Abstract
Background
Reactive oxygen species (ROS) are known to be related to cardiovascular diseases. Many studies have demonstrated that angiotensin-converting enzyme inhibitors have beneficial effects against ROS. We investigated the antioxidant effect of captopril and enalapril in nitric oxide mediated vascular endothelium-dependent relaxations.
Materials and Methods
Isolated rabbit abdominal aorta ring segments were exposed to ROS by electrolysis of the organ bath medium (Krebs-Henseleit solution) after pretreatment with various concentrations (range, 10-5 to 3×10-4 M) of captopril and enalapril. Before and after electrolysis, the endothelial function was measured by preconstricting the vessels with norepinephrine (10-6 M) followed by the cumulative addition of acetylcholine (range, 3×10-8 to 10-6 M). The relevance of the superoxide anion and hydrogen peroxide scavenging effect of captopril and enalapril was investigated using additional pretreatments of diethyldithiocarbamate (DETCA, 0.5 mM), an inhibitor of Cu/Zn superoxide dismutase, and 3-amino-1,2,4-triazole (3AT, 50 mM), an inhibitor of catalase.
Results
Both captopril and enalapril preserved vascular endothelium-dependent relaxation after exposure to ROS in a dose-dependent manner (p<0.0001). Pretreatment with DETCA attenuated the antioxidant effect of captopril and enalapril (p<0.0001), but pretreatment with 3AT did not have an effect.
Conclusion
Both captopril and enalapril protect endothelium against ROS in a dose-dependent fashion in isolated rabbit abdominal aortas. This protective effect is related to superoxide anion scavenging.
Keywords: Reactive oxygen species, Angiotensin-converting enzyme inhibitors, Captopril, Enalapril, Vasodilation
INTRODUCTION
Since the breakthrough discovery of the obligatory role of the vascular endothelial cells by Furchgott and Zawadzki in 1980 [1], endothelial pathophysiology has been intensely investigated, and the term "endothelial dysfunction" has been referred to in the scientific literature over 50,000 times (PubMed search, May 2012). It is well known that the endothelial dysfunction is associated with aging, hypertension, atherosclerosis, and numerous other physiological and pathophysiological processes [2].
Nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor (epoxyeicosatrienoic acid) are the three major substances involved in the endothelium-dependent vasodilator pathways [2]. The mechanism of endothelial dysfunction is largely due to the reduced bioavailability of endothelium-derived NO by oxidative stress [3]. Accordingly, the hallmark of endothelial dysfunction is impaired endothelium-dependent vasodilation, which is mediated by NO [4].
Oxidative stress is induced by free radicals. A free radical is defined as any species that contains one or more unpaired electrons. Reactive oxygen species (ROS) is a collective term that includes both oxygen free radicals, such as superoxide (O2·-), hydroxyl (OH·), peroxyl (ROO·), and hydroperoxyl (HO2·) radicals, and certain non-radical oxidizing agents, such as hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and ozone (O3), that can be readily converted to free radicals [5]. NO and peroxynitrite (ONOO-) are the most relevant reactive nitrogen species in the cardiovascular system [6].
Many studies have demonstrated that angiotensin-converting enzyme (ACE) inhibitors have induced many beneficial effects on the endothelial function in animal models and humans, have improved the endothelial function in hypertension, and have reduced the risk associated with cardiovascular disease [7].
There have been numerous attempts to decrease the major complications associated with cardiac surgery, and there is increasing evidence that ACE inhibitors hold promise as protective cardiovascular agents for cardiac surgery patients [8]. The mechanisms of the protective effects of ACE inhibitors involve multiple factors, including those relating to their antioxidant effects [9]. In several experimental studies, only sulfhydryl (SH)-containing ACE inhibitors such as captopril and zofenopril exerted the endothelium protective effect [10,11], but in other studies, the non-SH-containing ACE inhibitor also showed similar effects [7]. Therefore, the present study was designed to evaluate the antioxidant effects of ACE inhibitors with or without a SH group (captopril and enalapril, respectively) and to identify the involved mechanisms.
MATERIALS AND METHODS
All of the animal procedures were approved by the Animal Care and Use Committee of Hanyang University.
