Abstract
Objectives
Fibrocytes are integral in the development of fibroproliferative disease post lung transplantation. Undifferentiated fibrocytes (CD45+Col1+CXCR4+) preferentially traffic via the CXCR4/CXCL12 axis and differentiate into smooth muscle actin producing (CD45+CXCR4+αSMA+) cells. We postulated that an antibody directed against CXCL12 would attenuate fibrocyte migration and fibro-obliteration of heterotopic tracheal transplant allografts.
Methods
A total alloantigenic mismatch murine heterotopic tracheal transplant model of obliterative bronchiolitis was used. Animals were treated with either goat-anti-human CXCL12 F(ab′)2 or Goat IgG F(ab′)2. Buffy coat, bone marrow, and trachea allografts were collected and analyzed by flow cytometry. Tracheal luminal obliteration was assessed via hematoxylin/eosin and Direct Red 80 collagen stain.
Results
Compared to controls, anti-CXCL12 treated animals showed a significant decrease in tracheal allograft fibrocyte populations at 7 and 21 days post-transplantation. Bone marrow and buffy coat aspirates showed the same trend at 7 days. In anti-CXCL12 treated mice, there was a 35% decrease in luminal obliteration at 21 days (65.00 [interquartile range = 38]% vs. 100 [10]% obliterated; p=0.010) and decreased luminal collagen deposition at 21 and 28 days post transplantation (p=0.042 and 0.012, respectively).
Conclusions
Understanding the role of fibrocytes in airway fibrosis post lung transplantation may lead to a paradigm shift in treatment strategy. Anti-CXCL12 antibody afforded protection against infiltrating fibrocytes and reduced deterioration of tracheal allografts. Thus the CXCR4/CXCL12 axis is a novel target for the treatment of fibro-obliteration post lung transplantation and quantification of fibrocyte populations may provide clinicians with a biomarker of fibrosis allowing individualized drug therapy.
Introduction
Bronchiolitis obliterans syndrome (BOS) is a leading cause of morbidity and mortality post-lung transplantation (1). Lung fibroblasts and myofibroblasts are critical for the development of fibrosis and are thought to arise from three locations; 1) resident proliferation of fibroblasts, 2) epithelial to mesenchyme transition, and 3) from bone-marrow derived mesenchymal progenitor cell, fibrocytes (2).
Fibrocytes (CD45+Col1+CXCR4+) are bone marrow derived mesenchymal stem cells that are released into circulation in response to numerous inflammatory threats and microenvironmental cytokines and traffic to injured tissues via the CXCR4/CXCL12 chemokine axis. Fibrocytes differentiate into alpha smooth muscle actin (αSMA+) producing fibroblasts/myofibroblasts (2,3). Although fibrocytes have been shown to respond to several chemokines and express CCR3, CCR5, and CCR7 chemokines, the CXCR4/CXCL12 biological axis is the predominate driving force for fibrocyte trafficking following airway injury (4,5).
Fibrocytes were first described in the context of normal wound healing, however, their importance in the progression of fibrotic and asthmatic lung disease has since been demonstrated(6). Patients with exacerbations of idiopathic pulmonary fibrosis (IPF) have been shown to have an elevated proportion of peripheral blood fibrocytes as compared to patients with stable IPF. Furthermore, the total circulating fibrocyte population in IPF was found to be an independent predictor of death(7).
In a prospective study aimed at quantifying circulating fibrocyte populations (CD45+Col1+) in patients post lung transplantation, we found a statistically significant increase in circulating fibrocyte number in patients diagnosed with BOS (as defined by FEV1) as compared to patients without BOS (8.9×105 cells/ml vs. 2.96 ×105 cells/ml, respectively). Furthermore, we found significant incremental increases in circulating fibrocyte numbers with advancing BOS stage, suggesting a role for fibrocytes in BOS progression (8). This is corroborated by another study, which found a statistically significant increase in the number of fibrocytes upon staining for CXCR4/prolyl 4-hydroxylase in lung biopsy specimens from lung transplant patients with BOS as compared to controls(9).
Associations between fibrocyte numbers and BOS progression in human studies has suggested, but not yet proven, that fibrocytes have detrimental effects in pulmonary fibrosis. Given the correlation of fibrocyte number and clinical stage of BOS, as well as the importance of the CXCR4/CXCL12 axis in fibrocyte trafficking, we hypothesized that immune-therapy directed against CXCL12 would attenuate airway fibrosis and obliteration in a well-established murine heterotopic tracheal transplant model of obliterative bronchiolitis(10,11).
