Abstract
Introduction
RNA-dependent protein kinase (PKR) is an independent prognostic variable in patients with non-small cell lung cancer (NSCLC). In the present study, we investigated the correlation between PKR and 25 other biomarkers for NSCLC, identified the markers that could further improve the prognostic significance of PKR, and elucidated the mechanisms of interaction between these markers and PKR.
Methords
Tissue microarray samples obtained from 218 lung cancer patients were stained with an anti-PKR antibody and antibodies against 25 biomarkers. Immunohistochemical expression was scored and used for Kaplan-Meier survival analysis. The interaction between PKR and EphA2 in NSCLC cell lines was examined.
Results
We found that PKR was associated with EphA2 and that the prognostic information regarding NSCLC provided by the combination of PKR and EphA2 (P/E) was significantly more accurate than that provided by either marker alone. The 5-year overall survival rate in PKRlow/EphA2high patients (20%) was significantly lower than that of PKRhigh/EphA2low patients (74%), PKRhigh/EphA2high patients (55%), and PKRlow/EphA2low patients (55%) (p< 0.0001). We also found that the PKR:EphA2 (P/E) ratio was significantly associated with prognosis (p< 0.0001). Univariate and multivariate Cox analyses revealed that this P/E combination or ratio was an independent predictor of overall survival. In addition, induction of PKR expression reduced EphA2 protein expression levels in NSCLC cell lines.
Conclusions
PKR/EphA2 is a significant predictor of prognosis for NSCLC. PKR/EphA2 may be a promising approach to improving screening efficiency and predicting prognosis in NSCLC patients.
Keywords: PKR, EphA2, Biomarker, Lung cancer
The progression of non-small cell lung cancer (NSCLC), is driven by a variety of cell signaling pathways. NSCLC is molecularly heterogeneous, leading to differences in clinical outcome and requires the identification of multiple biomarkers of NSCLC to accurately predict outcome.1, 2 One potential biomarker of NSCLC is RNA-dependent protein kinase (PKR), a serine/threonine kinase initially identified as an innate immune antiviral protein that has a role in other cellular functions, including apoptosis, growth regulation, cell proliferation, and inflammation.3-7 We previously demonstrated that a PKR pathway is necessary for induction of cell death in various cancer cells after different treatments.8-11 In addition, autophosphorylated PKR can catalyze the phosphorylation of target substrates of eIF2α, thereby dramatically inhibiting protein synthesis, inducing apoptosis, and increasing the expression of proapoptotic factors such as Fas.3, 4 PKR may have a role as a tumor suppressor in patients with leukemia or other hematologic malignancies.12-14 PKR is a prognostic marker of several human cancers, including lung, liver, colon, and head and neck cancers.15, 16 What’s more, high PKR expression predicts favorable outcomes in lung cancer patients.17, 18 However, new biomarkers that can further improve the prognostic significance of PKR must be identified.
In the current study, we used immunohistochemical analysis to investigate the association between PKR and 25 biomarkers and found that the combination of PKR and EphA2 is a significantly more accurate prognostic indicator for NSCLC than either marker alone. EphA2 expression is frequently elevated in several different types of cancer cells, including lung, breast, colon, esophageal, ovarian, pancreatic, and prostate cancer cells.19, 20 High EphA2 protein expression predicts poor survival and disease recurrence in patients with these cancers.21, 22 These opposing functions of PKR and EphA2 led us to investigate the mechanisms of these two biomarkers’ interaction in NSCLC cells.
MATERIAL AND METHODS
Patients and Tissue Samples
218 NSCLC (119 adenocarcinomas and 99 squamous cell carcinomas) patients with stage I-IV disease who were undergoing resection of their primary cancer at The University of Texas M. D. Anderson Cancer Center between 1997 and 2001 were used for this study. Patients were excluded from the study if they had neoadjuvant or adjuvant therapy. The specimens of which were obtained from Lung Cancer Specialized Program of Research Excellence Tissue Bank at The University of Texas MD Anderson Cancer Center under a protocol approved by the MD Anderson Institutional Review Board.17, 21, 23 Detailed clinical and pathologic information, including demographic data, smoking history (never- and ever-smokers), pathologic tumor-node-metastasis (TNM) stage, and overall survival data, were available for all patients. The tissue samples were selected for tissue microarray (TMA) construction. TMAs were constructed using triplicate 1-mm-diameter cores from each specimen.