1) Abdominal aorta preparation and priming procedure
Twenty-five male New Zealand white rabbits (KOATECH, Pyeongtaek, Korea) weighing between 2.0 and 2.5 kg were anesthetized with 3% to 5% sevoflurane in 4 L/min of 100% oxygen in a restraint chamber. The marginal ear vein was cannulated, and heparin (1,000 IU/kg) was administered intravenously. After 10 minutes, the rabbit was sacrificed by exsanguination from the carotid artery.
The infrarenal abdominal aorta was carefully excised and placed in a petri dish filled with 4℃ Krebs-Henseleit solution of the following composition (mM): NaCl 120.0, NaHCO3 25.0, KCl 5.0, NaH2PO4 1.4, MgSO4 1.2, CaCl2 2.5, and glucose 11.0. The vessel was cleaned of fat and connective tissue with extreme caution not to damage the endothelium or smooth muscle. The isolated aorta was cut into rings 3 to 4 mm in length.
The vessel rings were mounted between two L-shaped steel hooks in water-jacketed (37℃±0.5℃) organ baths filled with 5 mL of Krebs-Henseleit solution. The medium was continuously gassed with a mixture of 95% of O2 and 5% of CO2 throughout the experiment. The lower hook was fixed to the organ chamber, and the upper hook to the force transducer (TSD125C; BIOPAC Systems Inc., Goleta, CA, USA). The isometric tension was continuously recorded on a personal computer with a data acquisition system (MP100 system, BIOPAC Systems Inc.) via a transducer amplifier (DA100C, BIOPAC Systems Inc.). All the vessels were first equilibrated for 90 minutes, and the resting tension was set to 2 g gradually. During the equilibration period, the chamber medium was exchanged every 15 minutes.
2) Pretest
After the equilibration period, the endothelium integrity was assessed by precontracting the aortic ring with 10-6 M of norepinephrine (NE) and a subsequent relaxation with cumulative concentrations (3×10-8, 10-7, 3×10-7, and 10-6 M) of acetylcholine (ACh). An aortic ring with an endothelial function (relaxation) greater than or equal to 80% was considered to have an intact endothelium, and was included in the experiment. These data were used as the control values.
3) Electrolysis procedure
ROS were generated by electrolysis of the organ bath medium. A constant current of 15 mA was applied for 35 seconds using two circular platinum wire electrodes (each 7 mm in length) located at the bottom of the organ baths. The vessel ring was mounted over 1 cm apart from the electrodes to avoid direct electrical injury.
4) Effect of captopril or enalapril pretreatment
At 15 minutes of equilibration after the pretest and medium exchange, various concentrations (10-5, 3×10-5, 10-4, and 3×10-4 M) of captopril or enalapril were added and incubated for 15 minutes. After exposure to ROS generated by electrolysis, the medium was exchanged again. The contraction and relaxation curves were recorded using an identical procedure as the pretest. Changes of the aortic tone by ACh were expressed as percentages, and they were used as the experimental values.
5) Effect of antioxidant enzyme inhibition
Cu/Zn superoxide dismutase (SOD) and catalase, the two important antioxidant enzymes, were inhibited to investigate the mechanism of the antioxidant activity of captopril and enalapril. Diethyldithiocarbamate (DETCA) was used for Cu/Zn SOD inhibition, and 3-amino-1,2,4-triazole (3AT) for catalase inhibition. After the pretest and equilibration period (15 minutes), the vessels were pre-incubated with 0.5 mM of DETCA for 30 minutes or 50 mM of 3AT for 60 minutes. Captopril (3×10-4 M) or enalapril (3×10-4 M) was added and incubated during the last 15 minutes of pre-incubation, and the vessel rings were then exposed to electrolysis-induced ROS. After refreshing the medium, NE-induced contraction and the series of ACh applications were then repeated.
6) Drugs
NE, ACh, captopril, enalapril, DETCA, and 3AT were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA).
7) Data analysis
The responses to ACh are expressed as the percentage of the ACh-induced relaxation, in the same vessel, during the pretest. All of the data are presented as mean±standard error of the mean. When appropriate, the Student t-test or one-way analysis of variance (ANOVA) followed by a post test for linear trend and the Dunnett test were performed. All of the curve fittings and statistical analyses were performed using Prism ver. 5.0 (GraphPad Software Inc., San Diego, CA, USA). A p-value less than 0.05 was considered to be statistically significant.
