Summary
Dendritic cells (DCs) play an essential role in immunity against bacteria by phagocytosis and by eliciting adaptive immune responses. Previously, we demonstrated that human monocyte‐derived DCs (MDDCs) express a high content of cell surface α2,6‐sialylated glycans. However, the relative role of these sialylated structures in phagocytosis of bacteria has not been reported. Here, we show that treatment with a sialidase significantly improved the capacity of both immature and mature MDDCs to phagocytose Escherichia coli. Desialylated MDDCs had a significantly more mature phenotype, with higher expression of MHC molecules and interleukin (IL)‐12, tumour necrosis factor‐α, IL‐6 and IL‐10 cytokines, and nuclear factor‐κB activation. T lymphocytes primed by desialylated MDDCs expressed more interferon‐γ when compared with priming by sialylated MDDCs. Improved phagocytosis required E. coli sialic acids, indicating a mechanism of host–pathogen interaction dependent on sialic acid moieties. The DCs harvested from mice deficient in the ST6Gal.1 sialyltransferase showed improved phagocytosis capacity, demonstrating that the observed sialidase effect was a result of the removal of α2,6‐sialic acid. The phagocytosis of different pathogenic E. coli isolates was also enhanced by sialidase, which suggests that modifications on MDDC sialic acids may be considered in the development of MDDC‐based antibacterial therapies. Physiologically, our findings shed new light on mechanisms that modulate the function of both immature and mature MDDCs, in the context of host–bacteria interaction. Hence, with particular relevance to DC‐based therapies, the engineering of α2,6‐sialic acid cell surface is a novel possibility to fine tune DC phagocytosis and immunological potency.
Keywords: dendritic cells, immunological potency, phagocytosis, sialic acid
Introduction
Dendritic cells (DCs) are essential for defence against invading pathogens by triggering and regulating host immune responses. Immature DCs actively internalize microbial antigens and undergo a maturation program leading to the initiation of T‐lymphocyte responses. Phagocytosis is an important mechanism for bacterial internalization1 that encompasses several sequential, complex events initiated by the mutual interaction of multiple components at DC and bacterial cell surfaces.
Dendritic cell maturation is characterized by profound phenotypic and physiological changes, including cytokine secretion, up‐regulation of the expression of MHC and co‐stimulatory molecules and down‐regulation of further antigen internalization.2 These processes elevate the efficiency of bacterial antigen presentation to T lymphocytes, which is essential for the induction of specific responses to fight bacterial pathogens.3 Because of the pivotal role of DCs in the elicitation of adaptive immune responses, DC‐based procedures are now being exploited as therapeutics to boost immunity against pathogens.4,5,6 Nevertheless, further studies are warranted to better elucidate DC–pathogen interactions and to improve the efficacy of these novel therapeutics.
Sialic acids are a family of sugars that occur predominantly at the terminal position of oligosaccharide chains attached to a wide variety of proteins and lipids.7,8,9 Sialylated moieties mediate selective cell‐receptor recognition and play an immunomodulatory role in host–bacteria interactions.10–15 When expressed by bacteria, sialic acids work both ways: by evading host recognition16 and by favouring recognition through inhibitory host lectin receptors.17 Moreover, desialylation mediated by sialidases of either bacterial or endogenous sources may unmask immune host cell receptors, resulting in enhanced bacterial adherence and uptake or enhanced toxin penetration into the host cells.18 We previously demonstrated that the surfaces of human monocyte‐derived DCs (MDDCs) were highly sialylated, and removal of these sialic acids resulted in induction of MDDC maturation and concomitant decreased macropinocytosis capacity.19,20 Conversely, MDDC maturation also resulted in decreased native sialylation.19–24
Inactivation of either the ST3Gal.1 or the ST6Gal.1 sialyltransferase resulted in a more mature phenotype and subsequent macropinocytosis down‐regulation in murine DCs.19,22 Together, these observations suggest a tight correlation between DC maturation, macropinocytotic functions and the state of cell surface sialylation. Enforcing the idea of sialic acids as complex endocytic modulators, we also provided evidence for the existence of membrane ectosialyltransferases that can rapidly restore cell surface sialylation and modulate human MDDC macropinocytosis.25
In this paper we intended to highlight the effect of sialic acid deficiency in the phagocytic capacity and immunological function of DCs.
Materials and methods
Generation of human MDDCs
Monocytes were isolated by positive selection using anti‐CD14‐coated magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) from peripheral blood mononuclear cells of healthy volunteers, provided and ethically approved by the Portuguese Blood Institute. The collected monocytes (CD14+ peripheral blood mononuclear cells) were then cultured for 6 days in RPMI‐1640 (Sigma, St Louis, MO) supplemented with 2 mm l‐glutamine, 1% non‐essential amino acids, 1% pyruvate, 100 μg/ml penicillin/streptomycin (Gibco, Grand Island, NY), 50 μm 2‐mercaptoethanol, 10% fetal bovine serum (FBS) from Sigma and interleukin‐4 (IL‐4) and granulocyte–macrophage colony‐stimulating factor (GM‐CSF) from R&D Systems (Minneapolis, MN), to be differentiated into immature MDDCs, as described elsewhere.20,26 Whenever needed, mature MDDCs (mMDDCs) were induced at day 5 with 5 μg/ml lipopolysaccharide (LPS) (Sigma). Staining with anti‐human CD14, BDCA‐1 and HLA‐DR antibodies (BioLegend, San Diego, CA) followed by flow cytometry analysis was used to monitor monocyte isolation, MDDC differentiation and maturation state.
Bacterial strain and labelling
An Escherichia coli K12‐derived strain was mainly used in this work. In some experiments, pathogenic E. coli isolates from blood cultures and haemocultures obtained from different patients either with urinary infection or septicaemia and identified through a Vitek 2 system (Biomérieux, Durham, NC) were used. Overnight cultures were heated at 95° for 1 hr and fluorescently labelled with 0·1 mg/ml of FITC from Molecular Probes‐Invitrogen (Leiden, the Netherlands) in the presence of 0·1 m sodium carbonate buffer (pH 9·0). Bacteria were then incubated for 1 hr, with PBS, with continuous shaking and stored at −20°.
Sialidase treatment
Human MDDCs or mMDDCs were resuspended in FBS‐free medium (5 × 106 cell/ml) and treated with 200 mU/ml sialidase from Clostridium perfringens (Roche Diagnostics, Basel, Switzerland) for 90 min at 37°. In parallel, control samples were incubated without sialidase. Identical results were obtained with heat‐inactivated sialidase. Cell surface desialylation was confirmed by staining with FITC‐labelled Sambucus nigra lectin (SNA) and Maackia amurensis lectin (MAA) (Vector Laboratories, Peterborough, UK). In some experiments the bacterial surface was desialylated with 13·5 U/ml of a sialidase from Arthrobacter ureafaciens (Sigma), according to the manufacturer's instructions.
