INTRODUCTION
Failla, Marie Curie, and Columbia University
It was a particular pleasure to receive the Radiation Research Society Failla Award in Warsaw, Poland, the birthplace of Marie Skłodowska Curie. Gioacchino Failla was the first director of my own Institute, now called the Columbia University Center for Radiological Research (CRR). Starting in 1918, Failla was in charge of the Columbia University Center for an astonishing 43 years, before Harold Rossi and then Eric Hall took over the reins. Between them, Rossi and Hall led the CRR for a further 49 years. Perhaps radiation is linked to scientific longevity, if not to increased lifespan (1).
Failla was in fact one of Marie Curie’s graduate students, and he received his doctorate from the Sorbonne in 1923. Two years earlier, Marie Curie had visited Failla in New York City during her trip to the U.S. The New York Times recorded her arrival (Fig. 1) with the headline “Mme. Curie Plans to End All Cancers,” together with the memorable subheading “Motherly looking scientist in plain black frock gives thanks to Americans.”
FIG. 1.
New York Times, May 12 1921, describing Marie Curie’s arrival in New York City. Curie met with her student, Gioacchino Failla, during her visit.
The Two Two-Edged Swords
Radiation’s two two-edged swords that have dominated my own scientific thinking are:
Radiation can cure cancer versus radiation can induce cancer.
Radiotherapy needs physics research versus radiotherapy needs biological research.
The goal of this brief summary is to give some examples of these contrasts, and try to draw some conclusions about research directions, interleaved with some observations about the scientists who have tried to push me in the right directions over the years.
THE FIRST TWO-EDGED SWORD
Radiation Can Cure Cancer versus Radiation Can Induce Cancer
1. Radiation Can Cure Cancer; But What is the Dominant Mechanism?
That radiotherapy can cure cancer was established early in the twentieth century. Despite repeated claims for its imminent demise in cancer therapy, radiotherapy remains one of its staples, with more than half of all cancer patients receiving radiotherapy at some stage during their treatment. Despite more than a century of clinical radiotherapeutic experience, however, there is still much debate about how it actually works, and how it should be optimally used.
In the first two decades of the twentieth century the general view was that single large “castrating” doses would kill tumors more than surrounding normal tissues, and were thus the preferred modality (2). This prevailing view changed in the 1920s, in large part due to the work of Claudius Regaud at the Institute Curie in Paris (3). Regaud and colleagues used the testis, a self-renewing tissue with a proliferating cell compartment, as a model for a growing tumor, and the overlying skin as a model of dose-limiting normal tissue. Using these models in a variety of animals they demonstrated that fractionating the dose decreased skin damage but did not significantly reduce damage to the testis – the model for tumor. The implication was that fractionation would thus increase the therapeutic advantage between tumor control and complications. Henri Coutard, took these concepts into the clinic at the Radium Institute in Paris, where he clearly confirmed the radiotherapeutic advantages of dose fractionation (4).
Since the 1920s fractionation has remained central to all radiotherapy, both because of the gains that it gives in terms of tumor control compared to late sequelae, but also because of the subsequent realization that fractionation is necessary to deal with hypoxic tumor cells. By the 1980s these concepts had been quantified, through mechanistic models such as the linear-quadratic formalism.
Central to these models is the basic idea that radiotherapeutic tumor control is related primarily to direct radiation killing of tumor clonogens. Supporting this notion are a variety of different studies showing that radiotherapeutic response can be quantitatively predicted from radiation-induced killing of tumor clonogens, either from measurements (5, 6) or from cell-killing models (7, 8). The more general argument here is that radiation-induced cell killing and radiotherapeutic response have remarkably similar dependencies on both dose and dose fractionation (9).
Taking this a step further, we can use our understanding of how radiation kills tumor clonogens and surrounding normal tissue to design new radiotherapeutic protocols. Should such approaches work, it would provide further support for the notion that tumor control is indeed primarily related to direct radiation inactivation of tumor clonogens.
An example here is the recent interest in reducing the number of dose fractions (hypofractionation) in the treatment of prostate cancer. As mentioned above, one of the major rationales for fractionation is its differential effect on tumor and late-responding normal tissue – fractionation generally spares late-responding normal tissues more than tumors. Back in 2009, Eric Hall and myself [and, independently, Lester Peters and Gilian Duchesne in Australia (10)] were wondering what was the biological difference between tumors and late-responding normal tissue that was responsible for this differential response? Based on in vitro studies, both groups hypothesized that it was related to late-responding normal tissues containing smaller numbers of dividing cells relative to most tumors. This immediately raised the issue of prostate cancers, which are generally very slow growing tumors containing relatively few dividing cells. So would prostate tumors respond to changes in fractionation like other tumors, or, as we suspected, like late-responding normal tissue?
By this time there was a reasonable way to quantify radiotherapeutic response to changes in fractionation, through the α/β ratio (11, 12). α/β values of around 10 Gy are characteristic of most tumors, while α/β values around 3 Gy are characteristic of late-responding normal tissue. So what would the α/β ratio for prostate cancers be? Our first analysis of available clinical data (13) gave a value of 1.5 Gy [95% confidence interval: 0.8–2.2 Gy] “comparable with a typical α/β ratio for late-responding normal tissues.”(13) A better analysis a couple of years later (14), where we used only a single large clinical data set, gave a very similar result of α/β = 1.2 Gy [95% confidence interval: 0.03–4.1 Gy].
This prostate study was one of many done jointly with Eric Hall. Interacting with Eric has been one of the great pleasures of my scientific career. His ability to look beyond the details and see what’s really important, and his ability to communicate his ideas to slower learners like myself, has made for the most satisfying of scientific interactions over the years, as well as for a friendship that I deeply treasure.
