Abstract
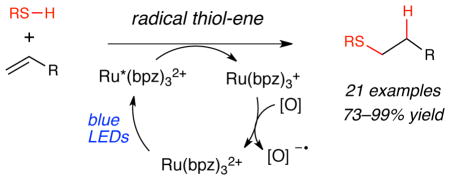
We describe the anti-Markovnikov hydrothiolation of olefins using visible light absorbing transition metal photocatalysts. The key thiyl radical intermediates are generated upon quenching of photoexcited Ru*(bpz)32 with a variety of thiols. The adducts of a wide variety of olefins and thiols are formed in excellent yield (73–99%).
Introduction
The construction of carbon–sulfur bonds is synthetically important because of the large number of sulfur-containing natural products and pharmaceuticals1 as well as the increasing importance of sulfur-containing ligands and chiral auxiliaries in synthetic chemistry.2 One of the most general methods for the construction of thioethers is the radical thiol-ene reaction, a prototypical “click” reaction3 that effects the anti-Markovnikov radical addition of a thiol S–H bond across an alkene (Scheme 1).4 This reaction is of particular significance in materials and biological applications due to the high efficiency of the bond-forming process and because of its compatibility with a wide range of polar functional groups.5 The thiol-ene is typically initiated by thermal or UV activation of a radical initiator or by direct irradiation with UV light.6 In this communication, we report that a ruthenium polypyridyl complex is an effective visible light photoinitiator of the radical thiol-ene reaction.7
Scheme 1.
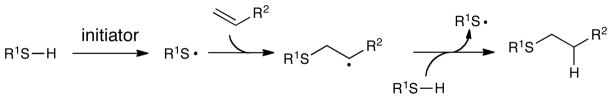
General mechanism of radical thiol-ene reactions.
Over the past several years, a number of labs including our own have been investigating the design of reactions that utilize the powerful photoredox properties of Ru(bpy)32+ and related transition metal chromophores.8 The ability of the photoexcited MLCT state of metal polypyridyl complexes to oxidize amines, alkenes, and arenes has been productively exploited in an impressively diverse array of atom transfer reactions,9 α-carbonyl functionalizations,10 carbon-carbon bond forming processes11 and amine oxidations.12 As part of an effort to broaden the range of transformations accessible using transition metal photoredox catalysis, we have been exploring the use of alternate electron donors that might enable the development of new synthetically useful processes. In particular, we envisioned that the one-electron photooxidation of a thiol by a ruthenium polypyridyl photocatalyst could produce a thiol radical cation, and deprotonation of its acidified S–H bond would generate an electrophilic thiyl radical. As an initial test of the ability of transition metal polypyridyl complexes to catalyze the formation of these reactive heteroatom-centered radical intermediates, we elected to use this approach to design a visible light-initiated radical thiol-ene reaction.
Results and Discussion
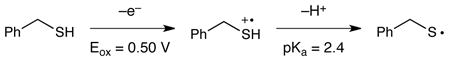
The ability of photoexcited ruthenium complexes to oxidize other sulfur-containing compounds has been documented,13 and a limited number of examples of organic reactions initiated by transition metal-catalyzed photooxidation of thioethers have been reported. The Zen and Guillo groups have developed photocatalytic conditions for oxidation of thioethers to sulfoxides.14 More recently, Li described the oxidation and subsequent cyclization of aromatic thioamides to produce benzothioazoles.15 To the best of our knowledge, however, the ability of ruthenium photocatalysts to oxidize thiols has not previously been reported. Indeed, Matsuda found that the fluorescence of Ru*(bpy)32+ is not quenched upon treatment with thiols.16 However, our experience with the use of ligand-modified ruthenium complexes with tailored electrochemical properties in the optimization of other photocatalytic reactions gave us confidence that our design plan would be successful.
 |
(1) |
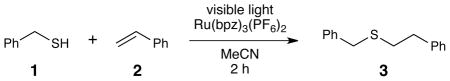
We initiated our studies by examining the reaction of benzyl mercaptan (Eox = +0.50 V vs SCE)16 with styrene. Irradiation in the presence of the canonical photocatalyst Ru(bpy)3Cl2 (Eox (2+*/+) = +0.77 V) produced only poor yields of the radical addition product (Table 1, entry 1). The use of the more powerfully oxidizing catalyst Ru(bpz)3(PF6)2 (Eox (2+*/+) = +1.35 V) led to a significant increase in reactivity (entry 2). The catalyst loading can be lowered to only 0.25 mol% without significant loss in yield (entry 3). Finally, we find that a four-fold excess of thiol enables full conversion after only 2 h of irradiation (entry 6). A control reaction in the absence of Ru(bpz)3(PF6)2 verified that the reaction is not promoted by irradiation with the LED alone. Irradiation with a broad-spectrum white CFL, on the other hand, produced significant background reaction in the absence of catalyst. In the presence of the catalyst, the CFL-irradiated reaction proceeds to completion, albeit at a slower rate than reactions irradiated with a monochromatic blue LED.