Generation of murine bone marrow DCs
To obtain bone‐marrow‐derived DCs (BMDCs), tibiae and femurs from C57BL/6J [wild‐type, from Jackson Laboratories, Bar Harbor, ME] and ST6Gal.1‐deficient (Siat1‐null) mice27–30 were removed and the bone marrow was flushed out with RPMI‐1640 with Glutamax‐I™ (Invitrogen, Grand Island, NY), as described previously.22 The collected cell suspension was strained and pelleted, and erythrocytes and platelets were lysed with a hypo‐osmotic solution. Cells were then cultured in RPMI‐Glutamax‐I™ supplemented with 5% (volume/volume) FBS, 50 μg/ml gentamicin sulphate (Cellgro, Mediatech Inc., Manassas, VA), 50 μm 2‐mercaptoethanol (Invitrogen) and 10 ng/ml murine GM‐CSF (R&D Systems) for 7 days. The resulting BMDCs were then monitored for proper CD11b, CD11c and MHC expression with flow cytometry assays.
Phagocytosis assay
Human MDDCs/mMDDCs or mouse BMDCs (5 × 105 cell/ml) were incubated with 5 × 106 FITC‐bacteria, for 1 hr, at 37° or 4°. Incubation time was terminated by adding trypan blue to quench surface‐attached fluorescence. The phagocytosis was analysed by flow cytometry or confocal microscopy. The internalized bacteria were estimated by flow cytometry, by measuring the mean fluorescence intensity (MFI) of the cells. When appropriate, the MFI values obtained at 4° were subtracted from the 37° values.
In some experiments, phagocytosis was conducted with human MDDCs incubated with 50 μg/ml of either SNA or MAA lectins, or, alternatively, in the presence of 10 μm cytidine 5′‐monophospho‐N‐acetylneuraminic acid (CMP‐5‐NeuAc) (Sigma). In competitive binding assays, 10 mm free synthetic sialic acid (Sigma) was added 30 min before the endocytic agent and left during the assay.
Confocal laser scanning microscopy
Human MDDCs were allowed to adhere to concanavalin‐A‐coated cover glasses and then, paraformaldehyde‐fixed and permeabilized with 0·1% Triton‐X (Sigma). The cell cytoskeleton was stained with phalloidine Alexa Fluor 633 (Molecular Probes‐Invitrogen). Images were acquired with a TCS SP2 AOBS confocal microscope (Leica Microsystem, Mannheim, GmbH, Deutschland) with × 40 oil immersion optics and 488 nm and 633 nm laser lines for FITC and Alexa Fluor 633 excitation, respectively. Images were assembled and analysed with the Leica Confocal software LCS Lite 2.6 (Leica Microsystem).
T‐lymphocyte priming assay
Human T‐lymphocytes were obtained during the monocyte isolation procedure (CD14− peripheral blood mononuclear cell fraction) and maintained in complete RPMI medium until autologous monocytes differentiated into MDDCs. After the phagocytosis assay, MDDCs were co‐cultured with T lymphocytes at a DC : T‐lymphocyte ratio of 1 : 4. T‐lymphocyte stimulation was assessed by determining interferon‐γ (IFN‐γ) gene expression after 48 hr of co‐culture.
Isolation of RNA and Real‐time PCR
Expression of cytokine genes was analysed by real‐time PCR. Total RNA was extracted using the RNeasy Mini Kit and the RNase‐Free DNase Set to eliminate genomic DNA, all from Qiagen (Manchester, UK). Using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA), 1 μg of total RNA was reverse transcribed with random primers. Real‐time PCR was performed with Master Mix, TaqMan probes and primers from Applied Biosystems. The assay IDs provided by the manufacturer were: Hs00174131_m1 (IL‐6); Hs00168405_m1 (IL‐12α); Hs00174128_m1 [Tumour necrosis factor (TNF)‐α]; Hs00174086 _m1 (IL‐10); Hs00174143_m1 (IFN‐γ) and 4352935E (β‐actin). The relative mRNA levels were normalized against the arithmetic mean of the β‐actin and GAPDH expression and calculated by the adapted formula 2−ΔCt × 1000, which infers the number of mRNA molecules of the gene of interest per 1000 molecules of the endogenous controls.31 ΔCt represents the difference between the cycle threshold of the target gene and that of the endogenous control genes. The efficiency for each primer/probe was above 95% (as determined by the manufacturer).
Nuclear factor‐κB translocation
Human MDDCs were adhered to cover‐slip glasses, fixed and then permeabilized, blocked with 3% BSA for 15 min and then stained with rabbit anti‐nuclear factor‐κB (NF‐κB) p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), diluted 1 : 100, for 1 hr at room temperature. After washing with a PBS + 0·05% Tween‐20 (Sigma) solution, goat anti‐rabbit 568 (Molecular Probes‐Invitrogen) diluted 1 : 400 was used as secondary antibody. The nucleus was stained with 4′,6‐diamidino‐2‐phenylindole (DAPI) (Molecular Probes‐Invitrogen). Fluorescent images were obtained with a DMRA2 fluorescent microscope (Leica Microsystem) and merged with imageJ software (National Institutes of Health, Bethesda, MD). The percentage of cells with translocation of the NF‐κB to the nucleus was determined through the analysis of at least 600 cells in each condition.
Rho GTPases activation assay
Human MDDCs were FBS‐starved for 24 hr, to set the basal state of the GTPase activation, and then treated under various conditions. Cell lysates (0·6 mg/ml of total protein) were prepared and the Rac1 and Cdc42 activation was determined using the G‐LISA Activation Assay Biochem Kit (Cytoskeleton, Denver, CO), according to the manufacturer's instructions. The final reaction was measured by absorbance at 490 nm. Parallel positive (Rac1 or Cdc42 protein) and negative (no protein) controls were also assayed. The amounts of activated Rac1 or Cdc42 (arbitrary units) are based on the optical density values obtained after subtracting negative control values.
Statistical analysis
Experimental data were analysed using the graphpad prism statistical analysis package (GraphPad Software, Inc., San Diego, CA). Statistical differences were analysed using Student's t‐test or one‐way analysis of variance at 95% confidence level, as appropriate. A P‐value < 0·05 was considered statistically significant. All data were expressed as means ± standard errors of independent assays, i.e. using cells from different donors.
