Abstract
Sera from patients with cancer contain antibodies which react with a unique group of autologous cellular antigens called tumor-associated antigens (TAAs). This study aimed to determine whether a mini-array of multiple TAAs would enhance antibody detection and be a useful approach in breast cancer detection and diagnosis. The mini-array of multiple TAAs was composed of ten TAAs, including Imp1, p62, Koc, p53, c-myc, survivin, p16, cyclin B1, cyclin D1 and CDK2 full-length recombinant proteins. An enzyme-linked immunosorbent assay (ELISA) was used to detect antibodies against these ten TAAs in 41 sera from patients with breast cancer, as well as 82 sera from normal individuals. The antibody frequency of the individual TAAs in breast cancer was variable and ranged between 7.3 and 22.0%. With the successive addition of TAAs to a final total of ten antigens, there was a stepwise increase in positive antibody reactions, reaching a sensitivity of 61.0% and a specificity of 86.6% in breast cancer. The positive and negative likelihood ratios were 5.545 and 0.438, respectively, which showed that the clinical diagnostic value of a parallel assay of eight TAAs was high. The positive and negative predictive values were 73.5 and 82.0%, respectively, indicating that the parallel assay of eight TAAs raised the diagnostic precision significantly. The agreement rate and κ-value were 79.7% and 0.52, respectively, while the Youden’s Index (YI) was 0.5, indicating that the observed value of this assay had a middle range coincidence with the actual value. The data from the present study further support our previous hypothesis that the detection of autoantibodies for the diagnosis of certain types of cancer may be enhanced using a mini-array of several TAAs as target antigens. A customized antigen mini-array using a panel of appropriately selected TAAs is able to enhance autoantibody detection in the immunodiagnosis of breast cancer.
Keywords: autoantibodies, tumor-associated antigens, immunodiagnosis, breast cancer
Introduction
Breast cancer is the most frequent malignant tumor and a leading cause of cancer mortality among females in the majority of Asian countries, including China (1). Despite significant progress, 40% of patients diagnosed with breast cancer succumb to the disease. The high mortality rate may be attributed in part to a lack of diagnostic methods allowing early detection. Another major cause of mortality among breast cancer patients continues to be the presence of the metastatic disease with ∼5% of patients exhibiting clinically detectable metastases at the time of the initial diagnosis and a further 30–40% of patients with no clinically detectable disease harboring occult metastases. Although mammograms are the most effective tool for detecting breast cancer, the US Food and Drug Administration reports that mammography is able to identify only ∼80% of breast cancers in females (2). Hence, there is a requirement for further understanding of tumor biology and host response mechanisms so that new diagnostic and therapeutic tools may be developed. Early diagnosis is essential for the optimal management of breast cancer. Thus, extensive studies are being conducted to identify and validate new biomarkers to add to current markers and increase the sensitivity and specificity of breast cancer detection.
Numerous studies have demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens called tumor-associated antigens (TAAs) (3,4). The types of cellular proteins that induce these autoantibody responses are varied and include tumor suppressors, such as p53 (5) and p16 (6), oncogene products, such as c-myc (7) and HER-2/neu (8), and other cancer-related proteins, such as Imp2/p62 (9), CRD-BP (10), CIP2A/p90 (11), survivin (12,13) and LEDGF (14). The various factors leading to the increased production of such autoantibodies are not completely understood. However, the available data show that a number of the target antigens are cellular proteins, such as p53, whose aberrant regulation or overexpression is capable of leading to tumorigenesis (5,15,16). The immune systems of certain cancer patients are able to sense these aberrant tumor-associated proteins as unknown antigens and have the capability to respond by producing autoantibodies (17). Thus, cancer-associated autoantibodies may be regarded as reporters identifying aberrant de novo or disregulated cellular mechanisms in tumorigenesis (3,4). The potential utility of TAA-autoantibody systems as early cancer biomarker tools to monitor therapeutic outcomes or as indicators of disease prognosis has been investigated. The present study evaluated whether a mini-array of multiple TAAs would enhance autoantibody detection and be an effective tool in the immunodiagnosis of breast cancer.
Materials and methods
Serum samples and antibodies
In the present study, sera from 41 patients with breast cancer and 82 normal individuals who had no clear evidence of malignancy were provided by our collaborator in China. Based on clinical information, all cancer sera were collected at the first time of diagnosis and patients did not receive any treatment with chemotherapy or radiotherapy. Normal control sera were collected during annual health examinations. The present study was approved by the Institutional Review Boards of the University of Texas at El Paso (UTEP) and collaborating academic institutions.