Table 1.
Optimization studies for radical thiol-ene reaction of benzyl mercaptan with styrene
| ||||
|---|---|---|---|---|
| entry | cat. loading (mol%) | 1:2 | light source | yield (%)a |
| 1 | 1.0%b | 1:1 | blue LED | 12% |
| 2 | 1.0% | 1:1 | blue LED | 37% |
| 3 | 0.25% | 1:1 | blue LED | 33% |
| 4 | 0.25% | 1:2 | blue LED | 30% |
| 5 | 0.25% | 2:1 | blue LED | 80% |
| 6 | 0.25% | 4:1 | blue LED | 98% |
| 7 | 0% | 4:1 | blue LED | 0% |
| 8 | 0% | 4:1 | 23 W CFL | 19% |
| 9 | 0.25% | 4:1 | 23 W CFL | 82% |
Yields determined by NMR analysis with reference to TMSPh as an internal standard. Remainder of mass is unreacted starting material.
Using Ru(bpy)3(PF6)2 as photocatalyst.
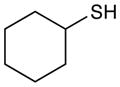
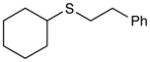
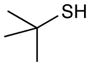
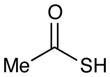
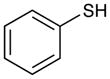
Table 2 summarizes experiments probing the scope of thiols that can be activated using these optimized conditions. Primary thiols such as benzyl mercaptan (entry 1) and methyl thioglycolate (entry 2) react efficiently to generate the hydrothiolated products in nearly quantitative yields. Bulkier thiols such as cyclohexyl (entry 3) and tert-butyl mercaptan (entry 4) require longer reaction times, yet still produce thiol-ene adducts in excellent yields. Functionalized thiols such as cysteine participate smoothly in this process (entry 5). The addition of thioacetic acid (entry 6) and thiophenol (entry 7) are high yielding under these conditions; however, these compounds possessing more acidic S–H bonds undergo background thiol-ene additions in the absence of photocatalyst.
Table 2.
Scope of thiol coupling partners.
| ||||
|---|---|---|---|---|
| entry | thiol | adduct | time | yielda |
| 1 |

|

|
2 h | 98% (0%) |
| 2 |

|
|
1.5 h | 96% (0%) |
| 3 |
|
|
8 h | 98% (0%) |
| 4 |
|

|
20 h | 86% (0%) |
| 5 |

|
|
1.5 h | 97% (0%) |
| 6 |
|
|
5 h | 90% (14%) |
| 7 |
|
|
1 h | 98% (99%) |
Isolated yields are the average of two reproducible experiments, numbers in parentheses are the yield obtained under standard reaction conditions where the catalyst is excluded.
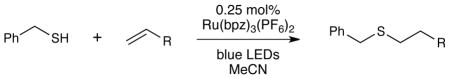
Table 3 summarizes the scope of alkenes that participate in this coupling process. Both aliphatic alkenes and styrenes with various substitution patterns react smoothly under these reaction conditions (entries 1–6); in all cases, the high regioselectivity observed is consistent with the anti-Markovnikov selectivity expected from radical thiol-ene additions. Alkynes, which are prone to multiple additions in other thiol-yne additions,17 undergo clean monoaddition to afford vinyl sulfides with high (E)-selectivity (entry 7). In accord with the high tolerance of the thiyl radical for polar functional groups, the functional group compatibility of this process is high, and esters (entry 8), unprotected alcohols (entry 9), and carbamates (entry 10) are not problematic. Particularly notable is the tolerance of this reaction to allylic and aryl halides which might be expected to participate in unproductive alkylation of the thiol (entry 11) or undergo photochemical decomposition upon UV irradiation (entries 12 and 13); however, these compounds participate in this visible light mediated radical thiol-ene process without competition from these undesired processes. Finally, we were pleased to observe that ethyl cinnamate exclusively produces the anti-Markovnikov adduct in high yields (entry 14). No trace of the complementary regioisomer arising from conjugate addition to the enone could be observed under these conditions.
Table 3.
Scope of alkene coupling partners.
| ||||
|---|---|---|---|---|
| entry | olefin | adduct | time | yielda |
| 1 |
|
|
1 h | 99% (0%) |
| 2 |
|

|
1 h | 98% (0%) |
| 3 |
|
|
2 h | 95% (0%) |
| 4 |
|
|
5 h | 90% (40%) |
| 5 |

|
|
6 h | 98% (0%) |
| 6 |

|
|
6 h | 73%, 5:1 dr (trans:cis) (0%) |
| 7 |
|
|
3 h | 90%, 10:1 E:Z (24%, 4:1 E:Z) |
| 8 |
|
|
2 h | 82% (0%) |
| 9 |
|
|
2 h | 86% (0%) |
| 10 |
|
|
3 h | 88% (0%) |
| 11 |
|
|
3 h | 80% (0%) |
| 12 |

|
|
2 h | 90% (<5%) |
| 13 |

|
|
2.5 h | 94% (65%) |
| 14 |
|

|
26 h | 93% (0%) |
Isolated yields are the average of two reproducible experiments, numbers in parentheses are the yield obtained under standard reaction conditions where the catalyst is excluded.