RESULTS
1) Effect of captopril or enalapril pretreatment
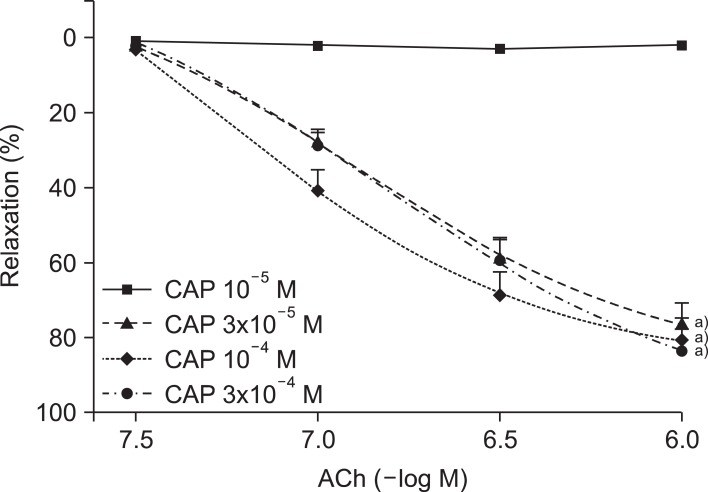
In a dose-dependent manner, the captopril-treated vessel rings preserved ACh-induced endothelium-dependent relaxation against the ROS attack (Fig. 1). At the highest concentration (10-6 M) of ACh, the 10-5, 3×10-5, 10-4, and 3×10-4 M captopril treated groups showed a relaxation rate of 1.9%±1.2%, 81.2%±1.9%, 86.0%±2.1%, and 89.1%±0.8%, respectively (n=15 for each group; ANOVA and post test for linear trend; both p<0.0001).
Fig. 1.
Concentration-response curves of acetylcholine (ACh)-induced, endothelium-dependent relaxation after electrolysis in the aortic rings pretreated with various concentrations (10-5, 3×10-5, 10-4, and 3×10-4 M) of captopril (CAP). Values are presented as mean±standard error of the mean (n=15). a)p<0.05 vs. CAP 10-5 M group.
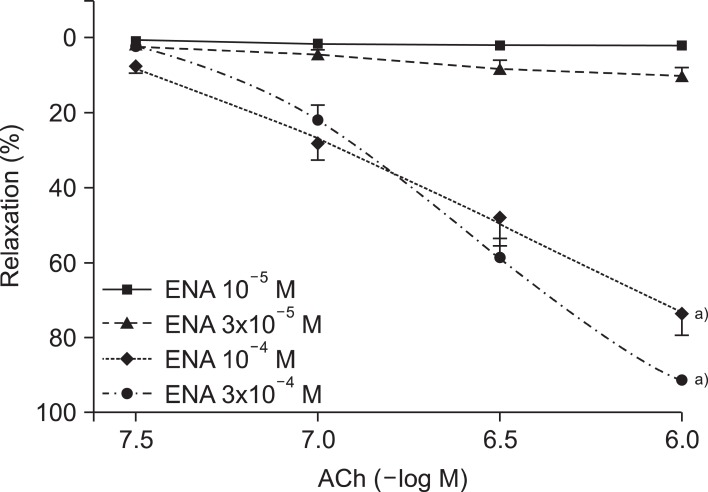
The treatment of enalapril showed a similar effect (Fig. 2). At the highest concentration (10-6 M) of ACh, the 10-5, 3×10-5, 10-4, and 3×10-4 M enalapril-treated groups had relaxation rates of 1.8%±1.2%, 10.0%±2.1%, 73.9%±5.7%, and 89.1%±0.8%, respectively (n, 13 to 15; ANOVA and post test for linear trend; both p<0.0001).
Fig. 2.
Concentration-response curves of acetylcholine (ACh)-induced, endothelium-dependent relaxation after electrolysis in the aortic rings pretreated with various concentrations (10-5, 3×10-5, 10-4, and 3×10-4 M) of captopril (CAP). Values are presented as mean±standard error of the mean (n=15). a)p<0.05 vs. CAP 10-5 M group.