Histopathologic Evaluation
Immunohistochemical staining for PKR and the other 25 biomarkers was performed as described previously.17 Briefly, formalin-fixed and paraffin-embedded tissue histology sections (5 μm thick) were deparaffinized, hydrated, and heated in a steamer for 10 min with 10 mmol/L of sodium citrate (pH 6.0) for antigen retrieval. Peroxide blocking was done with 3% H2O2 in methanol at room temperature for 15 min, followed by 10% bovine serum albumin in TBS-t for 30 min. The slides were incubated with primary antibody at 1:400 dilution for 65 min at room temperature. After washing with PBS, incubation with biotin-labeled secondary antibody for 30 min followed. Finally, the samples were incubated with a 1:40 solution of streptavidin-peroxidase for 30 min. The staining was then developed with 0.05% 3′3-diaminobenzidinetetrahydrochloride prepared in 0.05 mol/L of Tris buffer at pH 7.6 containing 0.024% H2O2 and then counterstained with hematoxylin. Formalin-fixed and paraffin-embedded lung tissues with normal bronchial epithelia were used as a positive control. For a negative control, we used the same specimens used for the positive controls, replacing the primary antibody with PBS.
Immunohistochemical expression was quantified by two independent pathologists (Drs. Pataer A and Raso MG) blinded to the patient treatment and outcome. Immunohistochemical expression for TMA was quantified using a 4-value intensity score (0 for negative, 1 for weak, 2 for moderate, and 3 for strong) and the percentage of tumor cells within each category was estimated. A final score was obtained by multiplying both intensity and extension values (0 × % negative tumor cells + 1 × % weakly stained tumor cells + 2 × % moderately stained tumor cells + 3 × % strongly stained tumor cells). The score ranged from a minimum of 0 to a maximum of 300. Cases with discordant scores between observers were reevaluated.
Cell Lines and Reagents
H1299 and H322 lung cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). Both H1299 and H322 cancer cells and their treatment with various adenovirus vectors have been well standardized in our laboratory. In addition, we have extensive experience in performing in vivo gene transduction assays in these cell lines. We can transfected great than 95% of these cells with vectors. All cell lines were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 10 mM glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin (Life Technologies, Inc., Grand Island, NY) in a 5% CO2 atmosphere at 37°C.
A small interfering RNA (siRNA) targeting the EphA2 receptor and a control nontargeting siRNA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Ephrin-A1 Fc and control Fc were obtained from R&D Systems (Minneapolis, MN). An anti-PKR (K-17) antibody was obtained from Santa Cruz Biotechnology. An anti-phosphorylated PKR (p-PKR) (Thr446) antibody was obtained from Epitomics (Burlingame, CA); a mouse anti-EphA2 antibody (Clone D7) was obtained from Millipore (Billerica, MA); and a mouse anti-β-actin antibody, which was used as the control antibody, was obtained from Sigma (St. Louis, MO).
SiRNA Transfection
H1299 and H322 lung cancer cell lines were transfected with EphA2 siRNA or control nontargeting siRNA. Briefly, lung cancer cells were plated at a density of 2.0 × 105 cells/well in RPMI 1640 medium. 24 hours after plating, cells were treated with RPMI 1640 medium. Transfected cells were incubated for a total of 96 hours at 37°C before Western blot analysis.
Treatment of Ephrin A1 for the Deregulation of EphA2 Expression
The cells were seeded in 6-well plates (2×105 cells/well) in serum-deprived RPMI 1640 medium overnight and then stimulated with 1 μg/mL ephrin-A1 Fc or control Fc for 6 or 24 hours. Cells were collected at the end of stimulation for Western blot analysis.
Adenoviral Vector Transfection
For adenoviral vector transfection experiments, cancer cells were cultured at 70% confluence, transfected with adenoviral luciferase (Ad-luc) or adenoviral wild-type PKR (Ad-WtPKR) vectors for 72 hours, and then evaluated for PKR protein expression via Western blot analysis. Western blot analysis was performed as described previously.9
Statistical Analysis
Biomarkers were assigned to either low- or high-level groups based on the median score (cutoff point for biomarkers). In the univariate analysis, continuous and categorical variables were analyzed using the independent samples t-test and χ2 test, respectively. The Kaplan-Meier method was used to estimate survival probability as a function of time for the study patients. The log-rank test was used to measure between-group differences in patient survival time. The influence of biomarker expression on survival time was calculated using the multivariate Cox proportional hazards model with adjustment for clinical and histopathologic parameters (age, sex, smoking status, and tumor histologic subgroup). The two-sided t-test was used to test equal proportion between groups in two-way contingency tables. The generalized estimating equation approach was used to estimate differences in the means for the data between groups. Statistical significance was set at p< 0.05.
Results
The Association between PKR and Protein Markers
Using TMAs and immunohistochemical analysis, we previously found that PKR expression was significantly associated with overall survival in patients with stage I-IV NSCLC who had not received neoadjuvant or adjuvant therapy.17 Wistuba and colleagues performed expression studies of 25 different protein markers according to their interest by using the same TMAs (Table 1).17, 21, 23 We next attempt to explore the correlation between PKR and these markers. We analyzed associations between PKR and known NSCLC markers and found that PKR protein expression was significantly associated with DNA nucleotide methyltransferase 1 (DNMT1), thyroid transcription factor 1 (TTF-1) and EphA2 (Table 1).