A reasonable mechanism for this process is outlined in Scheme 4. Visible light photoexcitation of Ru(bpz)32+ affords a strongly oxidizing MLCT state that can undergo reductive quenching by a thiol to generate the thiyl radical cation and Ru(bpz)3+. Deprotonation of the radical cation generates a thiyl radical that adds across the alkene with anti-Markovnikov selectivity. The resulting alkyl radical then abstracts hydrogen atom from an unreacted thiol compound to generate the hydrothiolated product and another equivalent of thiyl radical. The Ru(bpz)3+ catalyst is likely reoxidized by a molecule of oxygen, which regenerates the photoactive photocatalyst (Scheme 4). Empirically, we observe that these reactions tend to be quite clean and produce no significant side products. These observations are especially surprising in that we do not detect disulfide formation under neutral conditions, even though they are often formed as side products of reactions in which thiyl radicals are intermediates.16
In conclusion, we have shown that radical thiol-ene reactions can be photoinitiated upon irradiation with visible light in the presence of Ru(bpz)32+. These reactions are high-yielding and show excellent generality for a variety of alkenes and thiols. Moreover, the reactions can be initiated using long-wavelength visible light sources that are fully compatible with a range of photosensitive functional groups. These results also demonstrate that thiols can be used as reductive quenchers of photoexcited ruthenium complexes to generate oxidized sulfur species under mild experimental conditions. The use of this principle in the design of new synthetically useful transformations is an ongoing goal of research in our lab.
Experimental Section
General Information
Photochemical reactions were irradiated with a 6-inch strip of blue LED lights purchased from Creative Lightings. Ru(bpy)3Cl2·6H2O was purchased from commercial sources and used without further purification. Ru(bpz)3(PF6)2 was synthesized using known methods.18 All other reagents were purchased from commercial sources and purified immediately prior to use. Chromatography was performed with Purasil 60 Å silica gel (230–400 mesh). All glassware was oven-dried for at least 1 h before use. 1H and 13C NMR data are referenced to TMS (0.00 ppm) and CDCl3 (77 ppm), respectively.
4-Iodostyrene
An oven-dried round-bottom flask containing 1 mL (7.65 mmol) 4-bromostyrene and 50 mL dry THF was cooled to −78 °C under nitrogen. 4.2 mL (1.2 equiv) n-BuLi was slowly added, and the reaction was stirred at −78 °C for 10 min before warming to room temperature. After 20 min, the flask was returned to −78 °C, and a solution of iodine in THF (0.6 M) was added dropwise until the red color persisted. At this point the reaction was warmed to room temperature, diluted with ethyl ether and quenched by washing with 50 mL water, 50 mL saturated Na2S2O3, 50 mL saturated NaHCO3 and finally 50 mL brine. The organic layer was dried with MgSO4, filtered and concentrated. Purification by chromatography (100% hexanes) afforded 1.65 g (7.17 mmol, 94%) of a light yellow solid. All spectroscopic data were consistent with reported values.19
General procedure for radical thiol-ene reactions
To an oven-dried 1.5 dram vial were added 1.00 mmol olefin, 4.00 mmol thiol, 3.0 μmol Ru(bpz)3(PF6)2, and 0.5 mL acetonitrile. The vial was sealed with a Teflon cap and irradiated with blue LEDs. Upon completion of the reaction, the solution was diluted with pentane. Reactions involving base-sensitive substrates were filtered through a short pad of SiO2 and concentrated in vacuo, and the residue was purified by flash column chromatography (pentanes to 30:1 pentane:Et2O eluent) to afford the thiol-ene adducts. Reactions without base-sensitive substrates were first extracted twice with 10% NaOH (aq) to remove unreacted thiol. The aqueous layers were extracted with Et2O, and the combined organic layers were dried over Na2SO4, filtered, and concentrated prior to column chromatography.
Benzyl(phenethyl)sulfane (Table 2, entry 1)
Colorless oil. Experiment 1: 210 mg (0.980 mmol, 98% yield). Experiment 2: 208 mg (0.969 mmol, 97% yield). All spectroscopic data were consistent with reported values.20
Methyl 2-(phenethylthio)acetate (Table 2, entry 2)
Colorless oil. Experiment 1: 200 mg (0.949 mmol, 95% yield). Experiment 2: 205 mg (0.975 mmol, 98% yield). IR (thin film): 1734,1647, 1283 cm−1; 1H NMR (500 MHz, CDCl3) 7.50 – 6.96 (m, 5H), 3.74 (s, 3H), 3.23 (s, 2H), 2.90 (apparent s, 4H); 13C NMR (126 MHz, CDCl3) δ 170.8, 140.0, 128.5, 126.4, 52.4, 35.6, 34.0, 33.4; HRMS (EI) calc’d for [C11H14O2S+NH4]+ requires m/z 228.1053, found m/z 228.1053.