Since this first estimate of α/β for prostate, there have been more than 50 publications in the literature on α/β values for prostate cancer, and the clinical consensus now (15, 16) is that the α/β value is indeed low for most prostate cancers, quite similar to the original estimates. Why such wide interest? The reason is that if the α/β ratio for prostate cancer is indeed comparable to that for late-responding normal tissue, we lose one of the fundamental rationales for using many radiotherapy fractions, and hypofractionation becomes a potential option. Why is that so important? First and foremost, reducing the number of treatments from about 40 to perhaps 5 or 10 is a major practical advantage to the patient. Second, it means being able to treat a prostate-cancer patient with considerably less resources. A decade after the first papers suggesting a low α/β ratio for prostate, results of several phase-III randomized studies of hypofractionation have already been published (17–19), and several more phase III studies are underway (20, 21) – a tribute to the ability of the community to react quickly to new ideas. The bottom line, to date, is that the results of hypofractionated prostate cancer radiotherapy seem quite similar, and almost certainly no worse, than conventional fractionation.
That prostate hypofractionation results are turning out reasonably as predicted is gratifying in its own right, but the second conclusion to be drawn here is that the consistency of the clinical results with the predictions of standard models provides further confirmation that tumor control must indeed be primarily related to direct radiation-induced killing of tumor clonogens. Other mechanisms are no doubt involved (22), and may even dominate at ultra high doses per fraction (23), but for conventional dose fractionation (~2 Gy/fraction) or even most hypofractionated protocols (2–5 Gy/fraction), it is likely that direct radiation-induced killing of tumor clonogens remains the dominant mechanism of tumor control.
2. Radiotherapy Can Induce Cancers: But How Does this Happen at Very High Doses?
The 15-year relative survival rate for patients treated for breast or prostate cancer is now about 75%, as compared, for example, to about 58% for breast just a decade ago. As younger patients are treated, and with longer life expectancy, radiotherapy-induced secondary malignancies are assuming increasing importance. Ironically, the whole issue of radiotherapy-induced secondary cancers is, to a significant extent, a testimony to the efficacy of modern day radiotherapy – truly the price of success.
Back in 2000, and stimulated by our first interactions with our much missed late colleague Elaine Ron, we undertook a large-scale tumor registry analysis, in which secondary cancers after prostate cancer radiotherapy were compared with secondary cancers after prostate cancer surgery (24). Prostate is probably unique in allowing such a direct comparison of secondary cancers after radiotherapy versus surgery – the advantage here being that all the study subjects had prostate cancer, whereas most secondary cancer studies compare cancer risks with the general population. The study showed (24) that prostate radiotherapy- induced secondary cancers often occurred well outside the treatment volume – at least for treatments regimens that were common some decades ago. So studies of radiotherapy- induced cancers should ideally include all sites, not just those in the treatment volume – and of course the range of studied times post exposure must be large – at least two decades to estimate lifetime risks.
Around 2000 was also the time when Intensity-Modulated Radiation Therapy (IMRT) was becoming increasingly popular; IMRT uses multiple pencil beams to produce improved dose distributions around the tumor. Compared to the older three-dimensional conformal radiotherapy (3D-CRT), modern IMRT techniques minimize the amount of normal tissue getting high doses. But IMRT does result in larger volumes of normal tissue getting lower doses. So the question arises: which is preferable in terms of secondary cancers? Small volumes of normal tissue getting high doses (3D-CRT), or larger volumes of normal tissue getting low doses (IMRT)?
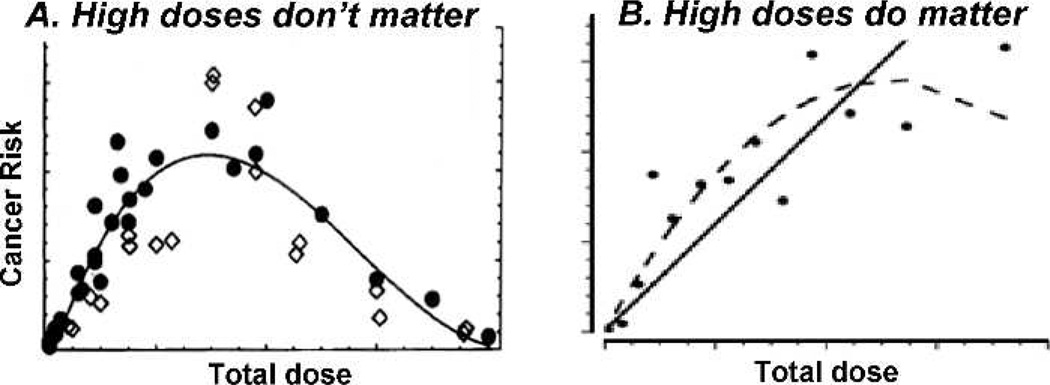
The answer, of course, depends on the shape of the dose-response curve. As illustrated in Fig. 2A, if the dose-response for radiation-induced cancer decreases sharply at high doses, then high doses do not matter from the perspective of radiation-induced cancer. This possible bell-shaped dose-response is in fact the standard “Gray model” (25), the one described in most text books, in which radiation-induced cancer at high doses is a result of competition between induced oncogenic transformation (increases risk) and cell killing (which decreases the risk at high doses). So in the context of IMRT, which essentially removes very high doses and replaces them with lower doses spread over larger volumes, if the standard Gray model (Fig. 2A) is correct and high radiation doses produce fewer secondary cancers, IMRT might result in more secondary cancers. On the other hand, if cancer risks do not decrease sharply at high doses (Fig. 2B), then IMRT would be more likely to be advantageous from the perspective of secondary cancers.
FIG. 2.
The significance of the shape of the dose-response curve for radiation-induced cancer at high doses. Panel A: Cancer risks decrease at high doses, due to cell killing – the Gray model: In this scenario high normal tissue doses will not contribute significantly to secondary cancer risks. Panel B: Cancer risks do not decrease sharply at high doses: In this scenario high normal-tissue doses may dominate secondary cancer risks.
