Abstract
Lipid peroxidation involves a cascade of reactions in which production of free radicals occurs selectively in the lipid components of cellular membranes. Polyunsaturated fatty acids easily undergo lipid peroxidation chain reactions, which, in turn, lead to the formation of highly reactive electrophilic aldehydes. Among these, the most abundant aldehydes are 4-hydroxy-2-nonenal (HNE) and malondialdehyde, while acrolein is the most reactive. Proteins are susceptible to post-translational modifications caused by aldehydes binding covalently to specific amino acid residues, in a process called Michael adduction, and these types of protein adducts, if not efficiently removed, may be, and generally are, dangerous for cellular homeostasis. In the present review, we focused the discussion on the selective proteins that are identified, by redox proteomics, as selective targets of HNE-modification during the progression and pathogenesis of Alzheimer disease (AD). By comparing results obtained at different stages of the AD, it may be possible to identify key biochemical pathways involved and ideally identify therapeutic targets to prevent, delay or treat AD.
Keywords: Lipid peroxidation, 4-hydroxy-2-nonenal, proteomics, enolase, collapsin regulatory protein 2, ATP synthase, oxidative stress
1. Lipid peroxidation and neurodegeneration
One of the major targets of lipid peroxidation process is the central nervous system (CNS). Indeed, the brain is highly sensitive to oxidative stress because this 1300 g organ consumes about 20–30 % of inspired oxygen, contains high levels of polyunsaturated fatty acids (PUFAs), ideal target of free radical attack, and high levels of redox transition metals. The latter play a crucial role in initiation/propagation of the cascade of reactions that start with the abstraction of an electron from the conjugate double bond system of fatty acid acyl chain. This process leads to the formation of a variety of free radical species, commonly grouped as reactive oxygen species (ROS), with slightly different reactivity. Altogether, ROS are highly unstable and easily react with all macromolecules such as proteins, nucleic acid and lipids. These events are further exacerbated in the brain by the relative inability of neuronal cell to neutralize free radicals due to paucity of both enzymatic and non-antioxidants.
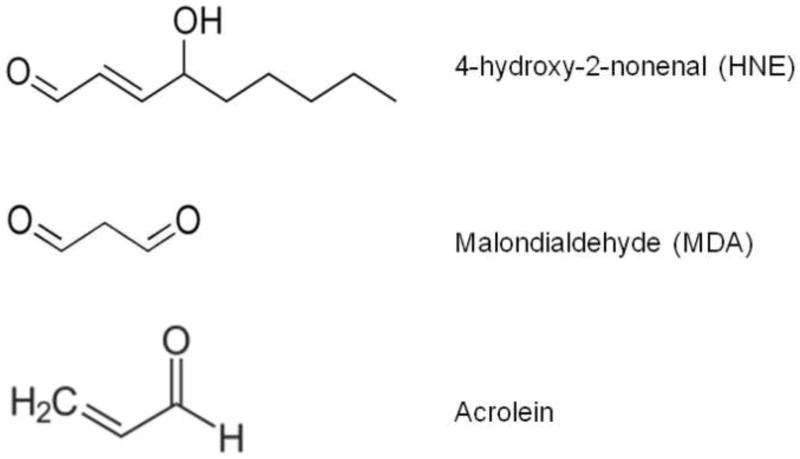
Lipid peroxidation is one of the major sources of free radical-mediated injury that directly damages neuronal membranes and yields a number of secondary products responsible of extensive cellular damage. Free radical attack to PUFAs leads to the formation of highly reactive electrophilic aldehydes, including malondialdehyde (MDA), 4-hydroxy-2-nonenal (HNE), the most abundant products, and acrolein, the most reactive (Figure 1) [1–3]. In addition to aldehyde formation, lipid hydroperoxyl radicals undergo endocyclization to produce fatty acid esters; two classes of these cyclized fatty acids are ispoprostanes and neuroprostanes (Figure 1) [4, 5].
Figure 1.
Chemical structure of 4-hydroxy-2-nonenal (HNE), malondialdehyde (MDA) and acrolein.
Peroxidation of arachidonic acid (AA) leads to the formation F2-isoprostanes (F2-IsoPs), while F4-neuroprostanes (F4-NPs) are the stable product of free radical damage to docosahexanoic acid (DHA). Once formed, F2-NPs and F4-NPs can undergo hydrolysis to free iso- and neuroprostanes that can be measured in body fluids [6], and F2-NPs and F4-NPs can undergo non-enzymatically additional conversions to form isochetals and neurochetals both of which are dangerous to cells [7, 8]
However, cells also are endowed with lipid antioxidants, especially lipid soluble vitamins and glutathione, glutathione-S-transferases, one isoform of glutathione peroxidase, and beta-alanyl-L-histidine, which can quench lipid oxidants including HNE. In addition, albumin and apolipoproteins in plasma can bind and buffer HNE. However, a specific repair process of lipid peroxidation does not exist as it is for proteins and DNA and this may explain why moderate levels of lipid peroxidation could have physiological significance for cell signaling and membrane remodeling [9].
Peroxidation of membrane lipids affects a variety of functions resulting in increased membrane rigidity, decreased activity of membrane-bound enzymes (e.g., sodium pump), impairment of membrane receptors and altered permeability [10, 11]. In addition to damage to phospholipids, radicals also can directly attack membrane proteins and induce lipid-protein and protein-protein crosslinking, all of which contribute to altered membrane integrity [12]. It is reasonable to hypothesize that perturbation of all the above-mentioned functions displayed by PUFAs and its metabolites, together with modification of proteins, affect neuronal homeostasis thus contributing to brain dysfunction.
The role of free radical mediated oxidative damage in the pathogenesis of neurodegenerative disorders has been firmly established [13–17]. In particular, markers of lipid peroxidation have been found to be elevated in brain tissues and body fluids in several neurodegenerative diseases, including Alzheimer disease (AD), Parkinson disease (PD), amyotrophic lateral sclerosis (ALS), Huntington disease (HD) and Down syndrome (DS) [17–21]. Consonant with these findings, several reports have documented increased levels of reactive products of lipid peroxidation in diseased regions of brain [17, 22, 23], but generally not in regions uninvolved in the disease [17]. This review focuses on the role of lipid peroxidation in brain of subjects at various stages of AD.
2. The chemistry of lipid peroxidation: focus on HNE
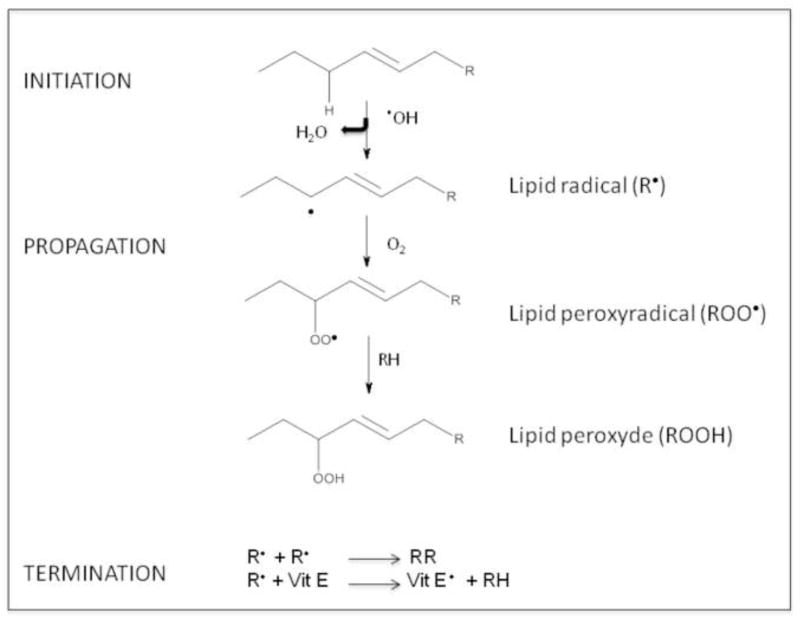
Lipid peroxidation involves a cascade of reactions which cause the degradation of lipids commonly described as a 5-step sequence (Figure 2).
Figure 2.
Lipid peroxidation process.
Step 1: Initiation, in which the free radical (hydroxyl HO•, alkoxyl RO•, peroxyl ROO•, and possibly HO2• but not H2O2 or O2−•) abstracts an allylic H from a methylene group in the acyl chain of phospholipids, followed by rearrangement of the double bonds to the conjugated diene form, and simultaneously producing a carbon-centered alkyl radical. Step 2: A peroxyl radical is produced when the alkyl radical reacts with paramagnetic molecular oxygen;
Step 3: Propagation, in which the peroxyl radical abstracts another allylic H atom to inititate a self-perpetuating chain reaction that ultimately lead to a variety of cyclic peroxides and hydroperoxides. These latter can be further degraded to hydrocarbons, alcohols, ether, epoxides, and aldehydes. Among these by-products, MDA, HNE and acrolein can cause irreversible modification of phospholipids, proteins, and DNA [24]; Step 4: Termination, by which different types of radicals react each other leading to formation of stable products; or
Step 5: Termination, by which reactions between the radicals and antioxidants give rise to non-radical products or unreactive radicals. Both exogenous and endogenous antioxidants such as vitamin E and vitamin C prevent the propagation of lipid peroxidation at the early stages of free radical attack [25, 26]. Vitamin E (α-tocopherol) is a “chain-breaking” antioxidant; when the allylic hydrogen is abstracted in step 1, and α-tocopherol radical forms (step 5), the tocopherol radical can be reverted back to vitamin E by the vitamin C (ascorbic acid) and glutathione. The protective effects exerted by antioxidant towards HNE and other toxic aldehydes have been investigated by many groups to test the possibility of a therapeutic use of free radical scavengers and antioxidants against lipid peroxidation-mediated toxicity. In additions to small molecules, antioxidant enzymes such as heme oxygenase-1 (HO-1), catalase, superoxide dismutase, peroxiredoxin, and glutathione reductase have been shown to lead to a significant decrease in lipid peroxidation products [27, 28].