Cyclohexyl(phenethyl)sulfane (Table 2, entry 3)
Colorless oil. Experiment 1: 214 mg (0.969 mmol, 97% yield). Experiment 2: 220 mg (0.998 mmol, 99% yield). IR (thin film): 2929, 2851, 1653, 1450 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.59 – 6.96 (m, 5H), 2.87, 2.78 (AA′BB′, 4H), 2.65 (m, 1H), 2.01–1.94 (m, 2H), 1.85–1.70 (m, 2H), 1.64–1.57 (m, 1H), 1.40–1.17 (m, 4H); 13C NMR (126 MHz, CDCl3) δ 140.8, 128.4, 126.2, 43.6, 36.7, 33.7, 31.6, 26.1, 25.8; HRMS (EI) calc’d for [C14H20S]+ requires m/z 220.1281, found m/z 220.1278.
tert-Butyl(phenethyl)sulfane (Table 2, entry 4)
Colorless oil. Experiment 1: 165 mg (0.848 mmol, 85% yield). Experiment 2: 167 mg (0.858 mmol, 86% yield). IR (thin film): 2967, 2865, 1504, 1467 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.50 – 6.87 (m, 5H), 2.86, 2.78 (AA′BB′, 4H), 1.33 (s, 9H); 13C NMR (126 MHz, CDCl3) δ 140.9, 128.4, 128.4, 126.2, 42.1, 36.4, 30.9, 29.9; HRMS (EI) calc’d for [C12H18S]+ requires m/z 194.1124, found m/z 194.1125.
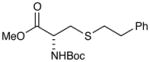
(S)-Methyl 2-((tert-butoxycarbonyl)amino)-3-(phenethylthio)propanoate (Table 2, entry 5)
Colorless semisolid. Experiment 1: 332 mg (0.977 mmol, 98% yield). Experiment 2: 327 mg (0.962 mmol, 96% yield). IR (thin film): 3432, 2979, 2253, 1708, 1498 cm−1; 1H NMR (500 MHz, CDCl3) 7.35 – 7.12 (m, 5H), 5.41 – 5.30 (m, 1H), 4.54 (s, 1H), 3.75 (s, 3H), 2.97 (t, J = 5.0 Hz, 2H), 2.78, 2.86 (AA′BB′, 4H), 1.44 (s, 9H); 13 C NMR (126 MHz, CDCl3) δ 171.5, 140.0, 128.4, 128.4, 126.4, 94.7, 80.1, 53.3, 52.5, 36.1, 34.6, 34.1, 28.3; HRMS (EI) calc’d for [C17H25NO4S+Na]+ requires m/z 362.1397, found m/z 362.1397.
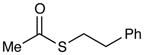
(S)-Phenethyl ethanethioate (Table 2, entry 6)
Colorless oil. Experiment 1: 167 mg (0.923 mmol, 92% yield). Experiment 2: 158 mg (0.877 mmol, 88% yield). All spectroscopic data were consistent with reported values.21
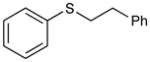
Phenethyl(phenyl)sulfane (Table 2, entry 7)
Colorless oil. Experiment 1: 210 mg (0.9803 mmol, 98% yield). Experiment 2: 208 mg (0.969 mmol, 97% yield). All spectroscopic data were consistent with reported values.22
Benzyl(octyl)sulfane (Table 3, entry 1)
Colorless oil. Experiment 1: 234 mg (0.989 mmol, 99% yield). Experiment 2: 235 mg (0.992 mmol, 99% yield). All spectroscopic data were consistent with reported values.23
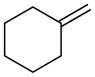
Benzyl(cyclohexylmethyl)sulfane (Table 3, entry 2)
Colorless oil. Experiment 1: 215 mg (0.974 mmol, 97% yield). Experiment 2: 219 mg (0.992 mmol, 99% yield). IR (thin film): 2925, 2852, 1497, 1450 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.72 – 7.07 (m, 5H), 3.68 (s, 2H), 2.30 (d, J = 6.8 Hz, 2H), 1.84 – 1.75 (m, 3H), 1.73 – 1.58 (m, 3H), 1.42 (dddddd, J = 3.4, 3.4, 3.4, 3.4, 3.4, 3.4, 3.4 Hz, 1H), 1.27 – 1.04 (m, 3H), 0.90 (ddd, J = 12.4, 2.1, 3.3 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 138.7, 128.8, 128.4, 126.8, 38.9, 37.6, 36.8, 32.8, 26.4, 26.1; HRMS (EI) calc’d for [C14H20S]+ requires m/z 220.1281, found m/z 220.1286.