Recombinant TAAs
All TAAs used in the present study, including Imp1, p62, Koc, p53, p16, c-myc, survivin, cyclin B1, cyclin D1, cyclin E and CDK2, were derived from our previous studies. The reactivities of these selected TAAs were determined with either polyclonal or monoclonal antibodies against the respective proteins.
Enzyme-linked immunosorbent assay (ELISA)
Purified recombinant TAAs were individually diluted in PBS to a final concentration of 0.5 μg/ml and 200 μl were pipetted into each well to coat Immulon 2 microtiter plates (Fisher Scientific, Houston, TX, USA) overnight at 4°C. The human serum samples were diluted at 1:200, incubated with the antigen-coated wells at 37°C for 90 min followed by washing with PBS containing 0.05% Tween-20. The samples were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-human IgG (Caltag Laboratories, Burlingame, CA, USA) as a secondary antibody diluted 1:2,000 for 90 min followed by washing with PBS containing 0.05% Tween-20. A solution of 3,3’,5,5’-tetramethyl benzidine (TMB)-H2O2-urea was used as the detecting agent. The OD of each well was read at 450 nm. Each sample was tested in duplicate. The cut-off value for determining a positive reaction was designated as the mean absorbance of the 82 normal human sera (NHS) plus 2 standard deviations (mean + 2SD). Since several hundred test sera were analyzed at various time periods, each run of the ELISA included at least 8 NHS samples and 2 positive control samples. These 8 NHS samples, representing a range of 2SD above and below the mean of the 82 NHS, were used in each experiment and the average value of the 8 NHS samples was used in each run to normalize all absorbance values to the mean of the entire 82 normal samples. In addition, all positive sera were confirmed with repeat testing, as were certain negative sera. The detailed protocol of the ELISA has been described previously (9,18).
Western blotting and slot blot analysis
Western blot analysis was used to confirm that the bands observed in SDS-PAGE were reactive with the reference antibodies. In brief, the purified TAAs were electrophoresed by SDS-PAGE and subsequently transferred to a nitrocellulose membrane. The individual strips were pre-blocked in PBS containing 0.05% Tween-20 (PBST) with 5% non-fat milk for 30 min at room temperature, then incubated for 90 min with patient sera diluted 1:100 and finally incubated with HRP-conjugated goat anti-human IgG diluted 1:3,000 for 90 min, followed by washing with PBST solution. The positive signals were recorded by autoradiography. Slot blot analysis was used to confirm the positive sera samples detected by ELISA. The method for the slot blotting was identical to that of the western blotting with the exception that the purified recombinant protein (100 ng/well) was applied directly to the nitrocellulose membrane using a vacuum source. Membranes were not cut into strips and therefore, the detection of autoantibodies to all the TAAs in an individual patient’s serum was performed in one blot simultaneously.
Statistical analysis
To determine whether the frequency of autoantibodies binding to selected TAAs in the cancer sera was significantly higher than that in NHS, the data were analyzed using the χ2 tests with Yates’ correction. Two levels of statistical significance (0.05 and 0.01) were used and P<0.05 was considered to indicate statistically significant differences. The comprehensive evaluations of the testing results for each anti-TAA antibody, including the methods for calculating the sensitivity, specificity, Youden’s index (YI), positive and negative likelihood ratio, positive (PPV) and negative predictive value (NPV), agreement rate and κ-value, were based on the methodology provided in the Epidemiology textbook (19).
Results
Prevalence of antibodies in a mini-array of multiple TAAs in breast cancer
In order to evaluate whether the combination of antibodies to multiple TAAs yields higher sensitivity for the diagnosis of breast cancer, the present study tested breast cancer sera for the presence of anti-TAA antibodies with a panel of ten selected recombinant TAAs using an ELISA and revealed that the combined the antibody frequency was 61.0% (25/41), significantly higher than the frequency (13.4%) in the sera from normal individuals (11/82). As shown in Table I, antibody frequency for any individual TAA in breast cancer was variable, ranging between 7.3 and 22.0%. The highest frequencies were against c-myc (22.0%), survivin (22.0%), cyclin B1 (17.1%) and cyclin D1 (17.1%), followed by p62 (12.2%), p53 (12.2%), p16 (12.2%), Imp1 (12.2%), CDK2 (9.8%) and Koc (7.3%). It was observed that, with the successive addition of TAAs to a total of eight antigens (c-myc, survivin, cyclin B1, cyclin D1, p62, p53, p12 and CDK2), there was a stepwise increase in the sensitivity, up to 61.0%, and the specificity was 89.0%. If additional antigens (Imp1 and Koc) were added to the panel, there was no further increase in the sensitivity (see Table II). These results indicate that an array of eight TAAs is able to serologically distinguish breast cancer patients from normal individuals at a sensitivity of 61.0%. However, it should be determined whether this TAA combination distinguishes breast cancer from other types of cancer. Positive results were also confirmed by slot blotting. Slot blot analysis of four representative breast cancer sera is shown in Fig. 1.