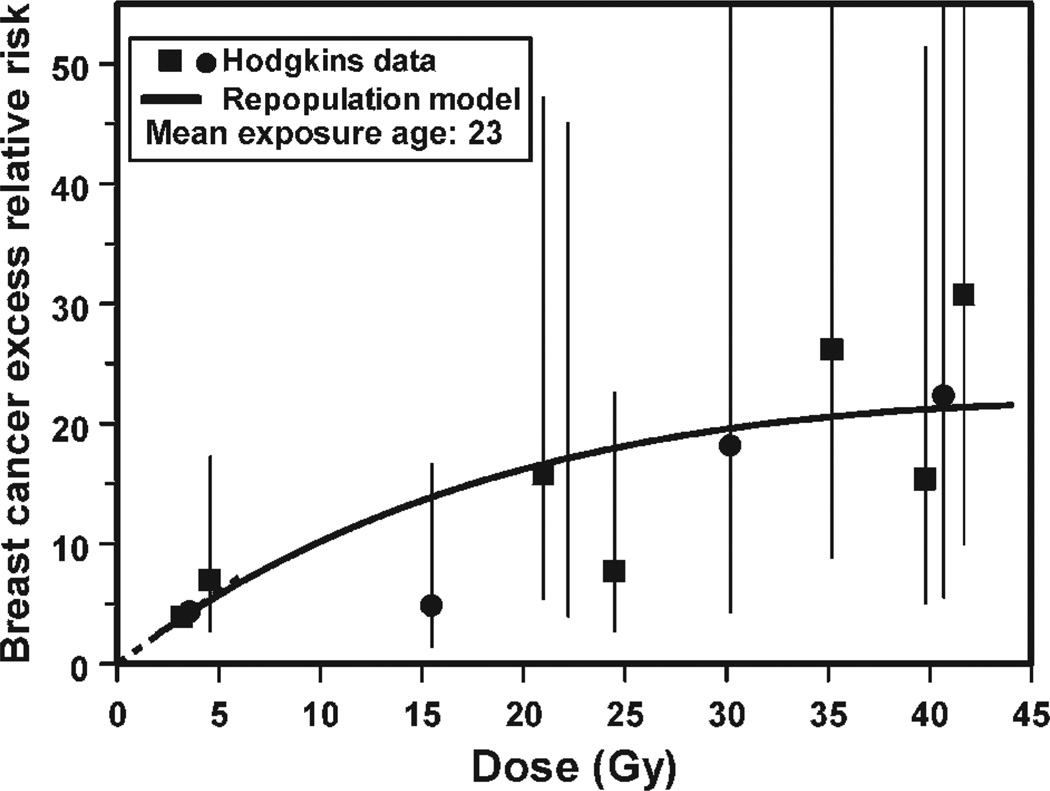
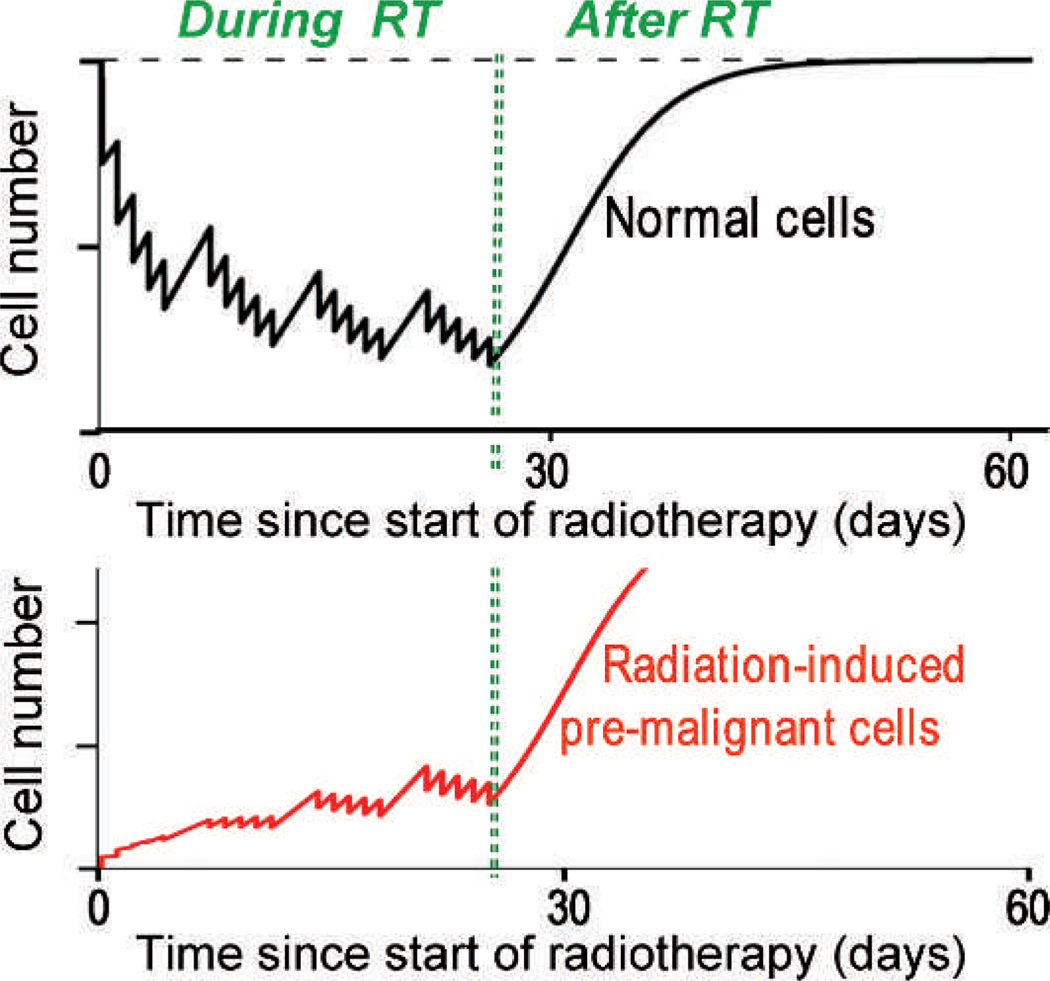
In fact recent epidemiology suggests that radiation-induced cancer risks are generally not small at large doses. An example is shown in Fig. 3 where secondary cancer risks continue to increase up to at least 45 Gy – which is quite inconsistent with the standard Gray model (Fig. 2A) where cell killing would result in very small cancer risks at these very high doses. Together with Rainer Sachs at UC Berkeley, we wondered what could be responsible for these significant secondary cancer risks at the large doses where cell killing would be expected to eliminate most radiation-induced pre-malignant cells. Finally we focused on repopulation: it is well known that surviving normal cells in heavily irradiated tissue proliferate rapidly under homeostatic control in the time period up to a few months after radiotherapy; so any radiation-produced pre-malignant cells would also be expected to proliferate with just the same kinetics, as illustrated in Fig. 4 (26). With what I thought was brilliant insight, Sachs showed that, in a first approximation, accelerated proliferation of pre-malignant cells can exactly cancel out the effects of cell killing, leaving a secondary cancer risk which will therefore not be negligible at high doses (26).
