The current issue of The Journal of Physiology contains two outstanding papers describing the pharmacological dissection of the compound inhibitory junction potential (IJP) (Gallego et al. 2012; Hwang et al. 2012). Both papers show that the IJP can be broken down to an initial purinergic component, followed by a later nitrergic component, in the circular muscle of murine colon. Furthermore, they show that the metabotropic P2Y1 receptor is the sole purinoceptor subtype mediating the initial component of the compound IJP, based on the outcome of experiments using P2Y1-selective antagonists and P2Y1-deficient tissues. Additionally, they describe the consequence of P2Y1 receptor deletion on patterns of motility at rest and during stimulation of motor nerves. It is rare that two papers submitted to The Journal should contain identical findings and reach identical conclusions. Thus, I speak of ‘resolution’ and ‘concordance’ in the title of this perspective, to signify the positive outcome of these studies and acknowledge a consensus on mechanistic detail.
Dissection of the IJP began with the proposal that ‘ATP or a related nucleotide’ is released by the inhibitory motoneurons in the gut (Burnstock et al. 1970). Accumulated evidence shows that the activation of a P2Y receptor subtype evokes an apamin-sensitive, calcium-dependent potassium current (via SK channels) to rapidly hyperpolarise smooth muscle. In turn, this nucleotide-evoked hyperpolarisation reduces the probability of opening of L-type Ca2+ channels, thereby reducing Ca2+ influx and intracellular Ca2+ levels and so promoting the active relaxation of smooth muscle. The purinoceptor concept for active relaxation of smooth muscle has become part of mainstream thought, although this concept was not wholly embraced in the past, and for good reasons, by myself and former colleagues in Glasgow (viz. A Bowman, KE Creed, A Gibson, JS Gillespie, W Martin, JC McGrath, TC Muir etc.) Each of us had reported much slower non-purinergic IJPs and/or active relaxations in the rat and rabbit anococcygeus and/or rabbit rectococcygeus, the so-called accessory muscles of defecation (for example, see King et al. 1977). In these two tissues, evidence was in favour of another inhibitory neurotransmitter released by the pelvic nerves (or ‘nervi erigentes’), the same nerves that bring about tumescence and penile erection. This inhibitory neurotransmitter was antagonised by haemoglobin and was eventually shown to be nitric oxide (NO). Ultimately it was proposed that released ATP accounts only for rapid IJPs whereas a mixture of NO and VIP (or the structurally related peptide, PACAP) may account for slower IJPs, particularly in smooth muscle preparations pre-treated with apamin (to remove the fast IJP) and/or Substance P (to remove the slow EJP) (He & Goyal, 1993) (see Fig. 1). The slow IJP is reduced and/or abolished by the nNOS inhibitor, l-NNA (Nω-nitro-l-arginine), a procedure which has been revisited in the two focus papers of this perspective.
Figure 1. Electrical stimulation of myenteric motoneurons produces a mixture of inhibitory and excitatory junction potentials in many types of gut smooth muscle.
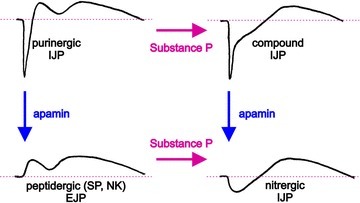
The initial fast hyperpolarization is a purinergic IJP and blocked by apamin (10−7m; SK channel inhibitor). The secondary hyperpolarization is the nitrergic IJP although, in some tissues, a small component may be mediated by VIP. The late, slow depolarization is a peptidergic EJP produced by neurokinins, and a component is desensitised by Substance P. These records (Brian F. King unpublished data) were made from circular muscle of guinea-pig ileum neuromuscularly blocked by atropine (10−6m) and nicardipine (10−6m). Analyses of synaptic events are based on data from Bywater & Taylor (1986).
