Abstract
Vitamin D (VD) has been implicated in type 1 diabetes (T1D) by genetic and epidemiological studies. Individuals living in regions with low sunlight exposure have an increased T1D risk and VD supplementation reduced the risk in human individuals and mouse models. One possibility of how VD influences the pathogenesis of T1D is its immunomodulatory effect on dendritic cells (DC), which then preferentially activate regulatory T cells (Tregs). In the present pilot study, we collected blood samples from a small cohort of patients with T1D at baseline and months 6 and 12. VD-deficient patients were advised to supplement with 1000 IU/day VD. We found a considerable variation in the VD plasma level at baseline and follow-up. However, with higher VD plasma levels, a lower frequency of interleukin (IL)-4-producing CD8 T cells was observed. We further performed a comprehensive genotyping of 13 VD-related polymorphisms and found an association between VD plasma level and the genotype of the VD binding protein (DBP). The frequency of DC and T cell subsets was variable in patients of all subgroups and in individual patients over time. Nevertheless, we found some significant associations, including the 1,25-dihydroxyvitamin D3 hydroxylase (CYP27B1) genotype with the frequency of DC subtypes. In summary, our preliminary results indicate only a limited influence of the VD plasma level on the immune balance in patients with T1D. Nevertheless, our pilot study provides a basis for a follow-up study with a larger cohort of patients.
Keywords: autoimmunity, dendritic cells, metabolism, polymorphism, regulatory T cells
Introduction
It is current opinion that human type 1 diabetes (T1D) is caused by a detrimental combination of genetic predisposition and environmental triggering factors. Genetically, T1D has been linked predominantly to certain human leucocyte antigen (HLA)-DR and -DQ alleles [1]. However, many other susceptibility genes have been identified including, among others, PTPN22, the insulin structural gene (INS), interleukin-2 receptor (IL-2R)-related genes, cytotoxic T lymphocyte antigen (CTLA)-4 and genes involved in vitamin D3 (VD) metabolism or binding [1]. Environmental factors, such as human pathogens, have been associated mainly with the induction and/or acceleration of T1D [2]. In particular, enteroviruses, such as coxsackie virus B, have emerged as potential triggers for T1D [3,4]. In contrast, according to the ‘hygiene hypothesis’ a more frequent encounter with human pathogens might lessen the risk of developing autoimmune diseases and allergies [2,5,6]. Indeed, autoimmune diseases, including T1D, are less frequent in regions with low sanitary standards [3]. In animal models, viruses have also been demonstrated to have protective properties by either direct abrogation of an ongoing aggressive immune response [7] or by induction of regulatory mechanisms [8,9].
Several observations suggest a role of VD as a protective factor in the pathogenesis of T1D. Epidemiologically, there is a higher prevalence of T1D in regions with low VD supplies, due to lower sunlight exposure or a reduced uptake of dietary VD (for review see [10]). The incidence of T1D follows a seasonal pattern and seems to be influenced by latitude and ultraviolet B (UVB) irradiance [10]. Further, VD supplementation in early childhood has been shown to reduce the risk for T1D [11], and several studies have demonstrated that children and young adults with T1D displayed significant lower plasma VD levels than age-matched healthy controls [12–14]. The main source for VD is the skin and results from the conversion of 7-dehydrocholesterol to vitamin D3 (cholecalciferol), induced by UVB irradiation. VD enters the circulation by binding to VD-binding protein (DBP). In the liver, VD is converted to 25-hydroxyvitamin D3 (25D, calcidiol) by several 25-vitamin D3 hydroxylases, including the cytochrome P450 (CYP), isoenzymes 27A1 (CYP27A1) and 2R1 (CYP2R1) [15,16]. In the kidney and in immune cells, 25D is further 1α-hydroxylated to the bioactive form, 1,25-dihydroxyvitamin D3 (1,25D, calcitriol), predominantly by the 25-hydroxyvitamin D3-1α hydroxylase CYP27B1 [15]. Finally, binding of 1,25D to the intracellular VD receptor (VDR) mediates VD signalling. Importantly, several polymorphisms of VD-related genes have been associated with T1D [10]. The polymorphic expression of genes of the VD-metabolizing enzymes CYP27B1 and CYP2R1 have been associated with susceptibility for T1D [16–19]. In contrast, no association has yet been found for the gene CYP24A1 encoding for the VD-catabolizing enzyme 1,25-dihydroxyvitamin D3 hydroxylase CYP24A1 [17,18], which nevertheless has been associated with VD levels in a genome-wide association study [20]. An association of VDR polymorphism with T1D has been inconsistent [21,22] However, a recent meta-analysis suggests that at least one of four known polymorphisms in the VDR gene is associated with a higher risk for T1D in Asians [23] Further, a link between DBP expression and T1D has been demonstrated [24].
The impact of the VD level on the immunopathogenesis of T1D appears to be multi-factorial, influencing both innate and acquired immunity at different stages, from resistance to virus infections to differentiation and activation of various cells of the immune system [25,26], almost all of which express the VDR [10]. Of particular interest is the effect of VD on dendritic cells (DC). The maturation of DCs is impaired in the presence of 1,25D leading to a reduced surface expression of major histocompatibility complex (MHC)-II and co-stimulatory molecules, and subsequently diminished antigen-presenting and T cell-activating properties [27]. Further, 1,25D treatment has been shown to induce apoptosis of mature DCs [28]. Most importantly, 1,25D seems to differentiate DCs into a tolerogenic state, where they induce regulatory T cells (Treg) preferentially [29,30].
In the present pilot study we intended to evaluate a possible association of plasma VD levels of patients with T1D with their immune status. In particular, we analysed the ratio of DC subtypes and the frequency of aggressive and regulatory T cells in the blood. Further, we correlated for the first time VD levels and immune status with the allelic expression of VD-related polymorphisms of the genes DBP, VDR, CYP2R1, CYP27B1 and CYP24A1. In an additional, longitudinal study we analysed VD levels and immune status over a period of 12 months in a small cohort of T1D patients with who were advised to supplement VD at 1000 IU/day provided that they were vitamin D-deficient.
Material and methods
Subjects
Blood was obtained from 63 T1D patients (30 males and 33 females) recruited from the endocrine out-patient clinics at the University Hospital, Frankfurt am Main. In order to avoid possible fluctuations in VD levels and immune status throughout the day, blood was always collected at the same time in the morning (between 8 a.m. and 10 a.m.). Type 1 diabetes mellitus was diagnosed according to the World Health Organization criteria. From day 27 a second blood sample and from day 17 a third blood sample were obtained after an average time of 6 and 12 months after the first sample was drawn, respectively (Fig. 1). The median age of the cohort was 44·6 years. Patients diagnosed as VD-deficient (< 20 ng/ml 25D) at the first visit were advised to supplement with 1000 IU/day of VD in the form of Viganatol oil or Vigantoletten 500 (both from Merck Serono GmbH, Darmstadt, Germany). The study protocol was approved by the Ethics Committee of the University Hospital Frankfurt am Main and written informed consent was obtained from all participants.
