Summary
Complement 5a (C5a) is a critical modulator of liver immunity. In this study, we investigated the role of C5a and its receptor in liver fibrosis in patients with hepatitis B virus infection. We found that plasma C5a concentration was significantly increased in patients with chronic hepatitis B, particularly in those patients with higher grade and stage scores. Further analysis indicated that the increased C5a concentration was positively correlated with clinical parameters reflecting liver fibrosis severity, including type IV collagen and procollagen type III N‐terminal peptide. Our in vitro data indicated that the C5a receptor is highly expressed in hepatic stellate cells (HSCs). Addition of C5a significantly activated HSCs and up‐regulated α‐smooth muscle actin, hyaluronic acid and type IV collagen expression. Also, addition of C5a could inhibit the spontaneous and soluble tumour necrosis factor‐related apoptosis‐inducing ligand‐induced apoptosis of HSCs. These findings highlight the potential role of C5a in the regulation of liver fibrosis.
Keywords: complement 5a, hepatic stellate cell, hepatitis B virus, liver fibrosis
Introduction
Hepatitis B virus (HBV) is a major human pathogen infecting about 400 million people worldwide.1 Chronic HBV infection leads to chronic hepatitis B (CHB) and liver cirrhosis, which can further develop into hepatocellular carcinoma. During this disease progression, liver fibrosis is generally considered to be a key event. Liver fibrosis can be considered as a wound‐healing response to persistent liver injury. An imbalance in extracellular matrix synthesis and degradation mediated primarily by activated hepatic stellate cells (HSCs) is regarded as the major cause of liver fibrosis.2 Following liver injury, HSCs undergo a phenotypic switch from quiescent, vitamin A storing cells to proliferative myofibroblast‐like cells that display the up‐regulated expression of α‐smooth muscle actin (α‐SMA) and collagen.3 Resolution of hepatic fibrosis, which generally occurs following clearance of the primary liver disease, is usually characterized by the loss of activation in HSCs.4 The biological function of HSCs is regulated by a substantial number of cytokines, including transforming growth factor‐β, platelet‐derived growth factor and interleukin‐8.5,6 It is of importance to elucidate the mechanisms contributing to hepatic fibrosis to provide a comprehensive portrait of fibrosis progression and regression.
Increasing evidence indicates that the complement system is actively involved in the pathogenesis of a variety of liver disorders, including liver injury and repair, and fibrosis. For example, complement 5 (C5) ‐deficient mice revealed an impairment in liver regeneration.7 C5 is also a quantitative trait gene that modifies liver fibrosis in mice and humans.8 Except for innate compounds, complement activation exerts its harmful roles through the generation of complement protein split products. C5a, one of the most potent inflammatory peptides, exerts its effects through two high‐affinity C5a receptors, C5aR and C5L2. C5L2 is considered to be the ‘default’ C5a receptor, and is thought to play an important role in balancing the biological effect of C5a.9 In rat HSCs, anaphylatoxin C5a exposure was associated with the up‐regulation of fibronectin, but not entactin, collagen IV or α‐SMA.10 Despite these intriguing studies, the association between C5a and liver fibrosis in human patients has not been previously examined.
In this study, we found that C5a was significantly increased in patients with CHB, and this increase was positively associated with liver fibrosis severity. Our in vitro data indicated that HSCs express high levels of C5aR, and that addition of C5a can up‐regulate α‐SMA expression and hyaluronic acid and type IV collagen production, and also inhibit HSC apoptosis. These findings highlight the role of C5a in liver fibrosis, and could potentially lead to the development of novel therapies targeting C5a/C5aR.
Materials and methods
Human subjects
Blood samples were collected from 73 HBV‐infected patients. The diagnoses of these patients were made using the diagnostic criteria of the 2010 Viral Hepatitis Management Scheme, issued by the Chinese Society of Infectious Diseases and the Chinese Medical Association of Hepatology. Individuals with concurrent hepatitis C virus, hepatitis G virus, or HIV‐1 infection were excluded. Patients with autoimmune liver diseases or who met clinical or biological criteria of bacterial or fungal infection were also excluded. The study protocol was approved by the ethics committee of the Beijing 302 hospital, and written informed consent was obtained from each subject. Seventeen age‐matched and sex‐matched healthy individuals were enrolled as controls. The basic clinical characteristics of these subjects are listed in Table 1.