TABLE 1.
Correlation between PKR and Biomarkers on NSCLC Patients
| Marker | Rho | P |
|---|---|---|
| 5T4 mem | −0.05 | 0.55 |
| BEK nuc | −0.01 | 0.93 |
| CAV-1 cyt | −0.03 | 0.79 |
| Amphiregulin cyt | −0.15 | 0.17 |
| SOX2 cyt | 0.04 | 0.68 |
| CD24 cyt | −0.03 | 0.79 |
| CD44 cyt | −0.06 | 0.56 |
| EZH2nuc | 0.09 | 0.30 |
| FEN1 nuc | 0.16 | 0.15 |
| FLG nuc | −0.07 | 0.52 |
| HER3 cyt | 0.14 | 0.09 |
| HEY1 cyt | −0.04 | 0.72 |
| IL11R cyt | 0.03 | 0.71 |
| JNK mem | 0.10 | 0.29 |
| IRAK-1 nuc | −0.07 | 0.51 |
| Jaggedl cyt | −0.02 | 0.85 |
| NFKB nuc | −0.09 | 0.26 |
| SDC1 | 0.15 | 0.17 |
| Her2/Neu nuc | 0.13 | 0.12 |
| RFC1 cyt | 0.04 | 0.67 |
| GLUT4 cyt | 0.14 | 0.10 |
| ki67 nuc | 0.09 | 0.27 |
| DNMT1 cyt | 0.41 | <0.0001 |
| TTF1 cyt | 0.28 | <0.0001 |
| EphA2 cyt | 0.45 | <0.0001 |
Mem, Membrane staining; Nuc, Nuclear staining; Cyt, Cytosol staining.
Complementary Prognostic Value of PKR and Markers
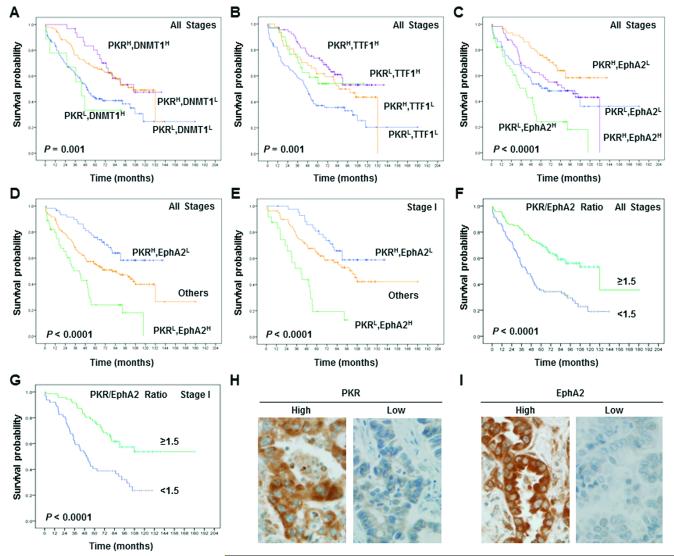
We sought to determine whether DNMT1, TTF-1 and EphA2 markers provided prognostic information for NSCLC in addition to that provided by PKR. We analyzed the combinations of PKR and DNMT1, PKR and TTF-1, and PKR and EphA2 in NSCLC patients. We combined PKR and each marker to stratify patients into four groups: high expression of both PKR and the biomarker (high/high); high PKR expression and low marker expression (high/low); low PKR expression and high marker expression (low/high); and low expression of both PKR and the biomarker (low/low). The NSCLC patients’ survival curves based on the expression of PKR and DNMT1 (Figure 1A), TTF-1 (Figure 1B), or EphA2 (Figure 1C) are shown in Figure 1. Since PKR/EphA2 was associated with the poorest survival durations in patients with NSCLC at stage I specifically (data not shown) and at all stages (Figure 1C), we focus on the relationship and mechanism between PKR and EphA2.
Figure 1.
Prognostic significance of markers as assessed using Kaplan-Meier survival estimates and the long-rank test. Kaplan-Meier survival curves showing the differences in survival duration using PKR combined with DNMT1 (A), TTF-1 (B), and EphA2 (C) in patients with all stages of NSCLC. The survival rate in patients with PKRlow/EphA2high was significantly lower than that in patients with PKRhigh/EphA2low, patients with PKRhigh/EphA2high, and patients with PKRlow/EphA2low at all NSCLC stages (D) and at stage I (E). The PKR:EphA2 ratio was significantly associated with prognosis for NSCLC at all stages (F) and at stage I (G). The survival rate in the patients with a PKR:EphA2 ratio of 1.5 less was significantly lower than that in patients with a PKR:EphA2 ratio of 1.5 or more than 1.5 (p< 0.0001). Immunohistochemical staining examples for the expressions of PKR (H) and EphA2 (I) in the NSCLC cells cytoplasm (original magnification x400).