Fig. 1.
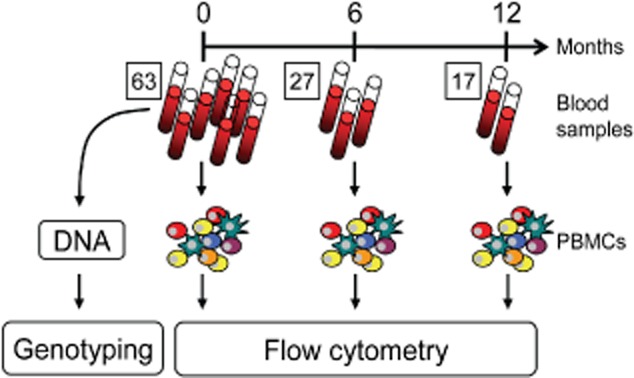
Experimental setup. Blood samples of 63 patients with type 1 diabetes (T1D) were collected and the peripheral blood mononuclear cells (PBMCs) were isolated and analysed by flow cytometry. For the longitudinal study second and third blood samples were collected with a mean interval of 6 and 12 months, respectively. Additionally, DNA isolated from granulocytes of the individuals was used for genotyping for T1D and vitamin D (VD) relevant genes (table 4.01).
Genes, polymorphisms and genotyping
The distribution of the cohort on the VD-related polymorphism is listed in Table 1. In total, 13 VD-related polymorphisms were investigated: two for CYP2R1 (rs12794714, rs10741657); three for CYP24A1 (rs2248137, rs2296241, rs927650); two for CYP27B1 (rs4646536, rs10877012); two for DBP (rs7041, rs4588); and four for VDR (rs7975232, rs1544410, rs10735810, rs731236). Polymorphism positions are given according to the National Center for Biotechnology Information (NCBI: http://www.ncbi.nlm.nih.gov). Genomic DNA was extracted from whole blood by the salting-out procedure [31] and used for restriction fragment length polymorphism and real-time polymerase chain reaction (PCR) methods. For the polymorphisms within the genes – CYP2R1 (rs12794714, rs10741657) CYP24A1 (rs927650), DBP (rs4588,rs7041) and VDR (rs7975232; rs1544410; rs10735810; rs731236) – the PCR products were digested with the respective restriction enzymes as described previously [16,32–34]. The polymorphisms rs10877012, rs4646536, rs2248137, rs2296241 were examined as described recently [17,32]. The HLA-DQA1 and -DQB1 genotyping was performed as described previously [35].
Table 1.
Distribution of vitamin D (VD)-related polymorphisms in the type 1 diabetes (T1D) patient cohort
| Polymorphism | Genotype | % |
|---|---|---|
| HLA DR/DQ | T1D risk | 75,5 |
| Other | 24,5 | |
| CYP24A1 | CC | 27,9 |
| rs2248137 | CG | 49,1 |
| GG | 23,0 | |
| CYP24A1 | AA | 36,1 |
| rs2296241 | AG | 54,1 |
| GG | 9,8 | |
| CYP24A1 | TT | 21,3 |
| rs927650 | TC | 50,8 |
| CC | 27,9 | |
| CYP27B1 | AA | 9,5 |
| rs10877012 | AC | 54,0 |
| CC | 36,5 | |
| CYP27B1 | TT | 48,3 |
| rs4646536 | TC | 43,3 |
| CC | 8,3 | |
| CYP2R1 | AA | 7,9 |
| rs1074657 | AG | 60,3 |
| GG | 31,8 | |
| CYP2R1 | AA | 17,7 |
| rs12794714 | AG | 43,5 |
| GG | 38,7 | |
| DBP | GG | 19,7 |
| Hae III | GT | 59,0 |
| rs7041 | TT | 21,3 |
| DBP | CC | 37,7 |
| Sty I | AC | 54,1 |
| rs4588 | AA | 8,2 |
| VDR | AA | 34,9 |
| Apa I | Aa | 42,9 |
| rs7975232 | aa | 22,2 |
| VDR | BB | 23,8 |
| Bms I | Bb | 44,4 |
| rs1544410 | bb | 31,7 |
| VDR | FF | 34,4 |
| Fok I | Ff | 45,9 |
| rs10735810 | ff | 19,7 |
| VDR | TT | 41,3 |
| Taq I | Tt | 38,1 |
| rs731236 | tt | 20,6 |
Human leucocyte antigen (HLA) type 1 diabetes (T1D) risk genotype: DQ2 (DQA*0501-DQB*0201) and DQ8 (DQA*0301-DQB*0302). DBP: VD-binding protein.
Quantification of VD plasma levels
For the determination of both VD metabolites, fresh ethylenediamine tetraacetic acid blood samples were centrifuged immediately by 600 g for 10 min and the separated plasma was stored at −20°C; 25D and 1,25D plasma levels were determined by radioimmunoassay (DiaSorin, Stillwater, Minnesota, USA and IDS, Frankfurt am Main, Germany, respectively).
Isolation of human peripheral blood mononuclear cells (PBMCs)
Approximately 10 ml of blood were centrifuged at room temperature (RT) for 10 min at 600 U/min. The resulting cellular pellet was diluted with phosphate-buffered saline (PBS) to a volume of 30 ml and overlaid on 15 ml Bicoll separation solution (Biochrom, Berlin, Germany). After 30 min centrifugation at 500 g (un-damped) the interphase was collected in a 50 ml Falcon tube, filled to 50 ml with PBS and spun at 500 g for 10 min at RT. Afterwards the pellet was washed twice more with 10 ml PBS and PBMCs were counted and frozen in fetal calf serum (FCS) containing 10% dimethylsulphoxide (DMSO). The remaining lower phase of the Bicoll centrifugation step was used for DNA isolation and subsequent determination of VD-related polymorphisms.
Flow cytometry of human PBMCs
PBMCs were resuspended in RPMI-1640 and 106 cells were transferred into a V-bottomed 96-well plate. For the analysis of DC subtypes, PBMCs were stained with the following antibodies: allophycocyanin (APC)-conjugated mouse anti-human CD303 [magnetic affinity cell sorting (MACS); Miltenyi Biotec, Bergisch Gladbach, Germany] fluorescein isithiocyanate (FITC)-conjugated Linage cocktail 3 (Lin3) (CD3, CD14, CD19, CD20), phycoerythrin (PE)-conjugated mouse anti-human CD123 (anti-IL-3Rα), PE-cyanin-5 (Cy5)-conjugated mouse anti-human CD11c, PE-Cy7-conjugated mouse anti-human CD4 and Horizon V450-conjugated mouse anti-human CD8 (all from BD Biosciences, Heidelberg, Germany) (Fig. 2). For the analysis of Tregs, due to parallel intracellular cytokine staining (see below) PBMCs were kept in culture in RPMI-1640 medium containing 10% FCS overnight prior to antibody staining with FITC-conjugated mouse anti-human CD25, PE-Cy7-conjgated mouse anti-human CD4 and Horizon V450-conjugated mouse anti-human CD8 (all from BD Biosciences). For forkhead box protein 3 (FoxP3) staining, cells were permeabilized with 50 μl at a 1 : 3 ratio of eBio Fix/Perm Concentrate and eBio Fix/Perm Diluent (both eBioscience, Frankfurt, Germany) for 30 min at 4°C and stained with PE-conjugated mouse anti-human FoxP3 antibody (BD Biosciences). Cells were analysed by flow cytometry using a BD FACS Canto II (BD Biosciences) (Fig. 2).
