Abstract
Virtually nothing is known about the effects on fetal physiology of xanthine oxidase inhibition. This is despite maternal treatment with the xanthine oxidase inhibitor allopurinol being considered in human complicated pregnancy to protect the infant's brain from excessive generation of ROS. We investigated the in vivo effects of maternal treatment with allopurinol on fetal cardiovascular function in ovine pregnancy in late gestation. Under anaesthesia, pregnant ewes and their singleton fetus were instrumented with vascular catheters and flow probes around an umbilical and a fetal femoral artery at 118 ± 1 dGA (days of gestational age; term ca. 145 days). Five days later, mothers were infused i.v. with either vehicle (n= 11) or allopurinol (n= 10). Fetal cardiovascular function was stimulated with increasing bolus doses of phenylephrine (PE) following maternal vehicle or allopurinol. The effects of maternal allopurinol on maternal and fetal cardiovascular function were also investigated following fetal NO blockade (n= 6) or fetal β1-adrenergic antagonism (n= 7). Maternal allopurinol led to significant increases in fetal heart rate, umbilical blood flow and umbilical vascular conductance, effects abolished by fetal β1-adrenergic antagonism but not by fetal NO blockade. Maternal allopurinol impaired fetal α1-adrenergic pressor and femoral vasopressor responses and enhanced the gain of the fetal cardiac baroreflex. These effects of maternal allopurinol were restored to control levels during fetal NO blockade. Maternal treatment with allopurinol induced maternal hypotension, tachycardia and acid–base disturbance. We conclude that maternal treatment with allopurinol alters in vivo maternal, umbilical and fetal vascular function via mechanisms involving NO and β1-adrenergic stimulation. The evidence suggests that the use of allopurinol in clinical practice should be approached with caution.
Key points
There is growing physiological and clinical interest in the role of the enzyme xanthine oxidase in the regulation of fetal cardiovascular function.
The xanthine oxidase inhibitor allopurinol is undergoing human clinical trials in complicated pregnancy to protect the fetal brain from injury by decreasing excessive generation of reactive oxygen species (ROS) and increasing nitric oxide (NO) availability. However, the effects on fetal cardiovascular physiology of xanthine oxidase inhibition are largely unknown.
We have previously reported that the balance between ROS and NO plays an important physiological role in the control of fetal cardiovascular function. Therefore, it seems likely that allopurinol might perturb this balance and alter fetal cardiovascular homeostasis.
Here, we report that maternal allopurinol treatment in late gestation ovine pregnancy has significant in vivo effects on umbilical blood flow and the cardiovascular system of the mother and fetus by altering NO and β1-adrenergic mechanisms.
The evidence suggests that xanthine oxidase has an important role in basal cardiovascular function in the fetus during late gestation. Therefore, further research is warranted before safe clinical application of maternal allopurinol during pregnancy in humans.
Introduction
Despite advances in obstetric practice, acute intra-partum fetal asphyxia remains one of the most common forms of fetal stress, with substantial morbidity and mortality (Low, 2004). One possible strategy to combat the detrimental effects of fetal asphyxia would be to decrease the associated excessive generation of reactive oxygen species (ROS) from stimulated pro-oxidant mechanisms within the cell, such as the xanthine oxidase pathway (Berry & Hare, 2004). Indeed, maternal treatment with the xanthine oxidase inhibitor allopurinol is being considered in human pregnancy complicated by intra-partum asphyxia in order to protect the infant from excessive generation of ROS (Peeters-Scholte et al. 2003; Benders et al. 2006; Chaudhari & McGuire, 2008; Torrance et al. 2009; Kaandorp et al. 2010). This clinical interest in antenatal maternal administration with allopurinol follows previous studies which reported that allopurinol treatment in the asphyxiated neonate improved neonatal outcome (Van Bel et al. 1998), but if the time interval between asphyxia and treatment had been too long, or when fetal asphyxia had been too severe, no reduction in serious morbidity or mortality was observed (Benders et al. 2006). Therefore, there has been growing clinical and scientific interest in establishing whether perinatal outcome might be improved in complicated labour if the window of treatment with allopurinol would be initiated before birth, for instance via maternal treatment to cover the actual period of fetal asphyxia (Kaandorp et al. 2010). It is now known that allopurinol given to the mother crosses the placenta (Derks et al. 2010), it suppresses superoxide anion (•O2−) production in the fetus (Masaoka et al. 2005) and it yields therapeutic levels in the fetus and neonate (van Kesteren et al. 2006; van Dijk et al. 2008b; Derks et al. 2010), justifying this route of administration for possible preventative therapy in clinical practice.
