Abstract
Increased oxidative stress has been implicated in both the onset and the progression of diabetes mellitus and its complications. The development of easy to measure biomarkers of oxidative stress would, therefore, help in determining in a prospective manner the impact of glycemic control on oxidative stress and macrovascular disease in patients with diabetes. We report the development and validation of a novel method to directly measure the urinary concentrations of the conjugated metabolites of vitamin E (α-tocopherol) and investigate whether the oxidized metabolite α-tocopheronolactone (α-TL) could be used as a biomarker of oxidative stress in children with type 1 diabetes. A novel method using liquid chromatography–tandem mass spectrometry was developed and used to measure directly and rapidly the urinary concentrations of the glucuronidated and sulfated metabolites of α-tocopherol in 32 young patients with type 1 diabetes and age-matched controls. The mean concentrations of the glucuronidated and sulfated conjugates of α-TL were all highly significantly increased in the children with type 1 diabetes (p<0.001). The results suggest that the measurement of the urinary concentrations of α-TL conjugates may provide a useful biomarker of oxidative stress in diabetes and possibly in other clinical conditions in which oxidative stress has been implicated.
1Abbreviations: α-CEHC, α-carboxyethylhydroxychroman; α-TL, α-tocopheronolactone; α-TTP, α-tocopherol transport protein; ACN, acetonitrile; BMI, body mass index; GC–MS, gas chromatography–mass spectrometry; HbA1c, glycosylated hemoglobin; LC–MS, liquid chromatography–mass spectrometry; MeOH, methanol; ODFR, oxygen-derived free radical; VLDL, very low density lipoprotein
Keywords: Oxidative stress, Diabetes mellitus, Urinary conjugated vitamin E metabolites, Sulfation, Glucuronidation, α-Tocopheronolactone, α-Carboxyethylhydroxychroman, Biomarker, Tandem mass spectrometry, Free radicals
Highlights
► A novel method was developed for measuring urinary conjugated vitamin E metabolites. ► α-Tocopheronolactone concentrations are highly significantly increased in diabetes. ► α-Tocopheronolactone is a potential biomarker of oxidative stress.
Introduction
Over the past 50 years a number of metabolites of vitamin E (α-tocopherol) have been described. These can be divided into two groups. The first includes α-tocopherylquinone, α-tocopheronic acid, and α-tocopheronolactone (α-TL)1, all of which result from the oxidation and opening of the chromanol ring of α-tocopherol [1,2]. The second group results from the successive shortening of the phytyl side chain of α-tocopherol, initially by ω-hydroxylation by a cytochrome P450-dependent mechanism followed by β-oxidation [3,4], and includes α-carboxymethylbutylhydroxychroman and α-carboxyethylhydroxychroman (α-CEHC) [5–7]. The principal reported urinary metabolites of α-tocopherol are α-CEHC and α-TL, both of which are excreted as their polar sulfate and glucuronide conjugates [5,8]. There is, however, doubt in the literature as to whether the α-TL observed in urine is a true metabolite or an artifact produced from the oxidation of α-CEHC during the methodological workup [5,9]. Confirmation of the authenticity of α-TL is important, as it could potentially be used as an ex vivo biomarker of oxidative stress.
Published methods for the measurement of urinary vitamin E metabolites typically involve deconjugation, extraction, and derivatization steps, which are time consuming and risk the artifactual formation of α-TL from α-CEHC [9]. We aimed to develop a new and rapid method utilizing tandem mass spectrometry for the direct assay of the intact conjugated vitamin E metabolites and to investigate whether there was evidence for increased urinary concentrations of α-TL conjugates under conditions where oxidative stress may be implicated, such as diabetes mellitus [10,11].
Diabetes is a major risk factor for cardiovascular disease. In patients with type 1 and type 2 diabetes, atherosclerosis occurs early in life (childhood and adolescence), leading to increased morbidity and mortality compared to the general population. In addition those with suboptimal metabolic control have a higher risk of developing macrovascular complications [12–14].
There seems to be a general agreement that the production of oxygen-derived free radicals (ODFRs) is implicated in both the onset and the progression of diabetes and its complications [10]. A number of mechanisms have been proposed to contribute to and thus explain the increased production of ODFRs. These include an increased flux through the polyol pathway, an increased formation of advanced glycation end products, activation of protein kinase C, and an increased production of superoxide by the mitochondrial electron transport chain [10,15]. As a result of an increased ODFR production, patients with diabetes are likely to have a greater antioxidant requirement [16] and there is some evidence suggesting that they have decreased concentrations of antioxidants [17–19], including lower plasma α-tocopherol concentrations [20,21].
The development of biomarkers of oxidative stress that can be easily measured would help determine prospectively the impact of glycemic control on oxidative stress and macrovascular disease in children and young people with type 1 diabetes. In this paper we report the development and validation of a method to measure urinary conjugated vitamin E metabolites, particularly the glucuronidated and sulfated conjugates of α-TL, and its application to children with type 1 diabetes. A summary of this work has been previously reported in abstract form [22].
Methods
Method development
The sulfated and glucuronidated conjugates of α-TL and α-CEHC used in the method development and validation studies were synthesized by Pope et al. [8,23].
The vitamin E metabolites were analyzed by mass spectrometry using a triple-quadrupole Micro Quattro instrument (MicroMass, Waters, UK) fitted with an electrospray ionization source. The source and desolvation gas temperatures were held constant at 150 and 350 °C, respectively, with flow rates of 950 and 60 liters of nitrogen per hour. The optimum gas cell pressure was set at 4 × 10−3 mbar.