Fig. 2.
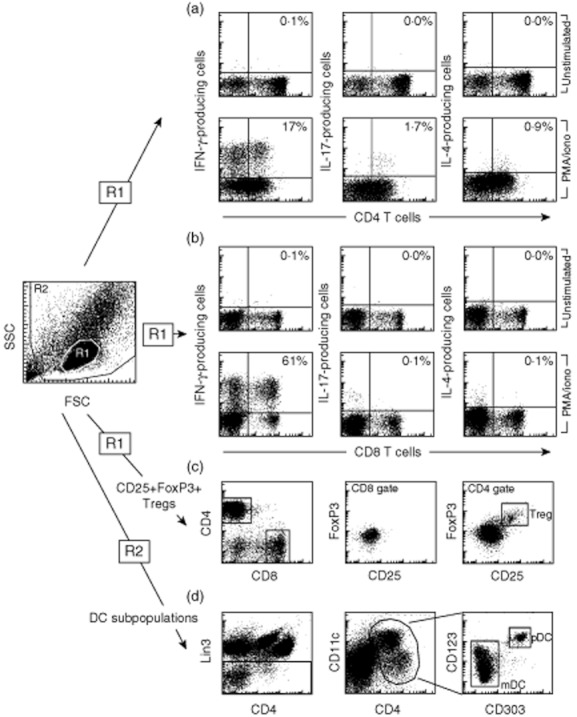
Flow cytometry of human PBMCs. (a,b) The frequency of T cells with a T helper type 1 (Th1), Th2 and Th17 phenotype was determined by stimulation of peripheral blood mononuclear cells (PBMCs) with phorbol mysristate acetate (PMA)/ionomycin in the presence of Bredfeldin A and staining with antibodies to CD4, CD8, interferon (IFN)-γ (Th1), interleukin (IL)-17 (Th17), and IL-4 (Th2). The gating strategy is displayed for one representative patient with type 1 diabetes (T1D). Numbers indicate the frequency of IFN-γ-, IL-17- or IL-4-producing cell in relation to the total number of CD4 or CD T cells. (c) In order to quantify the frequency of regulatory T cells (Tregs) PBMCs were stained with antibodies to CD4, CD8, CD25 and forkhead box protein 3 (FoxP3). Note that CD25, FoxP3 double-positive Tregs were detected only in the CD4 T cell population. (d) For the determination of dendritic cell (DC) subpopulations human PBMCs were stained with antibodies to Linage cocktail 3 (Lin3–), Lin3, CD303, CD123, CD11c and CD4 and gated for plasmacytoid dendritic cells (pDCs) (CD303+, CD123+ and CD11c+ of the Lin3 negative leucocytes) and myeloid dendritic cells (mDCs) (CD303–, CD123lo, CD11chi and CD4+ of the Lin3-negative leucocytes).
Intracellular cytokine stain (ICCS)
For intracellular cytokine staining, 106 human PBMCs were stimulated overnight with 50 μg/ml phorbol myristate acetate (PMA) and 5 μg/ml ionomycin in the presence of 1 μg/ml Brefeldin A. The cells were stained with PE-Cy7-conjgated mouse anti-human CD4 and Horizon V450-conjugated mouse anti-human CD8 antibodies (BD Biosciences), fixed, permeabilized and stained with FITC-conjugated mouse anti-human interferon (IFN)-γ, APC-conjugated mouse anti-human interleukin (IL)-4 or PE-conjugated mouse anti-human IL-17A antibodies (all from BD Biosciences). Cells were analysed by flow cytometry at a BD FACS Canto II (BD Biosciences) (Fig. 2).
Statistical analysis
Associations between polymorphism genotypes and VD plasma levels as well as parameters defining the immune status of T1D patients were analysed using the non-parametric, unpaired, two-tailed Mann–Whitney U-test, whereby two genotypes have always been compared with each other. Similar data were obtained using the non-parametric Kruskal–Wallis test comparing all three genotypes simultaneously (GraphPad Prism Software, San Diego, CA, USA). Associations between VD plasma levels and parameters characterizing the immune status of T1D patients were analysed using the non-parametric Spearman's correlation test, whereby P-values < 0·05 indicate significant correlations (GraphPad Prism Software).
Results
It was the aim of this pilot study to evaluate how the VD level correlates with the immune status of T1D patients. In addition, we wanted to assess the influence of genetic variations associated with either VD metabolism or binding on the frequency and activity of a broad variety of immune cells. In total, we analysed blood samples of 63 T1D patients. In order to investigate the immune status, we used the plasma for determination of the 25D and 1,25D content and isolated PBMC. Further, we obtained additional blood samples, one from 27 patients and two from 17 patients. These patients were supplemented with VD if the 25D level was below 20 ng/ml. The average time interval between the sample collections was 6 months (Fig. 1). In this longitudinal study design we wanted to assess how the immune status changes in individual patients over time.
Analysis of the VD levels exposes a high prevalence of VD deficiency in the cohort
First, determination of both 25D and 1,25D plasma levels revealed that 52·4% of the 63 patients were VD-deficient, with 25D levels of less than 20 ng/ml. Only 19% of patients displayed 25D levels within the normal range of 30–60 ng/ml (Fig. 3a). There was considerable variation, ranging from 4·6 to 47 ng/ml (median 20 ng/ml) and from 9·0 to 99 ng/ml (median 47 ng/ml) for 25D (Fig. 3a) and 1,25D (Fig. 3b), respectively.
