroid production. J Renin Angiotensin Aldosterone Syst 2002;3:214–221 [DOI] [PubMed] [Google Scholar]
- 5.Williams PJ, Mistry HD, Innes BA, Bulmer JN, Pipkin FB. Expression of AT1R, AT2R and AT4R and their roles in extravillous trophoblast invasion in the human. Placenta 2010;31:448–455 [DOI] [PubMed] [Google Scholar]
- 6.Pringle KG, Tadros MA, Callister RJ, Lumbers ER. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta 2011;32:956–962 [DOI] [PubMed] [Google Scholar]
- 7.Anton L, Brosnihan KB. Systemic and uteroplacental renin-angiotensin system in normal and pre-eclamptic pregnancies. Ther Adv Cardiovasc Dis 2008;2:349–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irani RA, Xia Y. The functional role of the renin-angiotensin system in pregnancy and preeclampsia. Placenta 2008;29:763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poston L. The control of blood flow to the placenta. Exp Physiol 1997;82:377–387 [DOI] [PubMed] [Google Scholar]
- 10.Speroff L, Haning RV, Jr, Levin RM. The effect of angiotensin II and indomethacin on uterine artery blood flow in pregnant monkeys. Obstet Gynecol 1977;50:611–614 [PubMed] [Google Scholar]
- 11.Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest 1981;68:468–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia Y, Wen HY, Kellems RE. Angiotensin II inhibits human trophoblast invasion through AT1 receptor activation. J Biol Chem 2002;277:24601–24608 [DOI] [PubMed] [Google Scholar]
- 13.Lenz R, Sealey JE, August P, James GD, Laragh JH. Tissue levels of active and total renin, angiotensinogen, human chorionic gonadotropin, estradiol, and progesterone in human placentas from different methods of delivery. J Clin Endocrinol Metab 1989;69:31–37 [DOI] [PubMed] [Google Scholar]
- 14.Lenz T, Sealey JE, Tewksbury DA. Regional distribution of the angiotensinogens in human placentae. Placenta 1993;14:695–699 [DOI] [PubMed] [Google Scholar]
- 15.Paul M, Wagner J, Dzau VJ. Gene expression of the renin-angiotensin system in human tissues. Quantitative analysis by the polymerase chain reaction. J Clin Invest 1993;91:2058–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper AC, Robinson G, Vinson GP, Cheung WT, Broughton Pipkin F. The localization and expression of the renin-angiotensin system in the human placenta throughout pregnancy. Placenta 1999;20:467–474 [DOI] [PubMed] [Google Scholar]
- 17.Downing GJ, Poisner R, Poisner AM. Beta-adrenoceptor activation stimulates, and phosphodiesterase inhibition potentiates, placental prorenin synthesis and release. J Clin Endocrinol Metab 1994;78:41–47 [DOI] [PubMed] [Google Scholar]
- 18.Poisner AM, Downing GJ, Poisner R. Prorenin secretion from villous placenta: regulation by cyclic AMP and angiotensin. Placenta 1994;15:487–499 [DOI] [PubMed] [Google Scholar]
- 19.Downing GJ, Yan B, Poisner AM. Beta-adrenoceptor activation-induced placental prorenin secretion is mediated by increased renin messenger RNA and protein synthesis. Mol Pharmacol 1997;51:201–208 [DOI] [PubMed] [Google Scholar]
- 20.Yagami H, Kurauchi O, Murata Y, Okamoto T, Mizutani S, Tomoda Y. Expression of angiotensin-converting enzyme in human placenta and its physiologic role in the fetal circulation. Obstet Gynecol 1994;84:453–457 [PubMed] [Google Scholar]
- 21.Knock GA, Sullivan MH, McCarthy A, Elder MG, Polak JM, Wharton J. Angiotensin II (AT1) vascular binding sites in human placentae from normal-term, preeclamptic and growth retarded pregnancies. J Pharmacol Exp Ther 1994;271:1007–1015 [PubMed] [Google Scholar]
- 22.Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, Barnes N, Ahmed A. Cellular localization of AT1 receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J Clin Invest 1998;101:442:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kingdom JC, McQueen J, Connell JM, Whittle MJ. Fetal angiotensin II levels and vascular (type I) angiotensin receptors in pregnancies complicated by intrauterine growth retardation. Br J Obstet Gynaecol 1993;100:476–482 [DOI] [PubMed] [Google Scholar]
- 24.Kalenga MK, De Gasparo M, Thomas K, De Hertogh R. Down-regulation of angiotensin AT1 receptor by progesterone in human placenta. J Clin Endocrinol Metab 1996;81:998–1002 [DOI] [PubMed] [Google Scholar]
- 25.Kalenga MK, de Gasparo M, Thomas K, de Hertogh R. Angiotensin II and its different receptor subtypes in placenta and fetal membranes. Placenta 1996;17:103–110 [DOI] [PubMed] [Google Scholar]
- 26.Kalenga MK, Thomas K, de Gasparo M, de Hertogh R. Determination of renin, angiotensin converting enzyme and angiotensin II levels in human placenta, chorion and amnion from women with pregnancy induced hypertension. Clin Endocrinol (Oxf) 1996;44:429–433 [DOI] [PubMed] [Google Scholar]
- 27.Petit A, Geoffroy P, Bélisle S. Expression of angiotensin II type-I receptor and phospholipase C-linked G alpha q/11 protein in the human placenta. J Soc Gynecol Investig 1996;3:316–321 [PubMed] [Google Scholar]
- 28.Shah DM. Role of the renin-angiotensin system in the pathogenesis of preeclampsia. Am J Physiol Renal Physiol 2005;288:F614–F625 [DOI] [PubMed] [Google Scholar]
- 29.Shah DM. Preeclampsia: new insights. Curr Opin Nephrol Hypertens 2007;16:213–220 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Pringle KG, Chen YX, Zakar T, Lumbers ER. Regulation of the renin-angiotensin system (RAS) in BeWo and HTR-8/SVneo trophoblast cell lines. Placenta 2012;33:634–639 [DOI] [PubMed] [Google Scholar]
- 31.Anton L, Merrill DC, Neves LA, Diz DI, Corthorn J, Valdes G, Stovall K, Gallagher PE, Moorefield C, Gruver C, Brosnihan KB. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology 2009;150:4316–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tewksbury DA. Angiotensinogen – Biochemistry and Molecular Biology. In: Laragh JH, Brenner BM, Eds. Hypertension: pathophysiology, diagnosis and management. New York, NY: Raven Press; 1990. pp. 1197–1216 [Google Scholar]
- 33.Tewksbury DA, Pan N, Kaiser SJ. Detection of a receptor for angiotensinogen on placental cells. Am J Hypertension 2003;16:59–62 [DOI] [PubMed] [Google Scholar]