We also stratified patients into three groups based on the combined PKR/EphA2 expression data: high PKR expression and low EphA2expression (high/low), low PKR expression and high EphA2 expression (low/high), and high or low expression of both proteins (others). Among patients with all stage of NSCLC, the 5-year overall survival rate in PKRlow/EphA2high patients (20%) was significantly lower than that of PKRhigh/EphA2low patients (74%), PKRhigh/EphA2high patients (55%), and PKRlow/EphA2low patients (55%) (Figure 1D). Among patients with stage I NSCLC, the 5-year overall survival rate in PKRlow/EphA2high patients (20%) was significantly lower than that in PKRhigh/EphA2low patients (81%), PKRhigh/EphA2high patients (61%), and PKRlow/EphA2low patients (61%) (Figure 1E). Our results also revealed that the PKR:EphA2 ratio was significantly associated with prognosis and was an independent indicator of survival duration in NSCLC patients at all stages (Figure 1F, p< 0.0001) and at stage 1 (Figure 1G, p< 0.0001). Immunohistochemical assays showed that both PKR and EphA2 proteins were primarily localized in the cytoplasm of tumor cells. Representative images of PKR and EphA2 expression in the cytoplasm of NSCLC cells are shown in Figure 1H and I.
Univariate Cox proportional hazards regression analysis revealed that age, pathologic TNM stage, pathologic T classification, pathologic N classification, PKR expression, EphA2 expression, and PKR/EphA2 expression significantly affected overall survival (Table 2). Multivariate Cox proportional hazards regression analysis revealed that the expression of PKR or EphA2 was significantly associated with overall survival rate after accounting for the effects of age and pathologic T and N classification (data not shown) and that PKR/EphA2 was an independent prognostic predictor of overall survival rate (p< 0.0001) (Table 3). Univariate and multivariate Cox proportional hazards regression analysis revealed that the PKR:EphA2 ratio was an independent prognostic predictor of overall survival rate (data not shown). We next investigated the relationships between PKR:EphA2 ratio and other clinicopathologic features. We did not detect any statistically significant correlations between PKR:EphA2 ratio and patient’s gender (p=0.2) and pathological stage (p=0.39) (Table 4). We observed that higher PKR:EphA2 ratio was associated with the adenocarcinomas subtype (p=0.003) and tobacco history (nonsmoker, p=0.001). In previous report, we have demonstrated that PKR is closely associated with its phosphorylation (p-PKR), and patients with high expression of both PKR and p-PKR had significantly longer survival than did those with other combinations of expression levels.18 In current study, we observed that higher PKR:EphA2 ratio was associated with the high p-PKR (phospho-PKR) expression (Table 4), and EphA2 protein expression negatively correlate with p-PKR protein expression (Rho= −0.41, p<0.0001). We found that patients with high expression of both PKR and p-PKR had lower EphA2 expression and patients with high expression of PKR and low expression of p-PKR had higher EphA2 expression. Our data suggest that p-PKR and EphA2 may negatively regulate each other in lung tumor.
TABLE 2.
Univariate Cox Hazards Analysis Results for Overall Survival in NSCLC Patients
| Characteristic | No. of Patients | HR (95% CI) | P |
|---|---|---|---|
| Age (Continuous) | 218 | 1.04 (1.02-1.06) | < 0.0001 |
| Gender | |||
| Female (Reference) | 113 | 1.00 | |
| Male | 105 | 1.27 (0.89-1.81) | 0.20 |
| Histology | |||
| Adenocarcinoma (Reference) | 119 | 1.00 | |
| Squamous Cell Carcinoma | 99 | 1.50 (1.04-2.14) | 0.03 |
| Tabacco History | |||
| No (Reference) | 38 | 1.00 | |
| Yes | 180 | 1.56 (0.91-2.69) | 0.11 |
| Pathological Stage | 0.04 | ||
| IA/IB (Reference) | 135 | 1.00 | |
| IIA/IIB | 47 | 1.20 (0.75-1.90) | 0.45 |
| IIIA/IIIB | 30 | 1.97 (1.20-3.26) | 0.01 |
| IV | 6 | 2.22(0.81-6.1) | 0.12 |
| PKR | |||
| Low (Reference) | 108 | 1.00 | |
| High | 110 | 0.51(0.36-0.74) | < 0.0001 |
| EphA2 | |||
| Low (Reference) | 116 | 1.00 | |
| High | 102 | 1.50(1.05-2.15) | 0.02 |
| PKR/EphA2 | < 0.0001 | ||
| PKRH, EphA2L (Reference) | 53 | 1.00 | |
| PKRL, EphA2H | 45 | 3.47 (1.99-6.03) | < 0.0001 |
| Others | 120 | 1.69 (1.03-2.75) | 0.03 |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval. (AJCC7)
TABLE 3.