Results
Desialylated MDDCs and mMDDCs exhibit an improved phagocytic capacity for E. coli
To investigate whether removal of cell surface sialic acids affected phagocytosis, we analysed the capacity of sialidase‐treated MDDCs to internalize a K12‐derived strain of E. coli. Flow cytometry analysis revealed that desialylated MDDCs phagocytosed significantly more E. coli (MFI = 499·90 ± 66·92) than fully sialylated MDDCs (MFI = 382·80 ± 38·94) (Fig. 1a). Sialidase intervention also resulted in an increase in the number of MDDCs that internalized bacteria (73·74% ± 3·9 as compared with 58·92% ± 3·8 in the control assays) (see Supplementary material, Fig. S1). To assess the effect of sialidase treatment in already mature MDDCs, i.e. mMDDCs, we desialylated MDDCs after stimulation with LPS, a potent inducer of MDDC maturation. An established hallmark of DC maturation is the loss of internalization capacity,2,32 so, as expected, pre‐stimulation of MDDCs with LPS (mMDDCs) decreased phagocytosis in ~ 60% (Fig. 1a). However, sialidase treatment significantly lowered the typical decrease in phagocytic capacity of LPS‐matured MDDCs (Fig. 1a). Equivalent phagocytosis results were obtained for TNF‐α‐matured MDDCs (see Supplementary material, Fig. S3). Together these observations suggest that phagocytosis can be altered by the removal of sialic acids regardless of the maturation state of the DCs.
Figure 1.
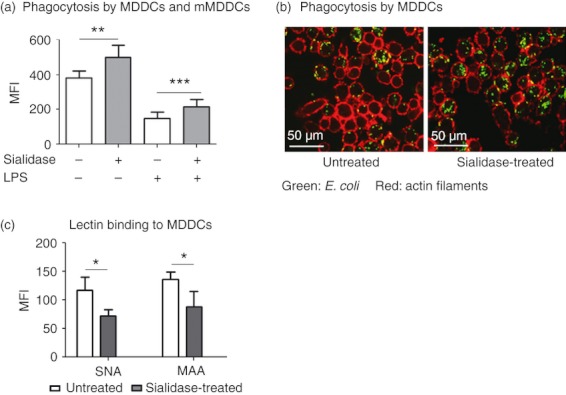
Sialidase treatment improves phagocytosis by human monocyte‐derived dendritic cells (MDDCs) and mature MDDCs. Immature MDDCs and lipopolysaccharide (LPS) ‐matured MDDCs (mMDDCs) were treated with sialidase or left untreated, following incubation with fluorescent Escherichia coli, for 1 hr at 4° or 37°. (a) The phagocytic capacity by MDDCs and mMDDCs was evaluated by flow cytometry as the mean fluorescence intensity (MFI) and values obtained at 4° were subtracted. Values represent the means of at least 20 independent assays. Statistical significance (**P < 0·001 or ***P < 0·0001) refers to the difference between untreated and sialidase‐treated MDDCs or mMDDCs. (b) Representative confocal microscopy images showing MDDCs with internalized E. coli (green). Actin filaments of the MDDCs’ cytoskeleton were stained with Phalloidin Alexa Fluor 633 (red). (c) MDDCs were stained with Sambucus nigra lectin (SNA; recognizing α2,6‐sialic acids) and Maackia amurensis lectin (MAA; recognizing α2,3‐sialic acids) lectins following sialidase treatment and analysed by flow cytometry. Values represent the means of the MFI of at least three independent assays. Statistical significance (*P < 0·05) refers to the difference between untreated and sialidase‐treated MDDCs.
The differences in phagocytosis were exclusively the result of antigen internalization and could not be attributed to differences in adhesion of the antigen to the cell surface, based on quenching experiments with trypan blue and negative control assays performed at 4°. Analysis of phagocytosis by confocal microscopy further confirmed the intracellular localization of the fluorescent bacteria (Fig. 1b and see Supplementary material, Fig. S2). Sialidase treatment led to desialylation of the MDDC surface, as confirmed by lectin‐staining assays, namely with SNA (binding to α2,6‐linked sialic acids) and MAA (binding to α2,3‐linked sialic acids). In fact, it was observed that sialidase treatment significantly decreased SNA and MAA binding to the MDDC surface (Fig. 1c), whereas no significant alterations in the level of lectin staining were observed in MDDCs due to the maturation induction by LPS or to the incubation with E. coli (results not shown).
Desialylation affects the MDDC cytoskeleton organization and the activation of Rho GTPases upon E. coli stimulation
Depending on the receptors involved, the phagocytosis often requires significant remodelling of the actin cytoskeleton and the activation of small GTPases of the Rho family, namely Cdc42 and Rac1.32,33 After observing that desialylation improves phagocytosis, we asked whether desialylation could affect the cytoskeleton organization or increase the level of Cdc42 and Rac1 GTPases activation. As shown in Fig. 2(a), desialylation did not affect the cytoskeleton organization of MDDCs. However, in the presence of E. coli, desialylated but not fully sialylated MDDCs showed a disorganization of actin filaments with a tendency to aggregate and form clusters (Fig. 2a). Sialidase treatment did not affect the basal level of active Cdc42 and Rac1 GTPases in MDDCs (Fig. 2b). However, no increase in the activation of Cdc42 and Rac1 upon E. coli phagocytosis was observed when the MDDCs were sialidase‐treated (Fig. 2b). Together these data indicate that increased phagocytosis upon removal of cell surface sialic acids by sialidase was not the result of improved MDDC cytoskeleton dynamics.
Figure 2.
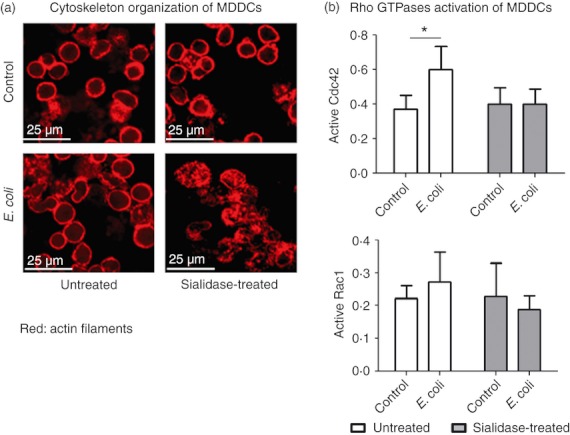
Sialidase affects the cytoskeleton organization and the activation of Rho GTPases. Monocyte‐derived dendritic cells (MDDCs) were treated with sialidase or left untreated, and then incubated or not (control) with Escherichia coli for 15 min at 37°. (a) The MDDC cytoskeleton was stained with Phalloidin Alexa Fluor 633 (red) and analysed by confocal microscopy. (b) The Rho GTPase activation level was measured in MDDC lysates, as described in the Materials and methods section. The level of active Rac1 or Cdc42 (in arbitrary units) is based in optical density values obtained at 490 nm after subtracting negative control values. Values represent the means of at least four independent assays. Statistical significance (*P < 0·05) refers to the difference between control MDDCs and MDDCs incubated with E. coli.
