Abstract
Vitamin D has been shown to modulate innate immune responses in vitro and ex vivo; however, human in-vivo data are lacking. At high latitudes, seasonal vitamin D deficiency is common due to alternating ultraviolet (UV)-B radiation exposure. In the present study, we investigated whether levels of 25 hydroxyvitamin D3 [25(OH)D3] and its active metabolite 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] are subject to seasonal variation and whether plasma levels of these vitamin D metabolites correlate with the in-vivo cytokine response during experimental human endotoxaemia [administration of lipopolysaccharide (LPS) in healthy volunteers]. Plasma levels of 25(OH)D3 and 1,25(OH)2D3 were determined in samples obtained just prior to administration of an intravenous bolus of 2 ng/kg LPS (derived from Escherichia coli O:113) in 112 healthy male volunteers. In the same subjects, plasma levels of the inflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10 were analysed serially after endotoxin administration. Plasma levels of 1,25(OH)2D3, but not 25(OH)D3, were subject to significant seasonal variation, with lower levels in autumn and winter. 25(OH)D3 and 1,25(OH)2D3 levels did not correlate with plasma cytokine responses. Furthermore, 25(OH)D3 deficient subjects (< 50 nmol/l) displayed an identical cytokine response compared with sufficient subjects. In conclusion, plasma levels of vitamin D are not correlated with the LPS-induced TNF, IL-6 and IL-10 cytokine response in humans in vivo. These findings question the direct role of vitamin D in modulation of the innate immune response.
Keywords: cytokines, endotoxin, human endotoxaemia, innate immune response, vitamin D
Introduction
Vitamin D has a plethora of functions in the human body, of which the regulation of calcium metabolism is the most well known [1,2]. Vitamin D3 (cholecalciferol) is obtained through de-novo synthesis in the skin under influence of ultraviolet (UV)-B exposure and dietary intake [3,4]. Vitamin D3 is hydroxylated in the liver into 25 hydroxyvitamin D3 [25(OH)D3], its main circulating metabolite that is used as a marker of vitamin D status [3,4]. Ultimately, 25(OH)D3 is converted by 1α-hydroxylase into its biological active metabolite 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] that can bind to the vitamin D receptor (VDR). 1,25(OH)2D3 is formed in many tissues/cells of the body, of which the kidney is the most important source [4,5]. Especially at higher latitudes, vitamin D status has been described to be subject to seasonal variation, with lower levels during the winter months and early spring due to little UV-B radiation exposure [6–9].
Recently, an important role for vitamin D in the modulation of the innate immune response has been identified [10]. 1,25(OH)2D3 limits production of proinflammatory cytokines such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 in lipopolysaccharide (LPS)- and Candida albicans-stimulated human peripheral blood mononuclear cells (PBMCs) in vitro [7,8]. Furthermore, seasonal variations in 25(OH)D3 and 1,25(OH)2D3 levels (in healthy volunteers living in the Netherlands at 52·5° N latitude) correlated with alterations in cytokine production by ex-vivo LPS-stimulated PBMCs, with lower TNF-α and IL-6 production in winter [7]. Encouraged by the increasing evidence of immunomodulatory effects of vitamin D, a role for seasonality/UV exposure and vitamin D levels in the prevalence of autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, and susceptibility towards influenza and viral upper respiratory tract infections during the winter season, has been implicated [11,12].
As outlined above, until now, the effects of vitamin D on the innate immune response have been established in vitro and ex vivo; however, in-vivo evidence in humans is lacking. In this retrospective study we investigated whether vitamin D plasma levels of healthy volunteers in the Netherlands are subject to seasonal variation and whether they correlate with the plasma cytokine response during experimental human endotoxaemia (LPS administration), a well-characterized, standardized model of the innate immune response in humans in vivo [13].
