Abstract
Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)-immunoglobulin (Ig) has immunosuppressive properties both in vivo and in vitro, but much is still unknown about the mechanisms by which CTLA-4-Ig exerts its immunosuppressive activities in vivo. The aim of this study was to investigate the effect of CTLA-4-Ig in a mouse model of contact hypersensitivity (CHS). The inflammatory response in the presence or absence of CTLA-4-Ig was evaluated by measuring the increase in ear thickness in sensitized animals after challenge. We observed a dose-dependent suppression of the ear swelling in both dinitrofluorobenzene (DNFB)- and oxazolone-induced CHS. The suppressive effect was still present 3 weeks after administration, even in the absence of circulating levels of CTLA-4-Ig. It was further shown that CTLA-4-Ig inhibits activation of T cells in the draining lymph node after sensitization and affects the maturation level of both dendritic cells and B cells. Furthermore, CTLA-4-Ig reduces infiltration of activated CD8+ T cells into the inflamed ear tissue and suppresses both local and systemic inflammation, as illustrated by reduced expression of cytokines and chemokines in the inflamed ear and a reduced level of acute-phase proteins in circulation. Finally, our results suggest that CTLA-4-Ig has a mainly immunosuppressive effect during the sensitization phase. We conclude that CTLA-4-Ig induces long-term immunosuppression of both DNFB- and oxazolone-induced inflammation and our data are the first to compare the effect of this compound in both DNFB- and oxazolone-induced CHS and to show that CTLA-4-Ig exerts an immunosuppressive effect on both local and systemic inflammatory mediators which is mediated principally during the sensitization phase.
Keywords: contact hypersensitivity, CTLA-4-Ig, immunosuppression, T cell activation, T cells
Introduction
Contact hypersensitivity (CHS) is a T cell-mediated immune reaction in which a presensitized animal is re-exposed to an antigen, thereby eliciting an immunological reaction at the site of antigen exposure. In mice, contact hypersensitivity has been studied in great detail using haptens such as dinitrofluorobenzene (DNFB) and oxazolone, and the immunological reaction is thought to encompass multiple cell types, including both Langerhans cells (LC) [1], dermal dendritic cells (DCs) [2], T cells [3], B-1 cells [4], natural killer T (NK T) cells [5], NK cells [6], granulocytes (in particular neutrophils) [7] and mast cells [8]. Furthermore, several cytokines and chemokines have been implicated in the process [9]. The CHS model in mice thus represents classical re-activation of antigen-specific T cells involving many different molecular and cellular pathways; thus, the CHS model is useful for studying the in vivo effect of modulating one or more of these pathways and therefore represents a mechanistic model of immune activation in general [9].
Activation of naive T cells is dependent on co-stimulation between CD80/CD86 on antigen-presenting cells (APCs) and CD28 expressed on T cells. This interaction triggers a signalling pathway that augments interleukin (IL)-2 production and T cell proliferation. To prevent excessive and uncontrollable activation, CD80/CD86 also binds to cytotoxic T lymphocyte-associated antigen-4 (CTLA-4, CD152), which is a negative regulator of T cell activation, and CTLA-4 plays an important role in the induction and maintenance of peripheral tolerance [10,11]. The soluble form of CTLA-4 [CTLA-4-immunoglobulin (Ig)] has been shown to induce T cell anergy in vitro, inhibit T cell-dependent antibody responses and prolong survival of allogeneic and xenogeneic grafts in vivo [12–15]. Furthermore, human CTLA-4-Ig induces long-term immune suppression of dinitrofluorobenzene (DNFB)-induced CHS [16], but the mechanism(s) by which CTLA-4-Ig exerts its action are not fully described. In this study, we confirm previous findings that CTLA-4-Ig mediates both short- and long-term immune suppression of the response in both DNFB- and oxazolone-induced CHS models. Furthermore, we extend previous findings by showing that CTLA-4-Ig inhibits activation of T cells in the draining lymph node after sensitization and reduces infiltration of activated CD8+ T cells into the inflamed ear after challenge. Additionally, we find that CTLA-4-Ig suppresses both local and systemic inflammation, as illustrated by reduced expression of certain cytokines and chemokines in the inflamed ear and a reduced level of acute-phase proteins in the serum. Finally, our results suggest that CTLA-4-Ig exerts its effect primarily during the sensitization phase of CHS and seems to be dispensable during the challenge phase. During the sensitization phase, CTLA-4-Ig is found to bind to DCs and to mediate a reduced expression of CD86 on both B cells and DCs. These results are useful to understand the mechanisms behind CTLA-4-Ig-mediated immune suppression in vivo.
Materials and methods
Mice
Female BALB/c mice were purchased from Taconic (Ry, Denmark). The mice were used at the age of 8–10 weeks. The mice had free access to water and to standard mouse chow (Altromin®, Lage, Germany) and were kept in a room with 12-h day/night cycle. All animal experiments were approved by the Danish Animal Inspectorate.
Contact hypersensitivity
CHS experiments were performed largely as described previously [17]. In brief, the mice were sensitized on day 0 by applying 20 μl 0·5% DNFB (1–fluoro-2·4-dinitrobenzene; Sigma, St Louis, MO, USA) or 100 μl 1% oxazolone (4-ethoxy-methylene-2-phenyl-3-oxazalin-5-one; Sigma), dissolved in 4:1 acetone (VWR)/olive oil (Sigma) on the shaved abdominal skin. Five (DNFB) or six (oxazolone) days later, the baseline ear thickness on the left ear was measured, after which both sides of the left ear were challenged by epicutaneous application of 20 μl 0·2% DNFB or 20 μl 0·75% oxazolone. The challenge treatment was performed under light anaesthesia with isoflurane. The ear thickness of the left ear was measured 24, 48 and 72 h after challenge with a dial thickness gauge from Mitutoyo (Mitutoyo Pocket Thickness Gages 7309; Kawasaki, Japan). The ear swelling (ΔT) was calculated as ear thickness 24, 48 or 72 h after challenge minus baseline ear thickness. It is expressed as the mean ± standard error (s.e.m.) in units of 10−2 mm. In the dose-titration studies with CTLA-4-Ig (see Fig. 1) one group was sensitized with acetone/olive oil alone but challenged with DNFB or oxazolone, which induced a non-specific irritative ear-swelling response. Another group was treated only with acetone/olive oil in both the sensitization and challenge phases, and together these two groups served as negative controls. For resensitization experiments, mice were repainted epicutaneously with 0·5% DNFB or 1% oxazolone on the shaved abdomen 3 weeks after the first sensitization. Five or 6 days later, 20 ul of 0·2% DNFB or 20 ul 0·75% oxazolone was applied to the left ear and ear thickness was measured 24, 48 and 72 h post-challenge. All groups always comprised five animals.