2.1 4-hydroxy 2-trans nonenal (HNE)
Among different types of lipid peroxidation by-products, the best characterized, mostly for its toxic role, is HNE. This alkenal is an α,β-unsaturated aldehyde that is formed by peroxidation of ω–6 polyunsaturated fatty acids such as linoleic acid, linolenic acid and mostly arachidonic acid (AA). Althoguh HNE is produced by non-enzymatic processes [29], Esterbauer’s group demonstrated that the formation of HNE from AA is greater in the presence of NADPH-dependent microsomal enzymes [30]. Degradation of PUFAs to HNE is further accelerated in the presence of iron ions. The cascade of reactions initiates with formation of a lipid peroxyl radical [31], which is further oxidized to a lipid peroxide. The C–O bond is broken by a hydration reaction, resulting in a 9-carbon alkenal, namely 4-hydroxy-2- nonenal (HNE). Once formed, HNE is highly reactive and can easily attach to proteins by Michael addition to Cys, His, and Lys residues [32–34]. HNE is not only a potent electrophile reacting with a variety of nucleophilic compounds, but also may act as a stress signaling molecule [1]. The concentration of HNE within cells may vary from 10 μM to 5 mM and causes a wide range of biological activities, including the suppression of basal and inducible NFkB activity [35], disruption of ions homeostasis such as Ca2+, impairment of Na+/K+ ATPase activity and activation of caspase pathways [1, 36, 37]. HNE can cause an impairment of glucose transport in cultured rat hippocampal neurons and an alteration of the glutamate transport in rat neocortical synaptosomes [37]. Therefore, uncontrolled and/or excessive production of HNE could interfere with normal cellular signaling and lead to development of pathological conditions as occur in several neurodegenerative diseases [17, 32, 36].
2.2 HNE-protein adducts
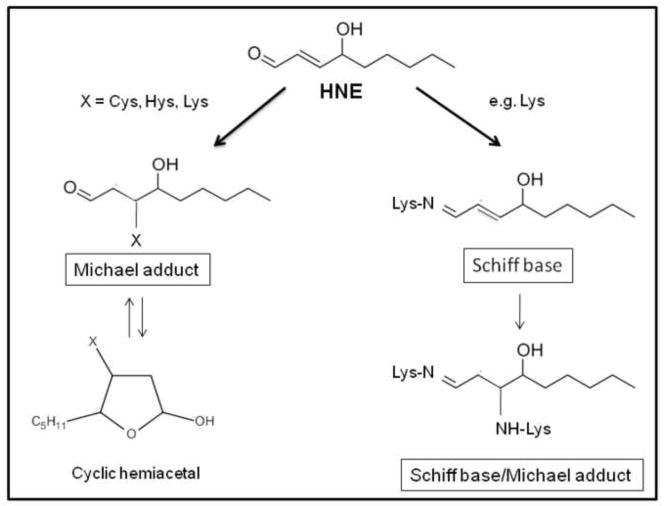
HNE is an amphiphilic compound, with both water-soluble and lipophilic properties. Since it has stronger hydrophobic nature, it is mostly associated with the membranes where it is produced but it can also diffuse to different cellular compartments and interact with many different substrates [38]. The high reactivity of HNE is due to its chemical structure, where the presence of three functional groups potentiates its electrophilic properties [39]. Indeed, a conjugated system of a C=C double bond and a CO carbonyl group provide a partial positive charge to carbon 3. This positive charge is further increased by the inductive effect of the hydroxy group at carbon 4. Therefore, nucleophilic attack, for example, by thiol or amino groups, occurs primarily at carbon 3 and second at the carbonyl carbon 1 [38, 40]..
It is not surprising that proteins are particularly vulnerable to HNE-induced modification [41, 42]. HNE forms adducts with three different side chains amino acids, namely Cys, His, and Lys via Michael addition (Figure 3), either with thiol (-SH) and amino (-NH2) groups of these amino acids. Cys residues displayed the highest reactivity in the following order: Cys>His>Lys. However, Cys residues are not always the preferential targets of HNE in proteins. The degree of reactivity mostly depend on the tertiary structure of the protein, that is the accessibility, and therefore reactivity, of amino acid residues towards exogenous chemicals. No reaction of HNE was detected with Glu [43]. Several proteins have been reported to be modified by HNE including: plasma membrane ion and nutrient transporters; receptors for growth factors and neurotransmitters; mitochondrial electron transport chain proteins; protein chaperones; proteasomal proteins; and cytoskeletal proteins [40, 44].
Figure 3.
HNE reacts with proteins either via Michael adduct, Schiff base formation or both.
Low levels of HNE-modification is sufficient to increase susceptibility of proteins to proteolysis and removal by the proteasomal system. But, while under normal conditions the proteasomal system is able to remove the majority of oxidatively damaged and modified proteins, under severe oxidative stress conditions accumulation of modified proteins occurs. This could take place either because of protein cross-linking or impairment of the aldehyde reductase, aldehyde oxidase and/or proteolytic machinery of the cell.
Interestingly, HNE can also react directly with amyloid beta-peptide (Aβ), the major component of senile plaques, a pathological hallmark of AD. This process is reported to affect formation of oligomers and to exacerbate Aβ aggregation and toxicity, which in turn causes oxidative stress, even more lipid peroxidation products, such as HNE, and more toxic Aβ oligomers [45]. Interestingly, in AD brain a protein involved in removing Aβ, LRP-1, is also covalently modified by HNE, and the consequent dysfunction of this efflux protein likely contributes to parenchymal accumulation of this neurotoxic peptide [46].
Cells possess different mechanisms to detoxify HNE and thereby prevent its damaging toxic actions. Glutathione (GSH) is the most powerful and it has the ability to rapidly bind HNE through its Cys residue. In addition, glutathione-S-transferases, together with the multidrug resistant protein protein (MRP1), contribute to regulate the intracellular level of HNE. The latter catalyzes the export the GSH conjugate of HNE out of neurons [47]. The dipeptide carnosine (β-alanyl-L-histidine) also can quench HNE via intra-molecular Michael addition [48].
Another major metabolic pathway for aldehyde detoxification is oxidation or reduction of the aldehyde to its corresponding acid or alcohol, respectively, by aldo-keto oxidoreductases [49]. Aldehyde oxidation to the corresponding acid is catalyzed by aldehyde dehydrogenases (ALDH; EC 1.2.1.3). Class 1 and 2 ALDH are NAD+ requiring enzymes that metabolize HNE to its corresponding acid, 4-hydroxy-(2)-nonenoic acid, while, class 3 ALDH utilizes either NAD+ or NADP+ as cofactors in vitro and does not metabolize HNE [50]. A decreased activity of ALDH2 has been reported in AD brain [51], and it may contribute for increased accumulation of toxic aldehydes.
Aldehyde reduction to the corresponding alcohol is catalyzed by members of the aldo-keto reductase superfamily (AKR), aldehyde reductase (AKR1), and aldose reductase (AKR1B1), as well as by 3 classes of alcohol dehydrogenases (AD). Aldehyde reductase is approximately 3-fold more active towards HNE than aldose reductase although both metabolize acrolein at the same rate [52]. Carbonyl reductase is a member of this enzyme family and it can reduce HNE [53]. Interestingly, carbonyl reductase is one the proteins found to be oxidatively modified in amnestic MCI brain by redox proteomics studies from our laboratory [54].
Other proteins expressed at high levels within or outside of cells, such as albumin [55] and apolipoprotein, may also play important roles in binding and thereby quenching HNE.
3. Redox proteomics: identification of HNE-modified proteins
In the last decade, development of new proteomics platforms has been a powerful tool to investigate the alteration of the proteome profile associated with a disease state. Thus, expression of specific proteins is often altered in disease conditions, and proteomics analysis is essential to help decipher biological processes and phenotyes of both normal and diseased cells. These differences become particularly intriguing when they are associated with a disease process. In addition to variation of protein expression levels, the activity of proteins is crucially regulated by post-translational modifications, including acetylation, phosphorylation, methylation, glutathionylation, among others. These modifications are reversible and are fundamental to regulate normal cellular functions. However, this tight regulation may be perturbed by a different set of post translational modifications which lead to often irreversible protein modifications, mostly with dangerous effects. Among these, oxidative modifications have been extensively investigated mostly in neurodegenerative diseases, a condition where increased oxidative stress is a constant treat for protein homeostasis. Oxidation results in impaired protein function and accumulation of oxidized proteins is a characteristic hallmark of AD [15, 16, 56] and other neurodegenerative diseases [9].
Redox proteomics, arguably pioneered in the Butterfield laboratory [57–60], is the branch of proteomics that leads the identification of oxidatively modified proteins, most often by coupling two-dimensional polyacrylamide gel electrophoresis (2D-PAGE), Western blot analyses and mass spectrometry (MS) [58]. Despite the intrinsic limitations of 2D-PAGE, as for example the challenges involved in studying membrane proteins, it is still the most performed separation tool when dealing with huge number of proteins. Oxidative stress response activates different signaling pathways which are responsible of different footprints in the cell, including oxidative modifications of proteins. Furthermore, the resolving power of proteomics allows identifying even the target of these oxidatively modified proteins. The study of the “redox proteome” is crucial to decipher such modifications occurring in “stressed cells” and relating oxidatively modified proteins to the clinical presentation and to the pathology of disease states.
The most common and abundant types of oxidative modifications to proteins are protein carbonylation, 3-nitrotyrosine formation, binding of HNE and glutathionylation. Redox proteomics can be applied to study all the above-mentioned modifications and many studies have been performed in our laboratory by following this approach. However, other non-gel based approaches that utilize liquid or affinity chromatography in combination with MS have also been developed for these modifications by other groups [58].
5. Alzheimer Disease
AD is the most common form of dementia in the elderly, characterized by neuronal degeneration in selective brain regions involved in cognition (hippocampus, entorhinal and frontal cortex) and emotional behaviors (amygdala, prefrontal cortex, hypothalamus). The major pathological hallmarks of the disease are deposition of extracellular senile plaques (SP) and intracellular neurofibrillary tangles (NFTs) and loss of synapses [61, 62]. The core of SP contains mostly Aβ, a 40–42 amino acid peptide that derives from the proteolysis (beta- and gamma-secretases) of the amyloid precursor protein (APP), surrounded by degenerating neuritis; NFTs are composed of aggregated hyperphosphorylated tau protein [63, 64].
Due to the progressive nature of AD, it is possible to define at least three stages: mild cognitive impairment (MCI), early-stage Alzheimer disease (EAD), and late-stage Alzheimer disease (LAD). Patients are usually diagnosed on the basis of the severity of symptoms during the transition into each progressing stage. Braak staging (score) characterizes the severity of the disease by assessing the distribution of NFTs and neurophil threads; there are six levels of Braak staging (the higher the stage, the greater the severity of AD).
Some patients showed a presymptomatic phase before MCI, namely preclinical Alzheimer disease (PCAD), characterized by absence of memory deficits and normal activities of daily living but pronounced AD neuropathology (Braak scores are III or higher) [65, 66]. It is difficult to study PCAD brain due to limits in collecting samples; thus experimental data on these subjects are lacking and will likely be available in the next future. However, studies from our laboratory showed proteomics changes in brain from subjects with PCAD [67], and in the transition from PCAD to amnestic MCI, including identification of carbonylated proteins that conceivably may be involved in memory loss of amnestic MCI compared to PCAD individuals [68].