2) Effect of antioxidant enzyme inhibition
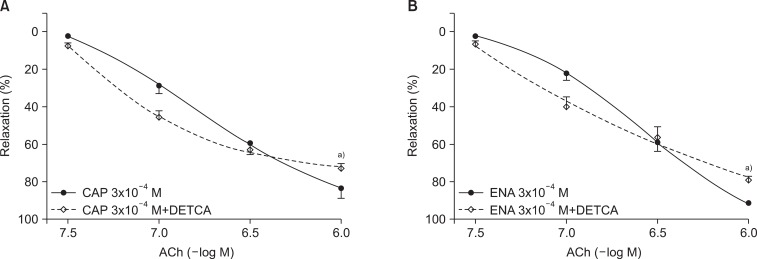
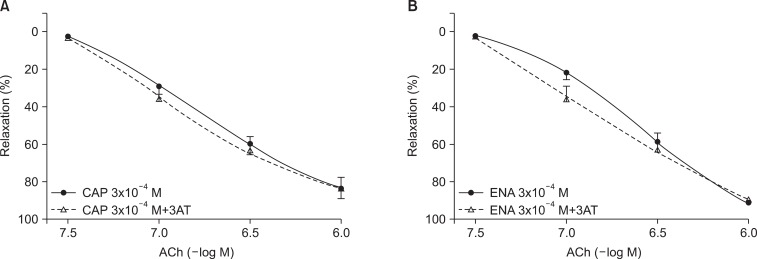
The relaxation rate of the DETCA+captopril group was significantly lower than the control (captopril-only) group after the ROS attack (73.0%±2.4% vs. 86.8%±1.1%; n=15; p<0.0001) (Fig. 3). DETCA exerted the same effect with enalapril (73.0%±2.4% vs. 86.8%±1.1%; n=15; p<0.0001) (Fig. 3). By contrast, 3AT pretreatment did not alter the relaxation rate of the captopril-treated group nor the enalapril-treated group (Fig. 4).
Fig. 3.
Concentration-response curves of acetylcholine (ACh)-induced, endothelium-dependent relaxation after electrolysis in the diethyldithiocarbamate (DETCA, 0.5 mM)-pretreated aortic rings incubated with captopril (CAP, 3×10-4 M) (A) or enalapril (ENA, 3×10-4 M) (B). Values are presented as mean±standard error of the mean (n=15). a)p<0.0001 vs. control group.
Fig. 4.
Concentration-response curves of acetylcholine (ACh)-induced, endothelium-dependent relaxation after electrolysis in the 3-amino-1,2,4-triazole (3AT, 50 mM)-pretreated aortic rings incubated with captopril (CAP, 3×10-4 M) (A) or enalapril (ENA, 3×10-4 M) (B). Values are presented as mean±standard error of the mean (n=15).
DISCUSSION
In the present study, both captopril and enalapril exerted an antioxidant effect against the electrolysis-induced ROS in the rabbit aortic rings. This antioxidant effect was partially reduced by DETCA pretreatment, but it was not affected by 3AT pretreatment.
ACh-induced vasorelaxation requires the integrity of the vascular endothelium, thus "endothelium-dependent" relaxation [1]. ACh binds to the muscarinic receptor of the endothelial cell, and the intracellular calcium level increases, which leads the endothelial nitric oxide synthase (eNOS) to produce NO. The produced NO diffuses to the vascular smooth muscle cell, and it activates the soluble guanylyl cyclase, which eventually converts the guanosine triphosphate to cyclic guanosine monophosphate (GMP) inducing smooth muscle relaxation. In addition, the cyclooxygenase (COX) pathway producing prostacyclin is also related to the endothelium-dependent vasorelaxation [2].
The endothelium is a major regulator of vascular homeostasis that maintains the balance between vasodilation and vasoconstriction, inhibition and promotion of the proliferation and migration of smooth muscle cells, prevention and stimulation of the adhesion and aggregation of platelets, as well as thrombogenesis and fibrinolysis. Upsetting this balance leads to endothelial dysfunction and damage to the arterial wall. These homeostatic effects are largely mediated by NO, a pivotal endothelium-derived substance [4]. NO is a diffusible substance with a half-life of a few seconds, and it is formed from L-arginine by oxidation of the guanidine-nitrogen terminal [12,13].