Fig. 1.
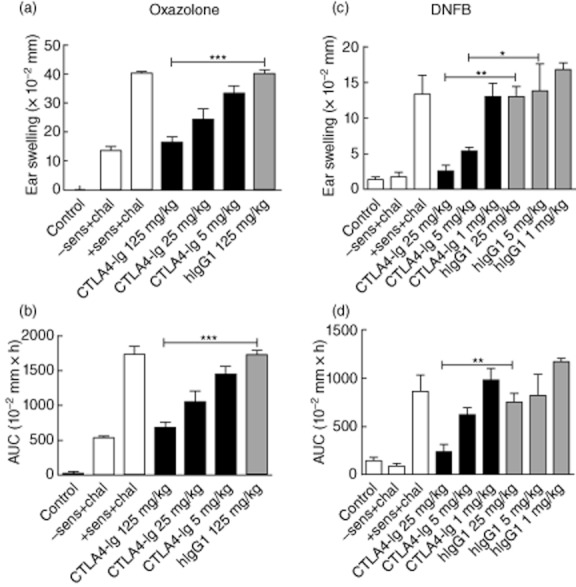
Treatment with cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) induces dose-dependent immune suppression in two hapten-induced inflammation models. (a+b) In the oxazolone model CTLA-4-Ig and the control treatment hIgG1 were injected in three different doses: 125 mg/kg, 25 mg/kg and 5 mg/kg intraperitoneally (i.p.) 1 day prior to sensitization. A dose-dependent effect on the ear-swelling response is observed. Ear swelling is shown at 24 h (a) and summarized as area under the curve (AUC) (b). A previous study showed that 25 mg/kg was not sufficient to block the oxazolone-induced inflammation completely (data not shown). (c,d) In the dinitrofluorobenzene (DNFB)-induced model mice were treated with CTLA-4-Ig or isotype control (hIgG1) at 25 mg/kg, 5 mg/kg and 1 mg/kg i.p. 1 day prior to sensitization. This study was performed twice with similar results. Ear swelling is shown at 24 h (c) and summarized as AUC (d). Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01; ***P < 0·001.
CTLA-4-Ig treatment
CTLA-4-Ig (Orencia®, Abatacept marketed by Bristol-Myers Squibb, New Hampshire, USA) was tested in doses of 1, 5, 25 or 125 mg/kg, as indicated. As controls, mice, injected with the Fc-part of a human IgG1 (BioXcell, Penzberg, Germany), in the same doses as CTLA-4-Ig, were included in all experiments. Serum levels of CTLA-4-Ig were determined by anti-human IgG1 enzyme-linked immunosorbent assay (ELISA) (Invitrogen, Carlsbad, CA, USA) 3 and 21 days after administration.
Flow cytometry
To examine the activation status of T cells after sensitization, inguinal lymph node was removed 24 h post-sensitization. Single-cell suspension was prepared by transferring the lymph node through a 70-μm cell strainer and washing cells with 1 × phosphate-buffered saline (PBS) (w/o Mg2+ and Ca2+; Gibco/Invitrogen). Cells were resuspended at 10 × 106 cells/ml and 1 × 106 cells/sample were used for staining. Additionally, the activation status of T cells in the inflamed ear was analysed 48 h after challenge. Briefly, the inflamed ear was divided into dorsal and ventral halves. Using a scalpel, the dermis was separated from epidermis and both parts were incubated subsequently with 2000 U/ml collagenase (Sigma) and 2000 U/ml DNAse (Roche, San Diego, CA, USA) for 60 min. Next, ear tissue was passed through a 70-μm cell strainer before cells were washed and resuspended in PBS (w/o Mg2+ and Ca2+; Gibco/Invitrogen). The cell suspensions were blocked with anti-CD32/CD16 (Fc block; BD Biosciences, San Jose, CA, USA) for 10 min and stained with the following anti-mouse monoclonal antibodies (mAb): CD45-eFluor605 (eBioscience, San Diego, CA, USA), T cell receptor (TCR)-β-phycoerythrin (PE)-cyanin-7 (Cy7) (Biolegend, San Diego, CA, USA), CD4-APC (BD Biosciences), CD8-fluorescein isothicyanate (FITC) (Santa-Cruz Laboratories, Santa Cruz, CA, USA), CD19-Q655 (Invitrogen), CD44-Pacific Blue (eBioscience), CD62L-Alexa-Fluor-700 (Biolegend), CD69-peridinin chlorophyll protein (PerCP)-Cy5·5 (BDBiosciences) and NKG2D-PE (eBioscience) for 30 min. Flow cytometric analysis of samples was analysed on a BD LSRII flow cytometer equipped with a blue, red and violet laser and data were analysed in BD FACS Diva software version 6·1.3.
Cytokine measurements
Ears were removed 24 and 48 h after challenge and a punch biopsy of 8 mm in diameter was collected from each ear, weighted and placed in 1 ml buffer [0·9% saline with 0·01% Triton X-100 (Sigma) + 1 protease inhibitor cocktail tablet (complete ethylenediamine tetraacetic acid-free from Roche)] on ice. The biopsies were subsequently homogenized and centrifuged at 4°C, 10 000 g for 15 min. The supernatants were centrifuged once more before being frozen at −80 degrees until use. Supernatants were analysed with Milliplex Map mouse cytokine/chemokine panel (Millipore, Billerica, MA, USA) using the Luminex detection method. Supernatants were analysed for the following cytokines and chemokines: IL-4, interferon gamma-induced protein (IP)-10, IL-12 (p40), macrophage inflammatory protein-2 (MIP-2), tumour necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1β, IL-10 and IL-6.
Acute-phase proteins
Serum samples taken 24 and 48 h after challenge were analysed for serum amyloid P (SAP) and haptoglobin using ELISAs according to the manufacturer's recommendations (Genway, San Diego, CA, USA).