More results have been obtained from subjects with amnestic MCI, which is considered a prodromal phase of AD, though not all AD patients have been previously diagnosed with MCI. MCI patients can be classified as having amnestic (memory-affecting) MCI or non amnestic MCI [69, 70]. Braak scores for MCI is typically III or IV, with deposition of NFTs detectable in the hippocampus and neocortex. Pathologically, amnestic MCI subjects shows mild degeneration of the hippocampus, entorhinal cortex, sulci, and gyri as evaluated by using magnetic resonance imaging technology (MRI) [71], while early and late stage AD patients show considerably greater loss in these areas [72]. It is estimated that the rate of conversion of amnestic MCI to AD is roughly 10–15% per year [73]; however, in some cases MCI individuals can revert to normal [70].
EAD, an intermediate stage between MCI and LAD, is characterized by increased frontal lobe atrophy, ventricular widening with progressive brain deterioration (Braak scores are between IV and V). Accordingly, these patients show decreased hippocampal volume which correlates with memory decline. There is a significant increase in the neurofibrillary tangle load in EAD subjects compared to MCI subjects in both the frontal and the temporal lobes [74], which correlates with impairments in verbal abilities, visuo-spatial functions, attention and executive functions. Similar to PCAD, experimental evidences on EAD are still limited because sample availability is quite rare.
LAD is the final stage of the disease: memory loss, dementia, behavioral changes are evident and significantly compromise all the activities of daily living. Braak score for these subjects is approximately IV–VI, as NFTs already caused substantial neuronal death in the hippocampus and neocortex. Accordingly, synapse loss, Aβ accumulation, and SP are profound [75]. Markers of oxidative stress including DNA oxidation, protein oxidation and lipid peroxidation are significantly higher in these patients [76–80]. In contrast, br rain levels of the antioxidant enzymes such as catalase, superoxide dismutase, glutathione S-transferase, and glutathione reductase are significantly decreased in LAD subjects.
5.1. Lipid Peroxidation in AD
In AD brain increased lipid peroxidation has been identified by measuring the levels of free and protein-bound HNE, F2-IsoP, F4-NP, isoprostane 8,12-iso-iPF2α-VI, malondialdehyde (MDA) and acrolein, [81–83]. Further, studies from our laboratory and other showed increased levels of lipid peroxidation markers such as thiobarbituric acid reactive substances, MDA, F2-IsoP, F4-NP, and protein-bound HNE also in subjects with MCI [82, 84, 85], suggesting that lipid peroxidation is an early event in the progression of AD. Pratico et al. showed an increased levels of the isoprostane, 8,12-iso-iPF2 α-VI in CSF in AD [86] and MCI [87] and suggested that this lipid peroxidation product conceivably could be used as a marker to identify MCI patients who are at increased risk to progress to symptomatic AD.
Lipid peroxidation products as mentioned above reacts with biomolecules resulting in the direct oxidation of amino acids, glycooxidation and lipoxidation in AD brain as indexed by the presence and concentrations of glutamic and aminoadipic semialdehydes, Nε-(carboxymethyl)-lysine, Nε-(carboxyethyl)-lysine, and Nε-(malondialdehyde)-lysine [88]. Pamplona et al., (2005) using redox proteomics approach found neurofilament L, alpha-tubulin, glial fibrillary acidic protein, ubiquinol-cytochrome c reductase complex protein I, and the beta-chain of ATP synthase as targets of Nε-(malondialdehyde)-lysine formation that correlated with increased levels of docosahexaenoic acid. Further, acrolein has been reported to react with DNA bases such as guanine to lead to increased formation of acrolein-deoxyguanosine in AD brain [89]. Quinn et al., (2004) showed elevated levels of F2-IsoPs, in CSF from AD patients [90]; further, these researchers showed that the patients receiving alpha-tocopherol and vitamin C had lower levels of CSF F2-IsoPs, suggesting that antioxidants may be a promising therapeutic strategy to treat AD [90].
5.2. HNE-Modified Common Proteins at Different Stages of AD
The presence of increased lipid peroxidation in different stages of this dementing disorder underscores the crucial role of this process in AD pathogenesis and progression. Redox proteomic studies led to the identification of a number of oxidatively modified proteins in AD and MCI brains that play important roles in regulating energy metabolism, cellular signaling, pH regulation, neuronal communication, antioxidant and detoxification, neurotransmitter regulation, tau hyperphosphorylation, and APP processing. These redox proteomics-identified brain proteins are consistent with the biochemical, histopathological and cognitive dysfunctions reported in AD [131; 132]. Table I shows the listing of proteins that have elevated HNE- modification in AD, EAD and MCI, compared to age-matched controls. In this review, we discuss enolase, hemeoxygenase 1 (HO-1), Collapsin Response Mediator Protein 2 regulatory protein-2 (CRMP2), and ATP synthase alpha, which are selectively modified during AD progression and contribute to illumination of the molecular events that drive neurodegenerative phenomena. These proteins are discussed in light of their role in AD pathogenesis and progression. It is reasonable to hypothesize that HNE modification may be not a random event but occurs on specific proteins, which, in turn, display altered functions. The biological effects of such modifications are also discussed.
Table I.
HNE-modified brain proteins identified in amnestic MCI, Early AD, and late-stage AD. Proteins highlighted in red are common targets of HNE modifications at different stages of AD.
| AD stage | HNE-Modified Proteins | Function | References |
|---|---|---|---|
| MCI | Neuropolypeptide h3 | Neuronal communication | 76, 109 |
| Collapsin response mediated protein 2 | Neuronal communication | ||
| Lactate dehydrogenase B | Energy metabolism | ||
| Phosphoglycerate kinase | Energy metabolism | ||
| Heat shock protein 70 | Stress response | ||
| carbonyl reductase | Antioxidant | ||
| ATP synthase alpha | Energy metabolism/mitochondrial function | ||
| Beta-actin | Cytoskeletal integrity | ||
| Alpha enolase | Energy metabolism | ||
| Pyruvate kinase | Energy metabolism | ||
| Eukaryotic Initiation factor alpha | Protein synthesis | ||
| Elongation factor-Tu | Protein synthesis | ||
| Hemeoxygenase 1 | Antioxidant | ||
| EAD | Manganese superoxide dismutase | antioxidant defense | 75 |
| Collapsin regulatory protien 2 | Neuronal communication and neurite outgrowth | ||
| Alpha-enolase | Energy metabolism | ||
| Malate dehydrogenase | Energy metabolism | ||
| Triosephosphate isomerase | Energy metabolism | ||
| F1 ATPase, alpha subunit | Energy metabolism | ||
| LAD | Aconitase | Energy metabolism | 77,109 |
| Aldolase | Energy metabolism | ||
| Peroxiredoxin VI | Antioxidant defense | ||
| Alpha-tubulin | Cytoskeletal integrity | ||
| Alpha-enolase | Energy metabolism | ||
| ATP synthase alpha | Energy metabolism/mitochondrial function | ||
| Glutamine synthase | Excitotoxicity | ||
| Superoxide dismutase 1 | Antioxidant | ||
| Collapsin regulatory protein 2 | Neuronal communication | ||
| Hemeoxygenase 1 | Antioxidant |
5.2.1. Enolase
Our redox proteomics studies performed on post mortem brain from annestic MCI, EAD and LAD subjects identified HNE-modified enolase in all the three stages of the disease. We analysed different brain regions, including the inferior parietal lobule, hippocampus, and frontal cortex, and also cerebellum, a brain region essentially devoid of pathology in AD. Compared with controls, the isoforms, α- and γ-enolase, were found to be excessively carbonylated [74, 91, 92], nitrated [93–95], HNE-modified [54, 96], and S-glutathionylated [97]. In addition to our findings, other studies reported α-enolase as one of the most frequently identified differentially expressed proteins in the brain from both human and animal studies [98].
Our results may raise the question whether or not oxidative modification is a specific modification involved in AD or simply a result of its structural susceptibility to oxidation. Further, enolase can be found in different regions of the cell in close proximity with redox reaction centers where its many active -Lys and -His residues can undergo oxidative modifications. To address this question, it is important to recall that one of the striking metabolic features of AD is the drastic reduction of ATP synthesis and glucose metabolism [99]. Typical findings in AD-like dementia include a significant, bilateral reduction in temporal and parietal glucose metabolism, as shown by Positron emission tomography (PET) studies. PET imaging with 2-[18F] fluoro-2-deoxy-D-glucose (FDG) as the tracer has long been used to track AD-related brain changes by providing qualitative and quantitative estimates of the cerebral metabolic rate of glucose. Recent evidence suggests that altered glucose metabolism is a very early change in AD and is an excellent correlate of the clinical disabilities in dementia [100]. At the molecular level, metabolic changes are intimately linked to glucose consumption and oxidative phosphorylation. In response to hypometabolism, up-regulation of glycolytic enzymes occurs to combat the mounting energy deficit and hypoxic environment [99]. Interestingly, in all studies of MCI, EAD, and LAD brain from our laboratory, enolase levels were increased [101]. In addition to up-regulation, we also showed that enolase was among the few proteins, glycolytic or otherwise, consistently oxidatively modified in the progression from MCI to LAD.
We suggest that oxidative modification and dysfunction of α-enolase disrupts neuronal energy metabolism and ATP-dependent ion homeostasis including functions of membrane ion-motive ATPases, glucose and glutamate transporters, loss of membrane asymmetry and signal transduction. Such oxidative and metabolic compromise may thereby render neurons vulnerable to excitotoxicity and apoptosis.
Recent studies demonstrated that enolase is not simply a glycolityc enzyme but also possesses a variety of different regulatory properties [102, 103]. In particular, enolase has been reported to be a neurotrophic factor, 14-3-2 [104, 105], a hypoxic stress protein [106], c-Myc binding protein and transcription factor [107] and a strong plasminogen (PGn) binding protein [108, 109] among others (Table II). This wide array of functions can be attributed to different DNA base sequences within enolase genes.
Table II.