Multivariate Cox Hazards Analysis Results for Overall Survival in NSCLC Patients
| Characteristic | No. of Patients | HR (95% CI) | P |
|---|---|---|---|
| Age (Continuous) | 218 | 1.05 (1.03-1.07) | < 0.0001 |
| Pathological Stage | 0.01 | ||
| IA/IB (Reference) | 135 | 1.00 | |
| IIA/IIB | 47 | 1.24 (0.77-1.99) | 0.37 |
| IIIA/IIIB | 30 | 1.84 (1.11-3.05) | 0.01 |
| IV | 6 | 3.97 (1.41-11.21) | 0.009 |
| PKR/EphA2 | < 0.0001 | ||
| PKRH,EphA2L (Reference) | 53 | 1.00 | |
| PKRL,EphA2H | 45 | 3.16 (1.8-5.55) | < 0.0001 |
| Others | 120 | 1.84 (1.12-3.02) | 0.01 |
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval. (AJCC7)
TABLE 4.
Relationships Between the PKR:EphA2 Ratio and Clinicopathologic Characteristics and Phospho-PKR Expression in 218 NSCLC patients
| Characteristics | Total Number (%) |
PKR:EphA2 Ratio |
P | |
|---|---|---|---|---|
| <1.5 | ≥1.5 | |||
| Gender | ||||
| Female | 113(52%) | 47 (45%) | 66 (58%) | 0.06 |
| Male | 105 (48%) | 57 (55%) | 42 (42%) | |
| Histology | ||||
| Adenocarcinoma | 119(55%) | 46 (44%) | 73 (64%) | 0.003 |
| Squamous Carcinoma | 99 (45%) | 58 (56%) | 41 (36%) | |
| Tobacco History | ||||
| No | 38(17%) | 9 (9%) | 29 (25%) | 0.001 |
| Yes | 180 (83%) | 95 (91%) | 85 (75%) | |
| Pathological Stage | 0.39a | |||
| I A/IB | 135 (62%) | 63 (61%) | 72 (63%) | |
| IIA/IIB | 47 (22%) | 24 (23%) | 23 (20%) | |
| IIIA/IIIB | 30 (14%) | 16(15%) | 14(12%) | |
| IV | 6 (2%) | 1 (1%) | 5 (5%) | |
| Phospho-PKR (p-PKR) | ||||
| High | 78 (47%) | 27 (33%) | 51 (61%) | <0.0001b |
| Low | 87 (53%) | 54 (67%) | 33 (39%) | |
The p-value was calculated between pathologic stage I and ll-IV.
Total 165 patients have all three markers data (PKR, EphA2 and phospho-PKR).
PKR Induction Inhibits EphA2 Protein Expression
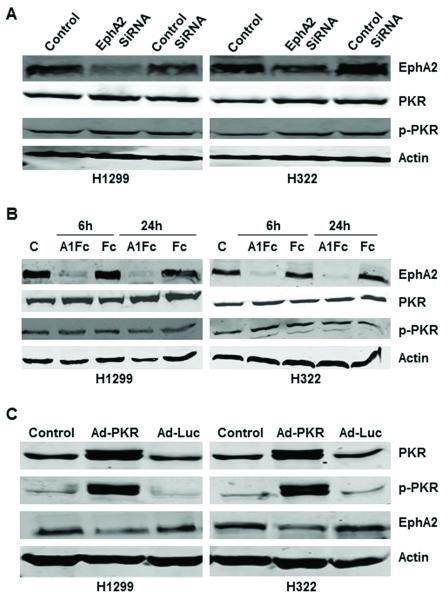
Since PKR has the strongest association with EphA2 (Table 1), and PKR/EphA2 combination was associated with the poorest survival durations in NSCLC patients (Figure 1C), we further investigated the mechanism of interaction between PKR and EphA2. We first determined whether knockdown (depletion) of EphA2 by EphA2 siRNA in human lung cancer cells (H1299 and H322) causes induction or activation of PKR. Our results showed that knockdown of EphA2 expression by EphA2 siRNA in H1299 and H322 human lung cancer cells did not affect PKR or p-PKR protein levels (Figure 2A).
Figure 2.