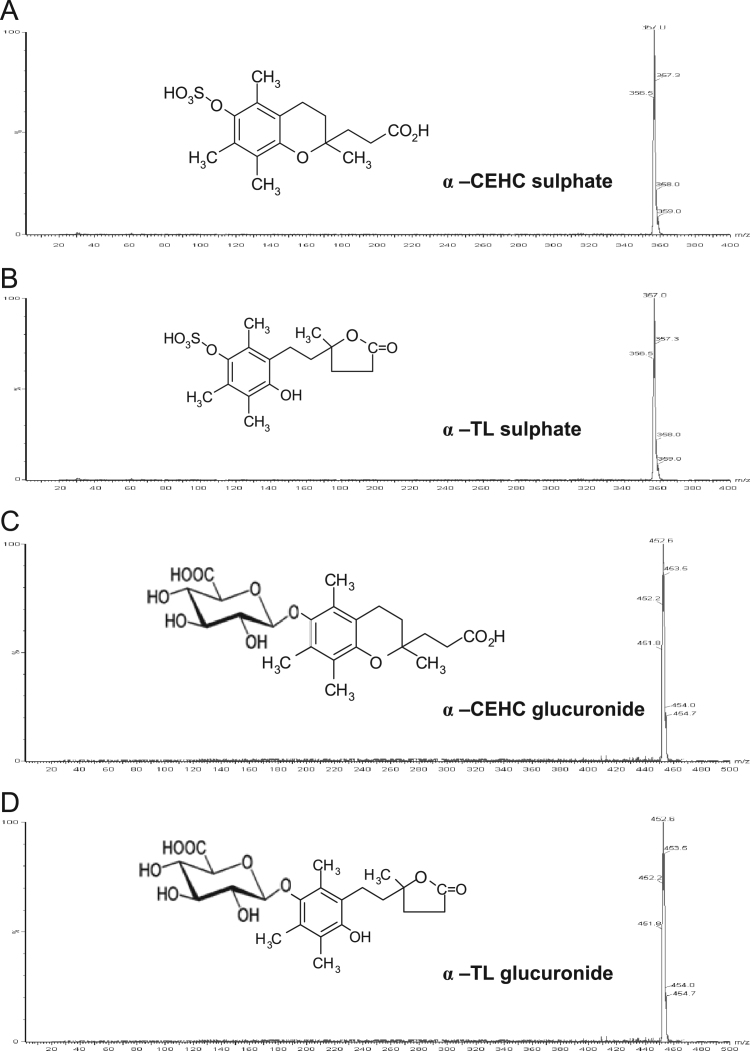
Fig. 1 shows the tandem mass spectral analysis of vitamin E metabolites obtained in scan mode over a mass range of 2–400 and 2–500 m/z [M−H−] for the sulfate and glucuronide metabolites, respectively. A mass was observed at m/z 357 [M−H−], which corresponded to the theoretical mass of the sulfated α-CEHC and α-TL (Figs. 1A and 1B). The mass observed at m/z 452.6 [M−H−] (Figs. 1C and 1D) corresponded to the theoretical masses of α-CEHC and α-TL glucuronide. α-CEHC and α-TL have the same molecular masses and are, therefore, isobaric.
Fig. 1.
Parent ion scans of vitamin E metabolites.
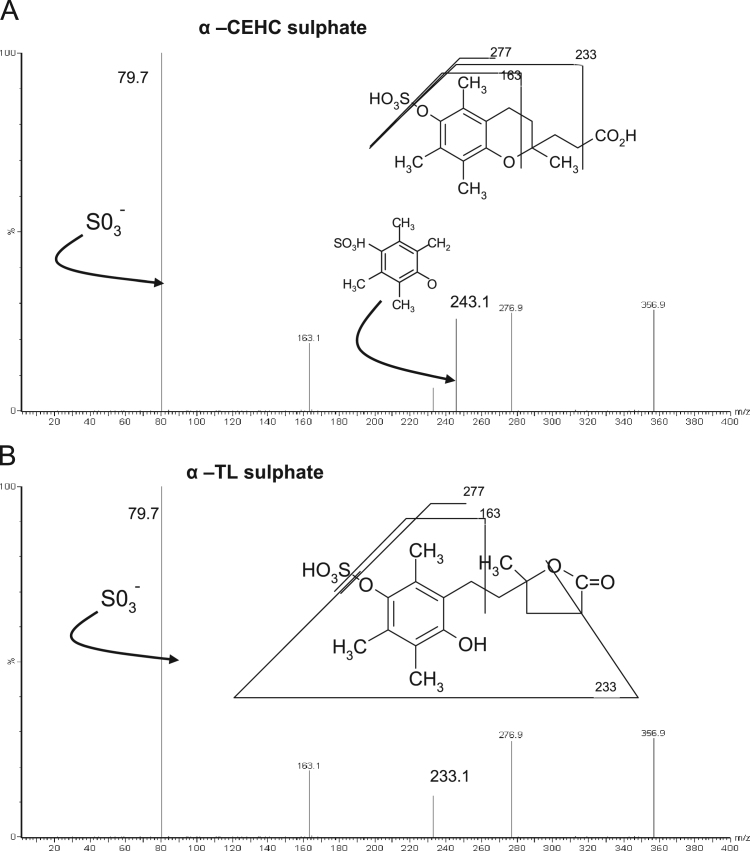
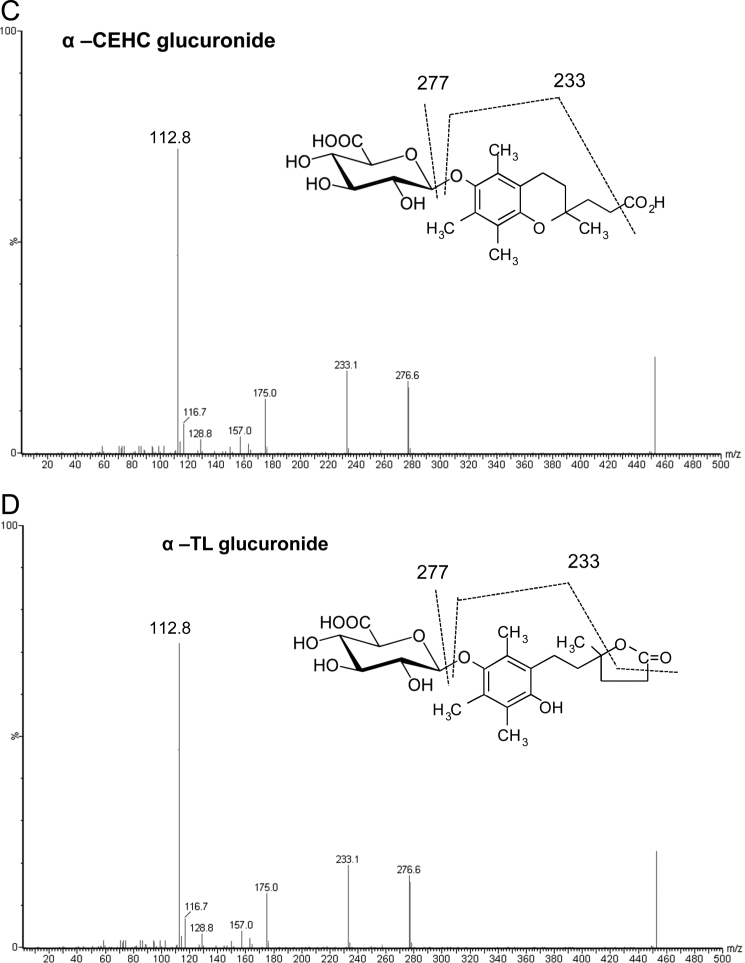
Each compound was further characterized by fragmentation studies using the mass spectrometer operating in the product ion scan mode. The fragmentation analysis for the sulfate metabolites was observed in a scan mode of 2–400 m/z (Fig. 2A and B). The fragmentation pattern of α-CEHC sulfate led to a “fingerprint” ion identified as a sulfite ion (SO3−) with an intensity of 79.7 m/z. Lesser intensity ions were observed at 276.9, 243.1, 233.1, and 163.1 m/z, which were attributed to the fragmentation of the metabolite as shown in Fig. 2A. Fragmentation analysis of α-TL sulfate (Fig. 2B) revealed the presence of a progeny and lesser intensity ions similar to that of the α-CEHC sulfate, except no ion at 243.1 m/z was observed.
Fig. 2.
Product ion scans of vitamin E metabolites.
The product ion analysis of the glucuronide metabolites was conducted in scan mode between 2 and 500 m/z. The highest intensity ion was observed at 112.8 m/z (Figs. 2C and 2D), which was formed by the loss of H2O and CO2 from the glucuronide moiety. Further fragmentation led to the formation of additional ions at 233.1 and 276.6 m/z. The fragmentations of the α-CEHC and α-TL glucuronides were very similar and thus the two metabolites could not be differentiated by mass spectral analysis alone.