List of different functions of enolase and their possible implications in disease.
| Enolase functional diversity | Disease |
| GLYCOLYSIS/GLUCONEOGENESIS | Various human diseases(cancer, neurological disease, autoimmunity, etc); |
| C-MYC BINDING PROTEIN | Tumar formation/Tumor marker; |
| PLASMINOGEN BINDING PROTEIN | Various human diseases(cancer, neurological disease, autoimmunity, etc); |
| HEAT-SHOCK PROTEIN | Saccharomyces cervesiae |
| IMMUNODOMINENT ANTIGEN | Candida infection |
| NEUROTROPHIC FACTOR | Activation of pro-survival ERK1/2 |
- = unknown function
5.2.1.1 Enolase, the plasminogen system and Amyloid β
The plasminogen system (PGn) is essential for maintenance of vascular potency and thrombolysis, by dissolving fibrin [110]. In order to exert its function, the glycoprotein PGn binds cell surface receptors via domains that recognize exposed C-terminal lysine residues. Therefore, virtually any surface protein exposing C-terminal lysines has the potential to bind and activate PGn processes. Enolase has frequently been reported as a strong PGn binding protein within the brain and is able to integrate into the cell membrane, although without possessing a specific signal sequence [109]. However, binding α-enolase alone cannot activate PGn conversion to plasmin; the PGn proteolytic cascade must begin with cleavage by either tissue-PGn activator (tPA) or urokinase-PGn activator (uPA) [111], both of which, can be found in human brain [112]. For example, when microglial and/or neuronal PGn binds membrane integrated enolase, PGn is rapidly activated through tPA proteolytic cleavage. Consequent production of plasmin activates a number of proenzymes, prohormones, progrowth factors, and procytokines as a result of the catalytic amplification of tPA/PGn signaling [113]. Moreover, binding enolase protects plasmin from inactivation from inhibitors, like α2-antiplasmin [111]. Therefore, it can be speculated that during AD progression, when excitotoxic events occur, the up-regulation of enolase may initially be an attempt to propagate neuronal-preservation pathways, that ultimately go amiss.
As it is well known, Aβ(1–42) has the ability to aggregate into fibrils in a β-sheet conformation, similar to the cross-beta-structure that fibrin peptides adopt during fibrinolysis [114]. Due to these structural similarities, which do not reflect sequence similarity, Aβ(1–42) can bind and activate tPA through its aggregated β-sheet structure, thereby substituting for fibrin in PGn activation by tPA, in the brain, where fibrin is not present [115]. However, through tPA cleavage of PGn, activated plasmin can degrade oligomeric and fibrillar Aβ, effectively blocking Aβ neuronal toxicity. Van Nostrand and Porter [116] further demonstrated that plasmin cleavage yields an N-terminal truncated form of Aβ with altered β-sheet properties that enhanced stimulation of tPA activity in a positive feedback-loop manner.
We suggest that the multifaceted roles of enolase in AD can be described in the following model, in which concomitant up-regualtion and oxidative modification of enolase occur. From one side, the upregulation and membrane integration of α-enolase, promotes surface-binding of the tPA/PGn complex, which results in the production of the protease plasmin. Plasmin, in turn, has the ability to degrade Aβ peptides associated with the bilayer and can also activate the MAPK/MEK/ERK1/2 pathway, promoting up-regulation of enolase transcription. This pathway would lead to increased production of enolase and would catalytically amplify an internal signal for cell survival during AD progression. However, in contradiction, enolase, once oxidized, becomes unable to facilitate the initiation of survival pathways, which would lead to the neuronal death in brain of subjects with MCI, EAD and LAD versus normal aged brain.
5.2.2. Hemeoxygenase-1 (HO-1)
Among the proteins we found to be HNE-modified in brains of subjects with amnesticMCI and AD compared with control brain, was heme oxygenase-1 (HO-1). Under conditions of oxidative stress the brain reacts by upregulating genes involved in cell stress response to counteract neuronal damage [117]. Indeed, activation of HO-1 is one of the earlier events in AD and plays a crucial role in the adaptive response to stress [118].
HO is a microsomal enzyme that exists in two isoforms: the inducible HO-1 and the constitutive HO-2 [119]. HO-1, also known as heat shock protein-32 (HSP32), is induced by various stimuli, including ROS/RNS, ischemia, heat shock, bacterial lipopolysaccharide and is primarily involved in the cell stress response. Conversely, HO-2 is involved in the physiological turnover of heme and is responsive to developmental factors and adrenal glucocorticoids [119, 120]. HO-1 is the rate-limiting enzyme in the production of bilirubin and catalyzes the degradation of heme in a multistep, energy-dependent way, resulting in equimolar amounts of carbon monoxide, ferrous iron, and biliverdin-IXα. Biliverdin-IXα is further reduced by the cytosolic enzyme biliverdin reductase-A (BVR-A) to bilirubin-IXα, the final product of heme catabolism and a potent antioxidant [121]. The activity of both HO-1 and BVR-A are regulated by the phosphorylation of serine/threonine/tyrosine residues [122]. In the CNS, HO-2 is ubiquitous in almost all brain areas [119], whereas the HO-1 is present at low levels in scattered groups of neurons [120] and also found in glial cells, where its expression can be induced by oxidative stress [123].
Increasing evidence suggests that the HO-1 gene is redox-regulated and contains in its promoter region the antioxidant responsive element (ARE), similar to other antioxidant enzymes. Since the expression of HSPs is closely related to that of amyloid precursor protein (APP), this family has been studied in brains of patients with AD [124]. Increased levels of HO-1 have been observed in association with neurofibrillary tangles and co-localized with senile plaques and glial fibrillary acidic protein-positive astrocytes in AD brains [124, 125]. It is conceivable that the high increase in HO-1 in AD may be a direct response to increased free heme associated with neurodegeneration and an attempt to convert the highly damaging heme into the antioxidants biliverdin and bilirubin.
Recent studies raised questions about the activation of the HO-1/BVR-A system in neurodegenerative disorders, opening a debate on its pathophysiological and clinical significance. Despite an upregulation of the HO-1/BVR-A system, a substantial protection against oxidative and nitrosative stress is not observed in AD brain. Furthermore, Hui et al. have recently proposed that excessive iron production mediated by HO-1 overexpression may be responsible of increased tau aggregation [126]. In addition, Schipper et al. showed that suppression of glial HO-1 hyperactivity may be an effective neurotherapeutic intervention in AD [127].
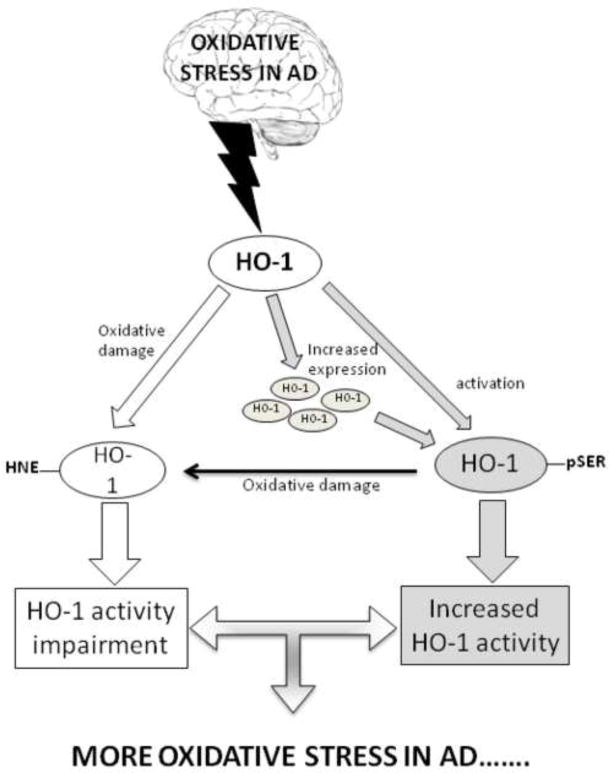
In this complex scenario, we have analyzed, in addition to expression levels, post translational modification of both HO-1 and BVR-A, which are crucial to regulate protein function, either neuroprotection or metabolic activity. In agreement with previous findings [128], we found increased levels of HO-1 in the hippocampus of AD subjects, whereas HO-2 protein levels were significantly decreased in both AD and MCI hippocampus. In addition, significant increases in Ser-residue phosphorylation together with increased HNE-modification of HO-1 were found in the hippocampus of AD subjects [129]. Because HO-1 is a stress-inducible protein, and phosphorylation on Ser residues seems to be important for its activation, the increase in oxidative stress levels in the hippocampus of AD subjects could lead to an increase in HO-1 protein levels and phosphorylation to promote its activity and its interaction with BVR [130]. At the same time, the increased oxidative stress results in HNE/HO-1 adduct formation, leading to altered protein structure and function impairment. With regard to amnestic MCI, levels of HO-1 protein did not show significant differences [131], while the formation of HNE adducts on HO-1 was already evident in the hippocampus of subjects with amnestic MCI compared with controls.
Based on our findings we propose the following scenario: (1) Increased oxidative stress conditions in the hippocampus of AD subjects promote the increase in HO-1 oxidative damage (HNE adducts on its structure). Consequently, the cell tries to restore the functionality of HO-1 by increasing Ser-residue phosphorylation. (2) Increased oxidative stress results in increased Ser phosphorylation to activate protein function, then HO-1 quickly becomes a target of oxidative posttranslational modifications, which in turn could impair its function [129].
Considering the importance of this defense mechanism in neuronal homeostasis, the impairment of HO-1 function as a consequence of oxidative modification, may leave neurons more susceptible to toxic stimuli and eventually to cell death. In addition, since oxidative modification of HO-1 occurs already in AD pathogenesis, i.e. amnestic MCI subjects, and continue to be robust in AD patients, oxidative damage to HO-1 may represent a putative marker of disease progression.
5.2.3. Collapsin Response Mediator Protein 2 (CRMP2)
CRMP2, also known as dihydropyrimidinase-related protein 2 (DRP2), is a ~62 to 75 kDa protein that plays an important role in cytoskeletal organization, membrane trafficking, axonogenesis, axon outgrowth, and neuronal polarity [132–134]. CRMP2 was found to be HNE modified in both MCI and AD brain [54, 96]. Hence, the oxidative modification of CRMP2 might play an important role in shortening of axons thereby impairing neuronal communication and as discussed below might also be a key contributor in the development of neuropathological hallmarks of AD, such as NFTs, and loss of synapses. Recent research suggests loss of synapses is an early feature of AD [61, 135]. Therefore, the oxidative modification of CRMP2 might be a driving force in the progression of AD.
CRMP2 is phosphorylated by kinases such as glycogen synthase kinase-3β (GSK-3β) [136], cyclin-dependent kinase 5 (cdk5) [136], Rho kinase [137], calmodulin dependent protein kinase II (CaMKII) [138] and the src family kinase Fyn [139]. Both CDK5 GSK-3β levels and activity were found to be altered in AD and MCI brain [140–142]. Phosphorylation of CRMP2 at Ser-522 and Thr-509/514 by CDK5, and GSK3β respectively, reduces its ability to interact with tubulin and consequently reduced stability of microtubule and axonal retraction [143, 144]. Hyperphosphorylated CRMP2 proteins co-exist with NFT, though the reasons for this are unclear [145, 146], we hypothesized that amyloid beta-peptide-induced oxidation of the regulatory protein, Pin1[74, 92, 147], leads to dysregulation of the activities of CDK5, and GSK3β and protein phosphotase 2A, leading to increased phosphorylation of tau protein thereby inhibiting tau function. Such changes could lead to impaired axonal transport. Inhibition of axonal anterograde and retrograde transport deprives synapses of energy-producting mitochondria, which likely contribute to synapse loss. This, in turn, may act as signal to recruit CRMP2 to axonal sites to promote neuronal sprouting and synapse formation. That CDK5 and GSK3 β are already recruited to this site might lead to hyperophosphorylation of CRMP2 protein consequently to the loss of synapses, an early feature in AD pathogenesis.