FIG. 3.
The data points show breast secondary cancer risks as a function of radiation dose at the location of the cancer, for Hodgkin’s lymphoma patients who underwent radiotherapy. Note the significance risks at very high doses where the standard Gray model (see Fig. 2A) would predict very small cancer risks, because of cell killing. The curve represents the prediction (26) of a model, which includes radiation-induced induction of pre-malignant cells and cell killing, and also accelerated proliferation of pre-malignant cells (see Fig. 4.) Figure redrawn from ref. (26).
FIG. 4.
Upper curve: cycles of cell killing and cellular proliferation of normal cells, during and after radiotherapy. Each “spike” corresponds to cell killing during one fraction followed by repopulation before the next treatment. After radiotherapy is completed the normal tissue cell number repopulates under homeostatic control to close to the pre-treatment number. Lower curve: Cycles of induction of pre-malignant cells, cell killing, and proliferation of premalignant cells. After the end of radiotherapy, the pre-malignant cells will proliferate with the same accelerated repopulation kinetics as the normal cells. Figure redrawn from ref. (26).
My scientific collaborations over the years with Ray Sachs have brought me enormous pleasure. Coming from a mathematical physics background [see the Sachs-Wolfe effect (27, 28)], Sachs has the analytic insights to develop and use biophysical mathematical models in what I think is the best way possible – investigating which are the dominant mechanism for any given phenomenon. Figuring out the dominant mechanism underlying a given phenomenon is often tough to do: like many scientists I too often get sidetracked towards interesting but second-order effects - and I am infinitely grateful to Sachs for setting me straight on numerous occasions.
So to summarize, there is a third mechanism which is critical for radiation-induced cancer at high doses. Beyond induction and killing of pre-malignant cells, as described by the standard Gray model, proliferation of premalignant cells is an additional important player at high doses. The outcome of this, in the context of IMRT, is that high doses generally do matter in terms of secondary cancer risks (Fig 2B). This being the case, it is likely that IMRT is reducing secondary cancer risks relative to the older 3D CRT techniques. This rather comforting conclusion does not, it should be said, take into account the whole body dose from treatment-head leakage radiation during IMRT: this is important because IMRT takes a much longer time to deliver than conventional 3D CRT – a fixable problem that needs to be fixed (29).
The bottom line here is that because it takes two decades or more to clinically assess secondary cancer risks from a new radiation modality, we have no choice but to use models. But we should use models which are (1) mechanistic – the days of curve fitting are hopefully behind us, and (2) validated as much as possible with what clinical radiation epidemiology we have right now. Using the standard Gray model, for example, would likely have led us to quite erroneous conclusions about IMRT.
THE SECOND TWO-EDGED SWORD
Radiotherapy Needs Physics Research versus Radiotherapy Needs Biological Research
1. Radiotherapy Needs Technology Research – But When is Enough?
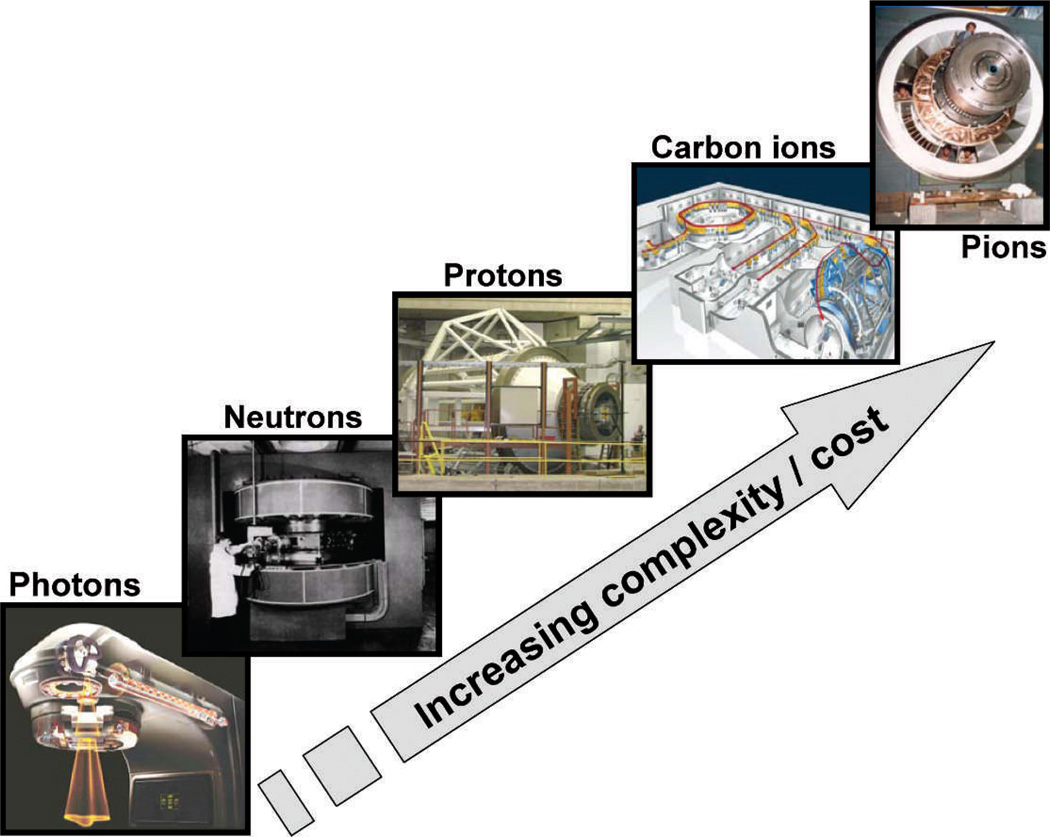
Since the 1940s, I think it is fair to say that most of the advances in radiotherapy have been driven by new technologies. Clearly the advent of medical linacs after World War II was a seminal event, arguably culminating in the extraordinary proliferation of proton therapy machines today. Culminating may not be the right word here because negative pi meson (pion) radiotherapy, which had its heyday in the 1980s, significant outstripped neutrons, protons, and even carbon ions, in terms of complexity and cost (Fig. 5). I was fortunate to have started my career working with pions, first for my graduate-school project at the Rutherford Laboratory in the UK, and subsequently as a postdoc at the Los Alamos National Laboratory.