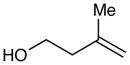
4-(Benzylthio)-3-methylbutan-1-ol (Table 3, entry 3)
Colorless oil. Experiment 1: 195 mg (0.930 mmol, 93% yield). Experiment 2: 202 mg (0.970 mmol, 97% yield). IR (thin film): 3384, 2927, 1494, 1454 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.37 – 7.19 (m, 5H), 3.69 (s, 2H), 3.66 – 3.58 (m, 2H), 2.42 (dd, J = 12.7, 6.1 Hz, 1H), 2.32 (dd, J = 12.7, 7.0 Hz, 1H), 1.84 – 1.74 (m, 1H), 1.71 – 1.63 (m, 1H), 1.48 (s, 1H), 1.45 – 1.36 (m, 1H), 0.98 (d, J = 6.7 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ138.5, 128.8, 128.4, 126.9, 60.7, 38.9, 38.8, 36.7, 29.7, 19.6; HRMS (EI) calc’d for [C12H18OS]+ requires m/z 210.1073, found m/z 210.1078.
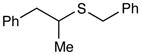
Benzyl(1-phenylpropan-2-yl)sulfane (Table 3, entry 4)
Colorless oil. Experiment 1: 213 mg (0.879 mmol, 88% yield). Experiment 2: 223 mg (0.920 mmol, 92% yield). IR (thin film): 3023, 1497, 1450 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.40 – 7.06 (m, 10H), 3.71 (s, 2H), 2.98 (dd, J = 13.4, 5.7 Hz, 1H), 2.91 – 2.81 (m, 1H), 2.63 (dd, J = 13.4, 8.6 Hz, 1H), 1.18 (d, J = 6.7 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 139.1, 138.5, 129.2, 128.8, 128.4, 128.2, 126.9, 126.2, 43.5, 40.6, 35.3, 20.4; HRMS (EI) calc’d for [C16H18S]+ requires m/z 242.1124, found m/z 242.1117.
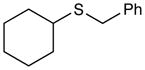
Benzyl(cyclohexyl)sulfane (Table 3, entry 5)
Colorless oil. Experiment 1: 206 mg (0.998 mmol, 99% yield). Experiment 2: 201 mg (0.974 mmol, 97% yield). IR (thin film): 2932, 2856, 1497, 1450 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.43 – 7.08 (m, 5H), 3.74 (s, 2H), 2.56 (ddd, J = 10.5, 6.8, 3.7 Hz, 1H), 2.01 – 1.85 (m, 2H), 1.74 (td, J = 6.0, 5.5, 2.9 Hz, 2H), 1.64 – 1.50 (m, 1H), 1.42 – 1.14 (m, 5H); 13C NMR (126 MHz, CDCl3) δ 141.6, 131.4, 131.1, 129.4, 45.6, 37.3, 36.1, 28.7, 28.5; HRMS (EI) calc’d for [C13H18S]+ requires m/z 206.1124, found m/z 206.1132.
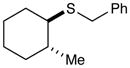
Benzyl(2-methylcyclohexyl)sulfane (Table 3, entry 6)
Colorless oil. Experiment 1: 155 mg (0.703 mmol, 70% yield, 5:1 dr, trans:cis). Experiment 2: 167 mg (0.759 mmol, 76% yield, 5:1 dr, trans:cis). IR (thin film): 3424, 2925, 2852, 1602, 1494, 1453 cm−1; 1H NMR (500 MHz, C6D6) δ 7.44 – 6.71 (m, 5H), 3.48 (d, J = 4.7 Hz, 2H), 3.26 (dd, J = 7.3, 1.2 Hz, 1H), 2.81 – 2.46 (m, 1H), 2.15 – 1.83 (m, 1H), 1.76 – 1.08 (m, 9H), 1.07 (d, J = 6.5 Hz, 3H), 1.00 (d, J = 6.9 Hz, 3H); 13C NMR (126 MHz, CDCl3) δ 138.9, 129.4, 128.8, 128.5, 128.3, 128.0, 127.4, 126.7, 50.8, 48.9, 43.2, 37.4, 36.0, 35.6, 34.8, 34.7, 34.3, 31.3, 30.6, 26.7, 25.8, 23.6, 23.2, 21.0, 17.4; HRMS (EI) calc’d for [C14H19S]+ requires m/z 219.1202, found m/z 219.1203.