In the fetus, the maintenance of cardiovascular function is vital for the effective delivery and distribution of nutrients and oxygen to the fetal tissues. Several studies have shown that NO is an important regulator of the fetal cardiovascular system and the gas is implicated in both femoral and umbilical vascular control, as blockade of NO synthesis leads to increased blood pressure and elevations in umbilical and femoral vascular resistance (Chang et al. 1992; Green et al. 1996; Gardner et al. 2001; Morrison et al. 2003). It is also now recognised that vascular NO bioavailability can be influenced by the physiological oxidant milieu. Increases in ROS may lead to a reduction in NO bioavailability, and the resulting increased ratio of •O2−:NO has been reported to promote vasoconstriction (Chen & Keaney, 2004; Valko et al. 2007). Recent evidence from our laboratory has begun to support the idea that this vascular redox status is active in fetal life and that it is important for the function of the fetal cardiovascular system under basal and stimulated conditions (Thakor et al. 2010a, b).
Given that the oxidant tone plays a significant role in the control of the fetal cardiovascular system, it is likely that maternal treatment with allopurinol in clinical practice may perturb fetal cardiovascular homeostasis. However, virtually nothing is known about the effects of xanthine oxidase inhibition on fetal cardiovascular control. Therefore, this study tested the hypothesis that xanthine oxidase has a role in the regulation of the fetal cardiovascular system. The hypothesis was tested by investigating the in vivo effects of maternal treatment with the xanthine oxidase inhibitor allopurinol on fetal cardiovascular function under basal and stimulated conditions in the chronically catheterised ewe and fetus in late gestation.
Methods
Surgical preparation
All procedures were performed under the UK Animals (Scientific Procedures) Act 1986 and were approved by the Ethical Review Committee of the University of Cambridge. Pregnant ewes and their singleton fetus were surgically instrumented at 118 ± 1 dGA (term ca. 145 days), as previously described in detail (Giussani et al. 1997; Fletcher et al. 2000). In brief, food, but not water, was withheld from the pregnant ewes for 24 h prior to surgery. Following induction with 20 mg kg−1i.v. sodium thiopentone (Intraval Sodium; Merial Animal Health Ltd; Rhone Mérieux, Dublin, Ireland), general anaesthesia (1.5–2.0% halothane in 50:50 O2:N2O) was maintained using positive pressure ventilation. Midline abdominal and uterine incisions were made, the fetal hind limbs were exteriorized and the right femoral artery and vein were isolated and catheterized (i.d., 0.86 mm; o.d., 1.52 mm; Critchly Electrical Products, NSW, Australia). The catheter tips were carefully advanced to the abdominal aorta and inferior vena cava, respectively. A further catheter was anchored onto the fetal hind limb for administration of antibiotics into the amniotic cavity (600 mg in 2 ml, benzylpenicillin; Crystapen, Schering-Plough, Animal Health Division, Welwyn Garden City, UK). On the contra-lateral side, a Transonic flow probe was placed around the femoral artery for measurement of blood flow (2R, Transonic Systems Inc.). In addition, another flow probe (4SB; Transonic Systems Inc., Ithaca, NY, USA) was placed around one of the umbilical arteries, close to the common umbilical artery, inside the fetal abdominal cavity, for continuous measurement of unilateral umbilical blood flow (Giussani et al. 1997; Gardner & Giussani, 2003). The fetal abdominal wall and skin were carefully closed in layers. The uterine incisions were then closed in layers, the combined dead space of the catheters was filled with heparinized saline (80 i.u. heparin ml−1 in 0.9% NaCl) and the catheter ends were plugged with sterile brass pins. A Teflon catheter (i.d. 1.0 mm, o.d. 1.6 mm, Altec, UK) was placed in the maternal femoral artery and extended to the descending aorta, in addition to a maternal venous catheter extended into the inferior vena cava (i.d., 0.86 mm; o.d., 1.52 mm; Critchly Electrical Products). The catheters and flow probe leads were then exteriorized via a keyhole incision in the maternal flank, coiled carefully and placed in a bag sutured to the flank of the ewe. Inhalational anaesthesia was withdrawn and intermittent positive pressure ventilation maintained until respiratory movements were observed in the ewe. The ewe was extubated when breathing spontaneously and moved to an individual recovery pen adjacent to other sheep with free access to food and water.