Vascular endothelial cells normally generate ROS as "second messengers" that might regulate endothelial cell growth and proliferation, endothelial barrier function, vasorelaxation, and vascular remodeling [14]. ROS are produced in the vasculature under both normal and stress conditions. ROS production has been known to occur in the endothelium, but also within the media and adventitia, all of which may impair NO signaling within vascular tissue to endothelium-dependent and endothelium-independent vasodilators [15].
Superoxide anion can be generated by various enzymes such as nicotinamide adenine dinucleotide phosphate oxidase, xanthine oxidase, COX, NO synthase, lipoxygenase, cytochrome P450 monooxygenases, and enzymes of the mitochondrial respiratory chain [2].
Superoxide anion is converted to hydrogen peroxide by SOD or spontaneously. Hydrogen peroxide is then reduced into water and oxygen by catalase or glutathione peroxidase. Superoxide anion and transition metals such as iron and copper react with hydrogen peroxide to form highly reactive hydroxyl radicals through the Fenton reaction (Fe2++H2O2→Fe3++OH·+OH-) and the Haber-Weiss reaction (O2·-+H2O2→OH·+OH-+O2) [2].
NO and superoxide anion rapidly interact to form peroxynitrite, a potent oxidant also capable of oxidizing SH groups and thioethers, as well as nitrating and hydroxylating aromatic groups, including tyrosine, tryptophan, and guanine [16]. At high concentrations, peroxynitrite is cytotoxic and may cause oxidative damage to proteins, lipids, and DNA [15].
Oxidative stress, however, may cause endothelial dysfunction or cell death through various pathways. Superoxide anions reduce the bioavailability of NO and directly inhibit its main target: soluble guanylyl cyclase [2]. The major target of ROS is NO, but the resultant formation of peroxynitrite also causes endothelial dysfunction by oxidation of the Zn-thiolate complexes within eNOS and the essential cofactor tetrahydrobiopterin (BH4). As a result of the oxidation of BH4, eNOS becomes "uncoupled" and produces ROS rather than NO [17]. In addition, peroxynitrite inhibits guanylyl cyclase, inactivates the prostacyclin synthase by tyrosine nitration, and further enhances oxidative stress by inhibiting SOD [2,15].
Hydroxyl radicals, another ROS, can induce cell damage through the peroxidation of lipids and SH groups [2].
On the other hand, oxidative stress can also induce apoptosis of endothelial cells by activation of apoptosis signal-regulating kinase 1 and c-Jun N-terminal kinase [18].
In the present study, we used the buffer solution electrolysis method to produce the oxidative stress to the vascular rings. The ROS generation system by electrolysis of a physiological salt solution has the advantage that a wide range of ROS are produced without addition of enzymes or chemicals, which themselves may influence the experiment [19]. Furthermore, the short duration of exposure to ROS allows the measurement of responses to various drugs, without the continuous inactivation of NO or the possible oxidation of drugs by ROS [20]. This electrolysis system generates superoxide anions, hydroxyl radicals, hydrogen peroxide, singlet oxygen, and hypochlorite [21].
The endothelium-dependent vasorelaxation is almost completely abolished by exposure to ROS [20]. In the present study, endothelium-dependent vasorelaxation was severely compromised in the aortic rings with low concentrations of captopril or enalapril pretreatment.
The vasoconstrictor response to α1 adrenoceptor stimulation is impaired by exposure to ROS [20]. Because we used NE for preconstriction of the vessels in the present experiment, we applied a duration and intensity (35 seconds and direct current 15 mA, respectively) of constant current electrolysis that compromised endothelium-dependent vasorelaxation but did not affect NE-induced vasoconstriction.
Meanwhile, enzymatic scavenging of superoxide anion is carried out by SOD. The predominant isoform Cu/Zn SOD is inhibited by the Cu2+ chelator DETCA [22]. In the present study, DETCA pretreatment partially impaired the antioxidant effect of captopril and enalapril. This represents that superoxide anion scavenging activity is related to the mechanism of the antioxidant effect of captopril and enalapril.