Desialylated MDDCs show enhanced immunological function
We then asked whether the immunological function of MDDCs following phagocytosis would change by removing sialic acids. Interestingly, we observed that desialylation and E. coli phagocytosis have an additive effect in the expression of both MHC (see Supplementary material, Fig. S4) and cytokines by MDDCs (Fig. 3a). The expression of IL‐10, IL‐12, TNF‐α and IL‐6 considerably increased following phagocytosis (Fig. 3a) but the highest level of cytokine expression was reached by the MDDCs challenged with E. coli that were previously desialylated (Fig. 3a). This remarkable observation suggests that removal of cell surface sialic acids beyond improving E. coli phagocytosis, also promotes MDDC cytokine expression.
Figure 3.
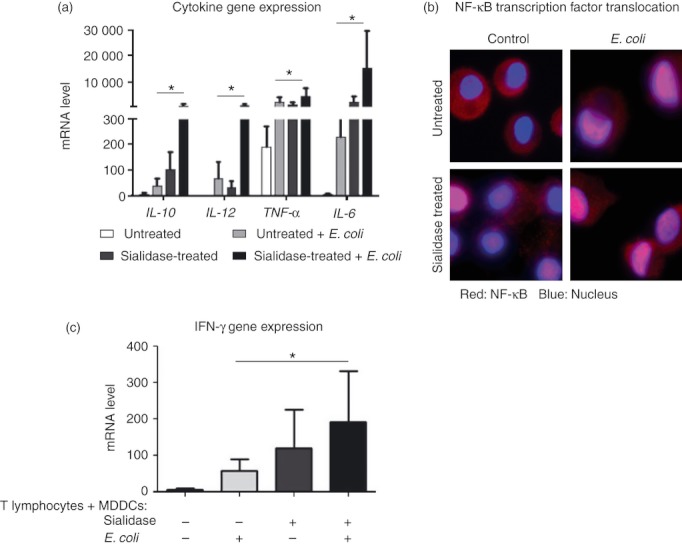
Sialidase treatment improves the immunological function of monocyte‐derived dendritic cells (MDDCs). MDDCs were treated with sialidase or left untreated, and then incubated or not (control) with Escherichia coli. (a) The expression of interleukin‐10 (IL‐10), IL‐12, tumour necrosis factor‐α (TNF‐α) and IL‐6 cytokine genes was evaluated by quantitative real‐time PCR in total RNA extracted MDDCs (following sialidase and 1‐hr incubation with E. coli). The mRNA levels of each cytokine are expressed as the permillage (‰) of the expression of the endogenous positive control, β‐actin. Values represent the means of at least six independent assays. Statistical significance (*P < 0·05) refers to the difference between untreated and sialidase‐treated MDDCs following E. coli phagocytosis. (b) Representative images of the nuclear factor‐κB (NF‐κB) transcription factor nuclear translocation. Translocation was assessed by labelling MDDCs (following sialidase and a 15‐min incubation with E. coli) with anti‐NF‐κB p65 (red) and staining the cell nucleus with DAPI (blue). Cells were then fixed and analysed by combining colours through microscopy. At least 600 cells in each condition were analysed. (c) Interferon‐γ (IFN‐γ) gene expression was evaluated by quantitative real‐time PCR in total RNA extracted from a 48 hr co‐culture of MDDCs, (following sialidase and a 1‐hr incubation with E. coli) and autologous T lymphocytes, in a DC : T‐lymphocyte ratio of 1 : 4. The IFN‐γ mRNA levels are expressed as the permillage (‰) of the expression of the endogenous positive control, β‐actin. Values represent the means of at least seven independent assays. Statistical significance (*P < 0·05) refers to the difference between untreated and sialidase‐treated MDDCs following E. coli phagocytosis.
To see how the sialidase treatment might influence the activation of NF‐κB, a key transcription factor of DC maturation,34,35 we followed the sub‐cellular distribution of p65 (RelA). RelA is a component of the NF‐κB transcription complex detectable in the cytoplasm of MDDCs that translocates to the nucleus upon DC maturation.36 Following phagocytosis, nuclear translocation of NF‐κB was observed in ~ 89% of sialidase‐treated MDDCs and in ~ 80% of untreated MDDCs. Upon sialidase treatment, NF‐κB nuclear translocation was limited to ~ 30% of the cells (Fig. 3b). The percentage of MDDCs with NF‐κB translocated to the nucleus was comparable with the cytokine levels mentioned above, suggesting the involvement of NF‐κB transcription factor in the sialidase‐induced increase of cytokine gene expression.
To assess how desialylation might influence the ability of MDDCs to stimulate a T‐lymphocyte response, we measured IFN‐γ mRNA levels in co‐cultures of MDDCs and autologous T lymphocytes (Fig. 3c). Interferon‐γ mRNA was not detected in unstimulated T lymphocytes or in MDDCs cultured alone, irrespective of them being challenged or not with E. coli (results not shown). In co‐cultures of T lymphocytes with untreated MDDCs that did not experience phagocytosis, IFN‐γ mRNA expression was also barely detectable. However, in T lymphocytes in the presence of MDDCs experiencing E. coli internalization, or pre‐treated with sialidase, or both, IFN‐γ mRNA was dramatically increased. In fact, desialylated MDDCs in response to E. coli exposure stimulate T lymphocytes to produce 70% more IFN‐γ mRNA than fully sialylated MDDCs. These results associate well with the expected downstream events accompanying maturation, including the increased cytokine and MHC expression observed in desialylated MDDCs following phagocytosis.
Enhanced phagocytosis induced by desialylation is mediated by sialic acid moieties
To further understand how MDDC cell surface sialic acids influence phagocytic activity, we examined the capacity of desialylated MDDCs to internalize E. coli in the presence of competitive free sialic acid. Interestingly, the enhanced phagocytosis observed in sialidase‐treated MDDCs was completely inhibited by the presence of free sialic acid (Fig. 4a). When SNA and MAA lectins were used to block cell surface α2,6‐linked and α2,3‐linked sialic acids, respectively, in lieu of cleaving sialic acids moieties by sialidase, the E. coli phagocytosis was not improved (Fig. 4b). These results suggest that the improved phagocytosis requires the removal of sialic acid and not the simple masking of cell surface sialic acid by lectins.
Figure 4.