Materials and Methods
Generation and purification of Anti-CXCL12 IgG F(ab′)2 fragments
Goat anti-human CXCL12 antibodies were purified using Melon™ Gel IgG purification Kit (Thermo Scientific, Rockford, IL). Normal goat IgG and purified goat anti-human CXCL12 IgG were digested with Immobilized Pepsin (Pierce, Rockford, IL). Fragments were recovered and dialyzed against PBS. F(ab′)2 were purified from Fc fragments using NUNC™ Protein G columns (NUNC, Rockford, IL).
Animals
All mice (Jackson Laboratory, Bar Harbor, ME) received humane care according to the “Principles of Laboratory Animal Care,” formulated by the National Society for Medical Research and The Guide for the Care and Use of Laboratory Animals. The study protocol was approved by the Animal Care and Use Committee at the University of Virginia.
Experimental group design
We used a heterotopic subcutaneous tracheal transplant model of BO as previously described(11). An MHC class I- and class II-mismatch was produced by transplanting four Balb/c (H-2d) tracheas into one C57BL/6 (H-2b) recipient. Four donor trachea allografts were used per recipient to ensure adequate tracheal tissue for fibrocyte determination and histology. Mice were divided into two groups: 1) IgG control: Recipients received IP injections of normal goat IgG F(ab′)2; 2) Anti-CXCL12: Recipients received IP injections of anti-CXCL12 F(ab′)2. Each group consisted of six recipients for a total of 120 donors and 30 recipients. Thus, there were a total of 240 donors and 60 recipients. All recipients were given IP injections at days −1, 0, 1, then every other day until the end-points. Mice were sacrificed and allografts were collected on days 3, 7, 12, 21, or 28. Tracheal allografts from five of the six animals at each time point were pooled for flow cytometry analysis, while the tracheal allografts from the sixth animal were used for histology and Sirius Red staining.
FACS analysis
Single cell suspensions isolated from bone marrow, peripheral blood, and trachea allografts were stained with perCP-labeled CD45, PB-labeled CXCR4 (BD Biosciences, San Diego, Ca), or isotype controls. Subsequently, cells were permeabilized using cytofix/cytoperm for staining with DyLight-488 conjugated anti-Collagen 1 (Col1), PE-labeled αSMA, or isotype controls. Anti-Col1 and a Rabbit IgG isotype control were conjugated using a DyLight-488 conjugation kit (Thermo Scientific, Rockford, IL). Four-color analysis of the stained cells was performed on a FACSCanto II flow cytometer using FACSDiva 6.0 software (BD Biosciences). All analyses were blinded.
Histology and Measurement of fibrosis and Luminal Obliteration
Allograft tracheal tissues were fixed, embedded, sectioned, and stained with hematoxylin & eosin (H&E). Allograft trachea sections were photographed at 4x magnification. Collagen deposition was quantified via percent luminal obliteration of the tracheal allografts using Image-Pro Plus software. Eight allografts were measured in each group.
Collagen staining and densitometry
Trachea sections were deparaffinized, rehydrated, and stained with Direct Red 80 (Sigma, St. Louis MO). Images were captured for quantification using the Image J software. Collagen signal in luminal fibro-obliteration tissue was semi-quantified using the same software but with a different parameter setting.
Data analysis
Fibrocytes from buffy coat, bone marrow, and tracheal single cells suspensions are presented as box and whisker plots. Each box an whisker plat at a given time point represents five animals. Continuous data are reported as either mean ± standard deviation or median [interquartile range]. Independent sample comparisons were performed using either Mann-Whitney U test or the Kruskal-Wallis test. Statistical significance was set to α<0.05. Group comparisons were unpaired, and p-values are two tailed. Analyses were performed using Predictive Analytics SoftWare.
Results
Attenuation of fibrocyte trafficking via an anti-CXCL12 F(ab′)2 antibody
We first assessed the ability of neutralizing anti-CXCL12 F(ab′)2 antibodies to attenuate fibrocyte trafficking and differentiation in the murine model of bronchiolitis obliterans. Bone marrow, buffy coat, and trachea allograft single cell suspensions from Balb/C mice subcutaneously transplanted with BL6 trachea, were analyzed for total undifferentiated (CD45+Col1+CXCR4+) and differentiated fibrocyte (CD45+Col1+αSMA+) cell populations.