Materials and methods
Subjects
The study population consisted of 112 young healthy non-smoking male student volunteers who participated in seven experimental human endotoxaemia trials performed in our institution from 2005 to 2010 (NCT00246714, NCT00513110, NCT00783068, NCT00785018, NCT00916448, NCT01349699, NCT01091571). We included only subjects who, apart from LPS, received either no intervention, a placebo or an intervention that did not influence their cytokine response and of whom sufficient plasma samples were left to analyse vitamin D and plasma cytokine levels. Subjects from each separate trial were not distributed equally over the four seasons, although there was overlap between two or even three seasons within four of the seven trials. As the protocol of the different LPS trials was identical, bias is unlikely. All subjects were Caucasian residents of the Netherlands (52·5° N latitude). We studied only male volunteers because of considerable differences in the LPS-induced cytokine response between males and females, and possible effects of hormonal variations due to the menstrual cycle [14]. The study protocols were approved by the Ethics Committee of the Radboud University Nijmegen Medical Centre and complied with the Declaration of Helsinki including current revisions and the Good Clinical Practice guidelines. Written informed consent was obtained from all study participants. Screening of the subjects within 14 days before the test revealed no abnormalities in medical history or physical examination. Volunteers were not taking any prescription medications, and were tested negative for hepatitis B surface antigen and human immunodeficiency virus infection. Based on the day of LPS administration (which was also the day that plasma for vitamin D analysis was collected), we divided the study population into the four seasons: spring (April–June), summer (July–September), autumn (October–December) and winter (January–March).
Experimental human endotoxaemia experiments
Subjects refrained from food 12 h before the start of the experiment, and caffeine- or alcohol-containing substances 24 h before the start of the experiment. The experiments were performed at the research unit of the intensive care department, with subjects in the supine position. After local anaesthesia (lidocaine HCl 20 mg/ml) the radial artery was cannulated using a 20-gauge arterial catheter (Angiocath; Becton Dickinson, San Jose, CA, USA) for blood sampling and arterial blood pressure monitoring (Edwards Lifesciences LLC, Irvine, CA, USA), connected to a Phillips IntelliVue MP70 monitor (Philips Medical Systems, Amsterdam, the Netherlands). A cannula was placed in the antecubital vein to permit infusion of 2·5% glucose/0·45% saline solution; subjects received a bolus of 1·5 l for 1 h before LPS infusion (prehydration), followed by 150 ml/h until 6 h after LPS infusion and 75 ml/h until the end of the experiment. US reference Escherichia coli endotoxin (E. coli O:113, Clinical Center Reference Endotoxin; National Institutes of Health (NIH), Bethesda, MA, USA) was used. Ec-5 endotoxin, supplied as a lypophilized powder, was reconstituted in 0·9% NaCl and vortex-mixed for at least 10 min after reconstitution. The endotoxin solution was administered as an intravenous bolus injection at a dose of 2 ng/kg of body weight. For safety reasons vital signs, including heart rate (HR) and mean arterial pressure (MAP), were monitored continuously throughout the study protocol.
25(OH)D3 and 1,25(OH)2D3 analysis
25(OH)D3 and 1,25(OH)2D3 levels were determined in plasma samples obtained just prior to LPS administration. To this end, ethylenediamine tetraacetic acid (EDTA) anti-coagulated blood was collected from the arterial line and centrifuged immediately at 2000 g for 10 min at 4°C, after which plasma was stored at −80°C until analysis. 25 OHD3 was measured in one batch using the Roche Cobas E601 analyser, according to the manufacturer's instructions [vitamin D3 (25-OH) assay with polyclonal antibody; Roche Diagnostics, Burgess Hill, UK]. 1,25(OH)2D3 levels were analysed in one batch using a radioimmunoassay (1,25 dihydroxyvitamin D kit; Immunodiagnostic Systems Ltd, Boldon, UK). Vitamin D deficiency was defined as plasma 25(OH)D3 levels of < 50 nmol/l, which is advocated by the American Institute of Medicine (2011).