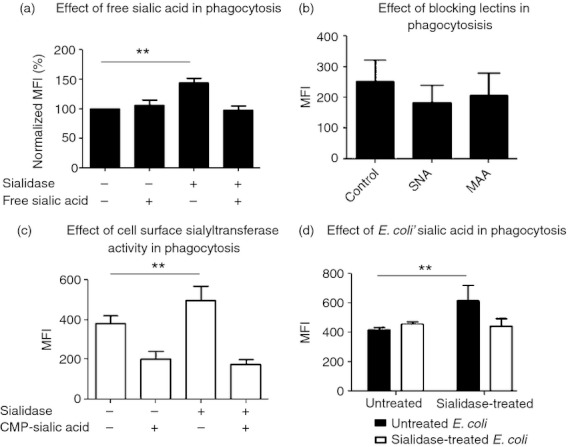
Escherichia coli phagocytosis is influenced by sialic acid moieties. Monocyte‐derived dendritic cells (MDDCs) were treated with sialidase or left untreated, and then incubated or not (control) with E. coli for 1 hr at 4° or 37°. (a) The influence of free sialic acid on phagocytosis was determined by evaluating the phagocytic capacity of MDDCs in the presence or absence of this sugar. Values, representing phagocytosis, calculated as the percentage (%) of the mean fluorescence intensity (MFI; obtained by flow cytometry) normalized with respect to untreated MDDCs incubated without free sialic acid. Values represent the means of at least five independent assays. Statistical significance (**P < 0·001) refers to the difference between sialidase‐treated and untreated MDDCs. (b) The effect of hiding surface α2,6‐linked and α2,3‐linked sialic acids in phagocytosis was determined by incubating untreated MDDCs in the absence (control) or in the presence of Sambucus nigra lectin (SNA) or Maackia amurensis lectin (MAA) blocking lectins. Values represent the means of MFI of at least three independent assays. (c) The influence of ectosialyltransferase activity in phagocytosis was assessed by conducting the assay in the presence or absence of the sialyltransferase substrate, cytidine 5′‐monophospho‐N‐acetylneuraminic acid (CMP‐5‐NeuAc). Values represent the means of MFI of at least five independent assays. Statistical significance (**P < 0·001) refers to the difference between sialidase‐treated and untreated MDDCs. (d) The participation of sialic acids from E. coli surface on phagocytosis was determined by comparing the capacity of MDDCs to internalize E. coli when sialidase‐treated or left untreated. Values represent the means of MFI of at least three independent assays. Statistical significance (**P < 0·001) refers to the difference between sialidase‐treated and untreated MDDCs.
If covalently linked cell surface sialic acids were involved in modulation of phagocytic activity, we predicted that restoration of sialic acids should undermine the sialidase activation of phagocytosis. To this end, we exploited the presence of an MDDC cell surface sialyltransferase activity that we had reported earlier,25 which would mediate the reconstruction of cell surface sialic acid linkages in the presence of the donor substrate, CMP‐5‐NeuAc. As expected, the presence of CMP‐5‐NeuAc abolished the improvement of E. coli phagocytosis by desialylated MDDCs (Fig. 4c), through the observed restoration of MDDC surface sialylation, and substantiated the idea that sialic acids act as phagocytic modulators.
A sialic acid‐dependent mechanism for the binding of sialylated bacteria to desialylated immune cells has been reported.10–15 As the E. coli‐K12‐derived strain used in the present work contains sialic acids (results not shown), we then wished to determine if improved phagocytosis was dependent on the E. coli surface sialic acids. As shown in Fig. 4(d), phagocytosis was not significantly improved upon desialylation of E. coli, suggesting a mechanism of phagocytosis mediated by sialic acids from both MDDC and E. coli.
Phagocytosis of pathogenic E. coli isolates is also improved in desialylated MDDCs
We then asked whether MDDC desialylation could improve the internalization of pathogenic strains. We used different pathogenic E. coli isolates (designated here as I, II, III, IV) obtained from urine cultures and haemocultures of patients either with a urinary infection or septicaemia. Interestingly, after treating MDDC with sialidase, the phagocytosis of all four pathogenic isolates was enhanced. In fact, as in the case of the K12‐derived strain, an improvement of 29%, 29%, 17% and 30% in the phagocytosis of pathogenic isolates I, II, III and IV, respectively, was observed when MDDCs were treated with sialidase (Fig. 5). These results suggest not only a role for sialic acid as a modulator of phagocytosis by MDDCs, but also that this property has potential therapeutic utility against pathogenic E. coli infections.
Figure 5.
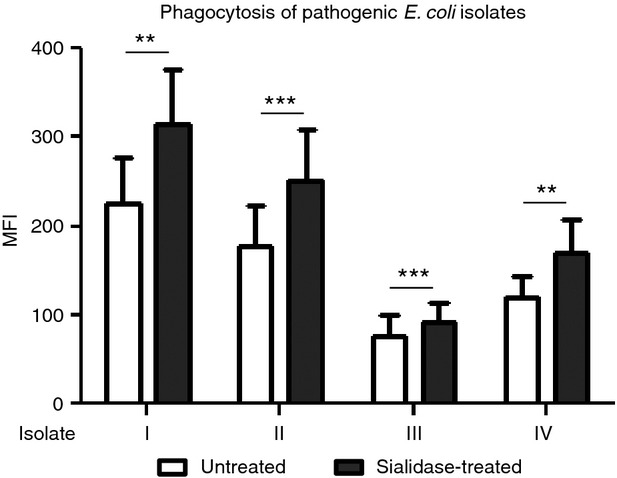
Sialidase treatment improves the capacity of monocyte‐derived dendritic cells (MDDCs) to phagocytose pathogenic Escherichia coli isolates. MDDCs were sialidase‐treated or left untreated and incubated for 1 hr, at 4° or 37°, with pathogenic E. coli isolates (I, II, III, IV). The phagocytic capacity was evaluated by flow cytometry as the MFI and values obtained at 4° were subtracted. Values represent the means of at least 10 independent assays. Statistical significance (**P < 0·001 or ***P < 0·0001) refers to the difference between sialidase‐treated and untreated MDDCs.
BMDCs from ST6Gal.1‐deficient mice have improved phagocytic capacity for E. coli
To further document the correlation between cell surface sialic acid and the phagocytic capacity of DCs without using sialidases, we used BMDCs harvested from sialyltransferase ST6Gal.1 knockout (Siat1‐null) mice,22 with a genetic inability to produce in α2,6‐linked sialic acid structures on galactose termini.27–29 As shown in Fig. 6, Siat1‐null BMDCs, showed an approximately twofold improvement in their capacity to phagocytose E. coli compared with BMDCs from the wild‐type mice. These results corroborate the data from human desialylated MDDCs, linking phagocytic capacity with cell surface sialylation status. Furthermore, the data suggest a specific contribution of α2,6‐sialic acids to this process.
Figure 6.