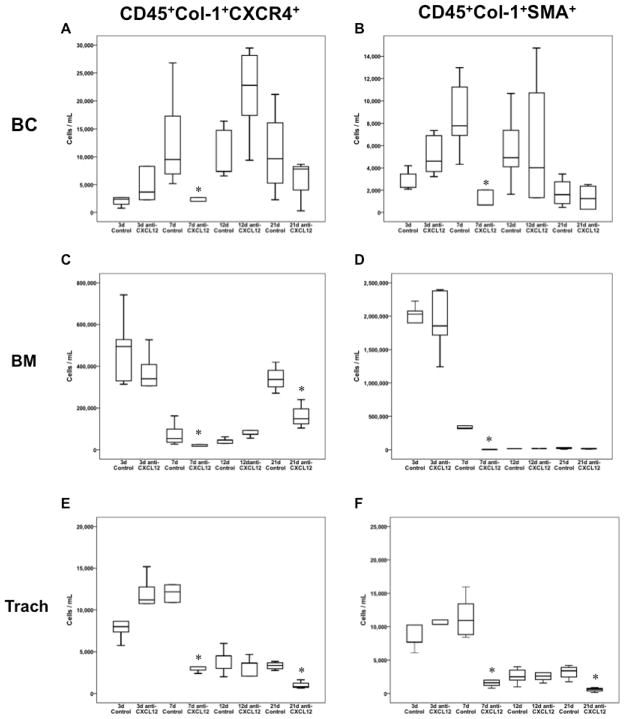
The comparison of buffy coat isolates from mouse whole blood preparations, revealed significant decrease in circulating undifferentiated fibrocytes in mice treated with anti-CXCL12 at 7 days (Figure 1a; p = 0.028) post trachea-transplantation. However, anti-CXCL12-treated mice at 12 days post transplantation (Figure 1a; p = 0.028) had increased undifferentiated fibrocytes as compared to controls. Figure 1b shows a concomitant increase in total differentiated fibrocytes in buffy coat preparations from anti-CXCL12 treated animals at 3 days (p = 0.047), with a decrease in differentiated fibrocyte population at 7 days (p = 0.008).
Figure 1.
Box and whisker plots depicting total fibrocyte populations in both anti-CXCL12 F(ab′)2 treated and control goat IgG F(ab′)2 treated animals following heterotopic transplant of allograft tracheas. Box and whiskers represent 25–75th and 10–90th percentiles, respectively; transverse lines represent the median. Each box and whisker plot at a given time point represents five animals, Panels A, C, and E represent undifferentiated, CD45+Col1+CXCR4+ fibrocyte populations over time in buffy coat (BC), bone marrow(BM), and tracheal allograft isolations (Trach), respectively. Panels B, D, and F represent differentiated, CD45+Col1+SMA+ fibrocyte populations in buffy coat, bone marrow, and trachea allograft isolations, respectively. *p<0.05 as determined by Mann-Whitney U test.
Bone marrow aspirates showed an increase in undifferentiated fibrocytes (Figure 1c) in anti-CXCL12 treated animals at 12 days post transplantation (p = 0.015) and a decrease in undifferentiated fibrocytes at 7 (p = 0.008) and 21 days post transplantation (p = 0.014) as compared to controls. Similarly, there was a significant increase in total differentiated fibrocytes (Figure 1d) in anti-CXCL12 treated animals at 12 days post transplantation (p = 0.004) with a decrease in the same population at 7 days post transplantation (p = 0.008).
As hypothesized, single cell suspensions isolated from tracheal allografts from anti-CXCL12 treated animals showed a significant decrease in both undifferentiated (Figure 1e) and differentiated (Figure 1f) fibrocytes at 7 days (p = 0.009 and p = 0.009, respectively) and 21 days post transplantation (p = 0.004 and p = 0.014, respectively) as compared to controls.
The mean ranks of undifferentiated and differentiated fibrocytes among buffy coat (H=18.95, 7d.f., p=0.008 and H=21.92, 7d.f., p=0.003, respectively), bone marrow (H=33.64, 7d.f., p<0.0001 and H=34.01, 7d.f., p<0.0001, respectively), and tracheal allograft single cell populations (H=30.29, 7d.f., p<0.0001 and H=33.02, 7d.f., p<0.0001, respectively) are significantly different among control and anti-CXCL12 treated animals. Thus, there is definite variation of fibrocyte number within each group.