Apart from tubulin, CRMP2 protein also interacts with various other proteins including: cytoskeletal proteins actin, vimentin, the Ca +-binding protein calmodulin (CaM) [148], N-methyl-D-aspartate receptors (NMDARs) subunits NR2A/2B [149], and N-type voltage-gated calcium channel (CaV2.2) [150], thereby playing an important role in endocytosis, vesicle recycling, synaptic assembly, calcium channel regulation, Ca+2 homeostasis, and neurotransmitter release [151]. Hence, oxidative modification of CRMP2 is consistent with altered neuronal functions as reported in MCI and AD [54, 96].
A recent study showed that in addition to the phospho-CRMP2 colocaliztion with neurofibrillary tangles (NFTs), another protein, the Wiskott-Aldrich syndrome protein family verprolin-homologous protein 1 (WAVE1) is also found at this site. WAVE1 is important for actin assembly at the distal end and thereby important in neurite outgrowth. Hence, the oxidative modification of CRMP2 alters the functions of protein that are downstream to it such as WAVE1 consequently leading to growth cone collapse. Knock out and RNA interference studies supported this observation [152, 153].
Studies conducted so far on CRMP2 suggest that post-translation modification of CRMP2 (oxidation and phosphorylation) might impact neurons by impairing axonal transport, affecting pathways involving CRMP2 and consequently leading to synapse loss [139]. Since the oxidation of CRMP2 was observed at the amnestic MCI stage, arguably the earliest stage of AD, targeting CRMP2 conceivably could prevent or delay the progression of this devastating disorder.
5.2.4. ATP synthase alpha
Mitochondrial dysfunction and energy metabolism deficiencies have been recognized as earliest events in AD and have been correlated with impairments of cognitive abilities in this disorder [154, 155]. The oligomeric form of Aβ species interfere in molecular and biochemical alterations in AD more so than the extracellular, insoluble amyloid deposits [156–158], suggesting that Aβ induced mitochondrial dysfunction can play a major role in AD progression and pathogenesis [159–161].
ATP synthase subunit α is a part of complex V, responsible for mitochondrial-resident ATP synthesis. As is well known, ATP synthase consists of a membrane spanning component called Fo and another part that projects out from the membrane into the mitochondrial matrix called F1. The F1 component binds to ADP and inorganic phosphate and synthesizes ATP on its surface. Subunits alpha along with subunit beta form the catalytic core in F1, and hence it is critical for the production of ATP. Further, studies showed that the F1FO ATP synthase also plays an important role in cellular response to antiangiogenic agents, intracellular pH and cholesterol homeostasis [162, 163].
In AD brain the activity of ATP synthase activity was reported to be decreased [164]. Further, low levels of complex V has been reported in the isolated AD mitochondria [164]. Studies from our laboratory showed that ATP synthase alpha undergoes HNE-modification in MCI and AD brain [54, 96], that likely explains the reduced activity of ATP synthase and reduced ATP levels in AD brain compared to age-matched controls. Lu et al., showed oxidative damage of the α subunit of the mitochondrial ATP-synthase gene promoter and resulting in decreased levels of the ATP-synthase [165]. Such changes would leads to reduced ATP synthesis and consequently to nuclear DNA damage of vulnerable genes [165]. In the hippocampus and parietal cortex of individuals with amnestic MCI, ATP-synthase lipoxidation has been reported, supporting the finding of oxidative damage of this protein reported from our laboratory and further supporting the fact that oxidative modification of key proteins plays an important role in the progression and pathogenesis of AD [54, 166]. The oxidation of ATP synthase alpha would not only compromise brain ATP synthesis but would also lead to increase production of ROS, further exacerbating the affect of its oxidative damage and mitochondrial dysfunction, with consequent neuronal damage.
Studies showed that the oligomeric form of the amyloid beta-peptide is present in mitochondria that can induce oxidative damage in mitochondria [159–161]. Further, mitochondria have been reported to alter APP metabolism, enhancing the intraneuronal accumulation of amyloid β-peptides and enhancing the neuronal vulnerability [167]. Like CRMP2, ATP synthase alpha was also found to be co-localized with the NFT, suggesting the oxidation of these proteins plays an important role in the progression and pathogenesis of AD [168]. A recent study by Vacirca et al. reported [169, 170] the presence of autoantibodies to ecto-F1-ATPase (ASabs) in sera and cerebrospinal fluids from patients with AD. Further, these researchers also showed that ASabs can increase cellular uptake of high density lipoprotein (HDL) via a mechanism involving the prototypical function of ecto-F1-ATPase. Hence, oxidation of ATP synthase could also lead to increase levels of HDL, one of the risk factors for the development of AD.
6. CONCLUSIONS
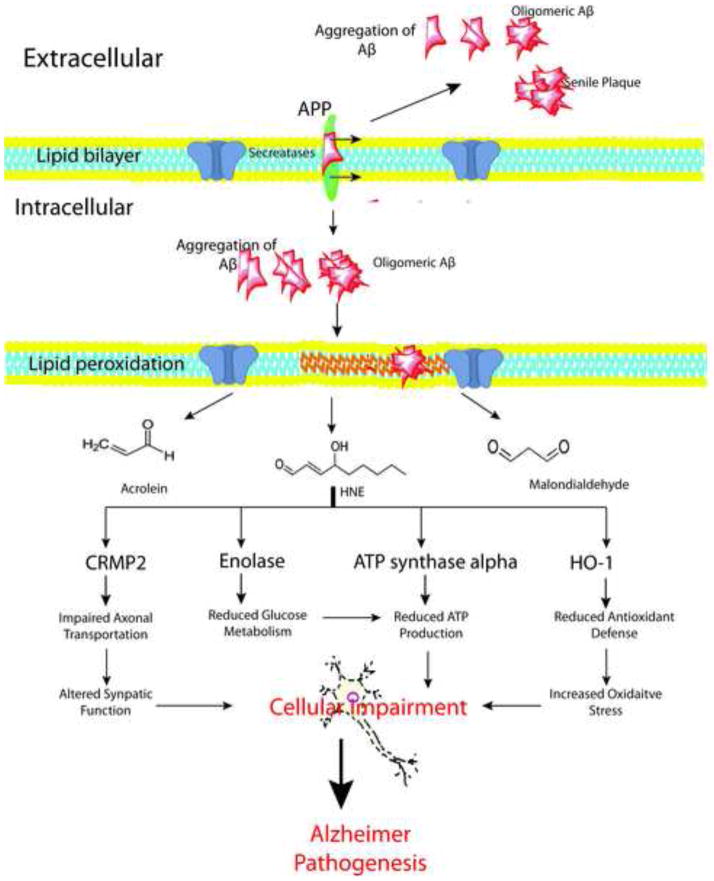
These cytotoxic metabolites of lipid peroxidation such as 4-HNE can have severe adverse effects on proteins function. The identification of specific HNE-modified proteins in the brain of subjects with AD, EAD and amnestic MCI provid an overview of the selective cellular functions that are altered and how they possibly relate to pathology and clinical presentations of both disorders. Studies conducted so far from our laboratory suggest that HNE-modification of enolase, HO-1, CRMP2 and ATP synthase are critical in the progression of AD (Figure 5). Further studies are needed to understand the link of these proteins and other specific pathways that are altered by products of lipid peroxidaiton at different stages of AD. The current literature suggests that targeting brain proteins in common in LAD, EAD, and amnestic MCI with HNE modification could possibly provide a therapeutic strategy to treat or prevent this devastating disorder. The identification of early markers of the AD is still difficult numerous factors, including, among others, pathology occurs prior to clinical symptoms and the dynamic range of plasma or CSF protein concentrations. Development of ever-increasing sophisticated experimental approaches together with advances in tissue sampling may help to detect subtle changes of protein oxidative modification, levels, and functions that may relate to a disease state, thereby helping in the treatment, or delay AD progression. Redox proteomics will be one method to achieve this goal.
Figure 5.
Amyloid β-peptide (Aβ) is generated by proteolytic cleavage of amyloid precursor protein (APP) by the action of secretases. Once generated Aβ undergo aggregations and is eventually deposited extracellularly as senile plaques. Oligomeric Aβ can insert itself into the lipid bilayer, subsequently initiating the lipid peroxidation process leading to the formation of highly reactive products such as malondialdehyde (MDA), 4-hydroxy 2-trans nonenal (HNE), and acrolein. HNE can react with the proteins forming HNE-protein adducts consequently altering the function of proteins. During the progression of the Alzheimer’s disease (AD), enolase, hemeoxygenase-1 (HO-1), collapsing response mediated protein 2 (CRMP2), and ATP synthase alpha are selective modified by HNE, eventually leading to cellular impairment and AD pathogenesis.
Figure 4.
Putative scenario of the oxidative stress-induced modification HO-1 in AD hippocampus. (1) White arrows: increased oxidative stress levels in AD leads to oxidative modification of HO-1 (e.g. HNE) thus resulting in impairment of its functions. (2) Gray arrows: in response to oxidative stress, cell activates HO-1 by upregulating protein synthesis or by Ser-phosphorylation. At the same time, activated” HO-1 is a target of oxidative modifications. Impairment of HO-1 functions may contribute to exacerbate oxidative damage in AD.
Highlights.