The tandem mass spectral analyses of the internal standards were obtained in a scan mode of 2–600 [M−H−]. Masses were observed at 455.3 and 465.3 m/z [M−H−], which were consistent with the internal standards lithocholic acid sulfate and androsterone glucuronide, respectively (not shown). The internal standards were further characterized by fragmentation studies. This led to the identification of a daughter ion (HSO4) of lithocholic acid sulfate at 97 m/z and a daughter ion of androsterone glucuronide at 112.8 m/z.
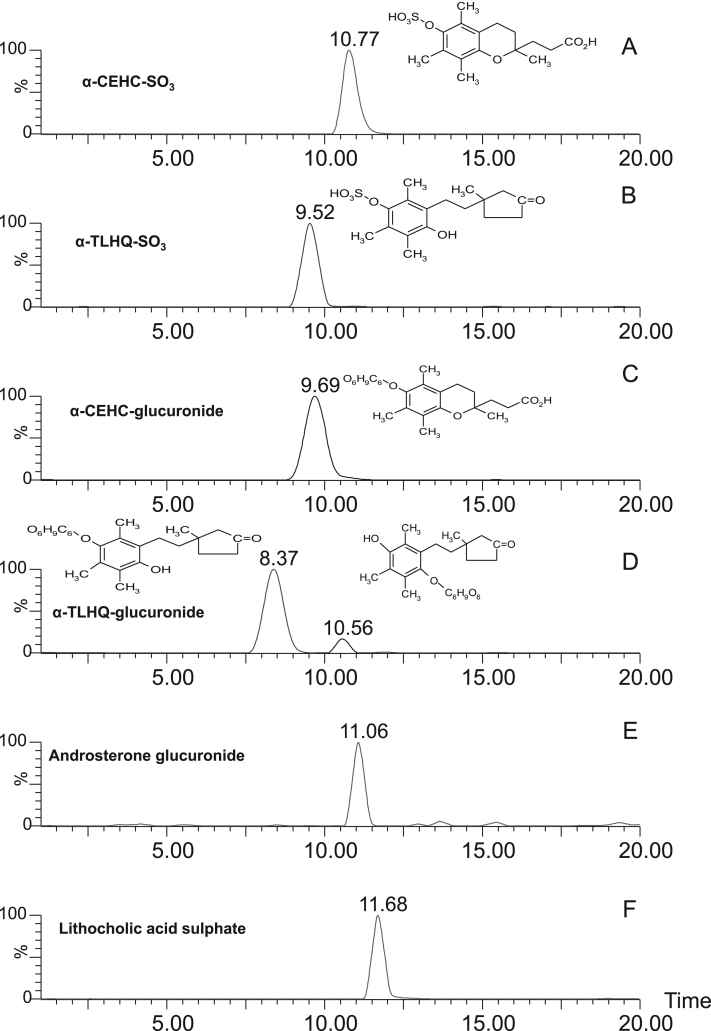
Because of the isobaric nature of α-CEHC and α-TL and their similar fragmentation patterns (with the exception of an ion at 243.1 m/z in the case of α-CEHC sulfate), high performance liquid chromatography was used together with tandem mass spectrometry to determine if it was possible to distinguish each vitamin E metabolite by its chromatographic retention time in addition to mass spectral analysis. Results from the use of a C8 column showed it to be 10-fold more sensitive than an HsF5 column, and a C8 column was, therefore, used in the method development. The chromatographic separations of the metabolites and internal standards are shown in Fig. 3. Two peaks were observed for the α-TL glucuronide, a major peak eluting at 8.37 min and a minor one at 10.56 min. The minor peak was postulated to be an isomer with the structure as illustrated on chromatogram in Fig. 3D and had been observed previously by Pope [23]. These major and minor peaks are referred to as α-TL glucuronide 1 and 2, respectively. The elution times meant that all the vitamin E metabolites and internal standards could be separated in a single run.