Evaluation of fibro-obliteration on tracheal allografts
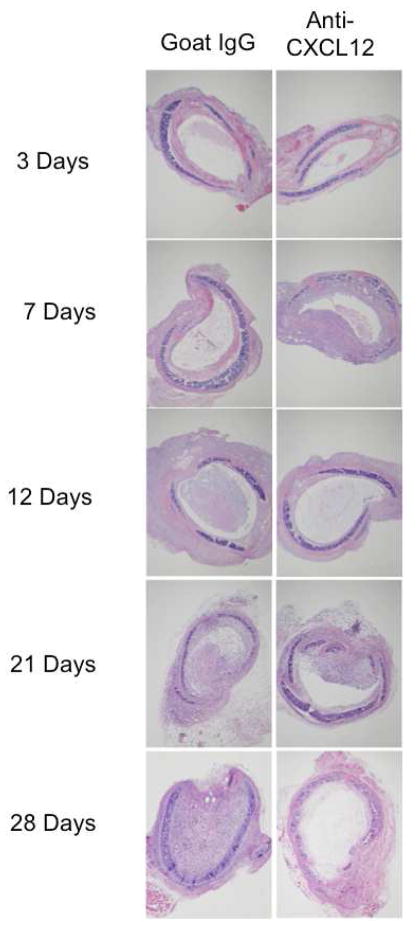
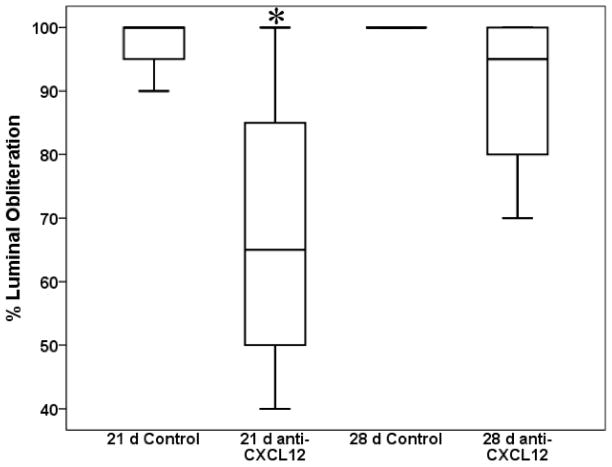
Representative H&E images of tracheal allografts from anti-CXCL12 and control animals at 3, 7, 12, and 21 days post transplantation are shown in Figure 2. Airway fibro-obliteration was scored in two ways. First, qualitative H&E analysis was reviewed blindly by a lung pathologist who determined percent luminal obliteration of tracheal allografts post tracheal transplantation (Figure 3). There was a significant decrease in median luminal obliteration at 21 days (p=0.010) in anti-CXCL12 treated animals (65.00 [38] % obliterated) as compared to controls (100 [10] % obliterated). This trend was not seen at 28 days post transplantation (p=0.153).
Figure 2.
Representative images of hematoxylin and eosin stained histopathologic sections of Goat IgG F(ab′)2 treated (Left column) or anti-CXCL12 F(ab′)2 treated (right column) animals at 3, 7, 12, 21, and 28 days post heterotopic transplantation of allograft trachea.
Figure 3.
Box and whisker plots depicting percent luminal fibrosis in tracheal allograft sections in both anti-CXCL12 F(ab′)2 treated and control goat IgG F(ab′)2 treated animals as assessed by a lung pathologist in a blinded fashion. Box and whiskers represent 25–75th and 10–90th percentiles, respectively; transverse lines represent the median. *p<0.05 as determined by Mann-Whitney U test.
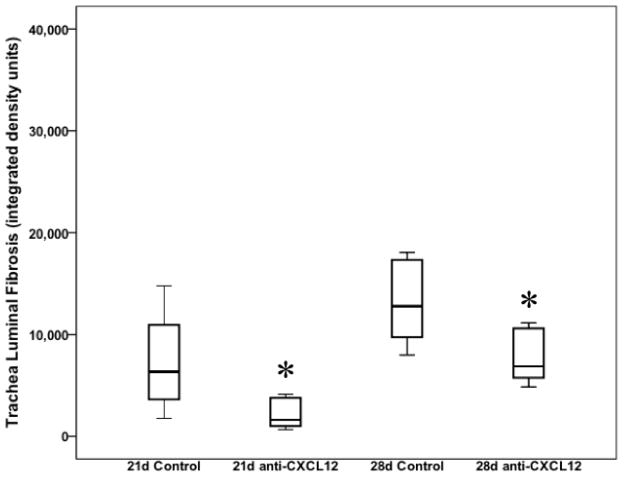
We next assessed the effect of anti-CXCL12 treatment on collagen deposition in trachea allograft lumens. Densometric quantitative measurement of direct red 80 collagen staining was used to determine total luminal collagen deposition over the same time. As expected, treatment with anti-CXCL12 resulted in a significant decrease in trachea allograft luminal collagen deposition at 21 days (1591.62 [3167.98] vs. 6314.97 [10725.91] integrated density units; p=0.042) and 28 days (6871.09 [5280.35] vs. 12775.80 [8346.05] integrated density units; p=0.0012) post transplantation (Figure 4). This is consistent with the hypothesis that inhibition of the CXCR4/CXCL12 biological axis results in attenuated fibrocyte trafficking to and differentiation in tracheal allografts.