FIG. 5.
Illustrating the increasing complexity and cost of accelerator-based radiotherapy systems that have been developed over the past 50 years.
Pions were a physicist’s dream (30). The idea was that, like protons, pions stop at a controllable distance inside the body, so giving an inherently better dose distribution than photons. But, unlike protons, when a negative pion stops, it is captured by a nearby atomic nucleus, which causes that nucleus to fragment into low-energy particles – a pion ‘star’. These stars consist of short-ranged high-LET particles, which mostly deposit their energy locally, i.e., in the tumor. And being high-LET, these star particles are expected to be able to efficiently kill hypoxic cells. The physicists’ dream: better dose distributions and the hypoxic cell problem, all solved with subatomic particles!
Pions were, sadly, an expensive failure. Expensive was a big part of the story, because just to make a practical pion beam, roughly 800 MeV protons are needed, almost four times the energy of current-day proton machines. That means a far bigger and far costlier machine than a proton therapy machine. Figure 6 shows the LAMPF accelerator used to make pions at Los Alamos – half a mile long!
FIG. 6.
The LAMPF linear accelerator, located on Mesita de Los Alamos in New Mexico. Protons were accelerated over LAMPF’s half-mile length to an energy of 800 MeV, producing negative pions (at the far end of the photograph) for pion therapy.
But space and cost were not the only reasons that pions failed. Two more reasons were the paucity of randomized trials (31), and the problem of a whole body dose of neutrons emanating from the pion interactions (32). All these problems – cost effectiveness, randomized trials, and neutrons – resonate with similar issues facing contemporary proton radiotherapy (33–35). Another striking similarity is the considerable emphasis at the time directed towards development of a radically smaller and cheaper accelerator for pion therapy, the PIGMI (36); that never panned out for pions, though high-field small-radius superconducting synchrocyclotron are looking promising for proton radiotherapy (37).
One of the great pleasures of working at the pion therapy facility at Los Alamos was to be the tail end of the trio of postdocs consecutively supervised by John Dicello (Fig. 7: Howard Amols, Marco Zaider, and then myself). The two smarter members of this trio both ended up in medical physics, and it has been a pleasure to interact with them over the years.
FIG. 7.
John Dicello and his three Los Alamos postdocs: Howard Amols, Marco Zaider and David Brenner.
Coming back to the theme of this section, there is little doubt that most of the gains in radiotherapy in the last half century have been technology driven. Has technology taken radiotherapy as far as it can? Probably so with the “big physics” approaches illustrated in Fig. 5. That being said, (somewhat) smaller technology approaches, such as the CyberKnife – a miniature X-ray machine mounted on the end of a robotic arm - continue to drive the field forward. But is it not time for radiobiologists to step up to the plate?
2. Radiotherapy Needs Radiobiology – But We’ve been Saying That for Fifty Years
When is radiobiology going to come through and really make a positive difference to radiotherapy? Looking back over the past half century, I think a skeptic could be forgiven for saying the question should be “will it?” rather than “when will it?” But I will make a couple of suggestions of where I think “when” could practically be “pretty soon."
In the past half century there have been three major themes as to where radiobiology can potentially help radiotherapy: Molecular targeting, alternate fractionation, and predictive assays. I will argue that molecular targeting is still a long way from making a clinical impact, but that perhaps there may be more immediate practical gains to be made in alternate fractionation and in predictive assays.
Molecular Targeting
Included here are targeting hypoxic cells and targeting the vasculature, and it is in these areas that most clinically-oriented radiobiological research has been directed. To date one would have to say that the resulting clinical impact has been very limited – with the big gains typically just around the corner. It does seems that there are some clinical gains to be had - a recent meta-analysis (38) suggests that hypoxic modification of radiotherapy (mainly hypoxic radiosensitizers and hyperbaric oxygen) can improve some survival rates by as much as 15%; but it seems that the enormous cost associated with developing therapeutic drugs [typically around a billion dollars over a decade (39)] may well be an insuperable impediment to practical implementation of molecular targeting in radiotherapy, unless the anticipated gains are exceedingly large.
Alternate Fractionation
Changing fractionation schemes is far less expensive than developing new drugs. The radiotherapy field has been experimenting with alternate fractionation schemes for more than a century (9), sometimes for better (4) and sometimes for worse (40). It was only in the 1970s, stemming largely from the pioneering work of Rod Withers, Lester Peters and Howard Thames, that this enterprise got on to a more mechanistically-based path. Withers, Peters and Thames showed that fractionation schemes can be optimized to take advantage of, among other factors, accelerated repopulation and the different α/β ratios shown by tumors relative to late-responding normal tissues (41). Again recent meta-analysis of multiple trials has shown clear clinical benefits, particularly for younger patients, accruing from optimized fractionation schemes (42), and here there are fewer impediments (though certainly not none!) to wider scale implementation.