Fig. 3.
The LC–MS/MS analysis of vitamin E metabolites and internal standards.
Final method
The sulfated and glucuronidated conjugates of α-TL and α-CEHC were synthesized by Pope et al. [8,23]. The internal standards (lithocholic acid sulfate and androsterone glucuronide) were purchased from Sigma–Aldrich Co. Ltd (Poole, Dorset, UK). All the solvents were liquid chromatography–mass spectrometer (LC–MS) grade or equivalent and were also obtained from Sigma–Aldrich Co. Ltd. Neat urine (150 μl) was spun for 5 min at 1500 rpm and spiked with 10 μl of the internal standards (100 μmol/L). Samples were vortexed and 15 μl was injected into the LC–MS for analysis. The concentration of each vitamin E metabolite in each patient urine sample was determined by comparison of the ratio of the response of the metabolite to the response of the internal standard and comparing it to a calibration curve created by spiking control urine with increasing amounts of each standard. The control urine had endogenous vitamin E metabolites removed by a cleanup procedure using C18 solid-phase extraction.
The urinary vitamin E metabolites were desalted and/or separated before mass spectrometry using a Waters 2795XE high-performance liquid chromatography unit fitted with a 100 × 2.1-mm (5 μ) HyPURITY C8 column plus a guard column containing the same stationary phase (Phenomenex UK). The LC gradient started with 5% methanol:acetonitrile (MeOH:ACN, 2:1) and 95% 4 mmol/L ammonium acetate (containing 0.01% formic acid) for 4 min. The ammonium acetate was then replaced by water (80%), which, together with MeOH:ACN (20%), was run for 1 min. This mixture was changed to water (55%) and MeOH:ACN (45%) for 5.5 min. It was at this gradient that all the vitamin E metabolites eluted between 7 and 10 min total run time. The column was then washed with 100% MeOH:ACN for 5.5 min before being reconditioned before the next injection with 5% MeOH:ACN and 95% ammonium acetate (4 mmol/L containing 0.01% formic acid) for 4 min. The flow rates varied from 0.25 to 0.55 ml/min. The total analysis time between each injection was 20 min. The mobile phase containing salts and other contaminants was diverted using the divert valve, away from the mass spectrometer, during the first 3 min. In this way at least 300 analyses could be performed before the ion source required routine maintenance.
The vitamin E metabolites were detected using multiple-reaction monitoring mode of the transitions at 356.9>79.7 and 453.0>112.8 m/z for the α-CEHC/TL sulfates and glucuronides, respectively. Data were acquired over a period of 2–20 min, in multiple-channel acquisition mode and with a dwell time for each ion species of 50 ms. The cone voltages were 42 and 25 V for the sulfates and glucuronides, respectively, and the collision voltages were 26 and 36 eV, respectively.
Validation of method
The urine samples were stored at either −20 or −80 °C before analysis. To investigate any effects of storage on α-TL conjugate concentrations, a batch of urine samples was stored at −20 °C and analyzed at monthly intervals for 3 months. There were no consistent or significant increases in concentrations of the α-TL conjugates or decreases in the α-CEHC conjugates.
To check the linearity of the method, increasing amounts of each of the vitamin E metabolites (0, 0.5, 1, 1.5, 2.5, 5, and 10 nmol) were added to constant amounts of the internal standard and ethanol before analysis. The ratio of the areas for the vitamin E metabolites over the corresponding internal standard was calculated for each metabolite using MassLynx software. The data obtained were analyzed by linear regression for which the best fit for the linear relationship was calculated using GraphPad Prism. The correlation coefficients (r2) were >0.99 for all the metabolites. In subsequent studies it was found that the linearity of the method ceased at amounts of approximately 15 nmol.
Recovery studies involved analysis before and after known amounts of the metabolites (0.2, 0.5, and 2.5 nmol) were added to 150 μl of a laboratory control urine containing a constant amount of internal standard. Triplicate injections at each concentration were performed. The ratio of the areas for the vitamin E metabolites over the corresponding internal standard was calculated for each metabolite. From the amount of the metabolite present, the percentage recovery of the added analyte was calculated. The recoveries for all the metabolites at the three concentrations varied from 90 to 100%.
To assess the reproducibility of the method, low, normal, and high amounts of the conjugates of α-CEHC and α-TL (0.1, 0.5, and 2.5 nmol) were added to a laboratory control urine. The intra-assay precision of the method was evaluated using 20 injections of the three concentrations of the metabolites in the same run on a single day. Interassay reproducibility was assessed by running a single injection of the three concentrations on 20 separate occasions over a period of 60 days. The ratio of the areas for the vitamin E metabolites over the corresponding internal standard was calculated for each metabolite. The intra-assay coefficient of variation for the metabolites ranged from 0.60 to 3.73% and the interassay coefficient of variation ranged from 1.18 to 4.32%.
The functional and biological limits of detection of the conjugated metabolites were determined by sequentially diluting a known amount of metabolite in ethanol (functional limit) and urine (biological limit). A signal-to-noise ratio of 5:1 was taken to be the limit of detection. The functional limit of detection for the metabolites varied from 0.01 to 0.20 nmol (0.06 to 1.3 μmol/L) and the biological limit of detection varied from 0.05 to 0.77 nmol (0.3 to 5.1 μmol/L).
The urinary concentrations of the vitamin E metabolites were expressed per urinary creatinine concentrations, which were measured by mass spectrometry using deuterated creatinine as the internal standard (unpublished method).
Unless stated otherwise the results are expressed as means±1 SEM. The significance of differences between mean concentrations was determined using the unpaired Student t test for analysis between groups.