Lipid peroxidation in brain is common among several neurodegenerative disorders
Among lipid peroxidation products is HNE, which can covalently bind to proteins
Proteins with HNE bound often have altered, mostly decreased, functions
HNE modification of HO-1, CRMP2 & ATP synthase may be critical in progression of AD
Acknowledgments
This work was supported by NIH grants to D.A.B. [AG-05119, AG-029839].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Pryor WA, Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autoxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–3. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 3.Loidl-Stahlhofen A, Hannemann K, Spiteller G. Generation of alpha-hydroxyaldehydic compounds in the course of lipid peroxidation. Biochim Biophys Acta. 1994;1213:140–8. doi: 10.1016/0005-2760(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 4.Morrow JD, Awad JA, Kato T, Takahashi K, Badr KF, Roberts LJ, 2nd, Burk RF. Formation of novel non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in carbon tetrachloride hepatotoxicity. An animal model of lipid peroxidation. J Clin Invest. 1992;90:2502–7. doi: 10.1172/JCI116143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musiek ES, Yin H, Milne GL, Morrow JD. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40:987–94. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 6.Roberts LJ, 2nd, Montine TJ, Markesbery WR, Tapper AR, Hardy P, Chemtob S, Dettbarn WD, Morrow JD. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273:13605–12. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 7.Montuschi P, Barnes P, Roberts LJ., 2nd Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 8.Roberts LJ, 2nd, Fessel JP, Davies SS. The biochemistry of the isoprostane, neuroprostane, and isofuran Pathways of lipid peroxidation. Brain Pathol. 2005;15:143–8. doi: 10.1111/j.1750-3639.2005.tb00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radak Z, Zhao Z, Goto S, Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med. 2011;32:305–15. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Anzai K, Ogawa K, Goto Y, Senzaki Y, Ozawa T, Yamamoto H. Oxidation-dependent changes in the stability and permeability of lipid bilayers. Antioxid Redox Signal. 1999;1:339–47. doi: 10.1089/ars.1999.1.3-339. [DOI] [PubMed] [Google Scholar]
- 11.Yehuda S, Rabinovitz S, Carasso RL, Mostofsky DI. The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol Aging. 2002;23:843–53. doi: 10.1016/s0197-4580(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 12.Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7:3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- 14.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007;35:7497–504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis. 2010;19:341–53. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 16.Martinez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–97. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:924–9. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kosaras B, Del Signore SJ, Cormier K, McKee A, Ratan RR, Kowall NW, Ryu H. Modulation of lipid peroxidation and mitochondrial function improves neuropathology in Huntington’s disease mice. Acta Neuropathol. 2011;121:487–98. doi: 10.1007/s00401-010-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiperez V, Darios F, Davletov B. Alpha-synuclein, lipids and Parkinson’s disease. Prog Lipid Res. 2010;49:420–8. doi: 10.1016/j.plipres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Sajdel-Sulkowska EM, Marotta CA. Alzheimer’s disease brain: alterations in RNA levels and in a ribonuclease-inhibitor complex. Science. 1984;225:947–9. doi: 10.1126/science.6206567. [DOI] [PubMed] [Google Scholar]
- 21.Shichiri M, Yoshida Y, Ishida N, Hagihara Y, Iwahashi H, Tamai H, Niki E. alpha-Tocopherol suppresses lipid peroxidation and behavioral and cognitive impairments in the Ts65Dn mouse model of Down syndrome. Free Radic Biol Med. 2011;50:1801–11. doi: 10.1016/j.freeradbiomed.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 22.Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer’s cortex show increased peroxidation in vitro. J Neurochem. 1990;55:342–5. doi: 10.1111/j.1471-4159.1990.tb08858.x. [DOI] [PubMed] [Google Scholar]
- 23.Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, MacGarvey U, Kowall NW, Brown RH, Jr, Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–74. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 24.Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–21. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 25.Behl C. Vitamin E and other antioxidants in neuroprotection. Int J Vitam Nutr Res. 1999;69:213–9. doi: 10.1024/0300-9831.69.3.213. [DOI] [PubMed] [Google Scholar]
- 26.Dhitavat S, Rivera ER, Rogers E, Shea TB. Differential efficacy of lipophilic and cytosolic antioxidants on generation of reactive oxygen species by amyloid-beta. J Alzheimers Dis. 2001;3:525–9. [PubMed] [Google Scholar]
- 27.Siow RC, Ishii T, Mann GE. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–5. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 28.Vander Jagt DL, Hunsaker LA, Vander Jagt TJ, Gomez MS, Gonzales DM, Deck LM, Royer RE. Inactivation of glutathione reductase by 4-hydroxynonenal and other endogenous aldehydes. Biochem Pharmacol. 1997;53:1133–40. doi: 10.1016/s0006-2952(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 29.Pratt DA, Tallman KA, Porter NA. Free radical oxidation of polyunsaturated lipids: New mechanistic insights and the development of peroxyl radical clocks. Acc Chem Res. 2011;44:458–67. doi: 10.1021/ar200024c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esterbauer H, Benedetti A, Lang J, Fulceri R, Fauler G, Comporti M. Studies on the mechanism of formation of 4-hydroxynonenal during microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:154–66. doi: 10.1016/0005-2760(86)90329-2. [DOI] [PubMed] [Google Scholar]
- 31.Schneider C, Porter NA, Brash AR. Autoxidative transformation of chiral omega6 hydroxy linoleic and arachidonic acids to chiral 4-hydroxy-2E-nonenal. Chem Res Toxicol. 2004;17:937–41. doi: 10.1021/tx049913n. [DOI] [PubMed] [Google Scholar]
- 32.Perluigi M, Coccia R, Butterfield DA. 4-Hydroxy-2-Nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uchida K, Stadtman ER. Covalent attachment of 4-hydroxynonenal to glyceraldehyde-3-phosphate dehydrogenase. A possible involvement of intra- and intermolecular cross-linking reaction. J Biol Chem. 1993;268:6388–93. [PubMed] [Google Scholar]
- 34.Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortical synaptosomal membrane proteins. J Neurochem. 1997;69:1161–9. doi: 10.1046/j.1471-4159.1997.69031161.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Sharma R, Sharma A, Awasthi S, Awasthi YC. Lipid peroxidation and cell cycle signaling: 4-hydroxynonenal, a key molecule in stress mediated signaling. Acta Biochim Pol. 2003;50:319–36. [PubMed] [Google Scholar]
- 36.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–33. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen WA, Cashman NR, Mattson MP. The lipid peroxidation product 4-hydroxynonenal impairs glutamate and glucose transport and choline acetyltransferase activity in NSC-19 motor neuron cells. Exp Neurol. 1999;155:1–10. doi: 10.1006/exnr.1998.6890. [DOI] [PubMed] [Google Scholar]
- 38.Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Advantage of Cell Aging Gerontology. 1997;2:161–91. [Google Scholar]
- 39.Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–21. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 40.Poli G, Biasi F, Leonarduzzi G. 4-Hydroxynonenal-protein adducts: A reliable biomarker of lipid oxidation in liver diseases. Mol Aspects Med. 2008;29:67–71. doi: 10.1016/j.mam.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 42.LoPachin RM, Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci. 2006;94:240–55. doi: 10.1093/toxsci/kfl066. [DOI] [PubMed] [Google Scholar]
- 43.Doorn JA, Petersen DR. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem Biol Interact. 2003;143–144:93–100. doi: 10.1016/s0009-2797(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 44.Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic Biol Med. 2004;37:937–45. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–10. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: implications for Abeta accumulation in AD brain. Free Radic Biol Med. 2010;49:1798–803. doi: 10.1016/j.freeradbiomed.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sultana R, Butterfield DA. Oxidatively modified GST and MRP1 in Alzheimer’s disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem Res. 2004;29:2215–20. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- 48.Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell DY, Petersen DR. The oxidation of alpha-beta unsaturated aldehydic products of lipid peroxidation by rat liver aldehyde dehydrogenases. Toxicol Appl Pharmacol. 1987;87:403–10. doi: 10.1016/0041-008x(87)90245-6. [DOI] [PubMed] [Google Scholar]
- 50.Picklo MJ, Olson SJ, Markesbery WR, Montine TJ. Expression and activities of aldo-keto oxidoreductases in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:686–95. doi: 10.1093/jnen/60.7.686. [DOI] [PubMed] [Google Scholar]
- 51.Ohta S, Ohsawa I. Dysfunction of mitochondria and oxidative stress in the pathogenesis of Alzheimer’s disease: on defects in the cytochrome c oxidase complex and aldehyde detoxification. J Alzheimers Dis. 2006;9:155–66. doi: 10.3233/jad-2006-9208. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor T, Ireland LS, Harrison DJ, Hayes JD. Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem J. 1999;343(Pt 2):487–504. [PMC free article] [PubMed] [Google Scholar]
- 53.Doorn JA, Maser E, Blum A, Claffey DJ, Petersen DR. Human carbonyl reductase catalyzes reduction of 4-oxonon-2-enal. Biochemistry. 2004;43:13106–14. doi: 10.1021/bi049136q. [DOI] [PubMed] [Google Scholar]
- 54.Reed T, Perluigi M, Sultana R, Pierce WM, Klein JB, Turner DM, Coccia R, Markesbery WR, Butterfield DA. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–20. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 55.Aldini G, Vistoli G, Regazzoni L, Gamberoni L, Facino RM, Yamaguchi S, Uchida K, Carini M. Albumin is the main nucleophilic target of human plasma: a protective role against pro-atherogenic electrophilic reactive carbonyl species? Chem Res Toxicol. 2008;21:824–35. doi: 10.1021/tx700349r. [DOI] [PubMed] [Google Scholar]
- 56.Sultana R, Perluigi M, Butterfield DA. Oxidatively modified proteins in Alzheimer’s disease (AD), mild cognitive impairment and animal models of AD: role of Abeta in pathogenesis. Acta Neuropathol. 2009;118:131–50. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002;33:562–71. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- 58.Dalle Donne I, Scaloni A, Butterfield DA. Redox Proteomics: From protein modifications to cellular dysfunction and diseases. John Wiley and Sons; Hoboken, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 59.Butterfield DA, Perluigi M, Reed T, Muharib T, Hughes CP, Robinson RA, Sultana R. Redox Proteomics in Selected Neurodegenerative Disorders: From Its Infancy to Future Applications. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4109. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perluigi M, Butterfield DA. The identification of protein biomarkers for oxidative stress in Down syndrome. Expert Rev Proteomics. 2011;8:427–9. doi: 10.1586/epr.11.36. [DOI] [PubMed] [Google Scholar]