Table 1.
Clinical characteristics of the study population
| Group | Chronic hepatitis B patients | Healthy control subjects |
|---|---|---|
| Cases | 73 | 17 |
| Sex (male/female) | 48/25 | 9/8 |
| Age (years) | 44 (18–52) | 30 (19–45) |
| ALT (U/l) | 93 (48–751) | ND |
| HBV DNA (IU/ml) | 3·42 × 107 (2·29 × 103–2·48 × 109) |
ND |
| HBeAg positive | 42 | 0 |
| HBeAb positive | 31 | 0 |
ALT, alanine transaminase; HBV, hepatitis B virus; HBeAg, hepatitis B ‘e’ antigen; HBeAb, hepatitis B ‘e’ antibody.
Data are shown as median and range.
These HBV‐infected patients were grouped according to their fibrosis stage (S) score, as determined by liver biopsy. There were 23 patients categorized as S1, 27 patients from S2 to S3, and 23 patients scored S4. All these patients were also re‐categorized by inflammation grade (G) score. There were 27 patients categorized as G1, 33 patients as G2, 13 patients were scored from G3 to G4.
Plasma measurements
Plasma was collected from patients with different grading and staging scores, and was used to measure the circulating concentration of C5a, laminin, hyaluronic acid, procollagen type III N‐terminal peptide (PIIINP) and collagen type IV. The plasma C5a concentration was detected using a commercial enzyme‐linked immunosorbent assay kit (Hycult Biotechnology, Uden, the Netherlands). The assay sensitivity was 0·3 ng/ml. The plasma concentrations of laminin, hyaluronic acid, PIIINP and collagen type IV were detected using a chemiluminescent quantitative immunoassay. Serum HBV DNA was quantified by using a commercial real‐time PCR kit (PG Biotech, Shen Zhen, China) according to the manufacturer's instructions.
Cell culture
LX‐2, a strain of human HSC, was maintained in our laboratory and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin G and 100 μg/ml streptomycin. The level of α‐SMA and C5aR expression on the surface of LX‐2 cells was measured at 0, 24, 48 and 72 hr in culture.
FACS analysis
All antibodies were purchased from BD Biosciences (San Jose, CA), except for allophycocyanin‐conjugated anti‐human C5aR (CD88; R&D Systems, Minneapolis, MN; clone FAB3648A) and phycoerythrin‐conjugated anti‐human‐α‐SMA antibody (R&D Systems; clone IC1420P). The surface and intracellular staining was performed according to our previously described protocols.11 FACS analysis was performed using facscalibur and cellquest software (Becton Dickinson, San Jose, CA).
Cell stimulation
LX‐2 cells were cultured in a six‐well plate in the presence of 50 nm C5a (Hycult Biotechnology; HC2101) for 48 hr. Then the cells were spun down and collected for measuring α‐SMA and C5aR expression. The supernatants were collected to measure the production of hyaluronic acid and collagen type IV. Alternatively, LX‐2 cells were cultured in medium and medium containing soluble tumour necrosis factor‐related apoptosis‐inducing ligand (TRAIL) (20 ng/ml) in a 12‐well plate in the presence of anti‐TRAIL (50 ng/ml) or C5a (50 nm) for 10 hr. Then, apoptotic HSCs were identified by measuring annexin V and 7‐aminoactinomycin D (7‐AAD; SouthernBiotech, Birmingham, AL; SBA 05‐10010‐09) through FACS analysis. In both experiments, untreated cells were used as controls.