Plasma cytokine analysis
EDTA anti-coagulated blood was collected from the arterial line and centrifuged immediately at 2000 g for 10 min at 4°C after which plasma was stored at −80°C until analysis. Concentrations of TNF-α, IL-6 and IL-10 were measured in one batch using a simultaneous Luminex Assay, according to the manufacturer's instructions (Milliplex; Millipore, Billerica, MA, USA). Detection range was 3·2–10 000 pg/ml for all cytokines.
Statistical analysis
Data are represented as mean or medians based on distribution (calculated by the Shapiro–Wilk test). Statistical tests used are indicated in the text and/or figure legends. Statistical analyses were performed using Graphpad Prism 5 (Graphpad Software, La Jolla, CA, USA).
Results
Seasonal variation in vitamin D plasma levels
Demographic characteristics of the study population of 112 healthy volunteers are listed in Table 1. Plasma levels of 25(OH)D3 did not differ significantly between seasons (Fig. 1a). However, 1,25(OH)2D3 levels were subject to seasonal variation, with lower concentrations found in autumn and winter (Fig. 1b). There was a weak but significant correlation between plasma levels of 25(OH)D3 and 1,25(OH)2D3 (Spearman's r = 0·23, P = 0·02).
Table 1.
Demographic characteristics of the study population
| Parameter | All subjects (n = 112) | Spring (n = 32) | Summer (n = 21) | Autumn (n = 34) | Winter (n = 25) |
|---|---|---|---|---|---|
| Age (years) | 22·2 ± 0·3 | 21·1 ± 0·3 | 22·9 ± 0·7 | 22·7 ± 0·4 | 22·5 ± 0·6 |
| BMI (kg/m2) | 22·8 ± 0·2 | 23·1 ± 0·5 | 22·2 ± 0·5 | 22·7 ± 0·4 | 22·9 ± 0·4 |
Data are presented as mean ± standard error of the mean. BMI: body mass index.
Fig. 1.
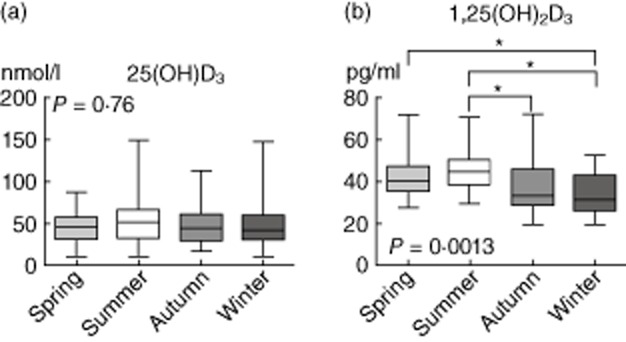
25 Hydroxyvitamin D3 [25(OH)D3] and 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] plasma levels, divided according to the four seasons: spring (April–June, n = 32), summer (July–September, n = 21), autumn (October–December, n = 34) and winter (January–March, n = 25). Data are represented as box and whiskers (range). P-values calculated using the Kruskal–Wallis test with Dunn's post-hoc test (* indicates P < 0·05).
Correlation of vitamin D levels with the LPS-induced plasma cytokine response
The pattern of LPS-induced plasma cytokine levels of the entire study population are depicted in Fig. 2. Plasma levels of 25(OH)D3 and 1,25(OH)2D3 did not correlate with the LPS-induced plasma cytokine response (correlations between vitamin D levels and both area under the curve (AUC) and peak plasma cytokine levels are listed in Table 2).
Fig. 2.
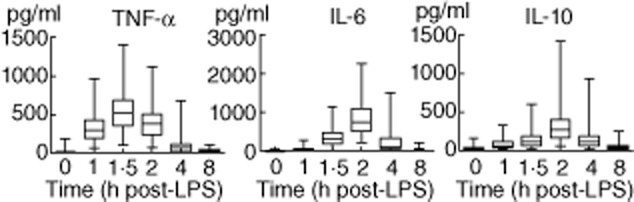
Time–course of lipopolysaccharide (LPS)-induced plasma cytokines of the 112 subjects. Data are represented as box and whiskers (range) of 112 subjects.
Table 2.