Post-operative care
During recovery, ewes were housed in individual pens in rooms with a 12 h:12 h light:dark cycle where they had free access to hay and water and were fed concentrates twice daily (100 g sheep nuts no. 6; H & C Beart Ltd, Kings Lynn, UK). Antibiotics were administered daily to the ewe (0.20–0.25 mg kg−1i.m. Depocillin; Mycofarm, Cambridge, UK) and fetus i.v. and into the amniotic cavity (600 mg in 2 ml 0.9% NaCl, benzylpenicillin; Crystapen, Schering-Plough). Generally, normal feeding patterns were restored within 24–48 h of recovery. Following 48–72 h of post-operative recovery, ewes were transferred to metabolic crates where they were housed for the remainder of the protocol. The arterial and amniotic catheters were connected to sterile pressure transducers (COBE; Argon Division, Maxxim Medical, Athens, TX, USA). Whilst on the metabolic crates, the patency of the fetal and maternal vascular catheters was maintained by a slow continuous infusion of heparinized saline (80 i.u. heparin ml−1 at 0.1 ml h−1 in 0.9% NaCl) containing antibiotic (1 mg ml−1 benzylpenicillin).
Allopurinol infusion protocol and baseline cardiovascular function
Experimental protocols were performed following at least 5 days of post-operative recovery. Following a 20 min baseline period, animals were subjected to either vehicle (control, n= 11) or allopurinol (150 mg kg−1, n= 10) administered by maternal i.v. infusion over 30 min (Fig. 1). Allopurinol (Sigma Aldrich, UK) was dissolved in the minimum volume of 4 m sodium hydroxide (Sigma) and made up with saline to 90 ml (van Dijk et al. 2008). Control vehicle was saline treated with 4 m NaOH to achieve the same pH of the allopurinol solution (pH 13.8). Cardiovascular data were recorded continuously. Data for fetal and maternal arterial blood pressure, amniotic pressure and fetal femoral and umbilical blood flow were recorded continuously by a custom-built data acquisition system [M-PAQ (Maastricht-Programmable AcQuisition system), Maastricht Instruments, The Netherlands, 500 Hz sample rate]. Fetal arterial blood pressure was corrected for amniotic pressure. Fetal and maternal heart rates were calculated from the systemic pressure recordings. Fetal femoral vascular resistance and umbilical vascular conductance were calculated on line by applying Ohm's law to the circulation. During the experimental protocols, descending aortic blood samples (0.3 ml) were taken using sterile techniques from the mother and fetus at set time points (0, 50 and 120 min; Fig. 1) to determine arterial blood gas and pH status (ABL5 Blood Gas Analyser, Radiometer; Copenhagen, Denmark; measurements corrected to 38°C for maternal and 39.5°C for fetal blood). Values for percentage saturation of haemoglobin with oxygen (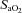 ) were determined using a haemoximeter (OSM3; Radiometer).
) were determined using a haemoximeter (OSM3; Radiometer).
Figure 1. Experimental protocol.
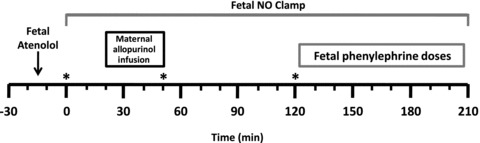
Maternal allopurinol (150 mg kg−1) infusion occurred between 20–50 min. Fetal treatment with the phenylephrine doses (5, 12.5, 25, 37.5 and 50 μg) started at 120 min. When used, atenolol was given 15 min prior to the start of the protocol, and the NO clamp was established before the start of the protocol and maintained for the duration of the protocol. *Time at which a blood sample was taken.
Stimulated cardiovascular function
Previous studies in our laboratory have shown that peak concentrations of oxypurinol (the active metabolite of allopurinol) can be measured in fetal plasma approximately 100–150 min following the end of maternal administration with allopurinol with a similar dosing regimen (Derks et al. 2010). Therefore, the stimulated cardiovascular function in the fetus was assessed during this period (Fig. 1). The change in fetal arterial blood pressure and heart rate, femoral and umbilical blood flow and resistance were recorded following increasing bolus doses of phenylephrine (5, 12.5, 25, 37.5 and 50 μg, l-phenylephrine; P-6126; Sigma). Suitable dose ranges were derived from pilot experiments and previous studies in the literature (Dawes et al. 1980; Itskovitz & Rudolph, 1982). Each dose was dissolved in 0.5 ml of saline (0.9% NaCl), and exogenously administered to the fetus, via the femoral artery over 2–3 s following at least 2 min of stable baseline recording. Catheters were flushed with 2 ml of saline immediately following the administration of each dose, and no further injections were given until fetal arterial blood pressure and heart rate had returned to baseline values (typically 30 min). The changes in fetal arterial pressure and in fetal heart rate obtained during the α1-adrenergic dose response experiments were used to construct a cardiac baroreflex analysis. The gradient of the relationship was used to quantify baroreflex gain, as previously described (Thakor & Giussani, 2009).