Immunofluorescent and immunohistochemical staining
For the detection of C5aR and α‐SMA expression, LX‐2 cells grown in chamber slides or liver frozen sections (4 μm) were fixed with cold acetone (−20°) for 5 min, and then rapidly rehydrated, permeabilized and blocked with blocking solution (PBS with 10% BSA). Subsequently, the samples were incubated with anti‐human α‐SMA at a dilution of 1 : 200 (ZhongShang Goldenbridge Biotech, Beijing, China) and anti‐human C5aR antibody at a dilution of 1 : 100 (Hycult Biotechnology; HM2095). The samples were incubated with those antibodies at 4° overnight and subsequently incubated for 30 min at room temperature with Alexa Fluor 488 goat anti‐rabbit and Alexa Fluor 594 goat anti‐mouse antibodies. Images were acquired with a Nikon Eclipse E600 fluorescence microscope (Nikon, Tokyo, Japan).
Paraffin‐embedded, formalin‐fixed liver tissue (≥ 5 μm) obtained from patients was deparaffinized, rehydrated and blocked with 3% hydrogen peroxide. Then, anti‐α‐SMA antibodies (blue fluorescence) and anti‐C5aR antibodies (red fluorescence) were used for double staining. Positively stained cells were counted using a high‐power microscope (400 × magnification) according to standard protocols.
RNA extraction and reverse transcription‐PCR
Total RNA was isolated from LX‐2 cells using the TRIzol method (Life Technologies Inc., Rockville, MD). Reverse transcription was performed with 5 μg RNA using the Superscript II RNase H Reverse Transcriptase (Gibco‐BRL Inc., Grand Island, NY). The primers used for amplifying C5aR were as follows: 5′ primer: 5′‐TCCTGCCCTCCCTCATC‐3′, and 3′ primer: 5′‐GCTGTAGTCCACGCCAC‐3′. The gene expression level of C5aR in LX‐2 cells was analysed by reverse transcription (RT‐) PCR. The RT‐PCR product size was confirmed by electrophoresis of samples using a 1·5% agarose gel.
Statistical analysis
All data were analysed using spss 13.0 for Windows software (SPSS Inc., Chicago, IL). Comparisons between various individuals were performed using the Mann–Whitney U‐test. Comparison between the same individual was performed using the Wilcoxon matched‐pairs t‐test. Correlation analysis was evaluated by the Spearman rank correlation test. For all tests, two‐sided P < 0·05 was considered to be statistically significant.
Results
Increased plasma C5a levels are positively correlated with liver injury and fibrosis in CHB patients
The plasma concentration of C5a was measured in patients with CHB and in healthy controls. We found that the plasma concentration of C5a was significantly higher in CHB patients compared with that in healthy controls (Fig. 1a). Further analysis indicated that the plasma concentration of C5a was significantly higher in CHB patients with G3 scores than that in patients with G2 and G1 scores. When patients were grouped according to fibrosis S scores, the plasma C5a concentration was also higher in patients with S4 scores than in patients with S1–S3 scores (Fig 1b). These data indicated that the plasma C5a concentration was significantly increased in patients with CHB compared with the healthy controls, particularly in patients with higher G and S scores.
Figure 1.