Induction of PKR expression inhibited EphA2. A and B, Western blot analysis of PKR, p-PKR, and EphA2 protein expression in H1299 and H322 cancer cells after knockdown of EphA2 expression by siRNA and ephrin A1Fc. Treatment with EphA2 targeting SiRNA (A) or 1 μg/mL ephrin A1Fc or Fc for 6 and 24 hours (B) did not affect PKR or p-PKR protein expression levels. C, control; A1Fc, ephrin A1Fc. Fc used as control. C, Western blot analysis of PKR, p-PKR, and EphA2 protein expression in H1299 and H322 cancer cells 72 hours after treatment with Ad-Luc (2500 viral particles/cell) and Ad-PKR (2500 viral particles/cell).
We next determined whether inhibition of the Ras pathway by ephrin-A1 in NSCLC cells caused induction of PKR or p-PKR. Ephrin-dependent EphA2 activation inhibits Ras-Raf-MEK-ERK signaling by activating the RasGTPase-activating protein, p120RasGAP, and downregulates EphA2 expression by causing receptor internalization and degradation.24 Ephrin-A1, which is a major ligand for EphA2, is anchored to the plasma membrane by a glycosylphosphatidylinositol linkage, but soluble forms of this ligand released from the cell surface can also activate EphA2.24-26 Our experiment revealed that degradation of EphA2 by ephrin-A1 Fc in H1299 and H322 lung cancer cells did not increase PKR or p-PKR expression (Figure 2B). We next determined whether using expression vectors to induce PKR or p-PKR expression in lung cancer cells reduced EphA2 protein expression levels. Compared with control and Ad-Luc, Ad-PKR inhibited EphA2 protein expression in H1299 and H322 cancer cell lines 72 hours after treatment (Figure 2C).
Discussion
In a previous study, Wistuba and colleagues used TMAs to measure the expression of 25 protein markers in NSCLC samples.21, 23 Using the same TMAs, we found that the loss of PKR expression was correlated with an aggressive disease course and that high PKR expression predicted a subgroup of patients with a better survival.17 In the present study, we investigated the association between PKR and the 25 markers and sought to identify the markers that could provide prognostic information in addition to that provided by PKR. We found that PKR protein expression was significantly correlated with DNMT1, TTF-1, and EphA2 expression and that PKR/EphA2 expression was a significant predictor of overall survival duration in NSCLC patients. We also found that the PKR:EphA2 ratio was an independent predictor of survival duration in NSCLC patients.
The present study’s findings not only identify PKR/EphA2 as a novel predictor of prognosis but also suggest that targeting PKR and/or EphA2 has therapeutic potential in NSCLC patients. PKR’s phosphorylation of eIF2α inhibits protein synthesis in host NSCLC cells, thereby causing apoptosis.7, 27, 28 High PKR expression indicates a favorable prognosis for a variety of malignancies, suggesting that PKR plays an important role effecting apoptosis in cancer cells and suppressing tumor progression.28 We previously found that high PKR or p-PKR expression in NSCLC cells correlated with a favorable prognosis, which is consistent with previous observations that PKR activation is associated with apoptosis induction.8, 9, 11, 17, 29 Several compounds can induce PKR-dependent apoptosis in cancer cells,30, 31 indicating that induction of expression or activation of PKR is one potential novel approach to treating cancer. EphA2 is detectable in many cancer cells, including ovarian, prostate, colon, and brain cancer cells. 32 In recent one study, Ishikawa M et al. reported that higher expression of EphA2 and ephrin A1 is related to favorable clinicopathological features in pathological stage I non-small cell lung carcinoma.33 However, presence of EphA2 has been associated with poor prognosis in many cancer patients.19-23,34 We anticipate that the PKR/EphA2 biomarker can be used to identify NSCLC patients who may benefit from antitumor therapy with EphA2 pathway inhibitors.
Recently, studies showed that MEDI-547, an EphA2 targeted fully human multiclonal antibody (1C1) conjugate, had a significant role in inhibiting tumor growth in mouse xenograft and rat syngeneic models of prostate and endometrial cancers.35,36 Furthermore, some peptides can selectively bind to the ligand-binding domain of EphA2 and an ephrin A1-Pseudomonas aeruginosa exotoxin A conjugate have shown promise in delivering drugs to tumors.37 Use of the combination of a PKR activator and an EphA2 inhibitor may be a promising therapy for NSCLC. In the future, we will investigate the use of combination therapy for NSCLC targeting both PKR and EphA2.