Clinical details
Thirty-two children and young people (16 male), with a mean age at study of 12.9 years (range 7.8–18.4), with type 1 diabetes were studied. Their mean age at diagnosis was 10.8 years and the average duration of diabetes was 5 years. None of the subjects were receiving any vitamin E supplements or pharmacological therapy at the time of the study and were receiving only appropriate insulin therapy. A urinary sample for measurement of vitamin E metabolites was obtained at a routine clinic visit and glycosylated hemoglobin (HbA1c) was measured as part of the standard clinic appointment using the Diabetes Control and Complications Trial-aligned Bayer 2000+ system (Siemens Healthcare Diagnostics, Deerfield, IL, USA).
The same 32 age- and sex-matched healthy control subjects were investigated throughout the study and were drawn from the University College London Fetal Growth Study. This study consists of 1650 consecutive mothers who delivered a singleton, Caucasian baby free of pregnancy complications and whose offspring's growth has been followed for the past 10 years. As part of the follow-up of these children urine samples were obtained contemporaneous with the type 1 diabetes study. There was no significant difference between the body mass indexes (BMIs) of the diabetic subjects and controls. The BMI expressed as a standard deviation score was 0.0 (SD 0.9) for the diabetic subjects and 0.1 (SD 0.8) for the controls.
Ethical approval was obtained from the Ethics Committees of University College London Hospital/University College London and Great Ormond Street Hospital for Sick Children/Institute of Child Health. Written informed consent was obtained from the parents and from the children and young people where appropriate.
Results
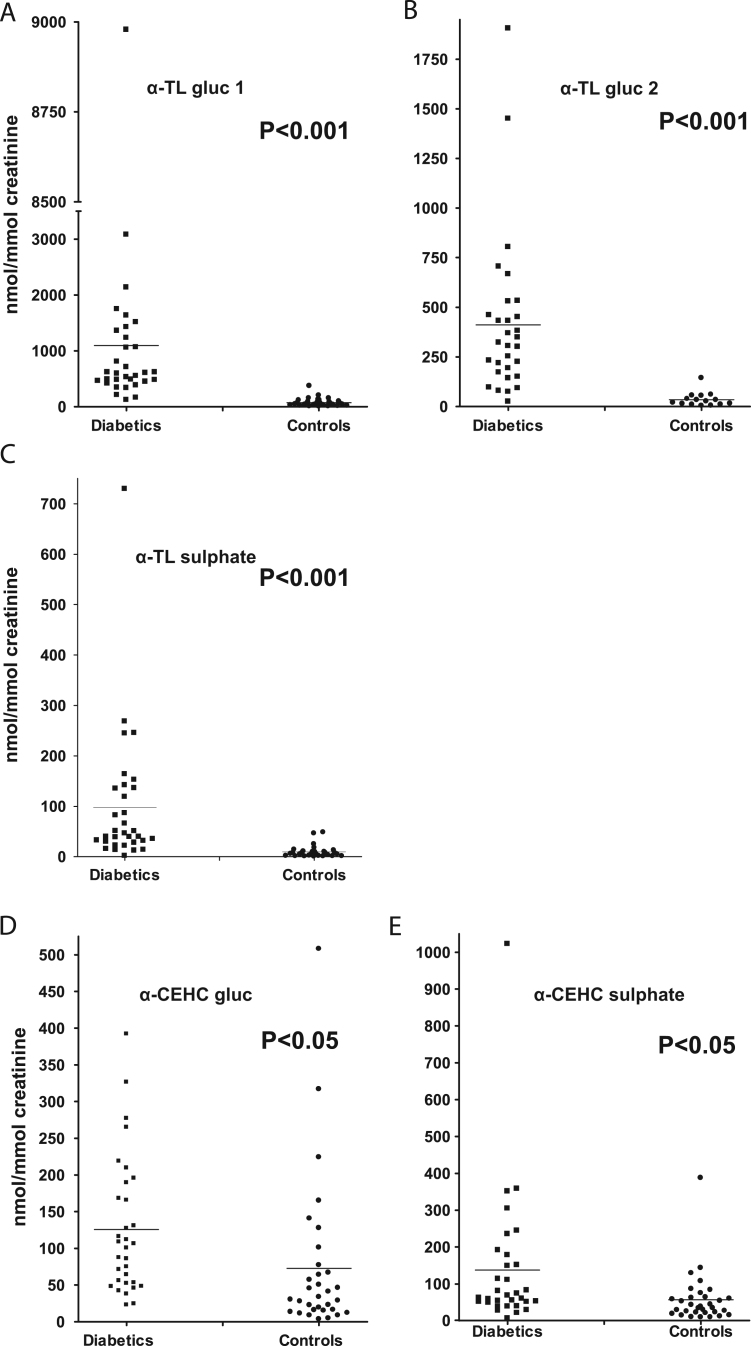
Concentrations of urinary vitamin E metabolites were quantified in 32 patients with type 1 diabetes mellitus and 32 age-matched controls. Fig. 4A–E show the range and mean concentration per creatinine for each of the metabolites with levels of significance between the subjects with diabetes and the controls. The results are summarized in Table 1. The mean concentrations of the α-TL conjugates are all highly significantly increased in the patients with diabetes (p<0.001). The concentrations of the α-CEHC conjugates were also significantly increased (p<0.05) in the patients with diabetes. The cohort with diabetes excreted approximately eightfold greater concentrations of the vitamin E metabolites than the control subjects. This was also true for the urinary concentrations of both total glucuronide (×10) and sulfate (×3.5) conjugates. In addition the concentrations of the glucuronide metabolites were greater than those of the sulfates in both cohorts. The concentrations of the glucuronidated and sulfated α-CEHC were similar within each cohort. There were, however, greater concentrations of both α-CEHC glucuronide and sulfate in the group with diabetes compared to controls. The control subjects excreted similar amounts of α-CEHC glucuronide and α-TL glucuronide.
Fig. 4.
Urinary concentrations of the conjugated metabolites of α-tocopherol (nmol/mmol creatinine).
Table 1.