Figure 4.
Box and whisker plots depicting percent tracheal luminal fibrosis as measured by Direct Red 80 collagen stain at 21 and 28 days post transplantation. Box and whiskers represent 25–75th and 10–90th percentiles, respectively; transverse lines represent the median. *p<0.05 as determined by Mann-Whitney U test.
Interestingly, the kinetics of total non-luminal tracheal collagen deposition shows a slightly different pattern from luminal deposition (data not shown). There is a significant decrease in tracheal fibro-obliteration in anti-CXCL12 treated mice at 12 (p<0.0001) and 21 days (p<0.0001) post transplantation as compared to controls, while no significant difference at 3, 7, and 28 days (p>0.05).
Discussion
The importance of fibrocyte biology has been established in multiple pan-corporeal fibrotic processes including normal wound repair, nephrogenic fibrosing dermatomyopathy, scleroderma, chronic pancreatitis, cystitis, liver fibrosis, and in multiple lung pathologies marked by recurring inflammation and repair (6). We have previously shown a correlation between increased circulating fibrocyte levels and the development and progression of BOS in patients following lung transplantation (8). These studies suggest that fibrocytes may contribute to the pathology of pulmonary fibrosis.
Given the potential clinical importance of fibrocytes in the progression of fibrotic pathology, we sought to identify the impact of an anti-CXCL12 F(ab′)2 antibody in mitigating tracheal fibro-obliteration. Despite its shortcomings, we utilized a murine tracheal transplant model of bronchiolitis obliterans as it is well described, less technically demanding and time consuming as compared to other models, and reproducible in its production of obliterative airway disease. Critics of this model point out that this is a large airway representation of a small airway disease and that it fails to utilize a vascularized or aerated allograft. Other models of BO have been developed, including the orthotopic tracheal model (12); however, like the heterotopic model, this is a large airway model and is not vascularized. Furthermore it is technically challenging and fails to uniformly develop histopathologic BO. Similarly, single lung transplantation has been successfully performed in mice, however these transplanted lungs allografts have not been shown to reliably develop histopathologic lesions of BO(13).
In this study, we demonstrated that an anti-CXCL12 antibody effectively attenuates fibrocyte trafficking to and differentiation in heterotopic trachea allografts. This antibody simultaneously mitigated graft disease progression and fibro-obliteration. Our findings are corroborated by evidence from other studies which found that blocking the CXCR4/CXCL12 axis with an anti-CXCL12 Ab (14) or a direct CXCR4 antagonist, AMD3100 (15), protected against pulmonary fibrosis in bleomycin-models of IPF; a process defined by cryptogenic fibro-obliteration of lung parenchyma.
Recent evidence points toward a duality in fibrocyte biology as a cell capable of both immune-modulation and ECM repair (16). The differentiation of fibrocytes into cells capable of fulfilling these roles is dependent on the cytokine milieu and progresses chronologically during the course of disease from an early role in an inflammatory phenotype to late differentiation into collagen producing reparative cells (17). The current study shows a temporal fibrocyte trafficking pattern consistent with this paradigm in which fibrocytes respond to tracheal tissue injury via CXCL12 chemotaxis and propagate a temporal fibrotic process. In the acute stages post-transplantation, there is an increase in bone marrow production of both undifferentiated (CD45+Col1+CXCR4+) and differentiated (CD45+Col1+αSMA+) fibrocytes in anti-CXCL12 treated and control animals. As expected, there is a lag in the appearance of both cell populations in buffy coat preparations.