An understanding of PKR/EphA2 protein interactions is vital to the study of lung cancer and clarifying their contribution to prognosis. In the present study, we observed no significant changes in the expression of PKR or p-PKR in the NSCLC cells in which EphA2 expression was downregulated, which indicates that EphA2 may not inhibit expression of PKR directly. One potential route of indirect inhibition of PKR is the Ras-Raf-MEK-ERK pathway, which negatively regulates PKR and positively regulates the EphA2 pathway.38-41 In the present study, however, treating NSCLC cells with eprhin A1, which downregulates both the EphA2 and Ras-Raf-MEK-ERK pathways,39 did not affect the expression of PKR or p-PKR, suggesting that the Ras pathway does not regulate PKR or EphA2 in these cells. We found that induction of expression of PKR by adenoviral expression of PKR inhibited expression of EphA2 protein in lung cancer cells. We observed that EphA2 protein expression positively correlate with PKR and negatively correlate with phospho-PKR (p-PKR) proteins. The possible explanation for this association is that the high expression of EphA2 may stimulate negative feedback (PKR induction), and activation of PKR (p-PKR) by others inhibit EphA2 protein expression. We also suspect that PKR and EphA2 regulate several common downstream targets and that cancer cell growth depends on the balance of PKR/EphA2 protein expression. Further study is needed to identify common targets of PKR and EphA2 pathways in NSCLC tumors.
In conclusion, PKR/EphA2 is a significant predictor of prognosis in NSCLC patients. Further studies of multiple NSCLC markers mayyield additional information about the prognostic significance of PKR/EphA2. PKR/EphA2 may be a promising approach to improving screening efficiency and predicting prognosis in NSCLC patients. We observed that induction of PKR expression in lung cancer cells inhibited EphA2 protein expression in vitro. The possible explanation is that a high PKR:p-PKR ratio turns off protein synthesis, decreases EphA2 expression, and inhibits tumor growth.
Acknowledgments
We thank Denise M. Woods and Lakshmi Kakarala for their technical assistance. We thank Joseph A Munch for editorial review.
Financial support: This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672 - Lung Program, a Specialized Program of Research Excellence (SPORE) Grant CA070907 (I.I.W.), a Department of Defense grant W81XWH-07-1-0306 (I.I.W.), the Homer Flower Gene Therapy Fund, the Charles Rogers Gene Therapy Fund, the Margaret W Elkins Endowed Research Fund, the Flora and Stuart Mason Lung Cancer Research Fund, the Phalan Thoracic Gene Therapy Fund, and the George P. Sweeney Esophageal Research Fund (S.G.S.).
Footnotes
Disclosure: The authors declare no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brower V. Biomarker studies abound for early detection of lung cancer. J Natl Cancer Inst. 2009;101:11–13. doi: 10.1093/jnci/djn483. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg AK, Lee MS. Biomarkers for lung cancer: clinical uses. Curr Opin Pulm Med. 2007;13:249–255. doi: 10.1097/MCP.0b013e32819f8f06. [DOI] [PubMed] [Google Scholar]
- 3.Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber GN. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–570. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- 5.Gil J, Alcami J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NF-kappaB. Mol Cell Biol. 1999;19:4653–4663. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams BR. Signal integration via PKR. Sci STKE. 2001;89:re2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 7.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 8.Pataer A, Hu W, Xiaolin L, et al. Adenoviral endoplasmic reticulum-targeted mda-7/interleukin-24 vector enhances human cancer cell killing. Mol Cancer Ther. 2008;7:2528–2535. doi: 10.1158/1535-7163.MCT-08-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pataer A, Vorburger SA, Barber GN, et al. Adenoviral transfer of the melanoma via up-regulation of the double-stranded RNA-dependent protein kinase (PKR) Cancer Res. 2002;62:2239–2243. [PubMed] [Google Scholar]
- 10.von Holzen U, Bocangel D, Pataer A, et al. Role for the double-stranded RNA-activated protein kinase PKR in Ad-TNF-alpha gene therapy in esophageal cancer. Surgery. 2005;138:261–268. doi: 10.1016/j.surg.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Vorburger SA, Pataer A, Yoshida K, et al. Role for the double-stranded RNA activated protein kinase PKR in E2F-1-induced apoptosis. Oncogene. 2002;21:6278–6288. doi: 10.1038/sj.onc.1205761. [DOI] [PubMed] [Google Scholar]
- 12.Hii SI, Hardy L, Crough T, et al. Loss of PKR activity in chronic lymphocytic leukemia. Int J Cancer. 2004;109:329–335. doi: 10.1002/ijc.11714. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Koromilas AE. Dominant negative function by an alternatively spliced form of the interferon-inducible protein kinase PKR. J Biol Chem. 2001;276:13881–13890. doi: 10.1074/jbc.M008140200. [DOI] [PubMed] [Google Scholar]