Adoptive transfer experiment
Where indicated, donor mice were treated with 25 mg/kg CTLA-4-Ig 1 day prior to sensitization and sensitized subsequently with DNFB on day 0 according to standard procedure. Five days later the donor mice were killed and the inguinal lymph node was isolated. Single cell suspension was prepared by transferring the lymph node through a 70-μm cell strainer and washing cells with 1 × PBS (w/o Mg2+ and Ca2+, Gibco/Invitrogen). Lymph node cells from each group, respectively, were pooled and resuspended in 1 × PBS. Subsequently, cells were injected intravenously (i.v.) into naive recipient mice, so each recipient mouse received lymph node cells from one donor mouse. The day before adoptive transfer, recipient mice were treated where indicated with 25 mg/kg CTLA-4-Ig. Five hours after adoptive transfer the recipient groups were challenged with DNFB by the standard procedure and ear swelling measured 24, 48 and 72 h post-challenge. A second adoptive transfer experiment was conducted where biopsies were taken from the inflamed ear 48 h post-challenge. These were analysed for their content of different cytokines and chemokines, as described previously, in order to investigate whether the changed cytokine and chemokine expression after CTLA-4-Ig treatment is due to a direct suppressive effect on the keratinocytes or if it can be explained by a decreased infiltration of effector cells after CTLA-4-Ig treatment.
Binding of CTLA-4-Ig on lymph node cells after sensitization with DNFB
To investigate binding of CTLA-4-Ig on lymph node cells in the inguinal lymph node after sensitization, groups of mice (n = 5) were treated with CTLA-4-Ig or isotype control (25 mg/kg). The next day all mice were sensitized with 0·5% DNFB, as described above. Subsequently, mice were killed 3, 4 and 5 days after sensitization and single cells from the inguinal lymph node were prepared for flow cytometric analysis as described above and the cell suspensions were blocked with anti-CD32/CD16 (Fc block; BDBiosciences) for 10 min and stained with the following anti-mouse monoclonal antibodies (mAb): anti-human IgG1-APC (Jackson Immunoresearch, West Grove, PA, USA), CD45-Efluor605 (eBiosciences), TCR-β-Qdot655 (Invitrogen), CD19-V450 (BDBiosciences), CD11c-PECy7 (BDBiosciences), I-A/E-FITC (eBiosciences) and CD86-PE (eBiosciences) for 30 min. Flow cytometric analysis of samples was analysed on a BD LSRII flow cytometer equipped with a blue, red and violet laser and data were analysed in BD fluorescence activated cell sorter (FACS) Diva software, version 6·1.3. DCs were gated as CD45+TCR-β–CD19−, MHCII+ and CD11c+, while B cells were gated as CD45+CD19+ cells, and the level of human IgG1+ DCs and B cells together with CD86+ DCs and B cells were investigated.
Results
Dose-dependent immune suppression by CTLA-4-Ig in two hapten-induced inflammation models
To investigate whether CTLA-4-Ig is able to suppress hapten-induced inflammation in vivo, two mouse models of contact hypersensitivity were analysed: the DNFB- and oxazolone-induced CHS models, respectively. BALB/c mice were treated with CTLA-4-Ig or control proteins (hIgG1Fc) and subsequently sensitized on day 0. Five (DNFB) or 6 (oxazolone) days later, mice were challenged with hapten, and ear thickness measured 24, 48 and 72 h later. Control groups included mice which were sensitized with acetone/olive oil but challenged with DNFB or oxazolone, and mice which were treated with only acetone/olive oil in both the sensitization and challenge phases. Figure 1 shows the ear-swelling response after 24 h (Fig. 1a,c) and summarized as area under the curve (AUC) from 0–72 h (Fig. 1b,d); the data confirm that CTLA-4-Ig mediates a dose-dependent suppression of the ear-swelling response in both models. Initial studies in the oxazolone model revealed no effect of 1 mg/kg and insufficient effect of 5 and 25 mg/kg (data not shown); hence, in the second study, mice were treated with CTLA-4-Ig or isotype control at 5, 25 and 125 mg/kg. Using these doses, a dose-dependent suppression of the response was observed with 125 mg/kg reducing the response to background levels (Fig. 1a,b). In the DNFB-induced model, CTLA-4-Ig inhibited the ear swelling in a dose-dependent manner and 25 mg/kg virtually inhibited the response completely (Fig. 1c,d). Taken together, these results show that CTLA-4-Ig mediates a dose-dependent immune suppression in both models and that the DNFB-induced model was responsive to lower doses of CTLA-4-Ig than the oxazolone-induced model.
Sustained immune suppression after CTLA-4-Ig treatment
Three weeks after the first sensitization and challenge, mice were resensitized and rechallenged with DNFB or oxazolone, respectively, without any further treatment with CTLA-4-Ig. As shown in Fig. 2a, mice in the DNFB-induced model dosed previously with 25 mg/kg still exhibited a significantly reduced ear-swelling response compared to the hIgG1 control group. In the oxazolone-induced model, the highest dose also exerted a suppressive effect 3 weeks after administration (Fig. 2b). Exposure analysis of circulating levels of CTLA-4-Ig 3 and 21 days after administration (Fig. 2c,d) were performed subsequently. Figure 2c shows serum levels 3 days after administration and clearly revealed detectable levels of CTLA-4-Ig. However, after 21 days the levels of CTLA-4-Ig in the serum samples were below the detection level of the assay (<0·43 μg/ml), suggesting that no or very low levels of CTLA-4-Ig were present in the serum (Fig. 2d). Based on this, we conclude that treatment with CTLA-4-Ig results in a sustained suppression of the ear-swelling response in both models independent of the presence of detectable, circulating levels of CTLA-4-Ig in the serum.
Fig. 2.