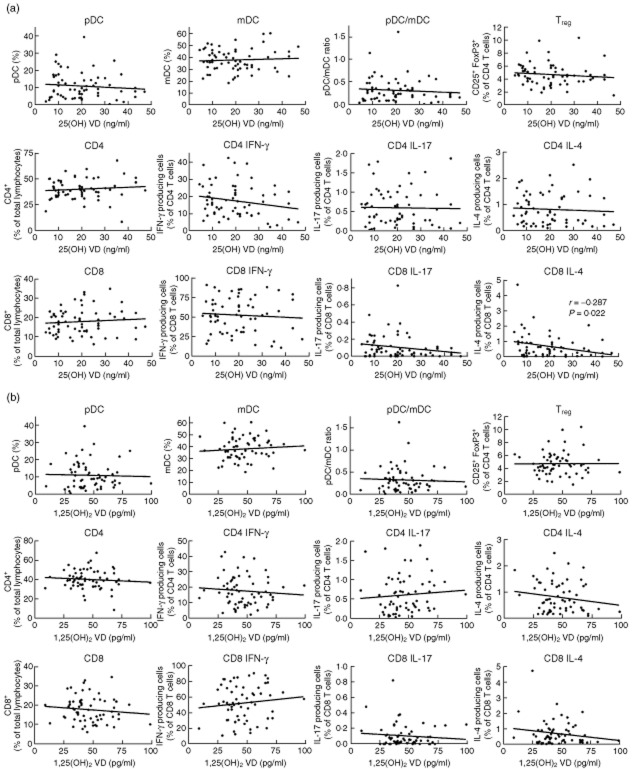
Fig. 3.
Correlation of vitamin D (VD) plasma levels and immune status exposed a significant association between the 25D and IL-4 producing CD8 T cells. Peripheral blood mononuclear cells (PBMCs) were stained for plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3–), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3–, CD303–, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and analysed by flow cytometry. In addition, PBMCs were stimulated with phorbol myristate acetate (PMA)/ionomycin overnight and intracellular cytokine staining was performed for interferon (IFN)-γ, interleukin (IL)-17 or IL-4 producing CD4 and CD8 T cells. The frequency of pDCs, mDCs, Tregs and IFN-γ, IL-17 and IL-4-producing CD4 and CD8 T cells were correlated to the 25D (a) and 1,25D plasma levels (b). Significant correlations as revealed by the non-parametric Spearman's correlation test are indicated (GraphPad Prism software).
Only marginal associations between VD plasma level and immune status of T1D patients
PBMC were isolated from 63 T1D patients and were assessed for the frequency of leucocyte subpopulations. In particular, we determined the frequencies of plasmacytoid (pDC) and myeloid dendritic cells (mDCs) and their ratio in order to evaluate the nature of the overall status of the main antigen-presenting cells that might be influenced by VD. In addition, we determined the frequency of FoxP3+ CD25+ CD4+ Tregs as well as CD4 and CD8 T cells producing IFN-γ, IL-4 or IL-17 by flow cytometry (Fig. 2). The values obtained were then correlated with the levels of 25D and 1,25D in individual patients (Fig. 3). The frequency of IL-4 producing CD8 T cells with higher 25D levels was reduced significantly (P = 0·022), and a trend towards a reduced frequency of IL-17-producing CD8 T cells (P = 0·134) was detected. However, no significant correlation was found between the 25D level and any of the other parameters analysed (Fig. 3a). Further, no correlation was found between 1,25D and any of the parameters analysed (Fig. 3b). These data indicate that in this small cohort of T1D patients the VD levels had almost no influence on the regulatory milieu in the blood. In particular, the ratio of DC subpopulation and the frequency of Tregs were not affected and the ratio of T cells producing type 1 (IFN-γ), type 2 (IL-4) or type 17 (IL-17) cytokines was only marginally dependent on the VD-levels.
Classification of T1D patients according to their VD-related polymorphisms reveals a moderate correlation between the CYP24A1 genotype and immune status
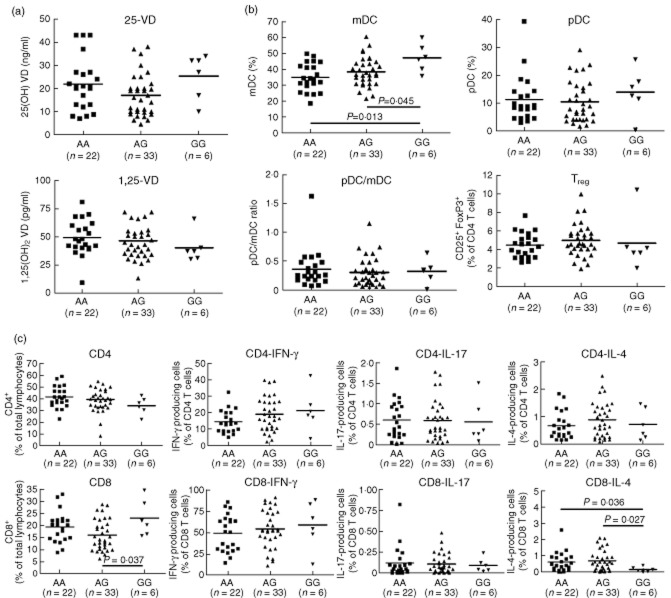
The cohort of T1D patients was subdivided according to their VD-related polymorphisms as well as HLA-DR/DQ. No differences in the VD levels and immune status were detected between patients carrying high-risk HLA genotypes (DR3, DR4, DQ2 and DQ8) and patients carrying other HLA alleles (Supporting information, Fig. S1). In contrast, significant differences were detected between patients carrying the GG genotype of the CYP24A1 rs2296241 polymorphism who showed a higher frequency of mDCs and CD8 T cells than patients with AA and AG genotypes (Fig. 4). In addition, these patients carried a significantly lower frequency of CD8 T cells that produced IL-4 upon unspecific stimulation with PMA/ionomycin. However, the frequency of IL-4-producing CD8 cells was below 1% for all genotypes. Further, we found a significant reduction of pDCs in patients with the GG genotype of the CYP24A1 rs2248137 polymorphism (Supporting information, Fig. S2) and an increased frequency of mDCs in patients with the TT genotype of the CYP24A1 rs927650 polymorphism (Supporting information, Fig. S3). No significant influence was found of the genotypes of the second 25-vitamin D3 hydroxylase CYP2R1 polymorphisms rs1074657 (Supporting information, Fig. S4) and rs12794714 (Supporting information, Fig. S5) on the VD levels or immune status. For the polymorphisms that affect the 25-hydroxyvitamin D3-1α hydroxylase CYP27B1, we found a lower frequency of Tregs in patients with the CC genotype of the rs4646536 polymorphism (Supporting information, Fig. S7), but no correlation with any genotype of the rs10877012 polymorphism (Supporting information, Fig. S6) with the VD levels of immune status.
Fig. 4.
Vitamin D (VD) plasma level and immune status in patients subgrouped according to the genotypes of the CYP24A1 rs2296241 polymorphism. Patients with type 1 diabetes (T1D) were subdivided into the three different genotypes of the CYP24A1 rs2296241 polymorphism (AA, AG, GG) and analysed for associations between the individual genotypes and (a) 25D and 1,25D plasma level; (b) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3–), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3–, CD303–, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (c) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
The most pronounced influence of genes expressed polymorphically was detected for DBP. In particular, patients with the TT genotype of the Hae III polymorphism had significantly reduced 25D-levels compared to patients with the GG or GT genotypes (Fig. 5). Similarly, patients with the AA genotype of the Sty I polymorphism showed significantly lower 1,25D levels and a tendency to lower 25D levels than patients with the CC and AC genotype (Fig. 5). However, the frequency of patients with the AA genotype of the Sty I polymorphism was somewhat low in our cohort. Nevertheless, the data confirm earlier studies that demonstrated lower VD plasma levels in individuals with the genotypes AA of Sty I and TT of Hae III [36,37]. In addition, patients with the TT genotype of the Hae III DBP polymorphism displayed an increased frequency of IL-4-producing CD4 and CD8 T cells (Supporting information, Fig. S8), and patients with the CC genotype of the Sty I DBP polymorphism showed a reduced frequency of IFN-γ-producing CD4 T cells (Supporting information, Fig. S9).