As the synaptic physiology of the compound IJP was unfolding, complementary studies led to the isolation of a single cDNA (clone 803) in chick brain. Clone 803 is present in the gastrointestinal tract and, subsequently, this cDNA was found to encode a G protein-coupled ATP receptor which we called P2Y1 (Webb et al. 1993). Recombinant P2Y1 receptors and ATP-mediated relaxations of gut smooth muscle were antagonised by pyridylisatogen tosylate (PIT) (King et al. 1996), a substance first used to differentiate between ATP- and adenosine-mediated inhibitory responses in the gut. PIT is now known to be a selective antagonist of P2Y1 receptors, without effect at P2Y2, P2Y4, P2Y6, P2Y11 or P2Y12 receptors (Gao et al. 2004). A better series of highly potent P2Y1 antagonists have since been discovered, based on: the adenosine bisphosphate scaffold, substitution of the ribose moiety by N-methanacarbo pseudosugars, and modifications at the C2 and N6 positions on the purine moiety. This series includes MRS2179 and MRS2279 (Nandanan et al. 2000) as well as MRS2500 (Hechler et al. 2006). ATP responses and purinergic IJPs are potently antagonised by these compounds, a procedure again revisited by the two focus papers of this perspective.
The last key element to the two focus papers is the availability of homozygotic P2Y1−/− mice, which were genetically engineered in the first instance to investigate the role of P2Y1 (Fabre et al. 1999) and thereafter P2X1 receptors (Fabre et al. 2001) in nucleotide-mediated thromboembolism of blood platelets. Up to the present time, wild-type and P2Y1−/− mice have been used side by side to compare the activity of P2Y1 antagonists (such as MRS2179, MRS2279 and MRS2500) and P2Y1 agonists (ADP, MRS2365 and β-NAD) and to examine the facilitatory role of the P2Y1 receptor in thromboembolism and vascular disease. It is therefore a radical departure from script to see P2Y1−/− mice and these pharmacological agents used now in investigations of the compound IJP.
So armed and prepared, the authors of the two current focus papers have provided us with a wealth of information on the roles of purinergic and nitrergic transmission in the gut. Their studies focused on the colon, principally an organ of storage involved in the periodic elimination of waste. These two focus papers show that ATP and NO are released tonically by inhibitory nerves and, alongside adrenergic tone, contribute to controlling the colon. Each appears to serve a different role: adrenergic tone controls motoneurons in the ENS; nitrergic tone controls the membrane potential; purinergic tone controls the amplitude of excitatory responses. One of the focus papers also reports a profound disturbance in colonic transit in P2Y1-deficient mice (Hwang et al. 2012).
The authors have raised two important, if controversial, issues. Both papers discuss the possibility that β-NAD, rather than ATP, may be the cognate ligand for the P2Y1 receptor. This is not a new proposal, but certainly one that will require further investigation. Both papers also discuss the possibility that P2Y1 receptors mediating the purinergic IJP may be located in the fibroblast-like (PDGRFα+) cells and not smooth muscle cells, and this too will require further investigation. Yet, both papers serve to stimulate the reader and challenge their audience to consider new concepts. For this reason, these are outstanding papers.
Lastly, readers must look to see which of these new discoveries can be applied to organs of storage such as the stomach or caecum. Both are heavily invested with inhibitory nerves which, for example, elicit fast IJPs in antral circular muscle to reset the slow wave firing frequency (King, 1994) or elicit fast and slow purinergic relaxations in longitudinal muscle of taenia to modify the storage capacity of the caecum (King & Townsend-Nicholson, 2008). We must be grateful to the authors of these two focus papers for launching a new era of gastrointestinal physiology, where there will be abundant opportunities for further research and which may help resolve currently irretractable diseases of gastrointestinal storage and transit.
References
- Burnstock G, Campbell G, Satchell D, Smythe A. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bywater RAR, Taylor GS. J Physiol. 1986;374:153–164. doi: 10.1113/jphysiol.1986.sp016072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre JE, King BF, Koller Drug Devel Res. 2001;52:150–155. [Google Scholar]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Nature Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Martinez-Cutillas M, Mañé N, Martin MT, Jiménez M. J Physiol. 2012;590:1943–1956. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. Biochem Pharmacol. 2004;68:231–237. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XD, Goyal RK. J Physiol. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. J Pharmacol Exp Ther. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sander KM, Ward SM. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF. Neurogastroenterol Motil. 1994;6:59–65. [Google Scholar]
- King BF, McKirdy HC, Wai SS. J Physiol. 1977;264:607–619. doi: 10.1113/jphysiol.1977.sp011685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Dacquet C, Ziganshin AU, Weetman DF, Burnstock G, Vanhoutte PM, Spedding M. Br J Pharmacol. 1996;117:1111–1118. doi: 10.1111/j.1476-5381.1996.tb16704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, et al. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, et al. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]