Withers, Peters and Thames correctly saw hyperfractionation as taking advantage of the differential α/β ratio between tumors and late-responding normal tissues; are there other biological differences between tumors and normal tissue that we can potentially take advantage of? In fact it has been strongly suspected for some times that DNA repair rates are typically much faster in tumors compared to late-responding normal tissue. Both from animal studies and from clinical studies, it appears that characteristic DNA repair rates in tumors are around 1 h or less, whereas characteristic DNA repair times in late-responding tissues are of the order of 5 h or more (43). Can we take advantage of this difference in repair rates to design optimized fractionation schemes, analogous to taking advantage of different α/β ratios?
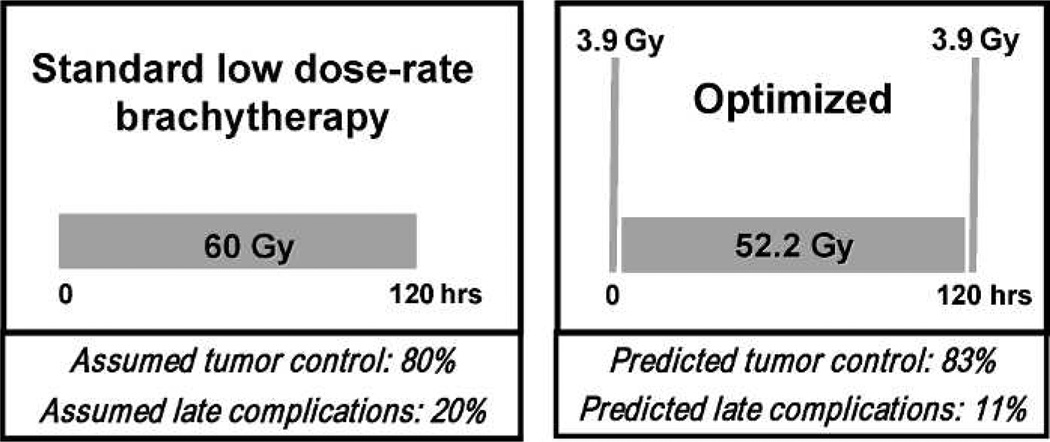
In fact we probably can, and Fig. 8 shows a standard low-dose rate brachytherapy scheme (60 Gy in 120 h), next to a “temporally optimized” scheme, which uses the same dose and the same overall time, but moves some of the dose to the beginning and to the end of treatment. It is not hard to show that one could significant improve the expected clinical outcome with this temporally optimized scheme (43), which could be relatively easily instituted in the clinic.
FIG. 8.
Taking advantage of the different DNA repair rates of tumors and late responding normal tissues. The left panel shows a standard 120 h/60 Gy low dose rate brachytherapy protocol, which we assume here to result in a tumor control probability of 80% and a late complication rate of 20%. If DNA repair rates are indeed faster in tumors than in late-responding normal tissue – and there is evidence to suggest this is the case – one can take advantage of this difference to design protocols with the same overall dose and overall time, but which give much reduced late complications. Specifically the temporally optimized protocol shown in the right panel features two short high-dose “spikes” at the beginning and the end of the treatment, and would be predicted to produce much reduced late complications (43) for similar tumor control.
Predictive Assays
Predictive assays, both for tumor control and radiotherapy, have been intensively studied for several decades. Lester Peters called them “the holy grail of radiobiology” (44). There is little doubt that if an effective predictive assay of normal-tissue radiosensitivity could be found, there would be a significant therapeutic gain for radiosensitive patients, and potentially for the remaining patient population, perhaps even opening the door to individualized radiotherapy dose prescriptions (45).
Why has no predictive assay worked? One answer is technical, and is to do with assay variability. Clearly if an assay result is not repeatable, i.e., has limited precision, then its predictive power will be limited. Several groups have quantified this observation (45, 46): for example Mackay and Hendry (45) concluded that to be useful as a predictive assay in the clinic, the assay variability needs to be at least 50% smaller (and ideally much smaller still) than the inherent variability in radiosensitivity among patients. This is not a trivial requirement, and most cellular assays that have been investigated (47) probably do not meet this criterion, in significant part because the assays are performed manually in a laboratory setting.
It is of course well established that the results of cellular assays which involve a significant number of manually-performed steps, such as pipetting, adding reagents, washing, microscope imaging etc, show considerable intra-laboratory variability (48, 49) – and one way to improve the precision, or repeatability, of cellular assays is with complete automation (50).
In fact assay automation is something that my own group has been working on for several years, in the context of high-throughput radiation biodosimetry (51, 52) after a large-scale radiological event. Here we have developed a completely automated robotically-based biodosimetry system called the RABiT [Rapid Automated Biodosimetry Tool, see Fig. 9)], which fully automates a number of cellular assays such as micronucleus, γ-H2AX, and (in progress) other cytogenetic endpoints. Complete robotic automation provides both high throughput and significantly improved repeatability. Given the availability of such tools, I would suggest that it is worth revisiting cellular predictive assays, given that we can now do much larger studies and with much improved assay precision – the two factors that I think doomed earlier predictive-assay research.
FIG. 9.
Breadboard version of the Rapid Automated Biodosimetry Tool [RABiT (51, 52)]. This device is designed to perform fully-automated high-throughput estimates of past radiation exposure, based on fingerstick blood samples. The RABiT is designed to automate established assays such as micronucleus, γ-H2AX and (in development) cytogenetic endpoints. Overall throughput is up to 30,000 samples per day, achieved through complete robotically-based automation of each assay, with in situ imaging in multi-well plates, together with innovations in high-speed imaging. In blinded studies, about 75% of RABiT dose estimates were within 0.5 Gy of the true dose, and 95% were within 1 Gy.
Discussing the RABiT system gives me an excuse to give my sincerest thanks to my own team at Columbia, mostly pictured in Fig. 10, whose dedication to good science, and whose patience with my own foibles, is really remarkable.
FIG. 10.