The nitric oxide clamp
To determine whether the mechanism mediating the effects of xanthine oxidase inhibition on fetal cardiovascular function were mediated via increased NO, the effects of maternal treatment with allopurinol was investigated following blockade of NO synthesis with the NO clamp in another group of animals (n= 6; Fig. 1). The NO clamp is a technique established in our laboratory that permits inhibition of de novo synthesis of NO while compensating for the tonic production of the gas, thereby maintaining basal cardiovascular function (Gardner et al. 2001; Gardner & Giussani, 2003; Morrison et al. 2003; Thakor & Giussani, 2005, 2009; Thakor et al. 2010a,b). In brief, a bolus dose of l-NAME (N-5751; Sigma; 100 mg kg−1 bolus dissolved in 2 ml heparinised saline) was injected via the fetal femoral artery. This was immediately followed by fetal i.v. infusion with the NO donor sodium nitroprusside (SNP; S-0501; Sigma; dissolved in heparinised saline) to return fetal arterial blood pressure, femoral blood flow and umbilical blood flow to pre-treatment levels. The rate of SNP infusion was titrated as required before the beginning of the protocol to obtain the pre-clamped baseline values; however, no adjustments were made once the dosing protocol had begun. At the end of the experimental protocol, the effectiveness of NOS blockade by the NO clamp and the persistence of l-NAME within the system were tested by withdrawal of the SNP infusion. This unmasked the influence of fetal treatment with l-NAME alone and led to generalised vasoconstriction and hypertension (Thakor et al. 2010a). The NO clamp was always preformed on the last day of any series of experiments. This was to ensure that l-NAME would have no effect on subsequent experiments, should its effects on the fetal circulation persist past 24 h.
Atenolol administration
Some of the effects of maternal allopurinol administration on fetal basal heart rate and umbilical vascular conductance were suspected to be mediated by β1-adrenergic stimulation. Therefore, in a third group of animals (n= 6), the effects of maternal treatment with allopurinol on fetal basal cardiovascular function was investigated following blockade with the selective β1-adrenoreceptor antagonist atenolol. A bolus dose of atenolol (2 mg kg−1i.v.; Sigma) was administered i.v. to the fetus 15 min prior to the start of the allopurinol infusion protocol, as its half-life is approximately 6–7 h (Lilja et al. 2005).
Plasma uric acid measurements
Xanthine oxidase catalyses the conversion of hypoxanthine to uric acid (Cao et al. 2010). Therefore, maternal and fetal plasma concentrations of urate were measured at 0, 50 and 120 min in control and allopurinol-treated pregnancies to confirm xanthine oxidase inhibition in the fetal circulation. Xanthine oxidase activity was measured by HPLC with electrochemical detection (Iriyama et al. 1984). In brief, aliquots of maternal and fetal plasma (acidified 1:1 with ice-cold 10% metaphophoric acid (MPA), centrifuged 3,500g and the supernatant stored at −80°C) were diluted 1:4 with ice-cold 5% MPA (final dilution of plasma 1 in 10). To this, 100 μl HPLC-grade heptane was added and following vortex mixing for 40 s, the samples were centrifuged (16,000g; 5 min) and the lower (aqueous) layer removed and treated with heptane again until the supernatant was clear. This clear supernatant was transferred to a 0.8 ml HPLC vial. An electrochemical detector (EG & G Instruments; Wokingham, UK) with a working electrode (set at 400 mV and sensitivity of 0.5 μA) was used for detection. Final concentrations for urate were calculated with external standards, which were run simultaneously. The limit of sensitivity for the assay was 0.1 μmol l−1 for urate, and the inter-assay coefficient of variation was less than 5%.