Hydrogen peroxide is scavenged by catalase, and this enzyme is inhibited by 3AT [23]. In the present experiment, pretreatment with 3AT did not affect the antioxidant effect of captopril and enalapril, suggesting that their endothelium protective effect is not associated with hydrogen peroxide scavenging action.
Many researchers have reported that only SH-containing ACE inhibitors possess an antioxidant effect [10,24-26]. The SH moiety easily undergoes oxidation and disulfide exchange reactions and can be converted into disulfides through the interaction with free radicals. By this process, it can serve as a free radical scavenger, thereby protecting the endothelial function [25].
On the other hand, some investigators have reported that non-SH-containing ACE inhibitors also exerted an endothelium protective effect against free radical injury [7,27,28]. The mechanism of this antioxidant effect is unclear. It was reported that ramiprilat, a non-SH-containing ACE inhibitor, protects against free radical injury in isolated working rat hearts [27]. The investigators explained that ramiprilat inhibits free radical-induced damages mainly by stimulation of prostacyclin synthesis and/or release, because their data showed that the addition of indomethacin (5 µmol/L) completely abolished the protective effects of ramiprilat. This interpretation seems confusing, however, because in a later study it was demonstrated that COX inhibitors, such as indomethacin, possess antioxidant properties [29]. In another experiment concerning this issue by Fujita et al. [28], they found that quinaprilat, a non-SH-containing ACE inhibitor, could ameliorate both apoptosis and necrosis through the up-regulation of constitutive eNOS via an increase of bradykinin, with the resulting increase of nitric oxide in the cultured human aortic endothelial cells. There are two identified kinin receptors: B1 and B2. The B2 receptor is constitutively expressed, but the B1 receptor is normally absent and is only induced under inflammatory conditions in humans [30]. Rabbit blood vasculature, however, is rich in B1 receptor sites; hence, this difference between species should be considered when interpreting the results [26].
One limitation of the present study is that the amount of ROS generated by electrolysis could not be measured due to the lack of methodologies. A device that can measure the real-time amount of ROS in the vicinity of the electrodes would be beneficial. This method may offer clues for elucidating the exact ROS composition ratio in the electrolyzed buffer solution. It remains to be investigated whether other ACE inhibitors, with or without a SH group, also protect endothelium against ROS. In addition, angiotensin receptor blockers might be good candidates for future experiments. Further studies on prostacyclin inhibition without any interactions with ROS may clarify the relevance of prostacyclin in the endothelium protective effects of ACE inhibitors. Performing these studies would more clearly explain the exact mechanism of the known beneficial effects of ACE inhibitors in cardiac surgery patients.
CONCLUSION
Both captopril (a SH-containing ACE inhibitor) and enalapril (a non-SH-containing ACE inhibitor) protect endothelium against free radical injury in a dose-dependent manner in isolated rabbit abdominal aortas. This protective effect is related to superoxide anion scavenging.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 3.Forstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459:923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 4.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 5.Bayir H. Reactive oxygen species. Crit Care Med. 2005;33(12 Suppl):S498–S501. doi: 10.1097/01.ccm.0000186787.64500.12. [DOI] [PubMed] [Google Scholar]
- 6.Gomes A, Costa D, Lima JL, Fernandes E. Antioxidant activity of beta-blockers: an effect mediated by scavenging reactive oxygen and nitrogen species? Bioorg Med Chem. 2006;14:4568–4577. doi: 10.1016/j.bmc.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Chen SX, Song T, Zhou SH, Liu YH, Wu SJ, Liu LY. Protective effects of ACE inhibitors on vascular endothelial dysfunction induced by exogenous advanced oxidation protein products in rats. Eur J Pharmacol. 2008;584:368–375. doi: 10.1016/j.ejphar.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Lazar HL. Role of angiotensin-converting enzyme inhibitors in the coronary artery bypass patient. Ann Thorac Surg. 2005;79:1081–1089. doi: 10.1016/j.athoracsur.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 9.Sun JZ, Cao LH, Liu H. ACE inhibitors in cardiac surgery: current studies and controversies. Hypertens Res. 2011;34:15–22. doi: 10.1038/hr.2010.188. [DOI] [PubMed] [Google Scholar]