Urinary concentrations of vitamin E metabolites.
| Patients with type 1 diabetes (D), n=32 | Controls (C), n=32 | pa | Ratio (D:C) | |
|---|---|---|---|---|
| α-TL glucuronide 1b | 1098±279 | 76±13 | <0.001 | 14.5 |
| α-TL glucuronide 2b | 441±73 | 34±9 | <0.001 | 13.0 |
| α-CEHC glucuronide | 126±16 | 73±19 | <0.05 | 1.7 |
| Total glucuronide | 1786 | 183 | 9.8 | |
| α-TL sulfate | 98±24 | 10±2 | <0.001 | 9.8 |
| α-CEHC sulfate | 138±33 | 57±12 | <0.05 | 2.4 |
| Total sulfate | 236 | 67 | 3.5 | |
| Total α-TL | 1758 | 120 | 14.7 | |
| Total α-CEHC | 264 | 130 | 2.0 | |
| Total metabolites | 2022 | 250 | 8.1 |
Concentrations are given in nmol/mmol creatinine; mean±SEM.
The significance of differences between mean concentrations was determined using the unpaired Student t test for analysis between groups.
Two peaks were observed for the α-TL glucuronide, a major peak eluting at 8.37 min and a minor one at 10.56 min; these major and minor peaks are referred to as α-TL glucuronide 1 and 2, respectively.
The concentrations of the urinary vitamin E conjugates were also compared after dividing the patients with diabetes into two groups according to their glycosylated hemoglobin concentration: 6–7.5% (42–58 mmol/mol) HbA1c (i.e., well controlled; n=8) and >7.5% (>58 mmol/mol) HbA1c (poorly controlled; n=24). The results are summarized in Table 2. With the exception of α-CEHC sulfate, the urinary concentrations of the α-tocopherol conjugates were similar in the two groups of patients with type 1 diabetes. The α-TL conjugates in both groups with diabetes remained highly significantly increased compared to the control group, whereas, with the exception of α-CEHC sulfate in the well controlled diabetic group, the concentrations of the α-CEHC conjugates were no longer significantly different from the controls.
Table 2.
Significance of difference between well and poorly controlled groups of diabetic subjects and controls and also between the two diabetic groups.
| 6–7.5 HbA1c% (42–58 mmol/mol)a, n=8 (p compared to controls) | >7.5 HbA1c% (>58 mmol/mol)a, n=24 (p compared to controls) | Interdiabetic pb | |
|---|---|---|---|
| α-TL-glucuronide 1c | 1034±351 (<0.001) | 1119±356 (<0.002) | NS |
| α-TL-glucuronide 2c | 305±86 (<0.002) | 443±91 (0.001) | NS |
| α-TL-sulfate | 110±43 (0.0002) | 68±12 (<0.0001) | NS |
| α-CEHC-glucuronide | 131±40 (NS) | 124±18 (NS) | NS |
| α-CEHC-sulfate | 174±60 (<0.005) | 90±13 (NS) | <0.05 |
Concentrations are given in nmol/mmol creatinine; mean±SEM. NS, not significant.
HbA1c mmol/mol=(HbA1c% − 2.15) × 10.929.
The significance of differences between mean concentrations was determined using the unpaired Student t test for analysis between groups.
Two peaks were observed for the α-TL glucuronide, a major peak eluting at 8.37 min and a minor one at 10.56 these major and minor peaks are referred to as α-TL glucuronide 1 and 2, respectively.
Discussion
The method described in this paper using LC–MS/MS for the direct measurement of the conjugated metabolites of vitamin E is novel as previous methods used gas chromatography–mass spectrometry (GC–MS) and were able to analyze only the free unconjugated metabolites. In addition the method has distinct advantages over previous methods. First it is direct and requires only minimal sample preparation, i.e., a centrifugation step to remove particulate matter. This is in contrast to the method of Pope et al. [9] and Lodge et al. [24] which required long deconjugation, extraction, and derivatization procedures before analysis by GC–MS. In addition because of the intact conjugate and short preparation time there is little risk of artifact formation. In contrast artifactual formation of α-TL from free α-CEHC can occur during the assay of the free metabolites [5,9]. It would have been ideal to use deuterated conjugated vitamin E metabolites as internal standards for this study but this was not possible. Thus type II internal standards (androsterone glucuronide and lithocholic acid sulfate) were used.
We have established and validated a simple, fast, and reproducible assay for directly quantitating the urinary conjugated vitamin E metabolites and shown unequivocally for the first time that conjugates of α-TL were real metabolites and not artifacts of the methodological procedure. Thus it was possible to investigate whether conjugated α-TL could be used as a biomarker of oxidative stress. The hypothesis put forward in this study was that the urinary concentrations of α-TL (sulfate and glucuronide) would be greater in patients with diabetes compared to age-matched controls, with less change in the concentrations of conjugated α-CEHC.
The total urinary concentrations of all the metabolites (sulfate and glucuronide) were found to be greater, by a factor of approximately 8, in the children with diabetes compared to their age-matched controls, with the total concentration of α-TL (glucuronide and sulfate) being approximately 15 times greater and the total α-CEHC (glucuronide and sulfate) being 2 times greater than in the control subjects. The ratio of α-TL:α-CEHC was approximately 7 in the cohort with diabetes compared to 0.9 in the controls. When the individual conjugated metabolites were compared in the two groups, the mean concentrations of the conjugated α-TL metabolites in the patients with diabetes were all highly significantly increased (p<0.001) compared to the controls, whereas the α-CEHC conjugates were not increased to the same degree of significance (p<0.05). This suggests that these results cannot be explained by a nonspecific increase in urinary excretion of the conjugated metabolites. Although plasma concentrations of α-tocopherol were not compared in the two groups, these results suggest an increased metabolism of vitamin E in patients with diabetes compared to controls, with oxidation of α-tocopherol being more prominent than chain shortening. This is compatible with an increase in oxidative stress in diabetes and suggests that conjugated α-TL may be a useful biomarker.