Table I.
Frequency of antibodies for ten TAAs in breast cancer.
| No. (%) of autoantibodies in:
|
||
|---|---|---|
| Autoantibodies to: | BC (41) | NHS (82) |
| c-myc | 9 (22.0)b | 0 (0) |
| survivin | 9 (22.0)b | 1 (1.2) |
| cyclin B1 | 7 (17.1)b | 1 (1.2) |
| cyclin D1 | 7 (17.1)a | 2 (2.4) |
| p62 | 5 (12.2)a | 1 (1.2) |
| p53 | 5 (12.2)a | 2 (2.4) |
| p16 | 5 (12.2)a | 2 (2.4) |
| Imp1 | 5 (12.2)a | 2 (2.4) |
| CDK2 | 4 (9.8)a | 1 (1.2) |
| Koc | 3 (7.3) | 1 (1.2) |
| Cumulative to ten antigens | 61.0 (25/41)b | 11 (13.4) |
P-values relative to NHS,
P<0.05,
P<0.01. TAA, tumor-associated antigen; BC, breast cancer; NHS, normal human sera.
Table II.
Sequential addition of antigen into the panel of ten TAAs in breast cancer.
| No. (%) of autoantibodies in:
|
||
|---|---|---|
| Antigen | BC (41) | NHS (82) |
| c-myc | 9 (22.0)a | 0 (0) |
| c-myc and survivin | 14 (34.1)a | 1 (1.2) |
| c-myc, surviving and cyclin B1 | 16 (39.0)a | 2 (2.4) |
| c-myc, survivin, cyclin B1 and cyclin D1 | 18 (43.9)a | 4 (4.9) |
| c-myc, survivin, cyclin B1, cyclin D1 and p62 | 20 (48.8)a | 5 (6.1) |
| c-myc, survivin, cyclin B1, cyclin D1, p62 and p53 | 21 (51.2)a | 7 (8.5) |
| c-myc, survivin, cyclin B1, cyclin D1, p62, p53 and p16 | 23 (56.1)a | 9 (11.0) |
| c-myc, survivin, cyclin B1, cyclin D1, p62, p53, p16 and CDK2 | 25 (61.0)a | 9 (11.0) |
| c-myc, survivin, cyclin B1, cyclin D1, p62, p53, p16, CDK2 and Imp1 | 25 (61.0)a | 11 (13.4) |
| c-myc, survivin, cyclin B1, cyclin D1, p62, p53, p16, Imp1, CDK2 and Koc | 25 (61.0)a | 11 (13.4) |
P-values relative to NHS:
P<0.01. TAA, tumor-associated antigen; BC, breast cancer; NHS, normal human sera.
Figure 1.
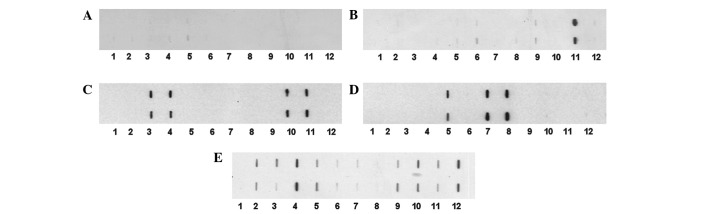
Mini-array of multiple TAAs with four representative breast cancer sera using slot blot analysis. Each blot represents a duplicate test for autoanti-bodies against a panel of eleven recombinant TAAs, with PBS as a negative control. Purified recombinant protein (100 ng per well) was applied directly to the nitrocellulose membrane using a vacuum device. Membranes were used for the simultaneous detection of autoantibodies in an individual patient’s serum to any of the eleven TAAs, following standard immunoblotting procedures. 1, PBS; 2, survivin; 3, p53; 4, p16; 5, cyclin B1; 6, cyclin D1; 7, cyclin E; 8, Koc; 9, Imp1; 10, p62; 11, CDK2; 12, c-myc. (A) Normal human serum showing no reactivity to any of the eleven TAAs. (B–E) Four representative breast cancer sera showing different antibody profiles with the 11 TAAs. TAA, tumor-associated antigen; PBS, phosphate-buffered saline.