Fig. 5.
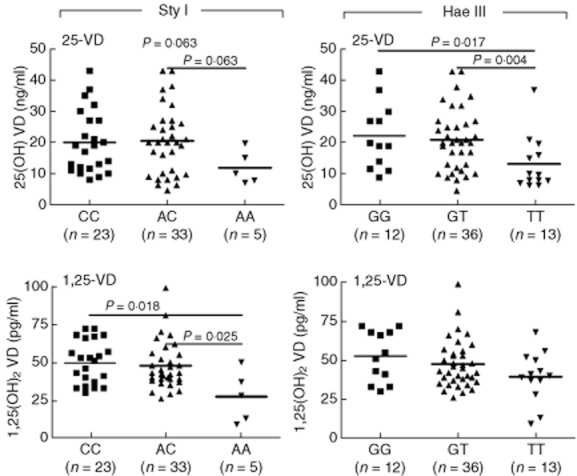
Vitamin D (VD) plasma levels are influenced by the genotypes of the VD-binding protein (DBP) polymorphisms Sty I and Hae III. Subjects were subdivided into the different genotypes of the (a) Sty I (CC, AC, AA) and (b) Hae III (GG, GT, TT) DBP polymorphisms and analysed for relations between genotypes and 25D and 1,25D level. Note that patients with type 1 diabetes (T1D) carrying the AA genotype of the Sty I polymorphism and the TT genotype of the Hae III polymorphism show a significant reduction in the VD plasma level. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Several associations have been found between the individual genotypes of four different VDR polymorphisms. The AA genotype of the VDR Apa I polymorphism is associated with an increased 25D level, a reduced CD4 T cell frequency and an increased CD8 T cell frequency (Supporting information, Fig. S10), the BB genotype of the VDR Bms I polymorphism with an increased 1,25D level and a reduced CD4 T cell frequency (Supporting information, Fig. S11), the FF genotype of the VDR Fok I polymorphism with an augmented frequency of IL-4-producing CD4 T cells (Supporting information, Fig. S12) and the tt genotype of the VDR Taq I polymorphism with an increased 1,25D level, a reduced CD4 T cell frequency and a increased CD8 T cell frequency (Supporting Fig. information, S13). These data indicate that individual genotypes of VD-related genes influence the frequency of cellular compartments that characterize the immune status of the patients. However, there is no clear picture emerging from these associations. The findings reflect our correlation data that, with the exception of the reduced frequency of IL-4-producing CD8 with higher 25D levels, revealed no significant correlation between the VD-levels and any of the parameters analysed (Fig. 3).
Longitudinal study of VD levels and immune status reveals no association
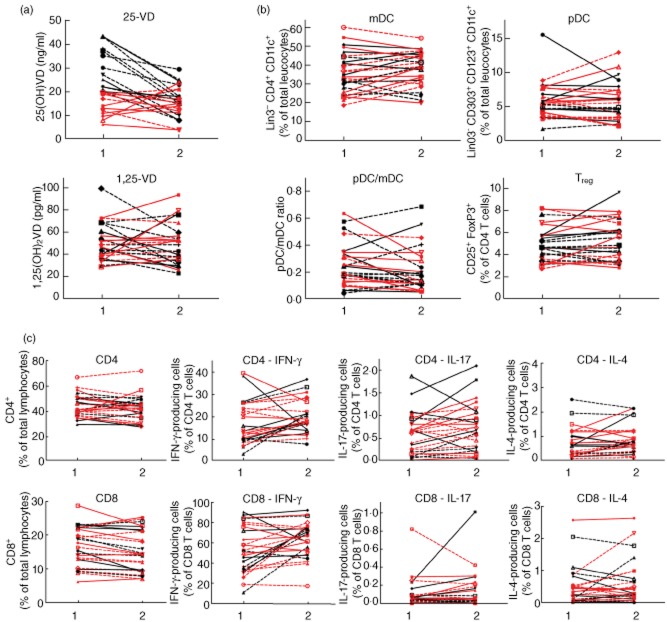
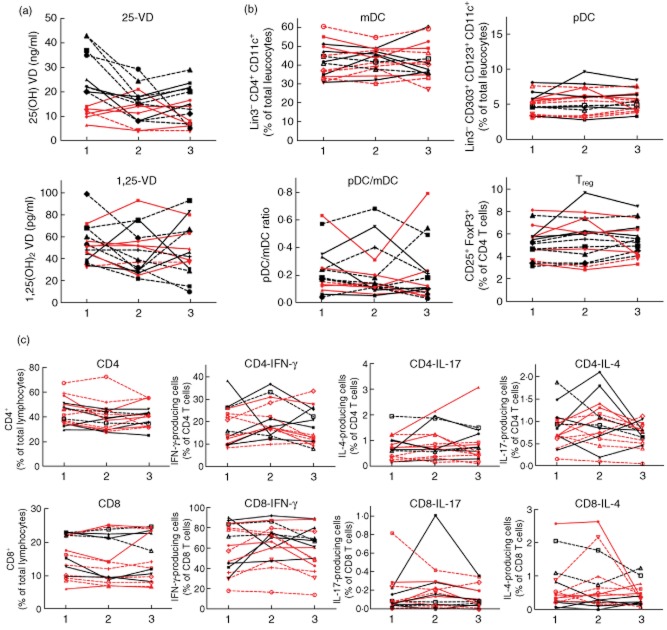
We obtained second and third blood samples of 27 and 17 T1D of the cohort of 63 patients, respectively. We performed a longitudinal assessment of the plasma VD levels and the immune status over time. For analysis the patients were grouped according to whether or not they were advised to supplement VD. For those patients from whom we obtained two blood samples in a mean interval of 6 months (Fig. 6) we found no significant different 25D and 1,25D plasma levels in the two samples, regardless of VD supplementation (Fig. 6a). Similarly, no significant differences have been detected in the distribution of DC and T cell subtypes (Fig. 6b,c). The mean time interval of the third blood samples obtained from 17 patients was 12 months after the first sample had been collected. The inclusion of this third sample demonstrated that the 25D and in particular the 1,25D plasma levels display a profound variability within these 12 months of observation (Fig. 7a). In contrast, the frequency of DC and T cell subtypes remained stable (Fig. 7b,c). No significant differences were detected in the groups that had or had not received VD supplementation. Therefore, we were able to detect an association of the plasma VD levels with immune status in this preliminary longitudinal study of 17 T1D patients. The number of individuals was too low for a detailed analysis of a possible influence of the VD-related genetic polymorphisms in the longitudinal study.