(E)-Benzyl(styryl)sulfane (Table 3, entry 7)
Colorless semi-solid. Experiment 1: 206 mg (0.911 mmol, 91% yield, 10:1 E:Z). Experiment 2: 200 mg (0.882 mmol, 88% yield, 9:1 E:Z). All spectroscopic data were consistent with reported values.24
2-(Benzylthio)ethyl acetate (Table 3, entry 8)
Colorless oil. Experiment 1: 170 mg (0.810 mmol, 81% yield). Experiment 2: 175 mg (0.830 mmol, 83% yield). All spectroscopic data were consistent with reported values.25
3-(Benzylthio)propan-1-ol (Table 3 entry 9)
Colorless oil. Experiment 1:155 mg (0.846 mmol, 85% yield). Experiment 2:158 mg (0.869 mmol, 87% yield). IR (thin film): 3426, 2908, 1647 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.39 – 7.17 (m, 5H), 3.73 (s, 2H), 3.73 – 3.68 (m, 2H), 2.54 (t, J = 7.0 Hz, 2H), 1.85 – 1.75 (m, 2H), 1.53 (bs, 1H); 13C NMR (126 MHz, CDCl3) δ 138.3, 128.8, 128.5, 127.0, 61.8, 36.3, 31.5, 28.0; HRMS (EI) calc’d for [C10H14OS+H]+ requires m/z 183.0839, found m/z 183.0833.
tert-Butyl (3-(benzylthio)propyl)carbamate (Table 3, entry 10)
White solid. Experiment 1: 248 mg (0.881 mmol, 88% yield). Experiment 2: 158 mg (0.869 mmol, 87% yield). IR (thin film): 3363, 2927, 2932, 2251, 1700, 1508 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.76 – 6.80 (m, 5H), 4.58 (s, 1H), 3.69 (s, 2H), 3.16 (q, J = 7.4, 7.0 Hz, 2H), 2.43 (t, J = 7.2 Hz, 2H), 1.96 – 1.53 (m, 2H), 1.43 (s, 9H); 13C NMR (126 MHz, CDCl3) δ 155.8, 138.3, 128.8, 128.4, 126.9, 79.1, 39.5, 36.2, 29.3, 28.4, 28.3.; HRMS (EI) calc’d for [C15H23O2NS+H]+ requires m/z 282.1523, found m/z 282.1527.
Benzyl(3-chloropropyl)sulfane (Table 3, entry 11)
Colorless oil. Experiment 1: 160 mg (0.801 mmol, 80% yield). Experiment 2: 159 mg (0.790 mmol, 79% yield). IR (thin film): 3030, 2921, 2258, 1497, 1450 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.43 – 7.17 (m, 5H), 3.71 (s, 2H), 3.61 (t, J = 6.4 Hz, 2H), 2.57 (t, J = 7.0 Hz, 2H), 1.98 (tt, J = 6.7, 6.7 Hz, 2H); 13C NMR (126 MHz, CDCl3) δ 140.9, 131.5, 131.2, 129.7, 46.1, 39.0, 34.5, 31.0; HRMS (EI) calc’d for [C10H13ClS]+ requires m/z 200.0421, found m/z 200.0411.
Benzyl(4-bromophenethyl)sulfane (Table 3, entry 12)
Colorless oil. Experiment 1: 273 mg (0.890 mmol, 89% yield). Experiment 2: 280 mg (0.910 mmol, 91% yield). IR (thin film): 3028, 2916, 1601, 1488, 1453 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.42 – 7.33 (m, 2H), 7.35 – 7.19 (m, 5H), 7.05 – 6.96 (m, 2H), 3.70 (s, 2H), 2.75, 2.61 (AA′BB′, 4H); 13C NMR (126 MHz, CDCl3) δ 139.5, 138.4, 131.6, 130.4, 129.0, 128.6, 127.2, 120.2, 36.6, 35.4, 32.6; HRMS (EI) calc’d for [C15H15BrS]+ requires m/z 306.0073, found m/z 306.0074.
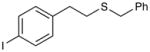
Benzyl(4-iodophenethyl)sulfane (Table 3, entry 13)
Colorless oil. Experiment 1: 330 mg (0.932 mmol, 93% yield). Experiment 2: 337 mg (0.951 mmol, 95% yield). IR (thin film): 3028, 2917, 1601, 1484, 1453 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.58 (d, J = 8.2 Hz, 2H), 7.43 – 7.22 (m, 5H), 6.87 (d, J = 8.3 Hz, 2H), 3.70, 2.61 (AA′BB′, 4H); 13C NMR (126 MHz, CDCl3) 140.0, 138.2, 137.4, 130.5, 128.8, 128.5, 127.0, 91.5, 36.4, 35.3, 32.4; HRMS (EI) calc’d for [C15H15IS]+ requires m/z 353.9934, found m/z 353.9923.
Ethyl 2-(benzylthio)-3-phenylpropanoate (Table 3, entry 14)
Colorless semisolid. Experiment 1: 275 mg (0.917 mmol, 92% yield). Experiment 2: 280 mg (0.933 mmol, 93% yield). All spectroscopic data were consistent with reported values.26
Supplementary Material
Scheme 2.