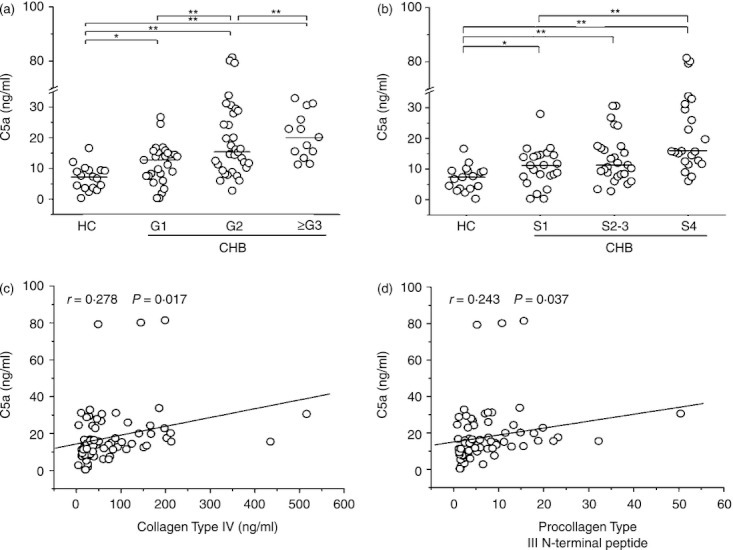
The plasma level of complement 5a (C5a) is associated with liver injury and fibrosis in patients with chronic hepatatis B (CHB). (a, b) The differential plasma C5a levels are shown in healthy controls and in CHB patients with varying Grade (a) and Stage (b) scores. HC, healthy controls; CHB, chronic hepatitis B; G, grade; S, stage. Each circle represents an individual subject. The horizontal lines are shown as median values. Comparisons between various groups were performed using the Mann–Whitney U‐test. *P < 0·05; **P < 0·01. (c, d) The plasma C5a level correlates with the clinical fibrotic markers, collagen IV (c) and procollagen type III N‐terminal peptide levels (d). Correlation analysis was evaluated by the Spearman rank correlation test. Solid line, linear growth trend; r, correlative coefficient. P‐values are shown.
Next, we analysed the correlation between the levels of plasma C5a and several clinical parameters, including HBV load, alanine transaminase (ALT) levels and markers of liver fibrosis such as hyaluronic acid, laminin, type IV collagen and PIIINP. We found a positive correlation between the levels of C5a and type IV collagen (Fig. 1c) and PIIINP levels (Fig. 1d). However, no correlation was observed between the plasma C5a concentration and ALT levels, HBV load, hyaluronic acid or laminin (data not shown). These data indicated that the plasma C5a concentration was positively associated with the severity of liver fibrosis in CHB patients.
Expression of C5aR by HSCs
C5a can exert its biological effect through binding to its high‐affinity receptor, C5aR. Interestingly, we found that LX‐2 cells, which are an immortalized human HSC line with features resembling activated HSCs, express C5aR (Fig. 2a). Using α−SMA expression as a marker of HSC activation, C5aR and α−SMA were found to be co‐expressed in LX‐2 cells (Fig. 2a). We were also able to detect C5aR mRNA in LX‐2 cells, confirming its expression in this cell line (Fig. 2b). In addition, it is found that HSC activation was accompanied by an increase in C5aR expression on the surface of LX‐2 cells (Fig. 2c). More importantly, the co‐expression of C5aR and α‐SMA was observed in the livers of patients with CHB through immunofluorescent and immunohistochemistry staining (Fig. 2d,e). These data clearly indicated that the HSCs could expressed C5aR.
Figure 2.
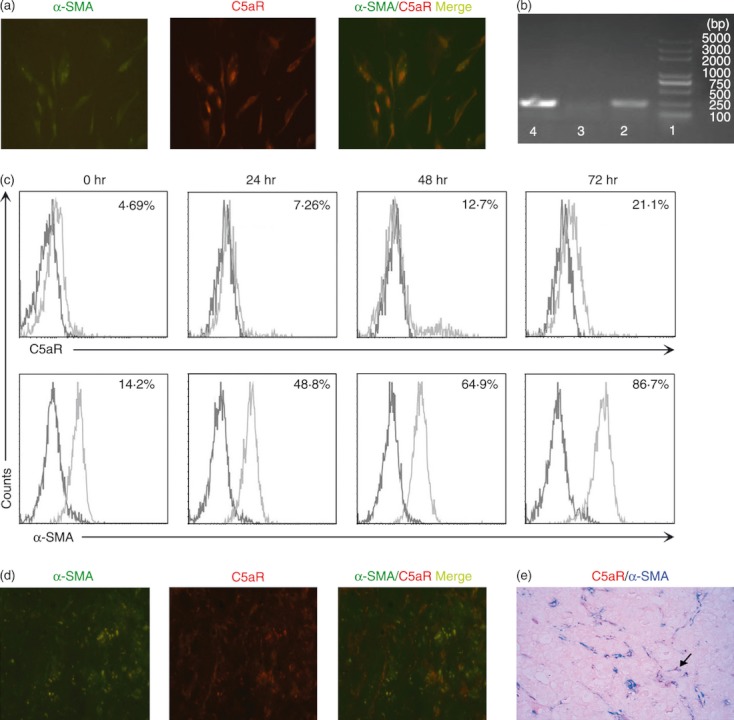