Essentially similar results were obtained when the patients with diabetes were divided on the basis of their glycosylated hemoglobin concentrations into those who were poorly and those who were well controlled. There was also no significant correlation between the concentrations of the α-TL conjugates and the HbA1c levels in all the diabetic patients. There was, therefore, no evidence from this study that the poorly controlled patients had an increased oxidative stress compared to the subjects with better control. To examine this further it will be necessary to follow a larger cohort of patients with diabetes longitudinally and compare urinary concentrations of conjugated α-TL with other well-recognized measures of diabetes control, such as HbA1c and urinary albumin concentrations. The results of this study strengthen the hypothesis put forward by Liebler et al. [25] and Schonfeld et al. [26] that α-TL could be an indicator/biomarker of oxidative stress but the nature of the relationship between clinical severity of diabetes and oxidative stress status requires further investigation.
We have no ready explanation as to why the children with type 1 diabetes excreted increased urinary concentrations of the conjugated vitamin E metabolites compared to the controls. They were not receiving any vitamin E supplementation or pharmacological therapy at the time of the study. We can only speculate that an increase in metabolism of vitamin E and particularly oxidation occurs as a result of the underlying condition. It is, however, of interest that a link between vitamin E and glucose metabolism has been reported [27]. In particular it was found that when mice deficient for the α-tocopherol transport protein (α-TTP) were supplemented with α-tocopherol there was an increase in their glucose tolerance.
The liver, and in particular the hepatic α-TTP, has a key role in discriminating between the various forms of vitamin E by loading α-tocopherol onto very low density lipoprotein (VLDL) and thereby maintaining plasma and tissue α-tocopherol concentrations (see Traber et al. [28]). Presumably those forms of vitamin E including metabolites of α-tocopherol that are not loaded onto VLDL are preferentially excreted.
The concentrations of the glucuronide metabolites were greater than the sulfates in both the cohorts, with the glucuronide:sulfate ratio being approximately 8 in patients with diabetes and 3 in the controls. This was expected as humans have a greater capacity for glucuronidation than sulfation [29] because of a high activity of the hepatic enzyme UDP-glucuronyltransferase [30]. Similar observations of preferential glucuronidation of the vitamin E metabolites were made by Pope when he conducted LC–MS/MS analysis on normal human urine [23].
An increase in the concentrations of other biomarkers of oxidative stress in patients with diabetes has been previously observed by a number of researchers. Thus Dandona et al. [31] observed an approximately fourfold greater concentration of 8-hydroxy-2′-deoxyguanosine in mononuclear cells of patients with diabetes compared to corresponding controls. This difference was statistically significant and demonstrated for the first time greater oxidative damage to DNA in such individuals. Davi et al. [32] were the first group to demonstrate that the increased 8-epi-PGF2α observed in both type 1 and type 2 diabetes patients could be normalized by vitamin E supplementation. Leonhardt et al. [33] had previously reported elevated levels of oxidized LDL and decreased concentrations of RRR-α-tocopherol in the plasma of patients with diabetes compared to healthy controls, making a case for investigating the role of vitamin E metabolites in diabetes. Others have reported reduced concentrations of antioxidants including vitamin E in diabetes (see [10]), and the role of vitamin E and oxidative stress in diabetes has recently been reviewed [34].
To fully validate the use of urinary conjugates of α-TL as a potential biomarker of oxidative stress, it will be necessary to synthesize the deuterated conjugates of α-TL and α-CEHC to use them as internal standards for the assay in place of the current type II internal standards (androsterone glucuronide and lithocholic acid sulfate) and to carry out the following additional studies. First, prospective longitudinal studies of urinary vitamin E metabolites will be required in subjects with diabetes, comparing the excretion of α-TL conjugates with other parameters of diabetic control such as plasma HbA1c and urinary albumin concentrations. Second, urinary concentrations of α-TL conjugates will need to be compared with other biomarkers of oxidative stress such as urinary isoprostanes. Finally, it will be important to investigate the urinary concentrations of α-TL conjugates in other conditions in which oxidative stress has been implicated.
Acknowledgments
G.S. thanks the Child Health Research Appeal Trust and the Szeben-Peto Foundation for financial support. S.M.O'R. was supported by a European Society for Paediatric Endocrinology Clinical Research Training Fellowship. The University College London Fetal Growth Study was supported by a grant from the British Heart Foundation. M.T.D. is funded by Great Ormond Street Hospital Children's Charity.
References
- 1.Simon E.J., Gross C.S., Milhorat A.T. The metabolism of vitamin E. I. The absorption and excretion of d-α-tocopherol-5-methyl-C14succinate. J. Biol. Chem. 1956;221:797–805. [PubMed] [Google Scholar]
- 2.Simon E.J., Eisengart A., Sundheim L., Milhorat A.T. The metabolism of vitamin E. II. Purification and characterisation of urinary metabolites of α-tocopherol. J. Biol. Chem. 1956;221:807–817. [PubMed] [Google Scholar]
- 3.Parker R.S., Sontag T.J., Swanson J.E. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 4.Sontag T.J., Parker R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism: novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 5.Schultz M., Leist M., Petrzika M., Gassmann B., Brigelius Flohe R. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2′-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 1995;62:1527S–15234S. doi: 10.1093/ajcn/62.6.1527S. [DOI] [PubMed] [Google Scholar]