Fig. 6.
Vitamin D (VD) plasma levels and immune status in VD-deficient and non-deficient patients with type 1 diabetes (T1D) over a period of 6 months. Two blood samples of VD-deficient (< 20 μg/ml 25D at begin of the study) (red) and non-deficient (black) patients with T1D collected over a mean interval of 6 months were analysed for the VD plasma levels and immune status. (a) 25D and 1,25D plasma levels; (b) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3–), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3–, CD303–, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (c) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Straight lines: male; dashed lines: female.
Fig. 7.
Vitamin D (VD) plasma level and immune status in VD-deficient and non-deficient patients with type 1 diabetes (T1D) over a period of 12 months. Three blood samples of VD-deficient (< 20 μg/ml 25D at beginning of the study) (red) and non-deficient (black) patients with T1D collected over a mean interval of 12 months were analysed for the VD plasma levels and the immune status. (a) 25D and 1,25D plasma levels; (b) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3–), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3–, CD303–, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (c) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Straight lines: male; dashed lines: female.
Discussion
It was the aim of this pilot study, with a small cohort of 63 T1D patients, to evaluate a possible association between plasma VD levels and immune status in patients with different genetic polymorphisms in VD-related genes. We found considerable variety in the VD plasma levels at the beginning of the study. VD-deficient patients who displayed 25D levels of below 20 ng/ml at the first visit were advised to supplement with VD at 1000 IU/day. However, this supplementation seems to have no direct effect on the 25D and 1,25D plasma levels at months 6 and 12 after beginning supplementation. Further, we correlated the plasma levels of 25D and 1,25D with immune status, including the frequency and ratio of mDC and pDC and the frequency of several T cell subtypes. It has been reported that 1,25D selectively induces tolerogenic properties in mDCs, but not pDCs [30]. In particular, it has been demonstrated that exposure of mDCs to 1,25D results in expression of the chemokine CCL22, which predominantly attracts FoxP3+, CD25+ Tregs [30]. The programmed cell death ligand 1 (PD-L1) seems to play a critical role in the process of mDC-induced Treg activation [38]. Further, aggressive T cells seem to be driven towards apoptosis upon interaction with 1,25D or analogue-differentiated mDCs [28]. It has also been shown that human autoreactive T cells can be redirected by in-vitro interaction with DCs that have been differentiated with a 1,25D analogue [39]. In the non-obese diabetic (NOD) mouse, it has been shown that administration of 1,25D alters the profile of bone marrow-derived DCs [27] and causes a decrease in islet cytokine and chemokine expression, a lower degree of insulitis and, most importantly, reduced T1D incidence [40,41]. All in all the bioactive form of VD, 1,25D, seems to play an important role in the differentiation of DCs and the activation of Tregs in vitro and in animal models for T1D. In our pilot study, we were not able to identify a clear-cut association between the plasma VD levels and frequency of DC subtypes and Tregs in PBMCs of T1D patients. Nevertheless, we found a decrease in the frequency of IL-4-producing CD8 T cells and a trend towards a reduced frequency of IL-17-producing CD8 T cells with a higher 25D level. However, it should be noted here that although the decreased frequency of IL-4-producing CD8 T cells is statistically significant (P = 0·022), it is dependent largely upon one patient with a very low 25D level and a somewhat high frequency of IL-4-producing CD8 T cells (Fig. 3). Removal of this outlier from the statistical analysis results in a P-value of 0·047. Nevertheless, our preliminary results suggest that, to a certain extent, VD might reduce the T helper type 2 (Th2) immune response. Although it is the general opinion that β cell destruction is mediated predominantly by a Th1/Th17 immune response, it has been shown in the past that T cells with a Th2 phenotype might also contribute to β cell damage or stress. In particular, transfer of islet-antigen-specific Th2-type T cells into immunocompromised NOD severe combined immunodeficient (SCID) mice resulted in ‘swarming pancreatitis’ with infiltrations in the endocrine and exocrine pancreas and, subsequently, T1D [42].
Fine-tuning of our data to the level of VD-related genetic polymorphisms revealed an association between the VD plasma level and the AA genotype of the DBP Sty I polymorphism and the TT genotype of the DBP Hae III polymorphism, both of which showed significantly reduced levels of VD. Even though the number of patients was low for some of the genotypes (i.e. n = 5 for the AA genotype of the DBP Sty I polymorphism), the data confirm earlier findings [36,37] and suggest that aberrant binding of free VD to DBP as occurring in individuals with the AA genotype of Sty I, the TT genotype of Hae III, results in lower plasma levels of bioactive 1,25D. A link between DBP expression and T1D has been demonstrated in the past [24]. However, in our pilot study we did not find an association between the DBP genotype and immune status. Because many studies have demonstrated an association between T1D and other VD-related polymorphisms, such as CYP27B1 and CYP2R1 [16–19], it is of interest that we did not find a significant difference in the immune status of patients who carry genotypes associated with a higher risk for T1D. In contrast, for CYP24A1 genotypes we found significant differences in the frequency of total CD8 T cells, IL-4-producing CD8 T cells and DC subsets. This observation may also be of interest in the context of other autoimmune disorders, as a recent genome-wide association study of multiple sclerosis (MS) patients showed a significant association with the CYP24A1 gene [43].
Controversially, an association of VDR polymorphism and T1D has been discussed. Some studies suggest that there is no association [21,22]. In contrast, a recent meta-analysis suggests that at least one of four known polymorphisms in the VDR gene is associated with a higher risk for T1D in Asians [23]. However, from our preliminary results we were not able to confirm a link of the VD plasma level to any of the four VDR-associated polymorphisms investigated.
Several reasons seem to account for our finding of a lack of a profound association between the VD plasma levels and immune status in our pilot study. One reason was clearly the small cohort of 63 T1D patients. In particular, subdivision of patients into individual subgroups according to the 13 VD-related polymorphisms resulted in somewhat small numbers of patients in some subgroups (see Supporting figures). Note that, due to the small number of patients, an association analysis of the genotypes with data from the longitudinal study was not possible. In addition, the variation in the VD plasma levels in all groups was considerable. The plasma was collected throughout the year from patients living in the greater Frankfurt area. Due to the reported seasonality of the VD status in individual healthy volunteers [44,45], such a variation is not entirely surprising. Of the 63 baseline samples in our cohort, 12 were collected in spring, 25 in summer, 16 in autumn and 10 in winter. However, there were no significant seasonal differences in the 25D plasma levels [spring, 22 (± 8·5) ng/ml; summer, 19 (± 11) ng/ml; autumn 20 (± 11) ng/ml; winter, 17 (± 11) ng/ml] and 1,25D [spring, 43 (± 11) pg/ml; summer, 50 (± 18) pg/ml; autumn, 49 (± 19) pg/ml; winter, 40 (± 7·8) pg/ml] in our cohort of T1D patients. An advised supplementation of 1000 IU/day VD in patients with a VD deficiency had no effect on the plasma 25D and 1,25D levels within a 12-month period. However, the VD supplement dose might have been too low, and we are currently performing a follow-up study supplementation of 4000 IU/day over a 3-month period.