Correlation between plasma levels of 25 hydroxyvitamin D3 [25(OH)D3)] or 1,25 dihydroxyvitamin D3 [1,25(OH)2D3] and AUC and peak plasma cytokines
| AUC TNF-α | Peak TNF-α | AUC IL-6 | Peak IL-6 | AUC IL-10 | Peak IL-10 | |
|---|---|---|---|---|---|---|
| 25(OH)D3 | −0·06 (0·50) | −0·04 (0·69) | −0·03 (0·78) | −0·06 (0·52) | 0·13 (0·17) | 0·14 (0·16) |
| 1,25(OH)2D3 | 0·06 (0·55) | 0·11 (0·25) | −0·07 (0·50) | −0·05 (0·61) | 0·13 (0·19) | 0·09 (0·36) |
Spearman's correlation coefficients (P-value) of 112 subjects are listed. AUC: area under the curve of plasma cytokines in time; IL: interleukin; TNF: tumour necrosis factor.
LPS-induced cytokine responses in vitamin D-deficient and -sufficient subjects
To investigate further the possible immunomodulatory effects of 25(OH)D3 we compared LPS-induced cytokine responses of 25(OH)D3-deficient subjects to those with sufficient levels. The majority of our study population was 25(OH)D3-deficient [65 deficient (58%) versus 47 sufficient (42%)]. However, as depicted in Fig. 3, there were no differences in cytokine responses between deficient and sufficient subjects (area under curve of plasma cytokine depicted in Fig. 3, peak cytokine levels showed similar results and are not shown). Finally, we analysed the plasma cytokine responses (AUC and peak levels) based on a division of 25(OH)D3 levels in quartiles, which would increase sensitivity in the case of a non-linear relation between 25(OH)D3 levels and cytokine responses, but again no differences were found (data not shown). To evaluate the power of this study, we performed a calculation to determine the minimum detectable difference in AUC TNF-α levels between 25(OH)D3-deficient and -sufficient subjects (based on the data from Fig. 3, left panel). Based on 65 deficient and 47 sufficient subjects and an alpha of 0·05, we would be able to detect a difference in TNF levels of 25% with a power of 80%.
Fig. 3.
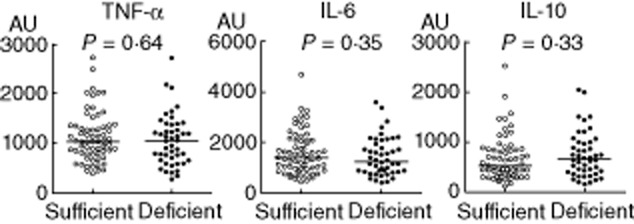
Area under curve of lipopolysaccharide (LPS)-induced plasma cytokines between 25 hydroxyvitamin D3 [25(OH)D3]-deficient (< 50 nmol/l, n = 65) and sufficient (≥ 50 nmol/l, n = 47) subjects. Lines indicate median values. P-values calculated using Mann–Whitney U-tests.
Discussion
In the present study, we investigated the seasonal variation of vitamin D plasma levels in young healthy males in the Netherlands and its relation with the plasma cytokine response induced by LPS administration in vivo. We did not find a significant seasonal variation in levels of 25(OH)D3, the main circulating metabolite. However, plasma concentrations of the active metabolite 1,25(OH)2D3 differed between seasons, with lower levels in autumn and winter. Furthermore, no relation between both vitamin D metabolites and the LPS-induced plasma cytokine response was found. The main finding of our study is that vitamin D status is not associated with the systemic inflammatory cytokine response during experimental human endotoxaemia.