Visible light photochemistry of ruthenium polypyridyl complexes and related photocatalysts.
Scheme 3.
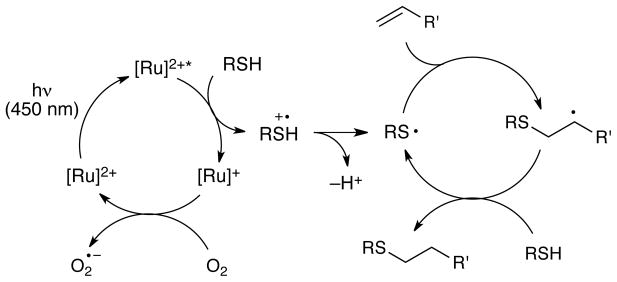
Proposed mechanism of the Ru(bpz)32+-catalyzed radical thiol-ene addition.
Acknowledgments
Financial support for this research was provided by the NIH (GM095666). The NMR spectroscopy facility at UW-Madison is funded by the NSF (CHE-1048642).
Footnotes
Supporting Information. 1H NMR, 13C NMR and NOE data for all relevant compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Parry JR. Tetrahedron. 1983;39:1215. [Google Scholar]; (b) Jacob C. Nat Prod Rep. 2006;23:851. doi: 10.1039/b609523m. [DOI] [PubMed] [Google Scholar]; (c) Clayden J, MacLellan P. Beilstein J Org Chem. 2011;7:582. doi: 10.3762/bjoc.7.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) McGarrigle EM, Myers EL, Illa O, Shaw MA, Riches SL, Aggarwal VK. Chem Rev. 2007;107:5841. doi: 10.1021/cr068402y. [DOI] [PubMed] [Google Scholar]; (b) Gómez RA, Carretero JC. Chem Commun. 2011;47:2207. doi: 10.1039/c0cc03978k. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Finn MG, Fokin VV. Chem Soc Rev. 2010;39:1231. doi: 10.1039/c003740k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Posner T. Ber. 1905;38:646. [Google Scholar]; (b) Kharasch MS, Read AT, Mayo FR. Chem Ind. 1938;57:752. [Google Scholar]; (c) Hoyle CE, Lowe AB, Bowman CN. Chem, Soc Rev. 2010;39:1355. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 5.For reviews, see: Hoyle CE, Lee TY, Roper TJ. Polym Sci Part A: Polym Chem. 2004;42:5301.Sletten E, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974. doi: 10.1002/anie.200900942.Hoyle CE, Bowman CN. Angew Chem Int Ed. 2010;49:1540. doi: 10.1002/anie.200903924.
- 6.(a) ten Brummelhuis N, Diehl C, Schlaad H. Macromol. 2008;41:9946. [Google Scholar]; (b) Rissing C, Son DY. Organometallics. 2008;27:5394. [Google Scholar]
- 7.For recent examples of radical thiol-ene chemistry using eosin as a visible light photoinitiator, see: DeForest CA, Anseth KS. Nat Chem. 2011;3:925. doi: 10.1038/nchem.1174.DeForest CA, Anseth KA. Angew Chem Int Ed. 2012;51:1816. doi: 10.1002/anie.201106463.
- 8.For reviews, see: Zeitler K. Angew Chem Int Ed. 2009;48:9785. doi: 10.1002/anie.200904056.Yoon TP, Ischay MA, Du J. Nature Chem. 2010;2:527. doi: 10.1038/nchem.687.Narayanam JMR, Stephenson CRJ. Chem Soc Rev. 2011;40:102. doi: 10.1039/b913880n.Teply F. Collect Czech Chem Commun. 2011;76:859.Tucker JW, Stephenson CRJ. J Org Chem. 2012;77:1617. doi: 10.1021/jo202538x.
- 9.For recent examples of atom transfer reactions see: Naraynam JMR, Tucker JW, Stephenson CRJ. J Am Chem Soc. 2009;131:8756. doi: 10.1021/ja9033582.Koike T, Akita M. Chem Lett. 2009;38:166.Nguyen JD, Tucker JW, Konieczynska MD, Stephenson CRJ. J Am Chem Soc. 2011;133:4160. doi: 10.1021/ja108560e.Su Y, Zhang L, Jiao N. Org Lett. 2011;13:2168. doi: 10.1021/ol2002013.Wallentin CJ, Ngyuen JD, Finkbeiner P, Stephenson CRJ. J Am Chem Soc. 2012;134:8875. doi: 10.1021/ja300798k.
- 10.For recent examples of α-carbonyl functionalizations see: Nicewicz DA, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976.Shih HW, Vander Wal MN, Grange RL, MacMillan DWC. J Am Chem Soc. 2010;132:13600. doi: 10.1021/ja106593m.