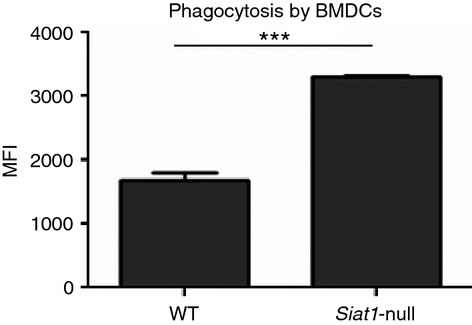
Siat1‐null bone marrow‐derived dendritic cells (BMDCs) have improved phagocytic capacity compared with wild‐type (WT). BMDCs were harvested from Siat1‐null and WT mice and incubated in presence of Escherichia coli, for 1 hr, at 4° and 37°. The data represent the means of the MFI values, obtained by flow cytometry; of at least four independent assays (values obtained at 4° were subtracted). Statistical significance (***P < 0·0001) refers to the difference between WT and Siat1‐null BMDCs.
Discussion
Antibiotic resistance among Gram‐negative pathogens has been on the rise and the current gold‐standard for the treatment of bacterial infections – the use of antibiotics – is under serious threat.37 Pathogenic variants of E. coli are known to easily acquire resistance, especially for antimicrobial agents that have long been in use in humans. Hence, while the search for new antibiotics continues, there is an urgent need for effective combat strategies against risk‐associated Gram‐negative bacteria. Reinforcement of the immune system through a synergy of cellular stimulation, anti‐microbial action, and long‐lasting anti‐bacterial protection is considered an interesting choice. Through their potent immunoregulatory capacities, DCs are promising therapeutic targets to boost sufficiently robust and long‐lasting immunity against pathogenic bacteria. Therefore, understanding the mechanisms that modulate the pivotal DC–pathogen interaction is, nowadays, a fundamental goal.
Recent reports show that human MDDCs are highly sialylated,20–24 and a growing body of evidence has implicated a role for their sialylated structures in the modulation of MDDC functions38 such as endocytosis mechanisms.22 Here, we addressed the question of whether MDDC sialic acids are particularly relevant for phagocytosis. Physiologically, it is already accepted that cell surface sialic acid content can be modulated by endogenous sialidases such as Neu139 and by sialidases from exogenous sources released by pathogenic bacteria or virus during the course of an infection. Regarding phagocytosis, it was reported that mouse cell surface Neu1 activates phagocytosis by macrophages and DCs through desialylation of cell surface receptors.18 In addition surface desialylation by influenza virus sialidase stimulates the internalization of target virus‐infected cells by mouse macrophages.40
In this work we reported for the first time that the phagocytosis by human MDDCs could also be modulated by cell surface sialic acids. We demonstrated that sialidase treatment significantly improved E. coli phagocytosis regardless of the state of DC maturation. As sialidase treatment led to actin cytoskeleton disorganization and did not lead to increased activation of the Rho family of GTPases, we inferred that desialylation influenced phagocytosis through an actin‐independent mechanism. However, further investigations are necessary to fully understand the supportive mechanisms.
We showed that the enhanced phagocytosis induced by desialylation was inhibited by free sialic acid and was dependent on E. coli sialic acids. Moreover, this phenomenon was not observed by simply hiding cell surface sialic acid by the use of lectins, suggesting that actual removal of cell surface sialic acid residues was needed for phagocytosis improvement. Concordantly, phagocytosis improvement could be reversed by regeneration of cell surface sialic acid linkages by means of an ectosialyltransferase activity present on DC surfaces.
One possible hypothesis to explain the sialidase phenomenon is the engagement of receptors that become more accessible after sialidase treatment. This mechanism is supported by previous suggestions that sialylation content, modulated by Neu1 sialidase, is a new important parameter controlling interactions between macrophage receptors, their ligands and signalling proteins.18
Bacterial pathogen‐associated molecular patterns are recognized by a variety of pathogen‐recognition receptors, which include several sialylated receptors and receptors that recognize sialylated structures. Toll‐like receptors (TLRs) recognize several microbial ligands, leading to the activation of intracellular signalling cascades. Interestingly, endogenous or exogenous sialidases have an essential role in LPS‐induced TLR4 activation by cleaving specific sialic acid residues.39–42 Sialic acid binding immunoglobulin‐like lectins (Siglecs) are receptors that specifically recognize sialic acids and are involved both in endocytosis and cellular signalling functions.43 Siglecs are usually masked by cis interactions with sialic acids expressed on the same cell membrane, which can be unmasked following exposure to sialidase or, in some cases, by cellular activation.17 Most Siglecs possess cytoplasmic tails harbouring immunoreceptor tyrosine‐based inhibitory motifs with an important immunoregulatory role. Indeed, some Siglecs from the CD33‐related family can suppress the TLR‐dependent production of TNF‐α and IL‐6 pro‐inflammatory cytokines and enhance the production of the anti‐inflammatory cytokine IL‐10.44 On a related note, many pathogens are known to possess, or acquire, sialic acid to interact with Siglec‐expressing leucocytes thus advantageously modulating the immune response.10,11,13 Taking these characteristics into account, and considering that E. coli exhibit some ligands for members of both TLRs and Siglecs (Guadalupe Cabral M and Paula A. Videira, unpublished data), these are candidate receptors involved in the observed improvement of E. coli phagocytosis by desialylated MDDCs. Nevertheless, it is probable that a complex combination of receptors is simultaneously engaged following MDDC desialylation resulting in the observed up‐regulation of phagocytosis.
Our studies with BMDCs deficient for ST6Gal.1, which mediates the synthesis of the α2,6‐sialyl linkages, strongly implicates the influence of specifically α2,6‐sialic acids in this process and further suggests a role for the expression of the ST6Gal.1 enzyme in regulating DC phagocytic activity. ST6Gal.1 is one of the most highly expressed sialyltransferases in human MDDCs.22 One of the best‐known products of ST6Gal.1 is the counter‐receptor of CD22 Siglec on B lymphocytes.45 Besides the humoral responses, the maintenance of myeloid homeostatic balance,27,28 T‐lymphocyte functionality,46 and integrin signalling47 are just a few of the physiological processes that also implicate ST6Gal.1 participation. The specific ST6Gal.1 acceptor substrates are still not identified in DCs but it is possible to involve key α2,6‐sialylated receptors or α2,6‐sialylated ligands engaged in cis‐interactions with endogenous receptors.
There is a possibility that E. coli receptors or components actively participating and interacting with desialylated MDDCs are irrelevant because, in this work, we adopted an experimental design that involved exclusively heat‐killed not live bacteria.