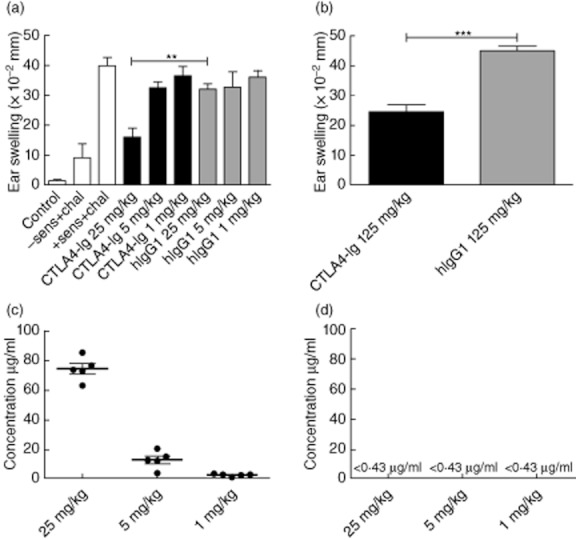
Cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) treatment leads to sustained suppression in both models. Three weeks after the first sensitization and challenge, mice were resensitized and rechallenged without any further treatment with CTLA-4-Ig. (a) Ear-swelling response after 24 h in the dinitrofluorobenzene (DNFB)-induced model dosed previously with 25 mg/kg, 5 mg/kg and 1 mg/kg. (b) Ear-swelling response after 24 h in the oxazolone-induced model dosed previously with 125 mg/kg. Exposure analyses of circulating levels of CTLA-4-Ig were detected by anti-human IgG1 enzyme-linked immunosorbent assay (ELISA). (c) Serum levels of CTLA-4-Ig 3 days after administration. (d) Serum levels of CTLA-4-Ig 21 days after administration; 0·43 μg/ml is the lower detection limit of the ELISA analysis. Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01; ***P < 0·001.
CTLA-4-Ig suppresses activation of T cells, DCs and B cells in the draining lymph node after sensitization
To investigate the mechanism by which CTLA-4-Ig exerts its suppressive function in greater detail, cells isolated from the inguinal lymph node draining the area of sensitized skin were stained for activation markers and analysed by flow cytometry 24 h post-sensitization (Fig. 3). CTLA-4-Ig treatment led to a reduced number of CD8+ and CD4+ T cells in the draining lymph node (Fig. 3a,b, right). This reduction was due to an overall lower number of cells in the lymph nodes, as the percentages of CD4+ and CD8+ T cells of CD45+ live cells were similar between the CTLA-4-Ig-treated and the isotype-treated group (Fig. 3a,b, left). Because inflammation in this model is dependent on CD8+ T cells [3], we investigated this cell population in greater detail. Figure 3c,d shows that CD8+ T cells in the draining lymph node have a less activated phenotype after CTLA-4-Ig treatment, as the number and percentage of CD44+CD62L–CD8+ T cells and CD69+CD8+ T cells were reduced significantly in the CTLA-4-Ig-treated mice compared to the control group. These results demonstrate that treatment with CTLA-4-Ig leads to reduced numbers of CD4+ and CD8+ T cells in the inguinal lymph node after sensitization and that CD8+ T cells in the draining lymph node have a less activated phenotype. We next analysed binding of CTLA-4-Ig on DCs and B cells after sensitization with DNFB. Mice were treated with 25 mg/kg of CTLA-4-Ig or control protein 1 day prior to sensitization. As shown in Fig. S1A, significant binding of CTLA-4-Ig to DCs could be detected on day 3. Furthermore, we found a significantly reduced expression of CD86 4 and 5 days after sensitization in CTLA-4-Ig-treated mice (Fig. S1B,C). In contrast, no specific binding of CTLA-4-Ig to B cells could be detected at either time-point examined (Fig. S1D), but expression of CD86 on B cells was strongly suppressed at every time-point after sensitization in the CTLA-4-Ig-treated group compared to treatment with isotype control (Fig. S1E,F). Together, these data suggest that CTLA-4-Ig binds preferentially to DCs in the draining lymph node after hapten sensitization, and that CTLA-4-Ig reduces the level of the maturation marker CD86 on both DCs and B cells.
Fig. 3.
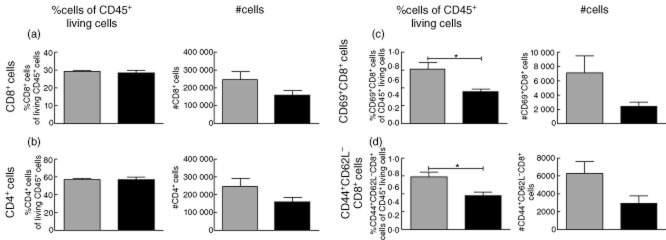
Flow cytometric analysis of activation status of T cells in the draining lymph node after sensitization with dinitrofluorobenzene (DNFB). Cells were isolated from the inguinal lymph node draining the sensitized abdominal skin 24 h post-sensitization, stained for activation markers and analysed by flow cytometry. (a) Left: %CD8+ cells of CD45+ living cells. Right: absolute number of CD8+ cells. (b) Left: %CD4+ cells of CD45+ living cells. Right: absolute numbers of CD4+ cells. (c) Left: %CD69+CD8+ cells of CD45+ living cells. Right: absolute numbers of CD69+CD8+ cells. (d) Left: %CD44+CD62L–CD8+ cells of CD45+ living cells. Right: absolute numbers of CD44+CD62L–CD8+ cells. Data are depicted as mean ± standard error of the mean, *P < 0·05.  : Isotype control-treated mice (hIgG1) (25 mg/kg);
: Isotype control-treated mice (hIgG1) (25 mg/kg);  : cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig)-treated mice (25 mg/kg).
: cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig)-treated mice (25 mg/kg).
Decreased infiltration of activated effector cells into the inflamed tissue after CTLA-4-Ig treatment
Having demonstrated a reduction of CD4+ and CD8+ T cell activation in draining lymph nodes in the presence of CTLA-4-Ig, we wanted to investigate the consequences for the inflammatory reaction in the tissue after challenge. Thus, infiltrating cells were isolated from the inflamed ear 48 h after challenge, stained for activation markers and analysed by flow cytometry. As shown in Fig. 4, CTLA-4-Ig treatment led to a significant reduction in both number and percentage of CD8+ T cells in the inflamed ear compared to controls (Fig. 4a). In contrast, the number of CD4+ T cells was not significantly different, but the percentage of CD4+ T cells was increased in the CTLA-4-Ig-treated group (Fig. 4b). More importantly, CTLA-4-Ig treatment resulted in a reduction in the number of activated CD8+ T cells in the inflamed ear compared to controls. Thus, we observed a decreased number and percentage of CD44+CD62L−CD8+ T cells and CD69+CD8+ T cells in the CTLA-4-Ig-treated group compared to controls (Fig. 4c,d). In conclusion, these results suggest that CTLA-4-Ig inhibits infiltration of activated CD8+ T cells into the challenged tissue.
Fig. 4.