In contrast to what others have found at both higher, lower and similar latitudes [6–9,15–17], there was no seasonal variation in levels of the main circulating vitamin D metabolite 25(OH)D3 in our study. Moreover, the majority of the study subjects was deficient for 25(OH)D3. The high prevalence of vitamin D deficiency in our healthy young male population was surprising with regard to the fact that deficiency is associated with low sun exposition, as seen at older age and for immigrant females. Interestingly, in a Norwegian study (60° N latitude) in elderly people, seasonal variation was found in a home-living group but not in a hospitalized group [18]. Furthermore, in the hospitalized group the prevalence of 25(OH)D3 deficiency was much higher compared to the home-living group [18]. It appears that in our study population, consisting of young male volunteers, virtually all of them students, UV-B exposure as the main resource as well as additional dietary intake may be relatively low. Unfortunately, we did not record information regarding vitamin D supplementation, outdoor activities or holidays in our study population.
In accordance with other studies [6,18], we found a weak but nevertheless significant correlation between 25(OH)D3 and1,25(OH)2D3 levels. The seasonal variation in 1,25(OH)2D3 levels as opposed to 25(OH)D3 was unexpected, as 25(OH)D3 is the main circulating metabolite of vitamin D and can be considered the source for 1,25(OH)2D3, and production of the latter is not influenced by UV-B radiation. The fact that 1,25(OH)2D3 levels were lower in autumn and winter and not in winter and spring is in agreement with an UV-B independent effect. Interindividual differences in 1α-hydroxylase activity, due possibly to variation in levels of parathyroid hormone (PTH) (which stimulates 1α-hydroxylase activity [19]), may represent a possible explanation for this discrepancy [6]. In this respect, low dietary calcium intake might be involved, as reduced calcium levels result in increased PTH levels and subsequent augmented 1α-hydroxylase activity [6]. Although we included only subjects who, besides LPS, received no intervention, a placebo or an intervention that did not influence their cytokine response, the interventions could potentially have influenced vitamin D levels. To address this, we also analysed seasonal variation in 25(OH)D3 and 1,25(OH)2D3 levels in the group of subjects that received no intervention or a placebo (n = 58), which revealed similar results (data not shown).
Two recent publications have found distinct anti-inflammatory effects of vitamin D; 1,25(OH)2D3 was shown to inhibit LPS-induced proinflammatory cytokine production by human PBMCs in vitro, and seasonal variation in cytokine production of ex-vivo LPS-stimulated PBMCs of healthy volunteers was found, which correlated with their 25(OH)D3 and 1,25(OH)2D3 levels [7,8]. In contrast, we did not find any correlations between 25(OH)D3 or 1,25(OH)2D3 levels and inflammatory cytokines after LPS administration in vivo. In accordance, no differences in plasma cytokine levels were found between vitamin D-sufficient and -deficient subjects. An explanation might be that the anti-inflammatory effects of 1,25(OH)2D3 in vitro were found at concentrations of 100 nmol/l [7,8], corresponding to 38 000 pg/ml, whereas the highest plasma concentration found in our study population was 72 pg/ml. Furthermore, higher plasma concentrations of 1,25(OH)2D3 (up to twofold higher) and especially 25(OH)D3 (up to fourfold higher) were found compared to our study [7,8]. In this respect, it might be speculated that 25(OH)D3 vitamin D levels in our subjects were too low to exert any anti-inflammatory effects. An alternative explanation is compartmentalization of the innate immune response. We have shown earlier that the cytokine production of ex-vivo LPS-stimulated leucocytes does not correlate with the plasma cytokine response elicited by LPS administration in vivo in the same subject [20,21]. In this respect, vitamin D levels might influence the leucocyte compartment, but not tissue-resident cells of the immune system such as macrophages, which are suggested to be the major cytokine producers after LPS administration in vivo [21]. It could be argued that our study was not powered adequately to detect more subtle (< 25%) differences in cytokine responses. However, the actual difference in AUC TNF-α levels between deficient and sufficient subjects was less than 4%. Irrespective of the relevance of such a small difference, to reach statistical significance of this difference with a power of 80%, a study population of almost 4500 subjects would be needed.