- 11.For recent examples of C-C bond forming reactions see: Ischay MA, Anzovino ME, Du J, Yoon TP. J Am Chem Soc. 2008;130:12886. doi: 10.1021/ja805387f.Ischay MA, Lu Z, Yoon TP. J Am Chem Soc. 2010;132:8572. doi: 10.1021/ja103934y.Lu Z, Shen M, Yoon TP. J Am Chem Soc. 2011;133:1162. doi: 10.1021/ja107849y.Hurtley AE, Cismesia MA, Ischay MA, Yoon TP. Tetrahedron. 2011;67:4442. doi: 10.1016/j.tet.2011.02.066.Lin S, Ischay MA, Fry CG, Yoon TP. J Am Chem Soc. 2011;133:19350. doi: 10.1021/ja2093579.Kalyani D, McMurtrey KB, Neufeldt SR, Sanford MS. J Am Chem Soc. 2011;133:18566. doi: 10.1021/ja208068w.Nagib DA, MacMillan DWC. Nature. 2011;480:224. doi: 10.1038/nature10647.Tyson EL, Farney EP, Yoon TP. Org Lett. 2012;14:1110. doi: 10.1021/ol3000298.Parrish JD, Ischay MA, Lu Z, Guo S, Peters NR, Yoon TP. Org Lett. 2012;14:1640. doi: 10.1021/ol300428q.Lin S, Padilla CE, Ischay MA, Yoon TP. Tetrahedron Lett. 2012;53:3073. doi: 10.1016/j.tetlet.2012.04.021.Ye Y, Sanford MS. J Am Chem Soc. 2012;134:9034. doi: 10.1021/ja301553c.
- 12.For recent examples of amine oxidations see: Miyake Y, Nakajima K, Nishibayashi Y. J Am Chem Soc. 2012;134:3338. doi: 10.1021/ja211770y.DiRocco D, Rovis T. J Am Chem Soc. 2012;134:8094. doi: 10.1021/ja3030164.Condie AG, Gónzalez-Gómez JC, Stephenson CRJ. J Am Chem Soc. 2010;132:1464. doi: 10.1021/ja909145y.Freeman DB, Furst L, Condie AG, Stephenson CRJ. Org Lett. 2012;14:94. doi: 10.1021/ol202883v.
- 13.(a) Deronzier A, Meyer TJ. Inorg Chem. 1980;19:2912. [Google Scholar]; (b) Miyashita T, Matsuda M. Bull Chem Soc Jpn. 1981;54:1740. [Google Scholar]; (c) Miyashita T, Matsuda M. Bull Chem Soc Jpn. 1985;58:3031. [Google Scholar]
- 14.(a) Zen JM, Liou SL, Kumar AS, Hsia MS. Angew Chem Int Ed. 2003;42:577. doi: 10.1002/anie.200390166. [DOI] [PubMed] [Google Scholar]; (b) Guillo P, Hamelin O, Batat P, Jonusauskas G, McClenaghan ND, Ménage S. Inorg Chem. 2012;51:2222. doi: 10.1021/ic2022159. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Yang J, Qu Y, Li P. Org Lett. 2012;14:98. doi: 10.1021/ol2028866. [DOI] [PubMed] [Google Scholar]
- 16.Bordwell FG, Zhang XM, Satish AV, Cheng JP. J Am Chem Soc. 1994;116:6605. [Google Scholar]
- 17.Fairbanks BD, Sims EA, Anseth KS, Bowman CN. Macromolecules. 2010;43:4113. [Google Scholar]
- 18.Rillema DP, Allen G, Meyer TJ, Conrad D. Inorg Chem. 1983;22:1617–1622. [Google Scholar]
- 19.Kang SK, Lee HW, Kim JS, Choi SC. Tetrahedron Lett. 1996;37:3723–3726. [Google Scholar]
- 20.Jean M, Renault J, van de Weghe P, Asao N. Tetrahedron Lett. 2010;51:378–381. [Google Scholar]
- 21.Sakai N, Moriya T, Konakahara T. J Org Chem. 2007;72:5920–5922. doi: 10.1021/jo070814z. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi N. J Org Chem. 2007;72:1241–1245. doi: 10.1021/jo062131+. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Ajiki K. Org Lett. 2005;7:1537–1539. doi: 10.1021/ol0501673. [DOI] [PubMed] [Google Scholar]
- 24.Ranjit S, Duan Z, Zhang P, Liu X. Org Lett. 2010;12:4134–4136. doi: 10.1021/ol101729k. [DOI] [PubMed] [Google Scholar]
- 25.Lou FW, Liu BK, Wu Q, Lv DS, Lin XF. Adv Synth Catal. 2008;350:1959–1962. [Google Scholar]
- 26.Xu F, Shi W, Wang J. J Org Chem. 2005;70:4191–4194. doi: 10.1021/jo050109v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