Data collection and statistical analysis
Minute by minute averages for infusion experiments and second by second averages for dose response experiments for all cardiovascular variables were imported into an Excel spreadsheet for analysis. For statistical analysis of basal cardiovascular function, summary measures analysis was applied to the serial data to focus the number of comparisons (Matthews et al. 1990). Summary measures represent the mean ± SEM for the absolute change from baseline during periods of baseline (B, 0–20 min), infusion (I, 20–50 min) and recovery (R1, 51–90 min and R2, 91–120 min). For the dose response experiments, baseline values for arterial blood pressure, heart rate, femoral blood flow and femoral vascular resistance were obtained by averaging the data over the 60 s preceding the administration of each bolus dose of phenylephrine. Maximum changes from baseline for each variable were then recorded. Baroreflex curves were constructed for each fetus by plotting for each dose the maximal decrease in heart rate against the maximal increase in arterial blood pressure and the gradient of this relationship was calculated. All data are expressed as mean ± SEM. All data were analysed statistically comparing the effect of dose, treatment and interactions between dose and treatment by two-way repeated-measures (RM) ANOVA with post hoc Student–Newman–Keuls test. For all comparisons, statistical significance was accepted when P < 0.05.
Results
Plasma uric acid
Basal maternal plasma uric acid levels were similar in all groups and were not significantly altered by maternal allopurinol infusion (Table 1). In marked contrast, basal fetal plasma uric acid levels were significantly higher than maternal levels, and maternal allopurinol treatment led to a significant decrease in fetal plasma uric acid levels (Table 1).
Table 1.
Maternal and fetal plasma uric acid
| Time (min) | |||||
|---|---|---|---|---|---|
| n | 0 | 50 | 120 | ||
| Maternal | Control | 5 | 4.3 ± 0.3 | 4.6 ± 0.2 | 5.0 ± 0.3 |
| Allopurinol | 6 | 4.5 ± 0.3 | 5.0 ± 0.4 | 4.4 ± 0.3 | |
| Allopurinol with NO clamp | 6 | 5.5 ± 0.5 | 6.1 ± 0.5 | 5.5 ± 0.5 | |
| Fetal | Control | 5 | 12.1 ± 0.6‡ | 12.1 ± 0.9‡ | 12.0 ± 0.7‡ |
| Allopurinol | 6 | 12.2 ± 0.4‡ | 12.4 ± 0.5‡ | 9.6 ± 0.4 *†‡ | |
| Allopurinol with NO clamp | 6 | 11.9 ± 0.6‡ | 11.6 ± 0.6‡ | 9.5 ± 0.4*†‡ | |
Values represent the mean ± SEM at pre-infusion (0), immediate post-infusion (50) and 70 min after the end of infusion (120). Significant differences (P < 0.05) are: *vs. pre-infusion (0), †vs. control, ‡vs. maternal (two-way RM ANOVA with post hoc Newman–Keuls test).
Blood gas and metabolic status
Maternal and fetal values for blood gases and acid–base status were within the normal limits for Welsh Mountain sheep at this stage of gestation at the commencement of the infusion protocol in all animals (Tables 2 and 3; see Fletcher et al. 2000; Derks et al. 2010). Maternal infusion with allopurinol led to increases in maternal acid–base excess and plasma HCO3− and significant decreases in maternal 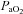 and in the oxygen percentage saturation of haemoglobin (Table 2). Application of the fetal NO clamp or fetal treatment with atenolol in addition to maternal allopurinol treatment did not alter these responses when compared with maternal allopurinol treatment alone (Table 2). Maternal allopurinol administration associated with the fetal NO clamp or fetal atenolol administration lowered fetal
and in the oxygen percentage saturation of haemoglobin (Table 2). Application of the fetal NO clamp or fetal treatment with atenolol in addition to maternal allopurinol treatment did not alter these responses when compared with maternal allopurinol treatment alone (Table 2). Maternal allopurinol administration associated with the fetal NO clamp or fetal atenolol administration lowered fetal 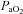 (Table 3).
(Table 3).
Table 2.