Human hepatic stellate cells (HSCs) express high levels of Complement 5a receptor (C5aR). (a) Immunofluorescence staining reflects the co‐localization (yellow) of C5aR (red) and α‐smooth muscle actin (α‐SMA; green) in activated LX‐2 cells. (b) Reverse transcription‐PCR amplification of C5aR mRNA in LX‐2 cells, neutrophils (positive controls) and Molt‐4 cells (negative control). Lane 1, DNA marker; lane 2, LX‐2 cells; lane 3, Molt‐4 cells; lane 4, neutrophils. **P < 0·01 (c) Representative histograms indicating the increased C5aR expression with the activation of LX‐2 cells in vitro at four time‐points (0, 24, 48 and 72 hr). The values showed the percentages of C5aR‐expressing and α‐SMA‐expressing LX‐2 cells. (d) Representative immunofluorescent staining for C5aR (red) and α‐SMA (green) double staining in the livers of patients with chronic hepatitis B (CHB), and (e), representative immunohistochemical staining for C5aR (red) and α‐SMA (blue) double staining in the livers from CHB patients showing the co‐localization of these markers. All images are shown in 400 × magnification.
Recombinant C5a promotes HSC activation and inhibits its apoptosis in vitro
We next investigated the role of C5a/C5aR in the regulation of human HSC activation. It was found that the addition of C5a significantly increased α‐SMA expression by LX‐2 cells in vitro (Fig. 3a,b). The addition of C5a also promoted HSCs to produce more hyaluronic acid and type IV collagen (Fig. 3c,d). Then we found that the treatment with C5a in vitro significantly reduced HSC spontaneous apoptosis, as indicated by decreased 7‐AAD and Annexin V expression (Fig. 4a). We found that sTRAIL could induce high levels of apoptosis of LX‐2 cells in vitro, and this apoptosis was completely abolished by anti‐TRAIL. Interestingly, the addition of C5a (50 nm) also significantly reduced 7‐AAD± Annexin V+ early apoptotic cells induced by sTRAIL (Fig. 4a). The pooled data further confirmed these observations (Fig. 4b). These data indicated that C5a actively involved liver fibrosis through activating HSC and maintaining their survival by reducing apoptosis.
Figure 3.
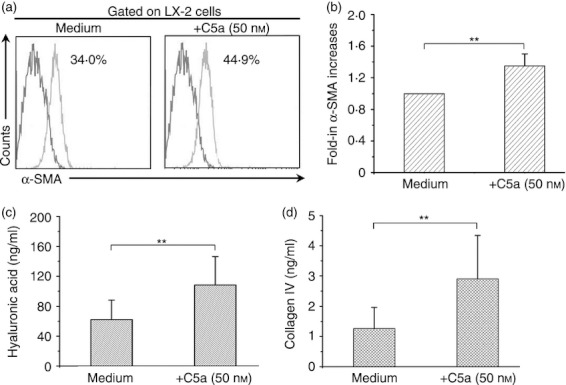
The effect of Complement 5a (C5a) exposure on activated hepatic stellate cells (HSCs) in vitro. (a) Representative histogram indicated that the addition of C5a significantly enhanced α‐smooth muscle actin (α‐SMA) expression in LX‐2 cells in vitro. The values are shown as the percentages of α‐SMA expression in LX‐2 cells (n = 4). (b) Pooled data indicating the relatively increased folds of α‐SMA expression in LX‐2 cells stimulated by C5a in relation to control cells (n = 4). (c, d) The concentration of hyaluronic acid (c) and type IV collagen (d) in the supernatants from C5a‐treated LX‐2 cells. The bars represent the means and standard deviations. **P < 0·01. The comparison was performed using the Wilcoxon matched‐pairs t‐test.