Cytokines produced by DCs are known to be important in regulating the adaptive immune response, directing it to either a pro‐inflammatory immune response or to an anti‐inflammatory immune response. It has been reported that Gram‐negative bacteria induce MDDCs towards a pro‐inflammatory response, with secretion of IL‐12, TNF‐α, IL‐6 and IL‐10.48 In fact, here we observed that desialylation of MDDCs followed by E. coli phagocytosis not only significantly improves the expression of IL‐12, IL‐6 and TNF‐α, which are potent pro‐inflammatory cytokines able to stimulate cytotoxic T cells, but also improves the expression of IL‐10, which helps the production of B‐cell immunoglobulin.48 This cytokine profile recalls the idea that sialidase triggers distinct intracellular signals, with relevant functional impact on the anti‐pathogenic response. We further confirm the high immunological potency of sialidase‐treated MDDCs following phagocytosis by demonstrating the high ability of these cells in priming T lymphocytes. In agreement with previous work,42 the matured phenotype of MDDCs, acquired through desialylation per se, activate the NF‐κB pathway, which explains the increased cytokine gene expression.
Of note, we found that desialylation also improved the capacity of MDDCs to internalize pathogenic E. coli isolates, suggesting the sialidase mechanism to be ubiquitous regarding other E. coli. According to the data shown above, this mechanism should be restricted, at least, to bacteria that express cell surface sialylated structures. However, further studies are necessary to elucidate if this mechanism is E. coli restricted or could be extended to other sialylated Gram‐negative bacteria.
In summary, data from the present study indicate that desialylation, and in particular depletion of α2,6‐sialic acids, improves the capacity of human MDDCs to internalize E. coli, independently of their maturation stage. Sialidase‐mediated cleavage of sialic acid residues remarkably potentiates DC maturation and cytokine production via NF‐κB translocation, resulting in an increased ability to activate T lymphocytes. Further studies are still required to illuminate the details of the involved mechanisms, but the present work lays the foundation insight for the importance of cell surface sialic acids of DC at the critical interface between bacterial challenges and the mobilization of host adaptive immune responses.
We believe that these findings are relevant in adding to the knowledge required for the establishment of efficient, antibiotic substitutes and immunotherapies, which are particularly important in fighting antibiotic‐resistant Gram‐negative pathogens.
Acknowledgments
This study was supported by the Fundação para a Ciência e Tecnologia, Portugal – PTDC/SAU‐MII/67561/2006 (PAV), CEDOC (PAV), SFRH/BPD/21619/2005 (MGC), FRH/BPD/41168/2007 (ZS) and SFRH/BD/61204/2009 (HC), and by National Institutes of Health grants R01AI38193 (JTL) and P01HL107146 (JTL). This research also used core facilities at RPCI supported in part by NCI‐funded Cancer Center Support Grant CA16056. We thank Manuela Correia for technical support.
Disclosures
The authors have no financial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Sialidase treatment improves the % of human monocyte‐derived dendritic cells (MDDCs) that phagocytosed Escherichia coli.
Escherichia coli are internalized by monocyte‐derived dendritic cells (MDDCs).
Sialidase treatment improves phagocytosis by human tumour necrosis factor‐α‐mature monocyte‐derived dendritic cells (MDDCs).
Sialidase treatment improves MHC expression in monocyte‐derived dendritic cells (MDDCs).
References
- 1.Norbury CC. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–51. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Colino J, Snapper CM. Dendritic cells, new tools for vaccination. Microbes Infect. 2003;5:311–9. doi: 10.1016/s1286-4579(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 5.Kis Z, Pallinger E, Endresz V, Burian K, Jelinek I, Gonczol E, Valyi‐Nagy I. The interactions between human dendritic cells and microbes; possible clinical applications of dendritic cells. Inflamm Res. 2004;53:413–23. doi: 10.1007/s00011-004-1274-0. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–24. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Nakahashi A, Taniguchi T, Miura N, Monde K. Stereochemical studies of sialic acid derivatives by vibrational circular dichroism. Org Lett. 2007;9:4741–4. doi: 10.1021/ol702042m. [DOI] [PubMed] [Google Scholar]
- 8.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–60. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Hanlon TP, Lau KM, Wang XC, Lau JT. Tissue‐specific expression of β‐galactoside α‐2,6‐sialyltransferase. Transcript heterogeneity predicts a divergent polypeptide. J Biol Chem. 1989;264:17389–94. [PubMed] [Google Scholar]
- 10.Avril T, Attrill H, Zhang J, Raper A, Crocker PR. Negative regulation of leucocyte functions by CD33‐related siglecs. Biochem Soc Trans. 2006;34(Pt 6):1024–7. doi: 10.1042/BST0341024. [DOI] [PubMed] [Google Scholar]
- 11.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec‐9 and dampen the innate immune response. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 13.Khatua B, Ghoshal A, Bhattacharya K, Mandal C, Saha B, Crocker PR. Sialic acids acquired by Pseudomonas aeruginosa are involved in reduced complement deposition and siglec mediated host‐cell recognition. FEBS Lett. 2010;584:555–61. doi: 10.1016/j.febslet.2009.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–14. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Severi E, Hood DW, Thomas GH. Sialic acid utilization by bacterial pathogens. Microbiology. 2007;153(Pt 9):2817–22. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 16.Vimr E, Lichtensteiger C. To sialylate, or not to sialylate: that is the question. Trends Microbiol. 2002;10:254–7. doi: 10.1016/s0966-842x(02)02361-2. [DOI] [PubMed] [Google Scholar]
- 17.Crocker PR. Siglecs in innate immunity. Curr Opin Pharmacol. 2005;5:431–7. doi: 10.1016/j.coph.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Seyrantepe V, Iannello A, Liang F, Kanshin E, Jayanth P, Samarani S. Regulation of phagocytosis in macrophages by neuraminidase 1. J Biol Chem. 2010;285:206–15. doi: 10.1074/jbc.M109.055475. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrascal M, Silva Z, Crespo H, Cabral MG, Videira PA. 1st edn. Vol. 37. Cambridge, UK: Royal Society of Chemistry; 2011. pp. 94–116. Sialylation and Dendritic Cells: Bridging Innate and Adaptive Immune Responses. In: Specialist Periodical Reports (SPR) Carbohydrate Chemistry. Vol. [Google Scholar]
- 20.Videira PA, Amado IF, Crespo HJ, Alguero MC, Dall'Olio F, Cabral MG. Surface α2‐3‐ and α2‐6‐sialylation of human monocytes and derived dendritic cells and its influence on endocytosis. Glycoconj J. 2008;25:259–68. doi: 10.1007/s10719-007-9092-6. et al. [DOI] [PubMed] [Google Scholar]