- 61.Scheff SW, Price DA, Schmitt FA, Scheff MA, Mufson EJ. Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2011;24:547–57. doi: 10.3233/JAD-2011-101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer’s disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 63.Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 64.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986;261:6084–9. [PubMed] [Google Scholar]
- 65.West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25:1205–12. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. PIB Imaging Predicts Progression from Cognitive Normal to Symptomatic Alzheimer’s Disease. Archives Neurology. 2009;66:1469–75. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aluise CD, Robinson RA, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide and proteomics. Neurobiol Dis. 2010;39:221–8. doi: 10.1016/j.nbd.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aluise CD, Robinson RA, Cai J, Pierce WM, Markesbery WR, Butterfield DA. Redox proteomics analysis of brains from subjects with amnestic mild cognitive impairment compared to brains from subjects with preclinical Alzheimer’s disease: insights into memory loss in MCI. J Alzheimers Dis. 2011;23:257–69. doi: 10.3233/JAD-2010-101083. [DOI] [PubMed] [Google Scholar]
- 69.Economou A, Papageorgiou SG, Karageorgiou C, Vassilopoulos D. Nonepisodic memory deficits in amnestic MCI. Cogn Behav Neurol. 2007;20:99–106. doi: 10.1097/WNN.0b013e31804c6fe7. [DOI] [PubMed] [Google Scholar]
- 70.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 71.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 72.Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;71:441–7. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rozzini L, Chilovi BV, Conti M, Bertoletti E, Delrio I, Trabucchi M, Padovani A. Conversion of amnestic Mild Cognitive Impairment to dementia of Alzheimer type is independent to memory deterioration. Int J Geriatr Psychiatry. 2007;22:1217–22. doi: 10.1002/gps.1816. [DOI] [PubMed] [Google Scholar]
- 74.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Merchant M, Markesbery WR, Butterfield DA. Redox proteomics identification of oxidized proteins in Alzheimer’s disease hippocampus and cerebellum: an approach to understand pathological and biochemical alterations in AD. Neurobiol Aging. 2006;27:1564–76. doi: 10.1016/j.neurobiolaging.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 75.Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging. 1990;11:29–37. doi: 10.1016/0197-4580(90)90059-9. [DOI] [PubMed] [Google Scholar]
- 76.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–67. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer’s disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–54. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 78.Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic Biol Med. 1997;23:134–47. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 79.Smith CD, Carney JM, Starkereed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess Brain Protein Oxidation and Enzyme Dysfunction in Normal Aging and in Alzheimer-Disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, Perry G. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of the carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem. 1998;46:731–5. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- 81.Lauderback CM, Hackett JM, Huang FF, Keller JN, Szweda LI, Markesbery WR, Butterfield DA. The glial glutamate transporter, GLT-1, is oxidatively modified by 4-hydroxy-2-nonenal in the Alzheimer’s disease brain: the role of Abeta1–42. J Neurochem. 2001;78:413–6. doi: 10.1046/j.1471-4159.2001.00451.x. [DOI] [PubMed] [Google Scholar]
- 82.Markesbery WR, Kryscio RJ, Lovell MA, Morrow JD. Lipid peroxidation is an early event in the brain in amnestic mild cognitive impairment. Ann Neurol. 2005;58:730–5. doi: 10.1002/ana.20629. [DOI] [PubMed] [Google Scholar]
- 83.Yao Y, Zhukareva V, Sung S, Clark CM, Rokach J, Lee VM, Trojanowski JQ, Pratico D. Enhanced brain levels of 8,12-iso-iPF2alpha-VI differentiate AD from frontotemporal dementia. Neurology. 2003;61:475–8. doi: 10.1212/01.wnl.0000070185.02546.5d. [DOI] [PubMed] [Google Scholar]
- 84.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 85.Butterfield DA, Reed T, Perluigi M, De Marco C, Coccia R, Cini C, Sultana R. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci Lett. 2006;397:170–3. doi: 10.1016/j.neulet.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 86.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis. 2004;6:171–5. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 87.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972–6. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 88.Pamplona R, Dalfo E, Ayala V, Bellmunt MJ, Prat J, Ferrer I, Portero-Otin M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. J Biol Chem. 2005;280:21522–30. doi: 10.1074/jbc.M502255200. [DOI] [PubMed] [Google Scholar]
- 89.Liu X, Lovell MA, Lynn BC. Development of a method for quantification of acrolein-deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal Chem. 2005;77:5982–9. doi: 10.1021/ac050624t. [DOI] [PubMed] [Google Scholar]
- 90.Quinn JF, Montine KS, Moore M, Morrow JD, Kaye JA, Montine TJ. Suppression of longitudinal increase in CSF F2-isoprostanes in Alzheimer’s disease. J Alzheimers Dis. 2004;6:93–7. doi: 10.3233/jad-2004-6110. [DOI] [PubMed] [Google Scholar]
- 91.Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002;82:1524–32. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- 92.Butterfield DA, Gnjec A, Poon HF, Castegna A, Pierce WM, Klein JB, Martins RN. Redox proteomics identification of oxidatively modified brain proteins in inherited Alzheimer’s disease: an initial assessment. J Alzheimers Dis. 2006;10:391–7. doi: 10.3233/jad-2006-10407. [DOI] [PubMed] [Google Scholar]
- 93.Castegna A, Thongboonkerd V, Klein JB, Lynn B, Markesbery WR, Butterfield DA. Proteomic identification of nitrated proteins in Alzheimer’s disease brain. J Neurochem. 2003;85:1394–401. doi: 10.1046/j.1471-4159.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 94.Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer’s disease brain using a redox proteomics approach. Neurobiol Dis. 2006;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Reed TT, Pierce WM, Jr, Turner DM, Markesbery WR, Butterfield DA. Proteomic identification of nitrated brain proteins in early Alzheimer’s disease inferior parietal lobule. J Cell Mol Med. 2009;13:2019–29. doi: 10.1111/j.1582-4934.2008.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Perluigi M, Sultana R, Cenini G, Di Domenico F, Memo M, Pierce WM, Coccia R, Butterfield DA. Redox proteomics identification of 4-hydroxynonenal-modified brain proteins in Alzheimer’s disease: Role of lipid peroxidation in Alzheimer’s disease pathogenesis. Proteomics Clin Appl. 2009;3:682–93. doi: 10.1002/prca.200800161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newman SF, Sultana R, Perluigi M, Coccia R, Cai J, Pierce WM, Klein JB, Turner DM, Butterfield DA. An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J Neurosci Res. 2007;85:1506–14. doi: 10.1002/jnr.21275. [DOI] [PubMed] [Google Scholar]
- 98.Petrak J, Ivanek R, Toman O, Cmejla R, Cmejlova J, Vyoral D, Zivny J, Vulpe CD. Deja vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics. 2008;8:1744–9. doi: 10.1002/pmic.200700919. [DOI] [PubMed] [Google Scholar]
- 99.Mielke R, Schroder R, Fink GR, Kessler J, Herholz K, Heiss WD. Regional cerebral glucose metabolism and postmortem pathology in Alzheimer’s disease. Acta Neuropathol. 1996;91:174–9. doi: 10.1007/s004010050410. [DOI] [PubMed] [Google Scholar]
- 100.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sultana R, Boyd-Kimball D, Cai J, Pierce WM, Klein JB, Merchant M, Butterfield DA. Proteomics analysis of the Alzheimer’s disease hippocampal proteome. J Alzheimers Dis. 2007;11:153–64. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- 102.Butterfield DA, Lange ML. Multifunctional roles of enolase in Alzheimer’s disease brain: beyond altered glucose metabolism. J Neurochem. 2009;111:915–33. doi: 10.1111/j.1471-4159.2009.06397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–20. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hattori T, Takei N, Mizuno Y, Kato K, Kohsaka S. Neurotrophic and neuroprotective effects of neuron-specific enolase on cultured neurons from embryonic rat brain. Neurosci Res. 1995;21:191–8. doi: 10.1016/0168-0102(94)00849-b. [DOI] [PubMed] [Google Scholar]
- 105.Takei N, Kondo J, Nagaike K, Ohsawa K, Kato K, Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J Neurochem. 1991;57:1178–84. doi: 10.1111/j.1471-4159.1991.tb08277.x. [DOI] [PubMed] [Google Scholar]
- 106.Aaronson RM, Graven KK, Tucci M, McDonald RJ, Farber HW. Non-neuronal enolase is an endothelial hypoxic stress protein. J Biol Chem. 1995;270:27752–7. doi: 10.1074/jbc.270.46.27752. [DOI] [PubMed] [Google Scholar]
- 107.Ray R, Miller DM. Cloning and characterization of a human c-myc promoter-binding protein. Mol Cell Biol. 1991;11:2154–61. doi: 10.1128/mcb.11.4.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakajima K, Hamanoue M, Takemoto N, Hattori T, Kato K, Kohsaka S. Plasminogen binds specifically to alpha-enolase on rat neuronal plasma membrane. J Neurochem. 1994;63:2048–57. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- 109.Pancholi V, Fischetti VA. alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J Biol Chem. 1998;273:14503–15. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- 110.Plow EF, Felez J, Miles LA. Cellular regulation of fibrinolysis. Thromb Haemost. 1991;66:32–6. [PubMed] [Google Scholar]
- 111.Bergman AC, Linder C, Sakaguchi K, Sten-Linder M, Alaiya AA, Franzen B, Shoshan MC, Bergman T, Wiman B, Auer G, Appella E, Jornvall H, Linder S. Increased expression of alpha-enolase in c-jun transformed rat fibroblasts without increased activation of plasminogen. FEBS Lett. 1997;417:17–20. doi: 10.1016/s0014-5793(97)01247-7. [DOI] [PubMed] [Google Scholar]
- 112.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–24. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 113.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]
- 114.Kranenburg O, Bouma B, Kroon-Batenburg LM, Reijerkerk A, Wu YP, Voest EE, Gebbink MF. Tissue-type plasminogen activator is a multiligand cross-beta structure receptor. Curr Biol. 2002;12:1833–9. doi: 10.1016/s0960-9822(02)01224-1. [DOI] [PubMed] [Google Scholar]
- 115.Kingston IB, Castro MJ, Anderson S. In vitro stimulation of tissue-type plasminogen activator by Alzheimer amyloid beta-peptide analogues. Nat Med. 1995;1:138–42. doi: 10.1038/nm0295-138. [DOI] [PubMed] [Google Scholar]
- 116.Van Nostrand WE, Porter M. Plasmin cleavage of the amyloid beta-protein: alteration of secondary structure and stimulation of tissue plasminogen activator activity. Biochemistry. 1999;38:11570–6. doi: 10.1021/bi990610f. [DOI] [PubMed] [Google Scholar]
- 117.Calabrese V, Cornelius C, Mancuso C, Barone E, Calafato S, Bates T, Rizzarelli E, Kostova AT. Vitagenes, dietary antioxidants and neuroprotection in neurodegenerative diseases. Front Biosci. 2009;14:376–97. doi: 10.2741/3250. [DOI] [PubMed] [Google Scholar]