- 14.Beretta L, Gabbay M, Berger R, Hanash SM, Sonenberg N. Expression of the protein kinase PKR in modulated by IRF-1 and is reduced in 5q-associated leukemias. Oncogene. 1996;12:1593–1596. [PubMed] [Google Scholar]
- 15.Haines GK, 3rd, Becker S, Ghadge G, Kies M, Pelzer H, Radosevich JA. Expression of the double-stranded RNA-dependent protein kinase (p68) in squamous cell carcinoma of the head and neck region. Arch Otolaryngol Head Neck Surg. 1993;119:1142–1147. doi: 10.1001/archotol.1993.01880220098012. [DOI] [PubMed] [Google Scholar]
- 16.Singh C, Haines GK, Talamonti MS, Radosevich JA. Expression of p68 in human colon cancer. Tumour Biol. 1995;16:281–289. doi: 10.1159/000217945. [DOI] [PubMed] [Google Scholar]
- 17.Pataer A, Raso MG, Correa AM, et al. Prognostic significance of RNA-dependent protein kinase on non-small cell lung cancer patients. Clin Cancer Res. 2010;16:5522–5528. doi: 10.1158/1078-0432.CCR-10-0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Correa AM, Raso MG, et al. The role of PKR/eIF2α signaling pathway in prognosis of non-small cell lung cancer. PLoS One. 2011;6:e24855. doi: 10.1371/journal.pone.0024855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ireton RC, Chen J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr Cancer Drug Targets. 2005;5:149–157. doi: 10.2174/1568009053765780. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 21.Brannan JM, Dong W, Prudkin L, et al. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res. 2009;15:4423–4430. doi: 10.1158/1078-0432.CCR-09-0473. [DOI] [PubMed] [Google Scholar]
- 22.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 23.Brannan JM, Sen B, Saigal B, et al. EphA2 in the early pathogenesis and progression of non-small cell lung cancer. Cancer Prev Res (Phila) 2009;2:1039–1049. doi: 10.1158/1940-6207.CAPR-09-0212. [DOI] [PubMed] [Google Scholar]
- 24.Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Jagus R, May WS. Interleukin 3 stimulates protein synthesis by regulating double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:7455–7459. doi: 10.1073/pnas.91.16.7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen AB, Stockhausen MT, Poulsen HS. Cell adhesion and EGFR activation regulate EphA2 expression in cancer. Cell Signal. 2010;22:636–644. doi: 10.1016/j.cellsig.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 27.Saelens X, Kalai M, Vandenabeele P. Translation inhibition in apoptosis: caspase-dependent PKR activation and eIF2-alpha phosphorylation. J Biol Chem. 2001;276:41620–41628. doi: 10.1074/jbc.M103674200. [DOI] [PubMed] [Google Scholar]
- 28.Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–138. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 29.Mankouri J, Dallas ML, Hughes ME, et al. Suppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistence. Proc Natl Acad Sci U S A. 2009;106:15903–15908. doi: 10.1073/pnas.0906798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu W, Hofstetter W, Wei X, et al. Double-stranded RNA-dependent protein kinase-dependent apoptosis induction by a novel small compound. J Pharmacol Exp Ther. 2009;328:866–872. doi: 10.1124/jpet.108.141754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian W, Liu J, Tong Y, et al. Enhanced antitumor activity by a selective conditionally replicating adenovirus combining with MDA-7/interleukin-24 for B-lymphoblastic leukemia via induction of apoptosis. Leukemia. 2008;22:361–369. doi: 10.1038/sj.leu.2405034. [DOI] [PubMed] [Google Scholar]
- 32.Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15:419–433. doi: 10.1016/j.cytogfr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa M, Miyahara R, Sonobe M, et al. Higher expression of EphA2 and ephrin-A1 is related to favorable clinicopathological features in pathological stage I non-small cell lung carcinoma. Lung Cancer. 2012;763:431–438. doi: 10.1016/j.lungcan.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Faoro L, Singleton PA, Cervantes GM, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem. 2010;285:18575–18585. doi: 10.1074/jbc.M109.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson D, Gooya J, Mao S, et al. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res. 2008;68:9367–9374. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]
- 36.Lee JW, Stone RL, Lee SJ, et al. EphA2 targeted chemotherapy using an antibody drug conjugate in endometrial carcinoma. Clin Cancer Res. 2010;16:2562–2570. doi: 10.1158/1078-0432.CCR-10-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wykosky J, Gibo DM, Debinski W. A novel, potent, and specific ephrinA1-based cytotoxin against EphA2 receptor expressing tumor cells. Mol Cancer Ther. 2007;6:3208–3218. doi: 10.1158/1535-7163.MCT-07-0200. [DOI] [PubMed] [Google Scholar]
- 38.Miao H, Wei BR, Peehl DM, et al. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 39.Macrae M, Neve RM, Rodriguez-Viciana P, et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell. 2005;8:111–118. doi: 10.1016/j.ccr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Smith KD, Mezhir JJ, Bickenbach K, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]