Figure 4.
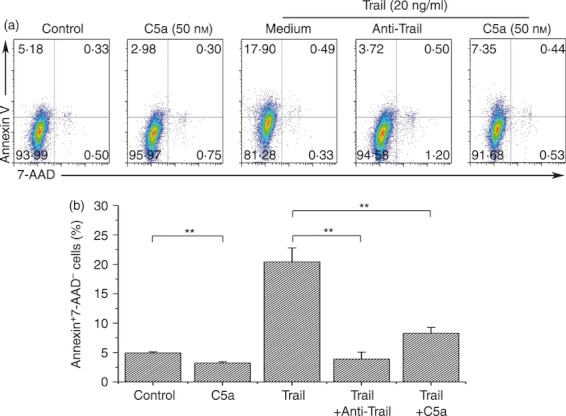
Exposure to C5a inhibited hepatic stellate cell (HSC) apoptosis in vitro. (a) Representative dot plots showing the expression of 7‐aminoactinomycin D (7‐AAD) and annexin V in LX‐2 cells in vitro. The values in the quadrants represent the percentages of LX‐2 cells that express 7‐AAD and Annexin V, respectively. (b) The pooled data indicate that the addition of C5a significantly reduced the proportion of annexin V‐positive and 7‐AAD‐negative apoptotic cells. The data were shown as the means and standard deviations (n = 5). **P < 0·01. The comparison was performed using the Wilcoxon matched‐pairs t‐test.
Discussion
Although complement has previously been shown to be involved in the pathogenesis of liver disease, the mechanistic role that C5a plays in liver fibrosis has not been fully identified. The present study uniquely found that elevated plasma levels of C5a were associated with liver inflammation and liver fibrosis severity, as indicated by G and S scores, as well as by fibrotic parameters such as collagen type IV and PIIINP in HBV‐infected CHB patients.
Several reviews have emphasized the essential role of HSC activation in the pathogenesis of hepatic fibrosis.12,13 In this regard, the current study highlights the possible mechanisms through which C5a might act as a mediator in liver fibrosis in patients with HBV infection. C5aR expression was originally described in myeloid cells including neutrophils, eosinophils, basophils and monocytes,14 as well as in a variety of non‐myeloid cells in several organs, particularly the lung and liver.15,16 In this context, we were able to demonstrate that C5aR is highly expressed in the human activated HSC line, LX‐2. It was also shown that C5aR is co‐expressed in α‐SMA‐positive human HSCs in the liver of CHB patients. More important, our data demonstrated the functional role of C5a/C5aR in the regulation of HSCs. On the one hand, C5a has the ability to activate HSCs in vitro, which was supported by our observation that HSCs exposed to recombinant C5a expressed higher levels of α‐SMA and showed an increased production of collagen type IV and PIIINP. On the other hand, C5a was also shown to have the potential to inhibit spontaneous and sTRAIL‐induced apoptosis of HSCs. Indeed, it has previously been shown that C5a modulates cytokine expression in various cell types,17 that it can enhance expression of cell adhesion molecules in neutrophils,14 induce thymocyte apoptosis,18 and reduce neutrophil apoptosis.19 Taken together, these data support the concept that C5a might actively function on C5aR‐expressing HSCs, and further possibly leading to the progression of liver fibrosis in CHB patients.
In summary, our data provide evidence to support the notion that C5a/C5aR is actively involved in the pathogenesis of liver fibrosis in patients with HBV infection. Given these interesting observations, future studies should focus on elucidating the cause and effect relationship between the high levels of C5a/C5aR and liver fibrosis progression, so as to further elucidate the potential role of C5a/C5aR in the treatment of patients with liver fibrosis.
Acknowledgments
We thank all HBV‐infected individuals and healthy participants in this study. This work was supported by grants from the National Key Basic Research Programme of China (No. 2012CB519005), The National Natural Science Foundation of China (No. 31170865), and the National Grand Programme on Key Infectious Disease (No. 2012ZX10002‐007‐002).