- 118.Poon HF, Calabrese V, Scapagnini G, Butterfield DA. Free radicals: key to brain aging and heme oxygenase as a cellular response to oxidative stress. J Gerontol A Biol Sci Med Sci. 2004;59:478–93. doi: 10.1093/gerona/59.5.m478. [DOI] [PubMed] [Google Scholar]
- 119.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 120.Mancuso C. Heme oxygenase and its products in the nervous system. Antioxid Redox Signal. 2004;6:878–87. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- 121.Mancuso C, Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–94. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 122.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–37. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 123.Dwyer BE, Nishimura RN, Lu SY. Differential expression of heme oxygenase-1 in cultured cortical neurons and astrocytes determined by the aid of a new heme oxygenase antibody. Response to oxidative stress. Brain Res Mol Brain Res. 1995;30:37–47. doi: 10.1016/0169-328x(94)00273-h. [DOI] [PubMed] [Google Scholar]
- 124.Calabrese V, Scapagnini G, Colombrita C, Ravagna A, Pennisi G, Giuffrida Stella AM, Galli F, Butterfield DA. Redox regulation of heat shock protein expression in aging and neurodegenerative disorders associated with oxidative stress: a nutritional approach. Amino Acids. 2003;25:437–44. doi: 10.1007/s00726-003-0048-2. [DOI] [PubMed] [Google Scholar]
- 125.Takeda A, Perry G, Abraham NG, Dwyer BE, Kutty RK, Laitinen JT, Petersen RB, Smith MA. Overexpression of heme oxygenase in neuronal cells, the possible interaction with Tau. J Biol Chem. 2000;275:5395–9. doi: 10.1074/jbc.275.8.5395. [DOI] [PubMed] [Google Scholar]
- 126.Hui Y, Wang D, Li W, Zhang L, Jin J, Ma N, Zhou L, Nakajima O, Zhao W, Gao X. Long-term overexpression of heme oxygenase 1 promotes tau aggregation in mouse brain by inducing tau phosphorylation. J Alzheimers Dis. 2011;26:299–313. doi: 10.3233/JAD-2011-102061. [DOI] [PubMed] [Google Scholar]
- 127.Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alzheimer Res. 2009;6:424–30. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- 128.Premkumar DR, Smith MA, Richey PL, Petersen RB, Castellani R, Kutty RK, Wiggert B, Perry G, Kalaria RN. Induction of heme oxygenase-1 mRNA and protein in neocortex and cerebral vessels in Alzheimer’s disease. J Neurochem. 1995;65:1399–402. doi: 10.1046/j.1471-4159.1995.65031399.x. [DOI] [PubMed] [Google Scholar]
- 129.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, Butterfield DA. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Salinas M, Wang J, Rosa de Sagarra M, Martin D, Rojo AI, Martin-Perez J, Ortiz de Montellano PR, Cuadrado A. Protein kinase Akt/PKB phosphorylates heme oxygenase-1 in vitro and in vivo. FEBS Lett. 2004;578:90–4. doi: 10.1016/j.febslet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 131.Di Domenico F, Sultana R, Tiu GF, Scheff NN, Perluigi M, Cini C, Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem. 2010;285:31918–22. doi: 10.1074/jbc.C110.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Quach TT, Duchemin AM, Rogemond V, Aguera M, Honnorat J, Belin MF, Kolattukudy PE. Involvement of collapsin response mediator proteins in the neurite extension induced by neurotrophins in dorsal root ganglion neurons. Mol Cell Neurosci. 2004;25:433–43. doi: 10.1016/j.mcn.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Hensley K, Christov A, Kamat S, Zhang XC, Jackson KW, Snow S, Post J. Proteomic identification of binding partners for the brain metabolite lanthionine ketimine (LK) and documentation of LK effects on microglia and motoneuron cell cultures. J Neurosci. 2010;30:2979–88. doi: 10.1523/JNEUROSCI.5247-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shankar GM, Walsh DM. Alzheimer’s disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cole AR, Causeret F, Yadirgi G, Hastie CJ, McLauchlan H, McManus EJ, Hernandez F, Eickholt BJ, Nikolic M, Sutherland C. Distinct priming kinases contribute to differential regulation of collapsin response mediator proteins by glycogen synthase kinase-3 in vivo. J Biol Chem. 2006;281:16591–8. doi: 10.1074/jbc.M513344200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hou ST, Jiang SX, Aylsworth A, Ferguson G, Slinn J, Hu H, Leung T, Kappler J, Kaibuchi K. CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity. J Neurochem. 2009;111:870–81. doi: 10.1111/j.1471-4159.2009.06375.x. [DOI] [PubMed] [Google Scholar]
- 139.Uchida Y, Ohshima T, Yamashita N, Ogawara M, Sasaki Y, Nakamura F, Goshima Y. Semaphorin3A signaling mediated by Fyn-dependent tyrosine phosphorylation of collapsin response mediator protein 2 at tyrosine 32. J Biol Chem. 2009;284:27393–401. doi: 10.1074/jbc.M109.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sultana R, Butterfield DA. Regional expression of key cell cycle proteins in brain from subjects with amnestic mild cognitive impairment. Neurochem Res. 2007;32:655–62. doi: 10.1007/s11064-006-9123-x. [DOI] [PubMed] [Google Scholar]
- 141.Camins A, Verdaguer E, Folch J, Canudas AM, Pallas M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–60. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- 142.Keeney JT, Swomley AM, Harris JL, Fiorini A, Mitov MI, Perluigi M, Sultana R, Butterfield DA. Cell Cycle Proteins in Brain in Mild Cognitive Impairment: Insights into Progression to Alzheimer Disease. Neurotox Res. 2011 doi: 10.1007/s12640-011-9287-2. [DOI] [PubMed] [Google Scholar]
- 143.Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–14. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- 144.Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–49. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 145.Williamson R, van Aalten L, Mann DM, Platt B, Plattner F, Bedford L, Mayer J, Howlett D, Usardi A, Sutherland C, Cole AR. CRMP2 hyperphosphorylation is characteristic of Alzheimer’s disease and not a feature common to other neurodegenerative diseases. J Alzheimers Dis. 2011;27:615–25. doi: 10.3233/JAD-2011-110617. [DOI] [PubMed] [Google Scholar]
- 146.Cole AR, Noble W, van Aalten L, Plattner F, Meimaridou R, Hogan D, Taylor M, LaFrancois J, Gunn-Moore F, Verkhratsky A, Oddo S, LaFerla F, Giese KP, Dineley KT, Duff K, Richardson JC, Yan SD, Hanger DP, Allan SM, Sutherland C. Collapsin response mediator protein-2 hyperphosphorylation is an early event in Alzheimer’s disease progression. J Neurochem. 2007;103:1132–44. doi: 10.1111/j.1471-4159.2007.04829.x. [DOI] [PubMed] [Google Scholar]
- 147.Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature. 1999;399:784–8. doi: 10.1038/21650. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Z, Majava V, Greffier A, Hayes RL, Kursula P, Wang KK. Collapsin response mediator protein-2 is a calmodulin-binding protein. Cell Mol Life Sci. 2009;66:526–36. doi: 10.1007/s00018-008-8362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–43. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, Hurley JH, Khanna M, Hammes NM, Samuels BC, White FA, Khanna R. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels (Austin) 2011;5:449–56. doi: 10.4161/chan.5.5.17363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hensley K, Venkova K, Christov A, Gunning W, Park J. Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol Neurobiol. 2011;43:180–91. doi: 10.1007/s12035-011-8166-4. [DOI] [PubMed] [Google Scholar]
- 152.Kawano Y, Yoshimura T, Tsuboi D, Kawabata S, Kaneko-Kawano T, Shirataki H, Takenawa T, Kaibuchi K. CRMP-2 is involved in kinesin-1-dependent transport of the Sra-1/WAVE1 complex and axon formation. Mol Cell Biol. 2005;25:9920–35. doi: 10.1128/MCB.25.22.9920-9935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–2. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 154.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blass JP, Gibson GE. Correlations of disability and biologic alterations in Alzheimer brain and test of significance by a therapeutic trial in humans. J Alzheimers Dis. 2006;9:207–18. doi: 10.3233/jad-2006-9212. [DOI] [PubMed] [Google Scholar]
- 156.Fernandez-Vizarra P, Fernandez AP, Castro-Blanco S, Serrano J, Bentura ML, Martinez-Murillo R, Martinez A, Rodrigo J. Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer’s disease. Histol Histopathol. 2004;19:823–44. doi: 10.14670/HH-19.823. [DOI] [PubMed] [Google Scholar]
- 157.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–29. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–52. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 159.Rhein V, Eckert A. Effects of Alzheimer’s amyloid-beta and tau protein on mitochondrial function -- role of glucose metabolism and insulin signalling. Arch Physiol Biochem. 2007;113:131–41. doi: 10.1080/13813450701572288. [DOI] [PubMed] [Google Scholar]
- 160.Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxid Redox Signal. 2007;9:1659–75. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- 161.Sultana R, Butterfield DA. Oxidatively modified, mitochondria-relevant brain proteins in subjects with Alzheimer disease and mild cognitive impairment. J Bioenerg Biomembr. 2009;41:441–6. doi: 10.1007/s10863-009-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burwick NR, Wahl ML, Fang J, Zhong Z, Moser TL, Li B, Capaldi RA, Kenan DJ, Pizzo SV. An Inhibitor of the F1 subunit of ATP synthase (IF1) modulates the activity of angiostatin on the endothelial cell surface. J Biol Chem. 2005;280:1740–5. doi: 10.1074/jbc.M405947200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chi SL, Pizzo SV. Cell surface F1Fo ATP synthase: a new paradigm? Ann Med. 2006;38:429–38. doi: 10.1080/07853890600928698. [DOI] [PubMed] [Google Scholar]
- 164.Schagger H, Ohm TG. Human diseases with defects in oxidative phosphorylation. 2. F1F0 ATP-synthase defects in Alzheimer disease revealed by blue native polyacrylamide gel electrophoresis. Eur J Biochem. 1995;227:916–21. doi: 10.1111/j.1432-1033.1995.tb20219.x. [DOI] [PubMed] [Google Scholar]
- 165.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 166.Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain Pathol. 2010;20:222–33. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Busciglio J, Pelsman A, Wong C, Pigino G, Yuan M, Mori H, Yankner BA. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron. 2002;33:677–88. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 168.Sergeant N, Wattez A, Galvan-valencia M, Ghestem A, David JP, Lemoine J, Sautiere PE, Dachary J, Mazat JP, Michalski JC, Velours J, Mena-Lopez R, Delacourte A. Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer’s disease. Neuroscience. 2003;117:293–303. doi: 10.1016/s0306-4522(02)00747-9. [DOI] [PubMed] [Google Scholar]
- 169.Vacirca D, Barbati C, Scazzocchio B, Masella R, Rosano G, Malorni W, Ortona E. Anti-ATP synthase autoantibodies from patients with Alzheimer’s disease reduce extracellular HDL level. J Alzheimers Dis. 2011;26:441–5. doi: 10.3233/JAD-2011-110350. [DOI] [PubMed] [Google Scholar]
- 170.Vacirca D, Delunardo F, Matarrese P, Colasanti T, Margutti P, Siracusano A, Pontecorvo S, Capozzi A, Sorice M, Francia A, Malorni W, Ortona E. Autoantibodies to the adenosine triphosphate synthase play a pathogenetic role in Alzheimer’s disease. Neurobiol Aging. 2012;33:753–66. doi: 10.1016/j.neurobiolaging.2010.05.013. [DOI] [PubMed] [Google Scholar]