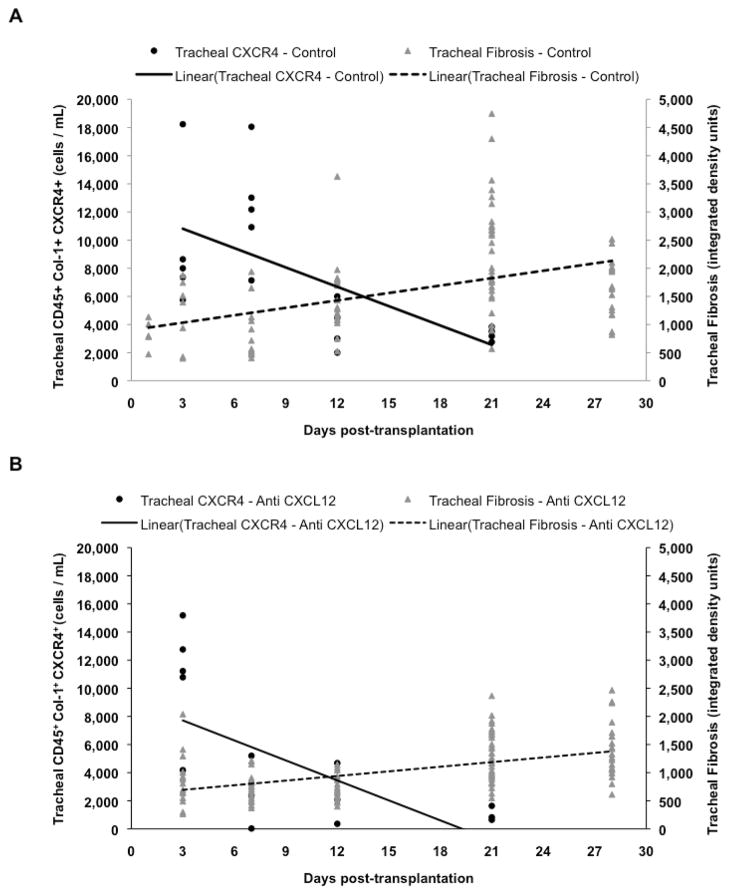
As demonstrated in figure 5a and 5b, there is an early accumulation of undifferentiated fibrocytes in tracheal allografts, which may result from the trafficking of fibrocytes already in circulation. It is noteworthy, however, that there is a lack of early concomitant fibrosis suggesting that these fibrocytes are responding to an inflammatory milieu that exists acutely post-transplantation by differentiating along antigen presenting or immune-modulatory cell lines as opposed to along a reparative cell lineage. A comparison of control and anti-CXCL12 animals demonstrates more rapid and ultimately more significant decrease in fibrocyte numbers in anti-CXCL12 treated mice and a more rapid and ultimately more significant increase in tracheal fibrosis in control animals. Kruskal-Wallis analysis demonstrated that both control and anti-CXCL12 fibrocyte populations (p<0.001) and tracheal collagen deposition (p<0.001) are significantly different.
Figure 5.
Combined kinetics of both undifferentiated, CD45+Col1+CXCR4+ fibrocytes in tracheal allografts, as determined by flow cytometry, and tracheal tissue collagen deposition, as determined by Direct Red 80 densitometry of tracheal allograft sections. Kinetics of control, Goat IgG F(ab′)2 treated animals (A) show a more gradual and less complete decrease in tracheal allograft undifferentiated fibrocyte number with a concomitant more rapid and greater increase in tracheal collagen deposition as compared to anti-CXCL12 F(ab′)2 treated animals (B). Kruskal-Wallis analysis demonstrates that both control and anti-CXCL12 fibrocyte populations (p<0.001) and tracheal collagen deposition (p<0.001) are significantly different.
Upon closer analysis, the difference between control and anti-CXCL12 treated animals is most pronounced at day 7, when anti-CXCL12 animals have a strikingly decreased number of both fibrocyte populations in buffy coat and trachea allograft isolations. While control animals continue to experience an increase in fibrocyte trafficking and differentiation into grafted airways, both fibrocyte populations in anti-CXCL12 treated animals greatly decrease. Therefore, day 7 might be significant in that it represents when the pressure to differentiate toward an inflammatory lineage subsides and the pressure to differentiate toward a reparative lineage increases. Thus, the larger fibrocyte populations on day 7 in control animals are able to respond toward this pressure and as a result, fibro-obliteration occurs more rapidly than in anti-CXCL12-treated animals.
Our data shows that inhibiting the CXCL12/CXCR4 axis has little effect on the acute bone marrow production of fibrocytes but instead appears more vital in preventing trafficking of fibrocytes from the bone marrow into the circulation and from the circulation into the extravascular compartment. Furthermore, the difference in kinetics of collagen deposition between tracheal tissue and lumen implicates the CXCL12/CXCR4 axis in the trafficking of fibrocytes through the tracheal tissue and into the tracheal lumen. At day 28, there was no difference in tracheal tissue collagen deposition between anti-CXCL12-treated mice and controls, but treated mice showed a significant decrease in total luminal collagen deposition indicating that while the anti-CXCL12 antibody did not completely prevent fibrotic disease from progressing in the tracheal allograft tissue, it mitigated total airway obliteration.