Disclosures
The authors declare no financial or commercial conflicts of interest.
References
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–72. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 3.Blomhoff R, Berg T. Isolation and cultivation of rat liver stellate cells. Methods Enzymol. 1990;190:58–71. doi: 10.1016/0076-6879(90)90009-p. [DOI] [PubMed] [Google Scholar]
- 4.Elsharkawy AM, Oakley F, Mann DA. The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis. 2005;10:927–39. doi: 10.1007/s10495-005-1055-4. [DOI] [PubMed] [Google Scholar]
- 5.Chamber RC, Lurent GJ. Coagulation cascade proteases and tissue fibrosis. Biochem Soc Trans. 2002;30:194–200. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 6.Mahmood S, Sho M, Yasuhara Y. Clinical significance of intrahepatic interleukin‐8 in chronic hepatitis C patients. Hepatol Res. 2002;24:413–9. doi: 10.1016/s1386-6346(02)00136-5. et al. [DOI] [PubMed] [Google Scholar]
- 7.Mastellos D, Papadimitriou JC, Franchini S, Tsonis PA, Lambris JD. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479–86. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- 8.Hillebrandt S, Wasmuth HE, Weiskirchen R. Complement factor 5 is a quantitative trait gene that modifies liver. Nat Genet. 2005;37:835–43. doi: 10.1038/ng1599. et al. [DOI] [PubMed] [Google Scholar]
- 9.Rittirsch D, Flierl MA, Nadeau BA. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–7. doi: 10.1038/nm1753. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaf G, Schmitz M, Heine I, Demberg T, Schieferdecker HL, Götze O. Upregulation of fibronectin but not of entactin, collagen IV and smooth muscle actin by anaphylatoxin C5a in rat hepatic stellate cells. Histol Histopathol. 2004;19:1165–74. doi: 10.14670/HH-19.1165. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Zhang S, Zou Z. Hypercytolytic activity of hepatic natural killer cells correlates with liver injury in chronic hepatitis B patients. Hepatology. 2011;53:73–85. doi: 10.1002/hep.23977. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gressner AM. WeiskirchenR. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF‐β as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alison MR, Vig P, Russo F, Bigger BW, Amofah E, Themis M, Forbes S. Hepatic stem cells: from inside and outside the liver? Cell Prolif. 2004;37:1–21. doi: 10.1111/j.1365-2184.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo RF, Riedemann NC, Laudes IJ, Sarma VJ, Kunkel RG, Dilley KA, Paulauskis JD, Ward PA. Altered neutrophil trafficking during sepsis. J Immunol. 2002;169:307–14. doi: 10.4049/jimmunol.169.1.307. [DOI] [PubMed] [Google Scholar]
- 15.Haviland DL, McCoy RL, Whitehead WT. Cellular expression of the C5a anaphyla‐ toxin receptor (C5aR): demonstration of C5aR on nonmyeloid cells of the liver and lung. J Immunol. 1995;154:1861–9. et al. [PubMed] [Google Scholar]
- 16.Schieferdecker HL, Schlaf G, Jungermann K, Götze O. Functions of anaphylatoxin C5a in rat liver: direct and indirect actions on nonparenchymal and parenchymal cells. Int Immunopharmacol. 2001;1:469–81. doi: 10.1016/s1567-5769(00)00038-2. [DOI] [PubMed] [Google Scholar]
- 17.Laudes IJ, Chu JC, Huber‐Lang M. Expression and function of C5a receptor in mouse microvascular endothelial cells. J Immunol. 2002;169:5962–70. doi: 10.4049/jimmunol.169.10.5962. et al. [DOI] [PubMed] [Google Scholar]
- 18.Riedemann NC, Guo RF, Laudes IJ, Keller K, Sarma VJ, Padgaonkar V, Zetoune FS, Ward PA. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 2002;16:887–8. doi: 10.1096/fj.02-0033fje. [DOI] [PubMed] [Google Scholar]
- 19.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3‐kinase‐signaling pathway. Kidney Int. 2002;61:456–63. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]