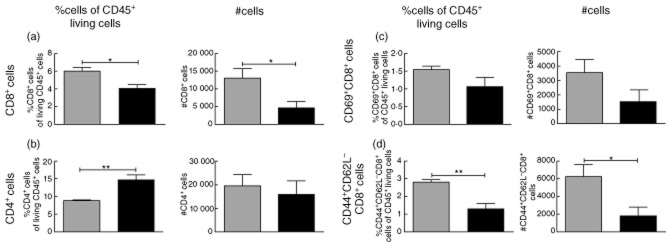
Flow cytometric analysis of activation status of infiltrating cells in the dinitrofluorobenzene (DNFB)-challenged ear. Infiltrating cells were isolated from the inflamed ear 48 h after challenge, stained for activation markers and analysed by flow cytometry. (a) Left: %CD8+ cells of CD45+ living cells. Right: absolute number of CD8+ cells. (b) Left: %CD4+ cells of CD45+ living cells. Right: absolute numbers of CD4+ cells. (c) Left: %CD69+CD8+ cells of CD45+ living cells. Right: absolute numbers of CD69+CD8+ cells. (d) Left: %CD44+CD62L–CD8+ cells of CD45+ living cells. Right: absolute numbers of CD44+CD62L–CD8+ cells. Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01.  : Isotype control-treated mice (hIgG1) (25 mg/kg);
: Isotype control-treated mice (hIgG1) (25 mg/kg);  : cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig)-treated mice (25 mg/kg).
: cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig)-treated mice (25 mg/kg).
Altered expression of cytokines and chemokines in the inflamed ear after CTLA-4-Ig treatment
To correlate the reduced cellular infiltration into the target tissue after CTLA-4-Ig treatment with the local production of cytokines and chemokines, homogenates of inflamed ear tissue from CTLA-4-Ig-treated and isotype control-treated animals were analysed for their content of a number of cytokines and chemokines including IL-4, CXCL10 (IP-10), IL-12 (p40), MIP-2, TNF-α, IFN-γ, IL-1β, IL-10 and IL-6. As shown in Fig. 5, IL-1β and IL-4 were suppressed significantly in the CTLA-4-Ig-treated group compared to the control group both in the DNFB- and in the oxazolone-induced models (Fig. 5a–d). Additionally, the concentrations of the chemokines MIP-2 and CXCL10 (IP-10) were reduced in both models (Fig. 5c,d,g,h) after CTLA-4-Ig treatment. In contrast, the measured amounts of TNF-α, IFN-γ, IL-6, IL-12 p40 and IL-10 were not affected significantly by treatment with CTLA-4-Ig compared to isotype treatment (data not shown). Taken together, we conclude that CTLA-4-Ig affects the level of cytokines and chemokines in the affected tissue by significantly reducing IL-4, IL-1β, MIP-2 and IP-10.
Fig. 5.
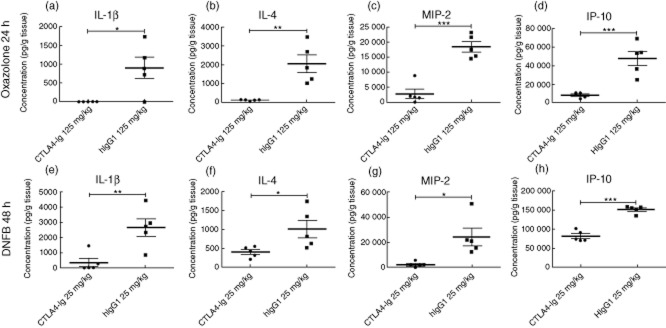
Changed expression of cytokines and chemokines in the inflamed ear after cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) treatment. Homogenates of inflamed ear tissue from CTLA-4-Ig-treated or isotype control-treated animals were analysed at 48 [dinitrofluorobenzene (DNFB)] or 24 (oxazolone) h for their content of a number of cytokines and chemokines including interleukin (IL)-4, interferon gamma-induced protein 10 (IP)-10, IL-12 (p40), macrophage inflammatory protein-2 (MIP-2), tumour necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1β, IL-10 and IL-6. (a–h). CTLA-4-Ig treatment led to a reduced expression of the following analytes in both models: IL-1β (a+e), IL-4 (b+f), MIP-2 (c+g) and IP-10 (d+h). Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01; ***P < 0·001.
CTLA-4-Ig suppresses systemic inflammation
To analyse the effect of CTLA-4-Ig on systemic inflammation, serum samples taken 24 and 48 h after challenge were analysed by ELISA for the acute-phase proteins SAP and haptoglobin. These factors have been shown to be reliable markers of inflammation in this model as their serum levels correspond to ear swelling (A.D.C. and C.H., data not shown). Furthermore, increased serum concentration of these components indicates systemic inflammation with involvement of the liver [18]. Figure 6b,d shows that serum levels of SAP and haptoglobin were reduced significantly following treatment with CTLA-4-Ig compared to control treatment at both 24 and 48 h after challenge in the DNFB-induced model, and in the oxazolone-induced model serum concentrations of haptoglobin were suppressed significantly after both 24 and 48 h (Fig. 6c). Similarly, SAP was reduced significantly after 48 h but not at 24 h (Fig. 6a). Based on these findings, we conclude that CTLA-4-Ig inhibits systemic inflammation as measured by circulating levels of SAP and haptoglobin.
Fig. 6.
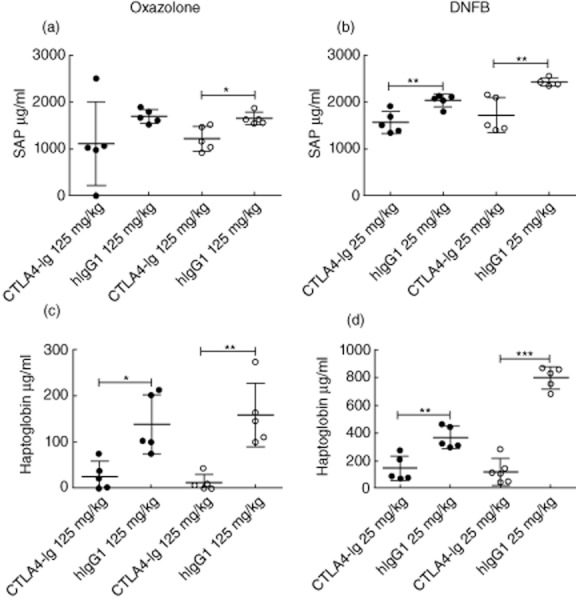
Cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) suppresses serum levels of serum amyloid P (SAP) and haptoglobin. Serum samples taken 24 and 48 h after challenge were analysed by enzyme-linked immunosorbent assay (ELISA) for the acute-phase proteins serum amyloid P (SAP) and haptoglobin. (a,c) Serum levels of SAP (a) and haptoglobin (c) from the oxazolone-induced model. (b,d) Serum levels of SAP (b) and haptoglobin (d) from the dinitrofluorobenzene (DNFB)-induced model. Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01; ***P < 0·001.  : 24 h;
: 24 h;  : 48 h.