Evaluation of diagnostic values of a mini-array of multiple TAAs in immunodiagnosis of breast cancer
The validity of a test is defined as its ability to distinguish between individuals who have a disease and those who do not. In order to address the question of how valuable the approach of antibody detection to a mini-array of multiple TAAs is in separating individuals with and without cancer, a group of parameters, including the sensitivity/specificity, YI and PPV/NPV, were calculated and are shown in Tables III and IV. Table III shows the comprehensive evaluation of antibodies to a panel of ten TAAs. With the successive addition of TAAs to a total of eight antigens, there was a stepwise increase in positive antibody reactions, up to 61.0%, as well as a slight decrease of specificity from 100% with one TAA to 89.0% with a panel of eight. If additional antigens (Imp1 and Koc) were added to the panel, there was no further increase in the sensitivity but a slight decrease of specificity from 89.0 to 86.6%. The sensitivity and specificity are consistent with the results of other two parameters (PPV/NPV). The PPV/NPVs were also variable in the various combinations of TAAs. In the panel with a total of eight TAAs, the PPV was 73.5% and the NPV was 82.0%. The YI was also increased from 0.220 with one TAA to 0.500 with eight TAAs. The positive and negative likelihood ratios were 5.545 and 0.438, respectively, indicating that the clinical diagnostic value of a parallel assay of five TAAs was high. This also suggests that a parallel assay of eight TAAs is able to raise the diagnostic accuracy significantly. The agreement rate and κ-value were 79.7% and 0.52, respectively, indicating that the observed value of this assay had a middle range coincidence with the actual value. Taken together, these data show the usefulness of the multiple antigen array in increasing the clinical diagnostic quality and value for cancer.
Table III.
Evaluation of antibodies for ten TAAs selected in the detection of breast cancer.
| Positive % (No.)
|
|||||||
|---|---|---|---|---|---|---|---|
| Panel of TAAs | BC (41) | NHS (82) | Sensitivity | Specificity | YI | PPV | NPV |
| c-myc | 22.0 (9/41)a | 0 (0/82) | 22.0 | 100.0 | 0.220 | 100.0 | 71.9 |
| c-myc+survivin | 34.1 (14/41)a | 1.2 (1/82) | 34.1 | 98.8 | 0.329 | 93.3 | 75.0 |
| c-myc+survivin+cyclin B1 | 39.0 (16/41)a | 2.4 (2/82) | 39.0 | 97.6 | 0.366 | 88.9 | 76.2 |
| c-myc+survivin+cyclin B1+cyclin D1 | 43.9 (18/41)a | 4.9 (4/82) | 43.9 | 95.1 | 0.390 | 81.8 | 77.2 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62 | 48.8 (20/41)a | 6.1 (5/82) | 48.8 | 93.9 | 0.427 | 80.0 | 78.6 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62+p53 | 51.2 (21/41)a | 8.5 (7/82) | 51.2 | 91.5 | 0.427 | 75.0 | 78.9 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62+p53+p16 | 56.1 (23/41)a | 11.0 (9/82) | 56.1 | 89.0 | 0.451 | 71.9 | 80.2 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62+p53+p16+CDK2 | 61.0 (25/41)a | 11.0 (9/82) | 61.0 | 89.0 | 0.500 | 73.5 | 82.0 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62+p53+p16+CDK2+Imp1 | 61.0 (25/41)a | 13.4 (11/82) | 61.0 | 86.6 | 0.476 | 69.4 | 81.6 |
| c-myc+survivin+cyclin B1+cyclin D1 +p62+p53+p16+Imp1+CDK2+koc | 61.0 (25/41)a | 13.4 (11/82) | 61.0 | 86.6 | 0.476 | 69.4 | 81.6 |
P-values relative to NHS:
P<0.01. TAA, tumor-associated antigen; BC, breast cancer; NHS, normal human sera; YI, Youden’s index; PPV, positive predictive value; NPV, negative predictive value.
Table IV.