The Columbia RABiT team. A truly interdisciplinary team of physicists and biologists.
CONCLUSION
The Failla prize was a real honor, and reminded me of Newton’s famous quote “If I have seen a little further it is by standing on the shoulders of giants.” But perhaps I should not have been too surprised to find out (53) that Newton actually borrowed the quote from Bernard of Chartres, half a millennium earlier, who wrote “I am like a dwarf on the shoulders of giants. If I can see more things at a great distance, it is not by virtue of any sharpness of sight on my part, but because I am carried high and raised up by their giant size.” Even more appropriate, I think, as illustrated in Fig. 11.
FIG. 11.
“Like a dwarf on the shoulders of giants”. German manuscript illustration, circa 1410. From the U.S. Library of Congress, Lessing J. Rosenwald Collection.
I have talked a little of my own scientific giants (Fig. 12), particularly my predecessors at Columbia University, Failla, Rossi, and Eric Hall, as well as my friend and teacher, Rainer Sachs at UC Berkeley. But I would like to end with a thank you to my late colleague and friend, Elaine Ron. Elaine was the consummate radiation scientist, combining scientific insight, compassion, and a complete intolerance for bad science. She is very much missed.
FIG. 12.
My own scientific giants.
REFERENCES
- 1.Cologne JB, Preston DL. Longevity of atomic-bomb survivors. Lancet. 2000;356:303–307. doi: 10.1016/S0140-6736(00)02506-X. [DOI] [PubMed] [Google Scholar]
- 2.Wintz H. Basic principles for successful Roentgen therapy of carcinoma. Radiology. 1938;30:35–42. [Google Scholar]
- 3.Regaud C, Ferroux R. Discordances des effets des rayons X, d’une part dans les peau, d’autre part dans les testicule, par le fractionnement de la dose: Diminution de l’efficacité dans le peau, maintien de l’efficacité dans le testicule. Compt Rend Soc Biol. 1927;97:431–434. [Google Scholar]
- 4.Coutard H. The results and methods of treatment of cancer by radiation. Ann Surg. 1937;106:584–598. doi: 10.1097/00000658-193710000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerweck LE, Zaidi ST, Zietman A. Multivariate determinants of radiocurability. I: Prediction of single fraction tumor control doses. Int J Radiat Oncol Biol Phys. 1994;29:57–66. doi: 10.1016/0360-3016(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 6.Fertil B, Malaise EP. Inherent cellular radiosensitivity as a basic concept for human tumor radiotherapy. Int J Radiat Oncol Biol Phys. 1981;7:621–629. doi: 10.1016/0360-3016(81)90377-1. [DOI] [PubMed] [Google Scholar]
- 7.Leborgne F, Leborgne JH, Fowler J, Zubizarreta E, Mezzera J. Accelerated hyperfractionated irradiation for advanced head and neck cancer: effect of shortening the median treatment duration by 13 days. Head Neck. 2001;23:661–668. doi: 10.1002/hed.1093. [DOI] [PubMed] [Google Scholar]
- 8.Akagi Y, Hirokawa Y, Kagemoto M, Matsuura K, Ito A, Fujita K, et al. Optimum fractionation for high-dose-rate endoesophageal brachytherapy following external irradiation of early stage esophageal cancer. Int J Radiat Oncol Biol Phys. 1999;43:525–530. doi: 10.1016/s0360-3016(98)00433-7. [DOI] [PubMed] [Google Scholar]
- 9.Thames HD, Hendry JH. Fractionation in Radiotherapy. London and New York: Taylor & Francis; 1987. [Google Scholar]
- 10.Duchesne GM, Peters LJ. What is the a/b ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1999;44:747–748. doi: 10.1016/s0360-3016(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 11.Thames HD, Jr, Withers HR, Peters LJ, Fletcher GH. Changes in early and late radiation responses with altered dose fractionation: implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 12.Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8:1981–1997. doi: 10.1016/0360-3016(82)90459-x. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low a/b ratio), similar to lateresponding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52:6–13. doi: 10.1016/s0360-3016(01)02664-5. [DOI] [PubMed] [Google Scholar]
- 15.Proust-Lima C, Taylor JM, Secher S, Sandler H, Kestin L, Pickles T, et al. Confirmation of a low alpha/beta ratio for prostate cancer treated by external beam radiation therapy alone using a posttreatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79:195–201. doi: 10.1016/j.ijrobp.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dosefractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta=1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 17.Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–1278. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 18.Arcangeli S, Strigari L, Gomellini S, Saracino B, Petrongari MG, Pinnaro P, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys Epub. 2012 doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 19.Pollack A, Walker G, Buyyounouski MK, Horowitz E, Price R, Feigenberg S, et al. Five year results of a randomized external beam radiotherapy hypofractionation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81:S1. [Google Scholar]
- 20.Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, Clark C, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. Lancet Oncol. 2012;13:43–54. doi: 10.1016/S1470-2045(11)70293-5. [DOI] [PubMed] [Google Scholar]
- 21.Kuban DA, Nogueras-Gonzalez GM, Hamblin L, Lee AK, Choi S, Frank SJ, et al. Preliminary report of a randomized dose escalation trial for prostate cancer using hypofractionation. Int J Radiat Oncol Biol Phys. 2010;78:S58–S59. [Google Scholar]
- 22.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 23.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiationinduced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 24.Brenner DJ, Curtis RE, Hall EJ, Ron E. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Gray LH. Cellular Radiation Biology; A symposium considering radiation effects in the cell and possible implications for cancer therapy, a collection of papers. Baltimore: William and Wilkins; 1965. Radiation biology and cancer; pp. 8–25. [Google Scholar]