: 48 h.
CTLA-4-Ig exerts its effect mainly during the activation phase
In the CHS model, it is not known whether CTLA-4-Ig exerts its effect in the sensitization phase alone or whether the presence of CTLA-4-Ig is also important in the effector phase. To test this, we set up an adoptive transfer system in which donor mice were sensitized in the presence or absence of CTLA-4-Ig. After 5 days, cells from the draining lymph node were transferred to recipient mice which had been treated with CTLA-4-Ig 24 h earlier or left untreated. Recipient mice were subsequently challenged with DNFB and ear swelling was measured 24, 48 and 72 h after challenge. As shown in Fig. 7, mice transferred with cells exposed to CTLA-4-Ig during both the sensitization phase and the challenge phase or during the sensitization phase alone (labelled +/+ and +/−, respectively) exhibited a significantly suppressed ear-swelling response compared to the untreated control group (labelled −/−). In contrast, the mice which were treated only with CTLA-4-Ig during the challenge phase (labelled −/+) exhibited ear swelling similar to the untreated mice. Taken together, these results indicate that CTLA-4-Ig exerts its immunosuppressive effect primarily during the sensitization phase. We next tested whether regulation of cytokines and chemokines in the inflamed tissue followed the same pattern as ear swelling by comparing levels of IL-1β, IL-4, IP-10 and MIP-2 in the adoptive transfer model treated with CTLA-4-Ig in the sensitization or challenge phase only. Interestingly, the pattern of regulation was not similar between the cytokines examined: whereas IL-1β was unaffected by the presence of CTLA-4-Ig in either sensitization or challenge phase alone, IL-4 and MIP-2 levels were suppressed strongly in both CTLA-4-Ig treatment protocols (Fig. S2). In contrast, levels of IP-10 correlated well with ear swelling, because only CTLA-4-Ig treatment in the sensitization phase – but not the challenge phase – could reduce the levels of IP-10. These data suggest that the release of IL-4, IL-1β, MIP-2 and IP-10 locally in the inflamed ear is regulated differently by CTLA-4-Ig; whereas IL-4, MIP-2 and IP-10 are suppressed when CTLA-4-Ig is present only in the sensitization phase, our data show that MIP-2 and IL-4 can also be suppressed when CTLA-4-Ig is present during the challenge phase alone.
Fig. 7.
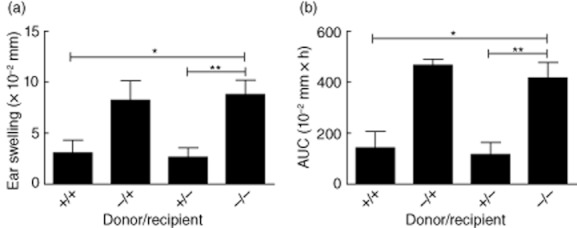
Cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) exerts its effect primarily during the sensitization phase. Donor mice were sensitized to dinitrofluorobenzene (DNFB)in the presence or absence of CTLA-4-Ig. After 5 days, cells from the draining lymph node were transferred to recipient mice which had been treated with CTLA-4-Ig 24 h earlier where indicated. Mice were subsequently challenged with DNFB and ear swelling measured 24, 48 and 72 h later. +/+: CTLA-4-Ig-treatment during both sensitization and challenge phases. +/−: CTLA-4-Ig treatment during sensitization phase alone. −/+: CTLA-4-Ig treatment during challenge phase alone. −/−: No treatment with CTLA-4-Ig. Ear swelling is shown after 24 h (a) and summarized as area under the curve (AUC) (b). Data are depicted as mean ± standard error of the mean, *P < 0·05; **P < 0·01.
Discussion
In this study we show that CTLA-4-Ig treatment suppresses hapten-induced inflammation in two skin inflammation models. The effect of CTLA-4-Ig has been shown previously in the DNFB-induced CHS model but not in the oxazolone-induced CHS model. The short-term effect on ear swelling was detected in both the DNFB-induced model, where 25 mg/kg was sufficient to suppress the response completely, while in the oxazolone model 125 mg/kg was necessary to obtain the same degree of suppression. DNFB has been described previously to induce a T helper type 1 (Th1)-mediated response [16,19], whereas oxazolone is shown to mediate a mixed phenotype characterized by both Th1 and Th2 cells [20]. The different efficacy of CTLA-4-Ig in the two models may be attributed to the notion that CTLA-4-Ig may suppress Th1 responses more efficiently than Th2 responses [16]. Furthermore, in our hands oxazolone-induced inflammation is dominated more by neutrophils than T cells (compared to the DNFB-model); thus, it is formally possible that the effect of CTLA-4-Ig is less efficient in the oxazolone model because of the considerable involvement of neutrophils. Alternatively, the need for a higher dose to suppress oxazolone-induced inflammation could also reflect the stronger overall response by oxazolone compared to DNFB (see Fig. 1).
In addition to the short-term effect, we found that CTLA-4-Ig induces a long-lasting suppression of inflammation in both models even in the absence of any detectable, circulating CTLA-4-Ig during the secondary response. The lack of circulating CTLA-4-Ig 3 weeks after administration is expected, as the half-life of human CTLA-4-Ig has been estimated to be 30 h in mice [13,21]. Interestingly, sustained immune modulation by CTLA-4-Ig has also been shown in other settings, including transplantation, where short-term CTLA-4-Ig therapy led to long-term tissue- and organ-graft survival and induction of tolerance [14,22–24]. Furthermore, it has been shown in vitro that CTLA-4-Ig induces a long-lasting hypo-responsiveness in human mixed leucocyte cultures (MLC) [12]. The precise mechanisms by which CTLA-4-Ig mediates the sustained suppression are not entirely clear. However, it has been suggested that CTLA-4-Ig is able to induce anergy in effector T cells [12], delete effector T cells by apoptosis [25] and/or prevent antigen-specific activation and proliferation of T cells [26]. Additionally, CTLA-4-Ig has been shown to induce production of indoleamine 2,3-dioxygenase (IDO) from APCs, which would inhibit T cell activation by tryptophan depletion [27,28]. Another potential immunosuppressive mechanism has been suggested by which CTLA-4-Ig can induce and increase the population of regulatory T cells both in vitro [29] as well as in collagen-induced arthritis in mice [30].