The inhibition of CXCL12 could be responsible for the decreased population of differentiated fibrocytes in trachea allografts and buffy coat isolations, as well as the decrease in luminal fibrosis in anti-CXCL12-treated mice. However, it is likely that these changes are in large part a consequence of diminished trafficking and extravasation of undifferentiated fibrocytes into the proper milieu for differentiation. While there was a drastic decrease in both buffy coat and tracheal undifferentiated and differentiated fibrocyte populations in anti-CXCL12-treated animals, there was a substantial increase in the same populations in control animals. This may suggest that in control animals, undifferentiated fibrocytes were more readily able to traffic to tracheal allografts and differentiate into fibroblastic, collagen producing cells. While the difference in fibrocytes populations may not account for the entire difference in fibro-obliteration between anti-CXCL12-treated and control animals, it does seem to be a major contributor to disease progression.
Yet, there is still an increasing accumulation of collagen deposition in tracheal allografts from anti-CXCL12 mice. This may be due, in part, to other less important chemokines (CCR7/CCR2) that are able to recruit fibrocytes into the allografts. Mouse fibrocytes have been shown to express CCR7, CXCR4, and CCR2 and respond to a number of different ligands (18). The CXCR4/CXCL12 axis is known to be the predominant receptor/ligand interaction for the trafficking to a grafted airway and subsequent extravasation of fibrocytes into tissue (5,18,19). These other receptor/ligand interactions may help to explain the differences in kinetics between the anti-CXCL12 treated and control groups. Furthermore, local differentiation of fibroblasts and epithelial to mesenchyme transition play a role in fibro-obliteration (2). The stimuli for fibrocyte production in the bone marrow and subsequent movement to blood is not clear, but it appears that other cytokine/receptor interactions may predominate. Thus blocking the CXCR4/CXCL12 axis, while being instrumental for inhibiting the progression of fibro-obliteration, does not, by itself, eliminate collagen deposition.
Our studies provide novel insight into the kinetics of fibrocyte trafficking during the progression of airway fibro-obliteration and demonstrate the potential that inhibiting this axis has for slowing the disease process. Anti-CXCL12 F(ab′)2 afforded protection against infiltrating fibrocytes and deterioration of allograft tracheal epithelium and collagen deposition. Thus, the CXCL12/CXCR4 axis provides a novel therapeutic target for the treatment of fibroproliferative disease. Given the clinical impact that fibrotic diseases have on morbidity and mortality further study is warranted in both animal models and humans to identify the role of this axis in molding fibrocyte differentiation in inflammatory and reparative milieus.
Newer immunomodulatory drugs directed toward inhibition of Mammalian target of Rapamycin (mTOR), like sirolimus and everolimus, offer significant hope in prevention of allograft rejection (20). Previous studies have shown that therapy with Rapamycin attenuates fibrocyte trafficking from bone marrow and prevents fibrocyte and collagen deposition in lungs challenged with bleomycin via altering CXCR4 expression (5). Others have shown everolimus to be a potent inhibitor of proliferation of ex vivo lung fibroblasts cultured from transbronchial biopsy samples harvested from patients post lung transplantation (21) and in two separate randomized control trials of lung transplant recipients, those patients receiving maintenance everolimus were less likely to have clinically suspected and biopsy proven acute graft rejection as compared to controls (22,23). Being that these drugs are currently FDA approved for prevention of solid organ rejection and their potency for immune-modulation, they may prove to be superior alternatives to anti-CXCL12 F(ab′)2. Despite this potential, there remain many therapeutic challenges to proper administration of these drugs. In lung transplant recipients receiving sirolimus, there was an increased risk of severe venous thromboembolism (20) and in separate trials, patients treated with sirolimus suffered from airway anastomosis dehiscence and generalized poor wound healing (24,25). As a result, many clinicians have decided to administer sirolimus post completion of bronchiolar wound healing (24) and as such, the use of sirolimus across all centers was 8% at 1 year and 18% at 5 years post transplantation (1).
An optimal outcome of mTOR inhibition would allow mitigation of fibro-proliferative disease while at the same time allowing normal wound repair. We suggest that given the integral nature of mTOR and the CXCR4 biological axis to the pathogenesis of fibrosis, monitoring fibrocyte populations could offer a new biomarker for optimizing mTOR inhibitor therapy. Quantification of circulating and lung allograft fibrocyte populations may provide the clinician with an individualized picture of fibrotic stage/progression in lung transplant patients. Using these populations as biomarkers, it might be possible to tailor mTOR inhibitor drug therapy to a dose that would both maximize gain and minimize unwanted side effects on an individual basis, effectively opening the door to a more personalized medical approach for patients.