Summary of the diagnostic value of antibodies for a panel of eight TAAs in breast cancer.
| Serum | Any TAA positive | All TAA negative | Total |
|---|---|---|---|
| BC | 25 (A) | 16 (C) | 41 (R1) |
| NHS | 9 (B) | 73 (D) | 82 (R2) |
| Total | 34 (C1) | 89 (C2) | 123 (N) |
Fourfold table χ2 tests: χ2=34.164, P=0.000. Sensitivity (%) = A/(A + C) = 25/41 = 61.0%. Specificity (%) = D/(B + D) = 73/82 = 89.0%. Youden’s index = Sensitivity + Specificity − 1 = 0.610 + 0.890 − 1 = 0.500. Positive (+) likelihood ratio = Sensitivity/(1 − Specificity) = 0.610/(1 − 0.890) = 5.545. Negative (-) likelihood ratio = (1 − Sensitivity)/Specificity = (1 − 0.610)/0.890 = 0.438. Percentage agreement = (A + D)/(A + B + C + D) × 100 = (25 + 73)/(25 + 16 + 9 +73) × 100 = 79.7%. κ = [N(A + D) − (R1C1 + R2C2)]/[N2 − (R1C1 + R2C2)] = 0.52. TAA, tumor-associated antigen; BC, breast cancer; NHS, normal human sera.
Discussion
Interest in the use of anti-TAA antibodies as serological markers for cancer diagnosis derives from the recognition that these antibodies are generally absent, or present in very low titers, in normal individuals and in non-cancer conditions (with the exception of autoimmune conditions). The persistence and stability of autoantibodies in the serum of cancer patients is an advantage over other potential markers, including the TAAs themselves, which are released by tumors but are rapidly degraded or cleared after circulating in the serum for a limited time (17). Furthermore, the widespread availability of methods and reagents to detect serum autoantibodies facilitates their characterization in cancer patients and assay development. However, in contrast to autoimmune diseases, where the presence of a particular autoantibody may have diagnostic value, individually evaluated cancer-associated autoantibodies have little diagnostic value primarily due to their low frequency, sensitivity and specificity. This drawback may be overcome using mini-arrays of carefully selected TAAs and different types of cancer may require different TAA arrays to achieve the sensitivity and specificity required to make immunodiagnosis a feasible adjunct to tumor diagnosis (18,20–25).
In the future we aim to increase the sensitivity and specificity of anti-TAA antibodies as diagnostic markers of cancer by expanding the TAA array to include antigens which may be more selectively associated with one specific type of cancer, such as breast cancer, and not with others. We expect that our mini-array of multiple TAAs may be used as a novel non-invasive approach to identify cancer in the normal population and high-risk individuals. Our concern is that the approach may be not suitable for distinguishing one type of cancer from another. The reason is that certain TAAs, such as p53, p16 and c-myc, which were used in the present mini-array approach, are associated with several types of cancer, including liver, colon, gastric, lung, ovarian and prostate cancer (18,20,22–25). For future studies, we propose that certain selected antibody-antigen systems may be unique to one type of cancer and others may not. A comprehensive analysis and evaluation of various combinations of selected antibody-antigen systems is likely to be useful for the development of autoantibody profiles involving various panels or arrays of TAAs and the results may be useful for diagnosis of certain other types of cancer. In the present study, a mini-array of multiple TAAs were used as coating antigens in an ELISA to detect autoantibodies against these antigens in 41 sera from patients with breast cancer and 82 sera from normal individuals. The antibody frequency to the individual TAAs in breast cancer was variable and ranged between 7.3 and 22.0%. This relatively low sensitivity using one individual anti-TAA antibody as a diagnostic marker does not meet the requirements of clinical early diagnosis of breast cancer. With the successive addition of TAAs to a total of eight antigens, there was a stepwise increase in positive antibody reactions, reaching a sensitivity of 61.0% and a specificity of 89.0% in breast cancer. The positive and negative likelihood ratios were 5.545 and 0.438, respectively, which showed that the clinical diagnostic value of a parallel assay of eight TAAs was high. The PPVs and NPVs were 73.5 and 82.0%, respectively, indicating that the parallel assay of eight TAAs raised the diagnostic precision significantly. The agreement rate and κ-value were 79.7% and 0.52, respectively, which indicated that the observed value of this assay had a middle range coincidence with the actual value.