In this study, we have shown further that activation and proliferation of T cells in the sensitized draining lymph node are inhibited after treatment with CTLA-4-Ig and that infiltration of activated effector CD8+ T cells in the inflamed tissue is reduced after challenge. The effect in the draining lymph node is in accordance with a study performed by Platt et al. [26], who demonstrated that in an ovalbumin (OVA)-specific T cell activation model, CTLA-4-Ig treatment leads to a reduced proliferation of T cells and reduced down-regulation of CD62L on OVA-specific T cells 3 days post-immunization together with a reduced expression of CD69 1 day post-immunization [26]. Less efficient down-regulation of CD62L on T cells in CTLA-4-Ig-treated mice is consistent with a reduced infiltration of effector cells into the inflamed ear tissue, as down-regulation of CD62L is needed for lymphocytes to exit the draining lymph node and to enter the site of inflammation [31]. Further, our data suggest that CTLA-4-Ig binds primarily to DCs but also mediates a strong inhibition of CD86 expression on B cells. Cytokines IL-4 and IL-1β, together with chemokines MIP-2 and IP-10, were suppressed after CTLA-4-Ig treatment. In the skin, a major source of both MIP-2 and IP-10 is keratinocytes [32,33] and it is currently not known how CTLA-4-Ig may suppress production of these two chemokines. It has been suggested that IP-10 production from keratinocytes attracts CD8+ T cells, which subsequently secrete IFN-γ, further stimulating keratinocytes to produce more IP-10 and thereby completing a positive feedback loop [34]. Because CTLA-4-Ig inhibits infiltration of CD8+ T cells into the challenged ear it is possible that the reduced infiltration of CD8+ T cells could lead to decreased release of IP-10, as found in our analysis. The data in the adoptive transfer studies show that both IP-10 and MIP-2 are suppressed when CTLA-4-Ig is present only in the sensitization phase – this is expected, as the presence of CTLA-4-Ig in the sensitization phase only also results in a reduced ear swelling and reduced influx of CD8+ T cells (Figs 4 and S2). However, it was surprising that MIP-2 but not IP-10 was suppressed when CTLA-4-Ig was present in the challenge phase alone, which does not reduce ear swelling (Fig. S2). Thus, regulation of IP-10 correlates with ear swelling and tissue inflammation, whereas regulation of MIP-2 is independent of ear swelling and suggests that MIP-2 is dispensable for the full elicitation of ear swelling, although we cannot completely exclude the possibility of subtle changes in the cellular composition in the tissue.
It is interesting to note that CTLA-4-Ig inhibits the systemic inflammatory response, as suggested by a reduced concentration of the acute-phase proteins SAP and haptoglobin levels in the blood. This may imply that CTLA-4-Ig affects systemic levels of the inflammatory cytokines IL-6, IL-1β and TNF-α, which are thought to stimulate the production of these acute-phase proteins from the liver, but this needs to be investigated further. To our knowledge, this is the first study to show that CTLA-4-Ig causes a reduced level of systemic inflammation markers in the CHS model but is in accordance with data from rheumatoid arthritis patients, where treatment with CTLA-4-Ig results in reduced serum levels of the acute-phase protein C-reactive protein (CRP) [35]. Our adoptive transfer study suggests that CTLA-4-Ig mainly mediates an immunosuppressive effect during the sensitization phase. This is in accordance with the fact that CTLA-4 is a negative regulator of T cell activation and thereby works primarily to dampen the inflammation during the activation phase. However, we cannot exclude that CTLA-4-Ig can modulate more subtle aspects of the secondary challenge response (e.g. chemokine or cytokine profiles).
In conclusion, our study shows that CTLA-4-Ig treatment suppresses inflammation measured by several different parameters, including reduced ear swelling, reduced activation of effector T cells in the skin-draining lymph node after sensitization, reduced infiltration of activated T cells into the inflamed ear after challenge, a decreased detection of certain cytokines and chemokines in the inflamed tissue and – on a systemic level – reduced serum levels of acute-phase proteins. Furthermore, our results suggest that CTLA-4-Ig mediates its effect primarily during the sensitization phase of CHS and is dispensable during the challenge phase.
Disclosure
A. D. C. and C. H. are employees of Novo Nordisk A/S.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) binds to dendritic cells (DCs) and down-regulates CD86 on both DCs and B cells in the draining lymph node after sensitization with dinitrofluorobenzene (DNFB). Groups of mice were treated with either CTLA-4-Ig or isotype control and sensitized with 0·5% DNFB the following day. Lymph node cells from the draining lymph node were stained with anti-human IgG1 and analysed by flow cytometry at days 3, 4 and 5 after sensitization for detection of binding of CTLA-4-Ig on lymph node cells. (A) %hIgG1+ cells of DCs gated as CD19–T cell receptor (TCR)-β–major histocompatibility complex II (MHC)II+CD11c+ cells 3, 4 and 5 days after sensitization. (B) %CD86+ cells of DCs. (C) Median fluorescence intensity (MFI) of CD86 phycoerythrin (PE) on CD19–MHCII+CD11C+ cells. (D) %hIgG1+ cells of B cells gated as CD19+ cells. (E) %CD86+ cells of B cells gated as CD19+ cells. (F) MFI of CD86 PE on CD19+ cells. Data are depicted as mean ± standard error of the mean, *P < 0·05, **P < 0·01 and ***P < 0·001. ‘Grey box' : Isotype control-treated mice (hIgG1) (25 mg/kg); ‘black box' : CTLA-4-Ig-treated mice (25 mg/kg).