- 10.Benzie IF, Tomlinson B. Antioxidant power of angiotensin-converting enzyme inhibitors in vitro. Br J Clin Pharmacol. 1998;45:168–169. doi: 10.1046/j.1365-2125.1998.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scribner AW, Loscalzo J, Napoli C. The effect of angiotensin-converting enzyme inhibition on endothelial function and oxidant stress. Eur J Pharmacol. 2003;482:95–99. doi: 10.1016/j.ejphar.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Luscher TF, Boulanger CM, Dohi Y, Yang ZH. Endothelium-derived contracting factors. Hypertension. 1992;19:117–130. doi: 10.1161/01.hyp.19.2.117. [DOI] [PubMed] [Google Scholar]
- 13.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 14.Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 15.Munzel T, Daiber A, Ullrich V, Mulsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25:1551–1557. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 16.Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97. doi: 10.1080/10623320490482619. [DOI] [PubMed] [Google Scholar]
- 17.Landmesser U, Dikalov S, Price SR, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Al-Lamki R, Bai L, et al. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 19.Jackson CV, Mickelson JK, Stringer K, Rao PS, Lucchesi BR. Electrolysis-induced myocardial dysfunction: a novel method for the study of free radical mediated tissue injury. J Pharmacol Methods. 1986;15:305–320. doi: 10.1016/0160-5402(86)90010-0. [DOI] [PubMed] [Google Scholar]
- 20.Peters SL, Mathy MJ, Pfaffendorf M, van Zwieten PA. Reactive oxygen species-induced aortic vasoconstriction and deterioration of functional integrity. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:127–133. doi: 10.1007/s002109900148. [DOI] [PubMed] [Google Scholar]
- 21.de Groot AA, Mathy MJ, van Zwieten PA, Peters SL. Antioxidant activity of nebivolol in the rat aorta. J Cardiovasc Pharmacol. 2004;43:148–153. doi: 10.1097/00005344-200401000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Wambi-Kiesse CO, Katusic ZS. Inhibition of copper/zinc superoxide dismutase impairs NO.-mediated endothelium-dependent relaxations. Am J Physiol. 1999;276(3 Pt 2):H1043–H1048. doi: 10.1152/ajpheart.1999.276.3.H1043. [DOI] [PubMed] [Google Scholar]
- 23.Margoliash E, Novogrodsky A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958;68:468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chopra M, Scott N, McMurray J, et al. Captopril: a free radical scavenger. Br J Clin Pharmacol. 1989;27:396–399. doi: 10.1111/j.1365-2125.1989.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldschmidt JE, Tallarida RJ. Pharmacological evidence that captopril possesses an endothelium-mediated component of vasodilation: effect of sulfhydryl groups on endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1991;257:1136–1145. [PubMed] [Google Scholar]
- 26.Mittra S, Singh M. Possible mechanism of captopril induced endothelium-dependent relaxation in isolated rabbit aorta. Mol Cell Biochem. 1998;183:63–67. doi: 10.1023/a:1006854313163. [DOI] [PubMed] [Google Scholar]
- 27.Pi XJ, Chen X. Captopril and ramiprilat protect against free radical injury in isolated working rat hearts. J Mol Cell Cardiol. 1989;21:1261–1271. doi: 10.1016/0022-2828(89)90672-x. [DOI] [PubMed] [Google Scholar]
- 28.Fujita N, Manabe H, Yoshida N, et al. Inhibition of angiotensin-converting enzyme protects endothelial cell against hypoxia/reoxygenation injury. Biofactors. 2000;11:257–266. doi: 10.1002/biof.5520110404. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes E, Costa D, Toste SA, Lima JL, Reis S. In vitro scavenging activity for reactive oxygen and nitrogen species by nonsteroidal anti-inflammatory indole, pyrrole, and oxazole derivative drugs. Free Radic Biol Med. 2004;37:1895–1905. doi: 10.1016/j.freeradbiomed.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Duchene J, Ahluwalia A. The kinin B(1) receptor and inflammation: new therapeutic target for cardiovascular disease. Curr Opin Pharmacol. 2009;9:125–131. doi: 10.1016/j.coph.2008.11.011. [DOI] [PubMed] [Google Scholar]