Acknowledgments
Grants
CLL is supported by a grant sponsored by the National Heart, Lung, and Blood Institute (1K08HL094704-01), the CVRC Partner’s Grant, and was the AATS John W. Kirklin Research Fellow.
Footnotes
Disclosure: There is no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011 Oct;30(10):1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009 Nov;136(5):1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- 4.Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. Journal of Clinical Investigation. 2007 Mar;117(3):549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009 Jul;41(8–9):1708–1718. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herzog EL, Bucala R. Fibrocytes in health and disease. Exp Hematol. 2010 Jul;38(7):548–556. doi: 10.1016/j.exphem.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009 Apr 1;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 8.Lapar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, et al. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Ann Thorac Surg. 2011 Aug;92(2):470–477. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson-Sjöland A, Erjefält JS, Bjermer L, Eriksson L, Westergren-Thorsson G. Fibrocytes are associated with vascular and parenchymal remodelling in patients with obliterative bronchiolitis. Respir Res. 2009;10(1):103. doi: 10.1186/1465-9921-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Lapar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, et al. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010 Dec;29(12):1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau CL, Zhao Y, Kron IL, Stoler MH, Laubach VE, Ailawadi G, et al. The role of adenosine A2A receptor signaling in bronchiolitis obliterans. Ann Thorac Surg. 2009 Oct;88(4):1071–1078. doi: 10.1016/j.athoracsur.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, et al. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007 Jun;7(6):1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 13.Hele DJ, Yacoub MH, Belvisi MG. The heterotopic tracheal allograft as an animal model of obliterative bronchiolitis. Respir Res. 2001;2(3):169–183. doi: 10.1186/rr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004 Aug;114(3):438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song JS, Kang CM, Kang HH, Yoon HK, Kim YK, Kim KH, et al. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med. 2010 Jun 30;42(6):465–472. doi: 10.3858/emm.2010.42.6.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmelli C, Alves MP, Steiner E, Zingg D, Peduto N, Ruggli N, et al. Responsiveness of fibrocytes to toll-like receptor danger signals. Immunobiology. 2007;212(9–10):693–699. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Reilkoff RA, Bucala R, Herzog EL. Nature Publishing Group. Nature Publishing Group; 2011. May 20, Fibrocytes: emerging effector cells in chronic inflammation; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007 Sep 1;82(3):449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Mora A, Shim H, Stecenko A, Brigham KL, Rojas M. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007 Sep;37(3):291–299. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahya VN, McShane PJ, Baz MA, Valentine VG, Arcasoy SM, Love RB, et al. HEALUN. 2. Vol. 30. Elsevier Inc; 2011. Feb 1, Increased risk of venous thromboembolism with a sirolimus-based immunosuppression regimen in lung transplantation; pp. 175–181. [DOI] [PubMed] [Google Scholar]
- 21.Azzola A, Havryk A, Chhajed P, Hostettler K, Black J, Johnson P, et al. Everolimus and mycophenolate mofetil are potent inhibitors of fibroblast proliferation after lung transplantation. Transplantation. 2004 Jan 27;77(2):275–280. doi: 10.1097/01.TP.0000101822.50960.AB. [DOI] [PubMed] [Google Scholar]
- 22.Kovarik JM, Snell GI, Valentine V, Aris R, Chan CKN, Schmidli H, et al. Everolimus in pulmonary transplantation: pharmacokinetics and exposure-response relationships. J Heart Lung Transplant. 2006 Apr;25(4):440–446. doi: 10.1016/j.healun.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Snell GI, Valentine VG, Vitulo P, Glanville AR, McGiffin DC, Loyd JE, et al. Everolimus Versus Azathioprine in Maintenance Lung Transplant Recipients: An International, Randomized, Double-Blind Clinical Trial. Am J Transplant. 2006 Jan;6(1):169–177. doi: 10.1111/j.1600-6143.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- 24.Groetzner J, Kur F, Spelsberg F, Behr J, Frey L, Bittmann I, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. HEALUN. 2004 May;23(5):632–638. doi: 10.1016/S1053-2498(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 25.King-Biggs MB, Dunitz JM, Park SJ, Kay Savik S, Hertz MI. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation. 2003 May 15;75(9):1437–1443. doi: 10.1097/01.TP.0000064083.02120.2C. [DOI] [PubMed] [Google Scholar]