To the best of our knowledge, there are no studies regarding the direct effects of vitamin D (supplementation) on systemic inflammation in vivo in humans or animals. However, several studies have shown that the human cationic anti-microbial peptide LL-37, one of the downstream targets of the VDR, decreases plasma cytokine levels and reduces mortality in rodent sepsis and endotoxaemia through neutralization of LPS [22–24]. As LL-37 also promotes innate immunity due to its direct antimicrobial effects, neutralization of LPS has been proposed as a compensatory mechanism to curtain excessive inflammation [23]. Because no correlations between 25(OH)D3/1,25(OH)2D3 levels and cytokine responses were found, and LL-37 can be induced by many other compounds/stimuli apart from 1,25(OH)2D3 [25], a possible relation between LL-37 and the plasma cytokine response in our study would be independent from vitamin D status, and therefore outside the study's scope.
Several limitations of our study deserve attention. First, we studied only young healthy male student volunteers living at the same latitude, which is an advantage with regard to the homogeneity of the study population but limits extrapolation to the general population. However, to our knowledge there are no data that indicate differential effects of vitamin D on the innate immune response between sexes or age classes. Secondly, we studied only a limited set of inflammatory mediators. Vitamin D has been shown to modulate production of other cytokines in response to LPS, notably IL-1β [7]. However, IL-1β is barely detectable during human endotoxaemia and was therefore not analysed in this study [14]. Thirdly, the human experimental endotoxaemia model is limited to activation of the innate immune system, while vitamin D has also been shown to modulate the T cell compartment and therefore adaptive immune responses [26–28]. Therefore, vitamin D might exert immunomodulatory effects that do not emerge during experimental endotoxaemia. Furthermore, due to the use of LPS, the human endotoxaemia model is confined to Toll-like receptor (TLR)-4 signalling. While LPS is one of the most potent activators of innate immunity, vitamin D may exert immunomodulatory effects upon activation of the innate immune system with other stimuli, such as other TLR ligands or Candida albicans [8]. Finally, this model does not represent an innate immune response against live bacteria in which multiple other factors that are possibly influenced by vitamin D, such as direct anti-microbial effects of LL-37 and neutrophil function, may play a role. Neutrophils, vital in killing of pathogens, express the vitamin D receptor and vitamin D has been shown to directly modulate both neutrophil chemotaxis and activity [29–31].
In conclusion, our study reveals that plasma levels of 25(OH)D3 and 1,25(OH)2D3 are not correlated to the systemic inflammatory cytokine response during experimental human endotoxaemia. These findings question the direct role of vitamin D in modulation of the innate immune system. Intervention studies in which vitamin D is supplemented before an inflammatory challenge could provide the definitive answer to the question of whether or not vitamin D exerts direct immunomodulatory effects in humans in vivo.
Acknowledgments
The authors would like to thank the Trees Jansen for the Luminex cytokine measurements and the research nurses of the department of Intensive Care Medicine for assistance in the conduct of human endotoxaemia experiments. The authors received no financial support for this study.
Disclosure
Authors declare that they have no conflicts of interest.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Brown AJ, Dusso A, Slatopolsky E. Vitamin D. Am J Physiol. 1999;277:F157–175. doi: 10.1152/ajprenal.1999.277.2.F157. [DOI] [PubMed] [Google Scholar]
- 5.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 6.Christensen MH, Lien EA, Hustad S, Almas B. Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest. 2010;70:281–286. doi: 10.3109/00365511003797172. [DOI] [PubMed] [Google Scholar]
- 7.Khoo AL, Chai LY, Koenen HJ, et al. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin Exp Immunol. 2011;164:72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoo AL, Chai LY, Koenen HJ, et al. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: impact of seasonal variation of immune responses. J Infect Dis. 2011;203:122–130. doi: 10.1093/infdis/jiq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusch J, Ackermann H, Badenhoop K. Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population. Horm Metab Res. 2009;41:402–407. doi: 10.1055/s-0028-1128131. [DOI] [PubMed] [Google Scholar]