- 21.Bax M, Garcia‐Vallejo JJ, Jang‐Lee J. Dendritic cell maturation results in pronounced changes in glycan expression affecting recognition by siglecs and galectins. J Immunol. 2007;179:8216–24. doi: 10.4049/jimmunol.179.12.8216. et al. [DOI] [PubMed] [Google Scholar]
- 22.Crespo HJ, Cabral MG, Teixeira AV, Lau JT, Trindade H, Videira PA. Effect of sialic acid loss on dendritic cell maturation. Immunology. 2009;128(Suppl 1):e621–31. doi: 10.1111/j.1365-2567.2009.03047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien S, Adriaenssens E, Ottenberg K, Furlan A, Courtand G, Vercoutter‐Edouart AS. ST6GalNAc I expression in MDA‐MB‐231 breast cancer cells greatly modifies their O‐glycosylation pattern and enhances their tumourigenicity. Glycobiology. 2006;16:54–64. doi: 10.1093/glycob/cwj033. et al. [DOI] [PubMed] [Google Scholar]
- 24.Trottein F, Schaffer L, Ivanov S. Glycosyltransferase and sulfotransferase gene expression profiles in human monocytes, dendritic cells and macrophages. Glycoconj J. 2009;26:1259–74. doi: 10.1007/s10719-009-9244-y. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabral MG, Piteira AR, Silva Z, Ligeiro D, Brossmer R, Videira PA. Human dendritic cells contain cell surface sialyltransferase activity. Immunol Lett. 2010;131:89–96. doi: 10.1016/j.imlet.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Silva Z, Tong Z, Cabral MG, Martins C, Castro R, Reis C. Sialyl Lewis(x)‐dependent binding of human monocyte‐derived dendritic cells to selectins. Biochem Biophys Res Commun. 2011;409:459–64. doi: 10.1016/j.bbrc.2011.05.026. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nasirikenari M, Chandrasekaran EV, Matta KL, Segal BH, Bogner PN, Lugade AA. Altered eosinophil profile in mice with ST6Gal‐1 deficiency: an additional role for ST6Gal‐1 generated by the P1 promoter in regulating allergic inflammation. J Leukoc Biol. 2010;87:457–66. doi: 10.1189/jlb.1108704. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nasirikenari M, Segal BH, Ostberg JR, Urbasic A, Lau JT. Altered granulopoietic profile and exaggerated acute neutrophilic inflammation in mice with targeted deficiency in the sialyltransferase ST6Gal I. Blood. 2006;108:3397–405. doi: 10.1182/blood-2006-04-014779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones MB, Nasirikenari M, Feng L, Migliore MT, Choi KS, Kazim L. Role for hepatic and circulatory ST6Gal‐1 sialyltransferase in regulating myelopoiesis. J Biol Chem. 2010;285:25009–17. doi: 10.1074/jbc.M110.104406. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–9. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Videira PA, Correia M, Malagolini N, Crespo HJ, Ligeiro D, Calais FM. ST3Gal.I sialyltransferase relevance in bladder cancer tissues and cell lines. BMC Cancer. 2009;9:357. doi: 10.1186/1471-2407-9-357. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrett WS, Chen LM, Kroschewski R, Ebersold M, Turley S, Trombetta S. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–34. doi: 10.1016/s0092-8674(00)00038-6. et al. [DOI] [PubMed] [Google Scholar]
- 33.Shurin GV, Tourkova IL, Chatta GS, Schmidt G, Wei S, Djeu JY. Small rho GTPases regulate antigen presentation in dendritic cells. J Imol. 2005;174:3394–400. doi: 10.4049/jimmunol.174.6.3394. et al. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, Hayden MS. New regulators of NF‐κB in inflammation. Nat Rev Immunol. 2008;8:837–48. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- 35.Patil S, Pincas H, Seto J, Nudelman G, Nudelman I, Sealfon SC. Signaling network of dendritic cells in response to pathogens: a community‐input supported knowledgebase. BMC Syst Biol. 2010;4:137. doi: 10.1186/1752-0509-4-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May MJ, Ghosh S. Signal transduction through NF‐κB. Immunol Today. 1998;19:80–8. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 37.Slama TG. Gram‐negative antibiotic resistance: there is a price to pay. Crit Care. 2008;12(Suppl 4):S4. doi: 10.1186/cc6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva Z, Konstantopoulos K, Videira PA. The role of sugars in dendritic cell trafficking. Ann Biomed Eng. 2012;40:777–89. doi: 10.1007/s10439-011-0448-5. [DOI] [PubMed] [Google Scholar]
- 39.Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R. Neu1 desialylation of sialyl α‐2,3‐linked β‐galactosyl residues of TOLL‐like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22:314–24. doi: 10.1016/j.cellsig.2009.09.038. et al. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe Y, Shiratsuchi A, Shimizu K, Takizawa T, Nakanishi Y. Stimulation of phagocytosis of influenza virus‐infected cells through surface desialylation of macrophages by viral neuraminidase. Microbiol Immunol. 2004;48:875–81. doi: 10.1111/j.1348-0421.2004.tb03619.x. [DOI] [PubMed] [Google Scholar]
- 41.Stamatos NM, Carubelli I, van de Vlekkert D. LPS‐induced cytokine production in human dendritic cells is regulated by sialidase activity. J Leukoc Biol. 2010;88:1227–39. doi: 10.1189/jlb.1209776. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatos NM, Curreli S, Zella D, Cross AS. Desialylation of glycoconjugates on the surface of monocytes activates the extracellular signal‐related kinases ERK 1/2 and results in enhanced production of specific cytokines. J Leukoc Biol. 2004;75:307–13. doi: 10.1189/jlb.0503241. [DOI] [PubMed] [Google Scholar]
- 43.Lock K, Zhang J, Lu J, Lee SH, Crocker PR. Expression of CD33‐related siglecs on human mononuclear phagocytes, monocyte‐derived dendritic cells and plasmacytoid dendritic cells. Immunobiology. 2004;209:199–207. doi: 10.1016/j.imbio.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Ando M, Tu W, Nishijima K, Iijima S. Siglec‐9 enhances IL‐10 production in macrophages via tyrosine‐based motifs. Biochem Biophys Res Commun. 2008;369:878–83. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands α2,6‐linked sialic acids. Int Immunol. 2006;18:603–11. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- 46.Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N‐glycans on CD45 to negatively regulate galectin‐1‐induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278:7469–75. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- 47.Woodard‐Grice AV, McBrayer AC, Wakefield JK, Zhuo Y, Bellis SL. Proteolytic shedding of ST6Gal‐I by BACE1 regulates the glycosylation and function of α4β1 integrins. J Biol Chem. 2008;283:26364–73. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram‐positive and gram‐negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671–8. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sialidase treatment improves the % of human monocyte‐derived dendritic cells (MDDCs) that phagocytosed Escherichia coli.
Escherichia coli are internalized by monocyte‐derived dendritic cells (MDDCs).
Sialidase treatment improves phagocytosis by human tumour necrosis factor‐α‐mature monocyte‐derived dendritic cells (MDDCs).
Sialidase treatment improves MHC expression in monocyte‐derived dendritic cells (MDDCs).