Khoo et al. recently demonstrated a change in the peripheral blood T cell compartment associated with the VD plasma level in 15 healthy individuals [45]. In contrast to our study, Khoo et al. performed a longitudinal study with healthy individuals collecting blood samples for the duration of 1 year and found that elevated plasma VD levels in the summer were associated with increased CD4 and CD8 T cells [45]. They further found a higher frequency of FoxP3-positive Tregs within the overall Treg population, and a reduced capacity of CD4 and CD8 T cells to produce proinflammatory cytokines that paralleled the elevated VD-plasma levels in the summer [45]. However, the data were restricted to a very small number of healthy male individuals, and a causative relation between the observed differences in the immune status and the VD plasma levels is still missing.
Another reason for the lack of a clear association of VD levels and immune status might be due to the complexity of the system, including the inherent variability of the immune status of patients as well as healthy individuals. Thus, a protective effect of VD administration as demonstrated in models with inbred mice [27,40,41] might be weakened in T1D patients. Alternatively, direct administration of in-vitro VD-differentiated DCs might circumvent such an ablation of a possible beneficial effect of VD. Indeed, it has been demonstrated that in-vitro VD-differentiated tolerogenic DCs transfer their regulatory properties via induction of autoreactive Tregs to proinflammatory DCs, indicating that VD-differentiated DCs may cause a form of infectious tolerance [46].
Our preliminary data provide insight into the complexity of the genetic polymorphism related to VD, on one hand, and the large variability of T1D patients with regard to VD supplementation on the other hand. Therefore, we believe that our pilot study provides a basis for further more comprehensive follow-up studies. Preclinical model systems, such as the NOD or the rat insulin promoter-lymphocytic choriomeningitis (RIP-LCMV) mouse [47], provide an excellent platform to evaluate further the mechanism behind a possible VD-induced protection. Additional clinical trials need to be performed in order to evaluate whether oral VD administration or direct transfer of VD-differentiated cell populations might be beneficial for T1D patients.
Acknowledgments
K.R. and E.R.L. were supported by the Else Kröner Fresenius Foundation. K.B. is supported by a grant from the European Community's Health Seventh Framework Program (FP7/2009–2014 under grant agreement 241447 with the acronym NAIMIT). U.C. is supported by the German Research Foundation (DFG).
Disclosure
The authors have nothing to disclose.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Vitamin D (VD) serum level and immune status in patients subgrouped according to the human leucocyte antigen (HLA)-DR/DQ genotype. Type 1 diabetes (T1D) patients were subdivided into two groups: group A includes individuals with T1D high-risk genotypes HLA-DR3, HLA-DR4, HLA-DQ2 and HLA-DQ8. Group B includes patients with other HLA genotypes not associated with an elevated risk to develop T1D. The two groups were analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], mDCs (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S2. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP24A1 rs2296237 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP24A1 rs2296237 polymorphism (CC, CG, GG) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid DCs (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S3. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP24A1 rs927650 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP24A1 rs927650 polymorphism (TT, TC, CC) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S4. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP2R1 rs1074657 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP2R1 rs1074657 polymorphism (AA, AG, GG) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S5. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP2R1 rs12794714 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP2R1 rs12794714 polymorphism (AA, AG, GG) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+], regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S6. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP27B1 rs10877012 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP27B1 rs10877012 polymorphism (AA, AC, CC) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S7. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the CYP27B1 rs4646536 polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the CYP27B1 rs4646536 polymorphism (TT, TC, CC) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S8. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the VD-binding protein (DBP) Hae III polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the DBP Hae III polymorphism (GG, GT, TT) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S9. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the VD-binding protein (DBP) Sty I polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the DBP Sty I polymorphism (CC, AC, AA) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S10. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the VDR Apa I polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the VDR Apa I polymorphism (AA, Aa, aa) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S11. Vitamin D (VD) serum level and immune status in patients sub-grouped according to the genotypes of the VDR Bms I polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the VDR Bms I polymorphism (BB, Bb, bb) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S12. VD serum level and immune status in patients subgrouped according to the genotypes of the VDR Fok I polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the VDR Fok I polymorphism (FF, Ff, ff) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], mDCs (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)-γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
Fig. S13. Vitamin D (VD) serum level and immune status in patients subgrouped according to the genotypes of the VDR Taq I polymorphism. Type 1 diabetes (T1D) patients were subdivided into the three different genotypes of the VDR Taq I polymorphism (TT, Tt, tt) and analysed for associations between the individual genotypes and (A) 25-VD and 1,25-VD serum level; (B) frequency of plasmacytoid dendritic cells (pDCs) [Linage cocktail 3 (Lin3−), CD303+, CD123+, CD11c+], myeloid dendritic cells (mDCs) (Lin3−, CD303−, CD123lo, CD11chi, CD4+), regulatory T cells (Tregs) (CD4+, CD25+, forkhead box protein 3 (FoxP3)+; CD8+, CD25+, FoxP3+) and (C) interferon (IFN)γ, interleukin (IL)-17 or IL-4-producing CD4 and CD8 T cells after overnight stimulation with phorbol myristate acetate (PMA)/ionomycin. Significant differences in the Mann–Whitney U-test are indicated (GraphPad Prism software).