- 10.Maruotti N, Cantatore FP. Vitamin D and the immune system. J Rheumatol. 2010;37:491–495. doi: 10.3899/jrheum.090797. [DOI] [PubMed] [Google Scholar]
- 11.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponsonby AL, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002;181–182:71–78. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- 13.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24(Suppl. 1):94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 14.van Eijk LT, Dorresteijn MJ, Smits P, van der Hoeven JG, Netea MG, Pickkers P. Gender differences in the innate immune response and vascular reactivity following the administration of endotoxin to human volunteers. Crit Care Med. 2007;35:1464–1469. doi: 10.1097/01.CCM.0000266534.14262.E8. [DOI] [PubMed] [Google Scholar]
- 15.Guillemant J, Taupin P, Le HT, et al. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int. 1999;10:222–225. doi: 10.1007/s001980050219. [DOI] [PubMed] [Google Scholar]
- 16.Davies PS, Bates CJ, Cole TJ, Prentice A, Clarke PC. Vitamin D: seasonal and regional differences in preschool children in Great Britain. Eur J Clin Nutr. 1999;53:195–198. doi: 10.1038/sj.ejcn.1600697. [DOI] [PubMed] [Google Scholar]
- 17.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55:1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 18.Mowe M, Bohmer T, Haug E. Serum calcidiol and calcitriol concentrations in elderly people: variations with age, sex, season and disease. Clin Nutr. 1996;15:201–206. doi: 10.1016/s0261-5614(96)80242-1. [DOI] [PubMed] [Google Scholar]
- 19.Walker AT, Stewart AF, Korn EA, Shiratori T, Mitnick MA, Carpenter TO. Effect of parathyroid hormone-like peptides on 25-hydroxyvitamin D-1 alpha-hydroxylase activity in rodents. Am J Physiol. 1990;258:E297–303. doi: 10.1152/ajpendo.1990.258.2.E297. [DOI] [PubMed] [Google Scholar]
- 20.Dorresteijn MJ, Draisma A, van der Hoeven JG, Pickkers P. Lipopolysaccharide stimulated whole blood cytokine production does not predict the inflammatory response in human endotoxemia. Innate Immun. 2010;16:248–253. doi: 10.1177/1753425909339923. [DOI] [PubMed] [Google Scholar]
- 21.Kox M, de Kleijn S, Pompe JC, et al. Differential ex vivo and in vivo endotoxin tolerance kinetics following human endotoxemia. Crit Care Med. 2011;39:1866–1870. doi: 10.1097/CCM.0b013e3182190d5d. [DOI] [PubMed] [Google Scholar]
- 22.Cirioni O, Giacometti A, Ghiselli R, et al. LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob Agents Chemother. 2006;50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 24.Robinson MW, Donnelly S, Hutchinson AT, et al. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog. 2011;7:e1002042. doi: 10.1371/journal.ppat.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez-Samperio P. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–1798. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Khoo AL, Koenen HJ, Chai LY, et al. Seasonal variation in vitamin D(3) levels is paralleled by changes in the peripheral blood human T cell compartment. PLoS ONE. 2012;7:e29250. doi: 10.1371/journal.pone.0029250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoo AL, Joosten I, Michels M, et al. 1,25-Dihydroxyvitamin D3 inhibits proliferation but not the suppressive function of regulatory T cells in the absence of antigen-presenting cells. Immunology. 2011;134:459–468. doi: 10.1111/j.1365-2567.2011.03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi K, Nakayama Y, Horiuchi H, et al. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol Immunotoxicol. 2002;24:335–347. doi: 10.1081/iph-120014721. [DOI] [PubMed] [Google Scholar]
- 30.Takano Y, Mitsuhashi H, Ueno K. 1alpha,25-Dihydroxyvitamin D(3) inhibits neutrophil recruitment in hamster model of acute lung injury. Steroids. 2011;76:1305–1309. doi: 10.1016/j.steroids.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Hirsch D, Archer FE, Joshi-Kale M, Vetrano AM, Weinberger B. Decreased anti-inflammatory responses to vitamin D in neonatal neutrophils. Mediat Inflamm. 2011;2011:598345. doi: 10.1155/2011/598345. [DOI] [PMC free article] [PubMed] [Google Scholar]