Figure S2. Cytotoxic T lymphocyte antigen-4 (CTLA-4)-immunoglobulin (Ig) treatment during challenge phase mediates a reduced release of interleukin (IL)-4 and macrophage inflammatory protein-2 (MIP-2). Donor mice were sensitized to dinitrofluorobenzene (DNFB) in the presence or absence of CTLA-4-Ig. After 5 days, cells from the draining lymph node were transferred to recipient mice which were treated with CTLA-4-Ig 24 h earlier where indicated. Mice were challenged 5 h later with DNFB and ear swelling measured 24 and 48 h later; 48 h after challenge homogenates of inflamed ear tissue were analysed for their content of IL-1β, IL-4, interferon gamma-induced protein (IP)-10 and MIP-2 (a). Ear swelling in the groups is shown in (b) after 24 h (upper) and as area under the curve (lower). +/−: CTLA-4-Ig treatment during sensitization phase alone; −/+: CTLA-4-Ig treatment during challenge phase alone; −/−: no treatment with CTLA-4-Ig. Data are depicted as mean ± standard error of the mean, *P < 0·05, **P < 0·01 and ***P < 0·001.
References
- 1.Bennett CL, Noordegraaf M, Martina CAE, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–6835. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 2.Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 4.Itakura A, Szczepanik M, Campos RA, et al. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 5.Campos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177:3686–3694. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 6.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 7.Engeman T, Gorbachev AV, Kish DD, Fairchild RL. The intensity of neutrophil infiltration controls the number of antigen-primed CD8 T cells recruited into cutaneous antigen challenge sites. J Leukoc Biol. 2004;76:941–949. doi: 10.1189/jlb.0304193. [DOI] [PubMed] [Google Scholar]
- 8.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 9.Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS. 2012;120:1–27. doi: 10.1111/j.1600-0463.2011.02832.x. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 12.Tan P, Anasetti C, Hansen JA, et al. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linsley PS, Wallace PM, Johnson J, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 14.Lenschow DJ, Zeng Y, Thistlethwaite JR, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA-4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 15.Wallace PM, Johnson JS, MacMaster JF, Kennedy KA, Gladstone P, Linsley PS. CTLA-4Ig treatment ameliorates the lethality of murine graft-versus-host disease across major histocompatibility complex barriers. Transplantation. 1994;58:602–610. doi: 10.1097/00007890-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Tang A, Judge TA, Nickoloff BJ, Turka LA. Suppression of murine allergic contact dermatitis by CTLA-4Ig. Tolerance induction of Th2 responses requires additional blockade of CD40-ligand. J Immunol. 1996;157:117–125. [PubMed] [Google Scholar]
- 17.Gaspari AA, Katz SI. Contact hypersensitivity. Curr Protoc Immunol. 2001 doi: 10.1002/0471142735.im0402s08. Chapter 4:4.2.1–4.2.5. [DOI] [PubMed] [Google Scholar]
- 18.Prowse KR, Baumann H. Interleukin-1 and interleukin-6 stimulate acute-phase protein production in primary mouse hepatocytes. J Leukoc Biol. 1989;45:55–61. doi: 10.1002/jlb.45.1.55. [DOI] [PubMed] [Google Scholar]
- 19.Dearman RJ, Basketter DA, Kimber I. Characterization of chemical allergens as a function of divergent cytokine secretion profiles induced in mice. Toxicol Appl Pharmacol. 1996;138:308–316. doi: 10.1006/taap.1996.0129. [DOI] [PubMed] [Google Scholar]
- 20.Thomson JA, Troutt AB, Kelso A. Contact sensitization to oxazolone: involvement of both interferon-gamma and interleukin-4 in oxazolone-specific Ig and T-cell responses. Immunology. 1993;78:185–192. [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace PM, Rodgers JN, Leytze GM, Johnson JS, Linsley PS. Induction and reversal of long-lived specific unresponsiveness to a T-dependent antigen following CTLA-4Ig treatment. J Immunol. 1995;154:5885–5895. [PubMed] [Google Scholar]
- 22.Lin H, Bolling SF, Linsley PS, et al. Long-term acceptance of major histocompatibility complex mismatched cardiac allografts induced by CTLA-4Ig plus donor-specific transfusion. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakkis FG, Konieczny BT, Saleem S, et al. Blocking the CD28-B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J Immunol. 1997;158:2443–2448. [PubMed] [Google Scholar]
- 24.Tran HM, Nickerson PW, Restifo AC, et al. Distinct mechanisms for the induction and maintenance of allograft tolerance with CTLA-4-Fc treatment. J Immunol. 1997;159:2232–2239. [PubMed] [Google Scholar]
- 25.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 26.Platt AM, Gibson VB, Patakas A, et al. Abatacept limits breach of self-tolerance in a murine model of arthritis via effects on the generation of T follicular helper cells. J Immunol. 2010;185:1558–1567. doi: 10.4049/jimmunol.1001311. [DOI] [PubMed] [Google Scholar]
- 27.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 28.Fallarino F, Grohmann U, Hwang KW, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 29.Razmara M, Hilliard B, Ziarani AK, Chen YH, Tykocinski ML. CTLA-4 Ig converts naive CD4+CD25− T cells into CD4+CD25+ regulatory T cells. Int Immunol. 2008;20:471–483. doi: 10.1093/intimm/dxn007. [DOI] [PubMed] [Google Scholar]
- 30.Ko HJ, Cho ML, Lee SY, et al. CTLA-4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34:111–120. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Tedder TF, Penta AC, Levine HB, Freedman AS. Expression of the human leukocyte adhesion molecule, LAM1. Identity with the TQ1 and Leu-8 differentiation antigens. J Immunol. 1990;144:532–540. [PubMed] [Google Scholar]
- 32.Sebastiani S, Albanesi C, De PO, Puddu P, Cavani A, Girolomoni G. The role of chemokines in allergic contact dermatitis. Arch Dermatol Res. 2002;293:552–559. doi: 10.1007/s00403-001-0276-9. [DOI] [PubMed] [Google Scholar]
- 33.Enk AH, Katz SI. Early molecular events in the induction phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tokuriki A, Seo N, Ito T, Kumakiri M, Takigawa M, Tokura Y. Dominant expression of CXCR3 is associated with induced expression of IP-10 at hapten-challenged sites of murine contact hypersensitivity: a possible role for interferon-gamma-producing CD8(+) T cells in IP-10 expression. J Dermatol Sci. 2002;28:234–241. doi: 10.1016/s0923-1811(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 35.Weisman MH, Durez P, Hallegua D, et al. Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol. 2006;33:2162–2166. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