- 26.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102:13040–13045. doi: 10.1073/pnas.0506648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachs RK, Wolfe AM. Perturbations of a cosmological model and angular variations of the microwave background. Astrophys J. 1967;147:73–90. [Google Scholar]
- 28.Stoeger WR, Ellis GF, Xu C. Observational cosmology. VI. The microwave background and the Sachs-Wolfe effect. Phys Rev D Part Fields. 1994;49:1845–1853. doi: 10.1103/physrevd.49.1845. [DOI] [PubMed] [Google Scholar]
- 29.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 30.Fowler PH, Perkins DH. The possibility of therapeutic applications of beams of negative pi-mesons. Nature. 1961;189:524–528. doi: 10.1038/189524a0. [DOI] [PubMed] [Google Scholar]
- 31.Elwood JM. The design of clinical trials comparing pi-meson therapy with conventional radiotherapy. J Can Assoc Radiol. 1979;30:79–82. [PubMed] [Google Scholar]
- 32.Brenner DJ, Dicello JF, Zaider M. An interpretation of some biological results obtained in range-modulated negative pion beams. Int J Radiat Oncol Biol Phys. 1982;8:121–126. doi: 10.1016/0360-3016(82)90396-0. [DOI] [PubMed] [Google Scholar]
- 33.Konski A, Speier W, Hanlon A, Beck JR, Pollack A. Is proton beam therapy cost effective in the treatment of adenocarcinoma of the prostate? J Clin Oncol. 2007;25:3603–3608. doi: 10.1200/JCO.2006.09.0811. [DOI] [PubMed] [Google Scholar]
- 34.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26:175–176. doi: 10.1200/JCO.2007.14.4329. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DJ, Hall EJ. Secondary neutrons in clinical proton radiotherapy: A charged issue. Radiother Oncol. 2008;86:165–170. doi: 10.1016/j.radonc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Knapp EA, Swenson DA. The Los Alamos Scientific Laboratory ion linear accelerator program. Int J Radiat Oncol Biol Phys. 1977;3:383–385. doi: 10.1016/0360-3016(77)90282-6. [DOI] [PubMed] [Google Scholar]
- 37.Schippers JM, Lomax AJ. Emerging technologies in proton therapy. Acta Oncol. 2011;50:838–850. doi: 10.3109/0284186X.2011.582513. [DOI] [PubMed] [Google Scholar]
- 38.Overgaard J. Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck–a systematic review and metaanalysis. Radiother Oncol. 2011;100:22–32. doi: 10.1016/j.radonc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 40.Bates TD, Peters LJ. Dangers of the clinical use of the NSD formula for small fraction numbers. Br J Radiol. 1975;48:773. doi: 10.1259/0007-1285-48-573-773-b. [DOI] [PubMed] [Google Scholar]
- 41.Thames HD, Jr, Peters LJ, Withers HR, Fletcher GH. Accelerated fractionation vs hyperfractionation: rationales for several treatments per day. Int J Radiat Oncol Biol Phys. 1983;9:127–138. doi: 10.1016/0360-3016(83)90089-5. [DOI] [PubMed] [Google Scholar]
- 42.Baujat B, Bourhis J, Blanchard P, Overgaard J, Ang KK, Saunders M, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev. 2010:CD002026. doi: 10.1002/14651858.CD002026.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner DJ, Hall EJ, Huang Y, Sachs RK. Optimizing the time course of brachytherapy and other accelerated radiotherapeutic protocols. Int J Radiat Oncol Biol Phys. 1994;29:893–901. doi: 10.1016/0360-3016(94)90583-5. [DOI] [PubMed] [Google Scholar]
- 44.Peters LJ. The ESTRO Regaud lecture. Inherent radiosensitivity of tumor and normal tissue cells as a predictor of human tumor response. Radiother Oncol. 1990;17:177–190. doi: 10.1016/0167-8140(90)90202-8. [DOI] [PubMed] [Google Scholar]
- 45.Mackay RI, Hendry JH. The modelled benefits of individualizing radiotherapy patients’ dose using cellular radiosensitivity assays with inherent variability. Radiother Oncol. 1999;50:67–75. doi: 10.1016/s0167-8140(98)00132-7. [DOI] [PubMed] [Google Scholar]
- 46.Brock WA, Tucker SL, Geara FB, Turesson I, Wike J, Nyman J, et al. Fibroblast radiosensitivity versus acute and late normal skin responses in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 1995;32:1371–1379. doi: 10.1016/0360-3016(95)00068-A. [DOI] [PubMed] [Google Scholar]
- 47.West CM. Invited review: intrinsic radiosensitivity as a predictor of patient response to radiotherapy. Br J Radiol. 1995;68:827–837. doi: 10.1259/0007-1285-68-812-827. [DOI] [PubMed] [Google Scholar]
- 48.Radack KL, Pinney SM, Livingston GK. Sources of variability in the human lymphocyte micronucleus assay: a population-based study. Environ Mol Mutagen. 1995;26:26–36. doi: 10.1002/em.2850260105. [DOI] [PubMed] [Google Scholar]
- 49.Bonassi S, Fenech M, Lando C, Lin YP, Ceppi M, Chang WP, et al. HUman MicroNucleus project: international database comparison for results with the cytokinesis-block micronucleus assay in human lymphocytes: I. Effect of laboratory protocol, scoring criteria, and host factors on the frequency of micronuclei. Environ Mol Mutagen. 2001;37:31–45. [PubMed] [Google Scholar]
- 50.Sturgeon C, Hill R, Hortin GL, Thompson D. Taking a new biomarker into routine use—a perspective from the routine clinical biochemistry laboratory. Proteomics Clin Appl. 2010;4:892–903. doi: 10.1002/prca.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garty G, Chen Y, Salerno A, Turner H, Zhang J, Lyulko O, et al. The RABiT: a rapid automated biodosimetry tool for radiological triage. Health Phys. 2010;98:209–217. doi: 10.1097/HP.0b013e3181ab3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garty G, Chen Y, Turner HC, Zhang J, Lyulko OV, Bertucci A, et al. The RABiT: a rapid automated biodosimetry tool for radiological triage. II. Technological developments. Int J Radiat Biol. 2011;87:776–790. doi: 10.3109/09553002.2011.573612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merton RK. On the shoulders of giants. Chicago: University of Chicago Press; 1965. [Google Scholar]