References
- 1.Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457–467. doi: 10.1016/j.immuni.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Christen U, von Herrath MG. Do viral infections protect from or enhance type 1 diabetes and how can we tell the difference? Cell Mol Immunol. 2011;8:193–198. doi: 10.1038/cmi.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracy S, Drescher KM, Jackson JD, et al. Enteroviruses, type 1 diabetes and hygiene: a complex relationship. Rev Med Virol. 2010;20:106–116. doi: 10.1002/rmv.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hober D, Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat Rev Endocrinol. 2010;6:279–289. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 5.Schubert C. News feature: the worm has turned. Nat Med. 2004;10:1271–1272. doi: 10.1038/nm1204-1271. [DOI] [PubMed] [Google Scholar]
- 6.Chatenoud L, You S, Okada H, et al. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: immune therapies of type 1 diabetes: new opportunities based on the hygiene hypothesis. Clin Exp Immunol. 2010;160:106–112. doi: 10.1111/j.1365-2249.2010.04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christen U, Benke D, Wolfe T, et al. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J Clin Invest. 2004;113:74–84. doi: 10.1172/JCI200417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippi CM, Estes EA, Oldham JE, et al. Immunoregulatory mechanisms triggered by viral infections protect from type 1 diabetes in mice. J Clin Invest. 2009;119:1515–1523. doi: 10.1172/JCI38503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diana J, Brezar V, Beaudoin L, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell–plasmacytoid dendritic cell interplay. J Exp Med. 2011;208:729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolden-Kirk H, Overbergh L, Christesen HT, et al. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 11.Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–1503. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 12.Mohr SB, Garland CF, Gorham ED, et al. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008;51:1391–1398. doi: 10.1007/s00125-008-1061-5. [DOI] [PubMed] [Google Scholar]
- 13.Sloka S, Grant M, Newhook LA. The geospatial relation between UV solar radiation and type 1 diabetes in Newfoundland. Acta Diabetol. 2010;47:73–78. doi: 10.1007/s00592-009-0100-0. [DOI] [PubMed] [Google Scholar]
- 14.Janner M, Ballinari P, Mullis PE, et al. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Swiss Med Wkly. 2010;140:w13091. doi: 10.4414/smw.2010.13091. [DOI] [PubMed] [Google Scholar]
- 15.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Lopez E, Bruck P, Jansen T, et al. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev. 2007;23:631–636. doi: 10.1002/dmrr.719. [DOI] [PubMed] [Google Scholar]
- 17.Bailey R, Cooper JD, Zeitels L, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56:2616–2621. doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper JD, Smyth DJ, Walker NM, et al. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60:1624–1631. doi: 10.2337/db10-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramos-Lopez E, Bruck P, Jansen T, et al. CYP2R1-, CYP27B1- and CYP24-mRNA expression in German type 1 diabetes patients. J Steroid Biochem Mol Biol. 2007;103:807–810. doi: 10.1016/j.jsbmb.2006.12.056. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nejentsev S, Cooper JD, Godfrey L, et al. Analysis of the vitamin D receptor gene sequence variants in type 1 diabetes. Diabetes. 2004;53:2709–2712. doi: 10.2337/diabetes.53.10.2709. [DOI] [PubMed] [Google Scholar]
- 22.Kahles H, Morahan G, Todd JA, et al. Association analyses of the vitamin D receptor gene in 1654 families with type I diabetes. Genes Immun. 2009;10(Suppl. 1):S60–S63. doi: 10.1038/gene.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Li W, Liu J, et al. Polymorphisms in the vitamin D receptor gene and type 1 diabetes mellitus risk: an update by meta-analysis. Mol Cell Endocrinol. 2012;355:135–142. doi: 10.1016/j.mce.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Ongagna JC, Pinget M, Belcourt A. Vitamin d-binding protein gene polymorphism association with IA-2 autoantibodies in type 1 diabetes. Clin Biochem. 2005;38:415–419. doi: 10.1016/j.clinbiochem.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 25.Jankosky C, Deussing E, Gibson RL, et al. Viruses and vitamin D in the etiology of type 1 diabetes mellitus and multiple sclerosis. Virus Res. 2012;163:424–430. doi: 10.1016/j.virusres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 27.van Etten E, Dardenne O, Gysemans C, et al. 1,25-Dihydroxyvitamin D3 alters the profile of bone marrow-derived dendritic cells of NOD mice. Ann NY Acad Sci. 2004;1037:186–192. doi: 10.1196/annals.1337.030. [DOI] [PubMed] [Google Scholar]
- 28.van Halteren AG, Tysma OM, van Etten E, et al. 1Alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J Autoimmun. 2004;23:233–239. doi: 10.1016/j.jaut.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Adorini L, Penna G, Giarratana N, et al. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J Cell Biochem. 2003;88:227–233. doi: 10.1002/jcb.10340. [DOI] [PubMed] [Google Scholar]
- 30.Penna G, Amuchastegui S, Giarratana N, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 31.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Penna-Martinez M, Ramos-Lopez E, Stern J, et al. Impaired vitamin D activation and association with CYP24A1 haplotypes in differentiated thyroid carcinoma. Thyroid. 2012;22:709–716. doi: 10.1089/thy.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurylowicz A, Ramos-Lopez E, Bednarczuk T, et al. Vitamin d-binding protein (DBP) gene polymorphism is associated with Graves’ disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes. 2006;114:329–335. doi: 10.1055/s-2006-924256. [DOI] [PubMed] [Google Scholar]
- 34.Ramos-Lopez E, Kurylowicz A, Bednarczuk T, et al. Vitamin D receptor polymorphisms are associated with Graves’ disease in German and Polish but not in Serbian patients. Thyroid. 2005;15:1125–1130. doi: 10.1089/thy.2005.15.1125. [DOI] [PubMed] [Google Scholar]
- 35.Badenhoop K, Walfish PG, Rau H, et al. Susceptibility and resistance alleles of human leukocyte antigen (HLA) DQA1 and HLA DQB1 are shared in endocrine autoimmune disease. J Clin Endocrinol Metab. 1995;80:2112–2117. doi: 10.1210/jcem.80.7.7608264. [DOI] [PubMed] [Google Scholar]
- 36.Fu L, Yun F, Oczak M, et al. Common genetic variants of the vitamin D binding protein (DBP) predict differences in response of serum 25-hydroxyvitamin D [25(OH)D] to vitamin D supplementation. Clin Biochem. 2009;42:1174–1177. doi: 10.1016/j.clinbiochem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Sinotte M, Diorio C, Berube S, et al. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89:634–640. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 38.Unger WW, Laban S, Kleijwegt FS, et al. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 39.van Halteren AG, van Etten E, de Jong EC, et al. Redirection of human autoreactive T-cells upon interaction with dendritic cells modulated by TX527, an analog of 1,25 dihydroxyvitamin D(3) Diabetes. 2002;51:2119–2125. doi: 10.2337/diabetes.51.7.2119. [DOI] [PubMed] [Google Scholar]
- 40.Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 41.Gysemans CA, Cardozo AK, Callewaert H, et al. 1,25-Dihydroxyvitamin D3 modulates expression of chemokines and cytokines in pancreatic islets: implications for prevention of diabetes in nonobese diabetic mice. Endocrinology. 2005;146:1956–1964. doi: 10.1210/en.2004-1322. [DOI] [PubMed] [Google Scholar]
- 42.Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoo AL, Chai LY, Koenen HJ, et al. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol. 2011;164:72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khoo AL, Koenen HJ, Chai LY, et al. Seasonal variation in vitamin D levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS ONE. 2012;7:e29250. doi: 10.1371/journal.pone.0029250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleijwegt FS, Laban S, Duinkerken G, et al. Transfer of regulatory properties from tolerogenic to proinflammatory dendritic cells via induced autoreactive regulatory T cells. J Immunol. 2011;187:6357–6364. doi: 10.4049/jimmunol.1101638. [DOI] [PubMed] [Google Scholar]
- 47.Christen U, Hintermann E, Holdener M, et al. Viral triggers for autoimmunity: is the ‘glass of molecular mimicry’ half full or half empty? J Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.