- 6.Traber M.G., Elsner A., Brigelius Flohe R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145–148. doi: 10.1016/s0014-5793(98)01210-1. [DOI] [PubMed] [Google Scholar]
- 7.Pope S.A., Burtin G.E., Clayton P.T., Madge D.J., Muller D.P. New synthesis of (+/-)-alpha-CMBHC and its confirmation as a metabolite of alpha-tocopherol (vitamin E) Bioorg. Med. Chem. 2001;9:1337–1343. doi: 10.1016/s0968-0896(01)00010-4. [DOI] [PubMed] [Google Scholar]
- 8.Pope S.A., Burtin G.E., Clayton P.T., Madge D.J., Muller D.P. Synthesis and analysis of conjugates of the major vitamin E metabolite, alpha-CEHC. Free Radic. Biol. Med. 2002;33:807–817. doi: 10.1016/s0891-5849(02)00974-7. [DOI] [PubMed] [Google Scholar]
- 9.Pope S.A., Clayton P.T., Muller D.P. A new method for the analysis of urinary vitamin E metabolites and the tentative identification of a novel group of compounds. Arch. Biochem. Biophys. 2000;381:8–15. doi: 10.1006/abbi.2000.1950. [DOI] [PubMed] [Google Scholar]
- 10.Rains J.L., Jain S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen E.J., Diamond-Stanic M.K., Marchionne E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011;51:993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krolewski A.S., Kosinski E.J., Warram J.H., Leland O.S., Busick E.J., Asmal A.C. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am. J. Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 13.Berenson G.S., Wattigney W.A., Tracy R.E., Newman W.P., Srinivasan III, Webber S.R. L.S.; et al. Atherosclerosis of the aorta and coronary arteries and cardiovascular risk factors in persons aged 6 to 30 years and studied at necropsy (The Bogalusa Heart Study) Am. J. Cardiol. 1992;70:851–858. doi: 10.1016/0002-9149(92)90726-f. [DOI] [PubMed] [Google Scholar]
- 14.Davi G., Chiarelli F., Santilli F., Pomilio M., Vigneri S., Falco A. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 2003;107:3199–3203. doi: 10.1161/01.CIR.0000074205.17807.D0. [DOI] [PubMed] [Google Scholar]
- 15.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 16.Sharma A., Kharb S., Chugh S.N., Kakkar R., Singh G.P. Evaluation of oxidative stress before and after control of glycemia and after vitamin E supplementation in diabetic patients. Metabolism. 2000;49:160–162. doi: 10.1016/s0026-0495(00)91117-x. [DOI] [PubMed] [Google Scholar]
- 17.Polidori M.C., Mecocci P., Stahl W., Parente B., Cecchetti R., Cherubini A. Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab. Res. Rev. 2000;16:15–19. doi: 10.1002/(sici)1520-7560(200001/02)16:1<15::aid-dmrr71>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Jain S.K., McVie R. Effect of glycemic control, race (white versus black), and duration of diabetes on reduced glutathione content in erythrocytes of diabetic patients. Metabolism. 1994;43:306–309. doi: 10.1016/0026-0495(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 19.Maritim A.C., Sanders R.A., Watkins J.B., III Diabetes, oxidative stress, and antioxidants: a review. J. Biochem. Mol. Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 20.Jain S.K., Levine S.N., Duett J., Hollier B. Reduced vitamin E and increased lipofuscin products in erythrocytes of diabetic rats. Diabetes. 1991;40:1241–1244. doi: 10.2337/diab.40.10.1241. [DOI] [PubMed] [Google Scholar]
- 21.Salonen J.T., Nyyssonen K., Tuomainen T.P., Maenpaa P.H., Korpela H., Kaplan G.A. Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. BMJ. 1995;311:1124–1127. doi: 10.1136/bmj.311.7013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma G., Muller D., O'Riordan S., Bryan S., Hindmarsh P., Dattani M. A novel method for the direct measurement of urinary conjugated metabolites of alpha-tocopherol and its use in diabetes. Mol. Nutr. Food Res. 2010;54:599–600. doi: 10.1002/mnfr.200900378. [DOI] [PubMed] [Google Scholar]
- 23.Pope S.A.S. The analysis and identification of urinary metabolites of vitamin E in man using mass spectrometry and chemical synthesis. University College London; 2001. [Ph.D. thesis]
- 24.Lodge J.K., Traber M.G., Elsner A., Brigelius-Flohe R. A rapid method for the extraction and determination of vitamin E metabolites in human urine. J. Lipid Res. 2000;41:148–154. [PubMed] [Google Scholar]
- 25.Liebler D.J., Burr J.A., Philips L. Gas chromatography–mass spectrometry analysis of vitamin E and its oxidation products. Anal. Biochem. 1996;236:27–34. doi: 10.1006/abio.1996.0127. [DOI] [PubMed] [Google Scholar]
- 26.Schonfeld A., Schultz M., Petrizka M., Gassmann B. A novel metabolite of RRR-tocopherol in human urine. Nahrung/Food. 2006;37:498–500. doi: 10.1002/food.19930370514. [DOI] [PubMed] [Google Scholar]
- 27.Birringer M., Kuhlow D., Pfluger P.T., Landes N., Schulz T.J., Glaubitz M. Improved glucose metabolism in mice lacking alpha-tocopherol transfer protein. Eur. J. Nutr. 2007;46:397–405. doi: 10.1007/s00394-007-0679-2. [DOI] [PubMed] [Google Scholar]
- 28.Traber M.G., Burton G.W., Hamilton R.L. Vitamin E trafficking. Ann. N. Y. Acad. Sci. 2004;1031:1–12. doi: 10.1196/annals.1331.001. [DOI] [PubMed] [Google Scholar]
- 29.Gibson G., Skett P. Blackie Academic and Professional; London/New York: 1994. Introduction to Drug Metabolism. [Google Scholar]
- 30.Mulder G.J. Glucuronidation and its role in regulation of biological activity of drugs. Annu. Rev. Pharmacol. Toxicol. 1992;32:25–49. doi: 10.1146/annurev.pa.32.040192.000325. [DOI] [PubMed] [Google Scholar]
- 31.Dandona P., Thusu K., Cook S., Snyder B., Makowski J., Armstrong D. Oxidative damage to DNA in diabetes mellitus. Lancet. 1996;347:4–287. doi: 10.1016/s0140-6736(96)90013-6. [DOI] [PubMed] [Google Scholar]
- 32.Davi G., Ciabattoni G., Consoli A., Mezzetti A., Falco A., Santarone S. In vivo formation of 8-iso-prostaglandin F2{alpha} and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 33.Leonhardt W., Hanefeld M., Muller G., Hora C., Meissner D., Lattke P. Impact of concentrations of glycated hemoglobin, [alpha]-tocopherol, copper, and manganese on oxidation of low-density lipoproteins in patients with type I diabetes, type II diabetes and control subjects. Clin. Chim. Acta. 1996;254:173–186. doi: 10.1016/0009-8981(96)06384-x. [DOI] [PubMed] [Google Scholar]
- 34.Pazdro R., Burgess J.R. The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 2010;131:276–286. doi: 10.1016/j.mad.2010.03.005. [DOI] [PubMed] [Google Scholar]