In conclusion, this preliminary study further supports our hypothesis and also suggests that additional breast cancer-specific TAAs are likely to be necessary to enhance the frequency of anti-TAA antibody detection using an array of multiple TAAs with potential immunodiagnostic value. Once a TAA array that is highly specific and sensitive to breast cancer is identified, we plan to develop a breast cancer-specific mini-array TAA chip for automated high-throughput breast cancer screening. Given that the presence of serum autoantibodies to TAAs may signal molecular events associated with tumorigenesis, it would be possible to use highly sensitive and specific TAA chips for screening populations at a high risk of developing breast cancer, which may lead to early preventive or therapeutic interventions aimed at suppressing or slowing the appearance of tumors.
Acknowledgments
The authors would like to thank Dr Xuanxian Peng at Sun Yat-sen University, China providing a number of the serum samples to this study. This study was supported by grants from the National Natural Science Foundation of China (#81172086) and the NIH of the USA (#SC1CA166016).
References
- 1.Shin HR, Joubert C, Boniol M, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010;21:1777–1785. doi: 10.1007/s10552-010-9604-8. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society . Cancer Facts & Figures 2007. ACS Publication; Atlanta GA: 2007. [Google Scholar]
- 3.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–340. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10:321–328. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 6.Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep. 2006;16:1105–1110. [PubMed] [Google Scholar]
- 7.Yamamoto A, Shimizu E, Takeuchi E, et al. Infrequent presence of anti-c-Myc antibodies and absence of c-Myc oncoprotein in sera from lung cancer patients. Oncology. 1999;56:129–133. doi: 10.1159/000011953. [DOI] [PubMed] [Google Scholar]
- 8.Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA. High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–1110. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle GA, Bourdeau-Heller JM, Coulthard S, Meisner LF, Ross J. Amplification in human breast cancer of a gene encoding a c-myc mRNA-binding protein. Cancer Res. 2000;60:2756–2759. [PubMed] [Google Scholar]
- 11.Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kDa ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–5015. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 13.Megliorino R, Shi FD, Peng XX, et al. Autoimmune response to anti-apoptotic protein survivin and its association with antibodies to p53 and c-myc in cancer detection. Cancer Detect Prev. 2005;29:241–248. doi: 10.1016/j.cdp.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Daniels T, Zhang J, Gutierrez I, et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate. 2005;62:14–26. doi: 10.1002/pros.20112. [DOI] [PubMed] [Google Scholar]
- 15.Crawford LV, Pim DC, Bulbrook RD. Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer. 1982;30:403–408. doi: 10.1002/ijc.2910300404. [DOI] [PubMed] [Google Scholar]
- 16.Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–4174. [PubMed] [Google Scholar]
- 17.Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133. doi: 10.1021/pr0500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang JY, Casiano CA, Peng XX, Koziol JA, Chan EK, Tan EM. Enhancement of antibody detection in cancer using panel of recombinant tumor-associated antigens. Cancer Epidemiol Biomarkers Prev. 2003;12:136–143. [PubMed] [Google Scholar]
- 19.Chen WQ. Assessing the validity and reliability of screening test. In: Li LM, editor. Epidemiology. Publishing Company of the People’s Health; Beijing: 2004. pp. 289–292. [Google Scholar]
- 20.Koziol JA, Zhang JY, Casiano CA, et al. Recursive partitioning as an approach to selection of immune markers for tumor diagnosis. Clin Cancer Res. 2003;9:5120–5126. [PubMed] [Google Scholar]
- 21.Zhang JY, Megliorino R, Peng XX, Tan EM, Chen Y, Chan EK. Antibody detection using tumor-associated antigen mini-array in immunodiagnosing human hepatocellular carcinoma. J Hepatol. 2007;46:107–114. doi: 10.1016/j.jhep.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Zhou Y, Qiu S, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289:32–39. doi: 10.1016/j.canlet.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W, Wang P, Li Z, et al. Evaluation of tumour-associated antigen (TAA) miniarray in immunodiagnosis of colon cancer. Scand J Immunol. 2009;69:57–63. doi: 10.1111/j.1365-3083.2008.02195.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Lin P, Qiu S, et al. Autoantibodies to Ca2+ binding protein Calnuc is a potential marker in colon cancer detection. Int J Oncol. 2007;30:1137–1144. [PubMed] [Google Scholar]
- 25.Li L, Wang K, Dai L, Wang P, Peng XX, Zhang JY. Detection of autoantibodies to multiple tumor-associated antigens in the immunodiagnosis of ovarian cancer. Mol Med Rep. 2008;1:589–594. [PMC free article] [PubMed] [Google Scholar]