Maternal arterial blood gases and metabolites during allopurinol treatment
| Time (min) | |||||
|---|---|---|---|---|---|
| n | 0 | 50 | 120 | ||
| pHa | Control | 11 | 7.53 ± 0.01 | 7.51 ± 0.01 | 7.54 ± 0.01 |
| Allopurinol | 10 | 7.50 ± 0.02 | 7.61 ± 0.02 | 7.57 ± 0.01 | |
| Allopurinol with NO clamp | 6 | 7.49 ± 0.02 | 7.62 ± 0.01 | 7.57 ± 0.01 | |
| Allopurinol with atenolol | 7 | 7.50 ± 0.01 | 7.59 ± 0.02 | 7.56 ± 0.02 | |
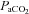 (mmHg) (mmHg) |
Control | 11 | 34.55 ± 0.82 | 35.27 ± 1.28 | 33.55 ± 0.90 |
| Allopurinol | 10 | 36.10 ± 1.69 | 33.50 ± 1.12 | 33.80 ± 0.79 | |
| Allopurinol with NO clamp | 6 | 37.67 ± 0.80 | 34.67 ± 0.80 | 37.00 ± 0.97 | |
| Allopurinol with atenolol | 7 | 38.29 ± 1.02 | 36.86 ± 1.06 | 38.00 ± 1.38 | |
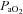 (mmHg) (mmHg) |
Control | 11 | 105.55 ± 2.58 | 100.18 ± 3.02 | 108.64 ± 4.20 |
| Allopurinol | 10 | 104.80 ± 2.38 | 85.90 ± 5.29*† | 107.00 ± 2.04 | |
| Allopurinol with NO clamp | 6 | 103.67 ± 1.93 | 77.17 ± 3.36*† | 100.00 ± 2.46 | |
| Allopurinol with atenolol | 7 | 107.79 ± 3.28 | 92.29 ± 6.75* | 111.29 ± 2.63 | |
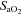 (%) (%) |
Control | 11 | 97.31 ± 0.73 | 96.52 ± 0.80 | 96.68 ± 0.71 |
| Allopurinol | 10 | 98.54 ± 1.54 | 96.15 ± 1.31* | 99.03 ± 1.29 | |
| Allopurinol with NO clamp | 6 | 100.22 ± 1.14 | 97.17 ± 1.19* | 100.63 ± 1.23 | |
| Allopurinol with atenolol | 7 | 103.59 ± 0.68† | 102.50 ± 1.11† | 103.80 ± 0.73 | |
| [Hb] (g dl−1) | Control | 11 | 9.55 ± 0.29 | 9.21 ± 0.27 | 9.48 ± 0.33 |
| Allopurinol | 10 | 9.45 ± 0.33 | 9.62 ± 0.41 | 8.88 ± 0.37 | |
| Allopurinol with NO clamp | 6 | 8.85 ± 0.29 | 9.58 ± 0.41 | 7.98 ± 0.51 | |
| Allopurinol with atenolol | 7 | 9.75 ± 0.36 | 10.13 ± 0.68 | 9.61 ± 0.49 | |
| ABE (meq l−1) | Control | 11 | 5.82 ± 0.52 | 5.73 ± 0.38 | 5.82 ± 0.60 |
| Allopurinol | 10 | 5.90 ± 0.67 | 11.70 ± 1.29*† | 9.50 ± 1.00*† | |
| Allopurinol with NO clamp | 6 | 6.17 ± 1.35 | 12.67 ± 1.41*† | 8.83 ± 1.74* | |
| Allopurinol with atenolol | 7 | 6.14 ± 1.42 | 12.29 ± 2.11*† | 10.86 ± 1.97*† | |
| [HCO3−] (mm) | Control | 11 | 28.09 ± 0.59 | 28.09 ± 0.58 | 27.91 ± 0.65 |
| Allopurinol | 10 | 28.60 ± 0.85 | 33.50 ± 1.50*† | 31.60 ± 1.28* | |
| Allopurinol with NO clamp | 6 | 25.83 ± 3.77 | 36.17 ± 1.14*† | 31.83 ± 2.09* | |
| Allopurinol with atenolol | 7 | 28.86 ± 1.53 | 34.86 ± 2.56*† | 33.57 ± 2.20*† | |
| [Lac] (mm) | Control | 11 | 0.39 ± 0.07 | 0.45 ± 0.08 | 0.47 ± 0.10 |
| Allopurinol | 10 | 0.44 ± 0.04 | 0.69 ± 0.12* | 0.68 ± 0.16* | |
| Allopurinol with NO clamp | 6 | 0.40 ± 0.03 | 0.57 ± 0.03 | 0.56 ± 0.06 | |
| Allopurinol with atenolol | 7 | 0.55 ± 0.04 | 0.84 ± 0.07*† | 0.74 ± 0.09* | |
Values represent the mean ± SEM at pre-infusion (0), immediate post-infusion (50) and 70 min after the end of infusion (120). Significant differences (P < 0.05) are: *vs. pre-infusion (0), †vs. control (two-way RM ANOVA with post hoc Newman–Keuls test). pHa, arterial pH; 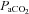 , arterial partial pressure of CO2;
, arterial partial pressure of CO2; 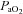 , arterial partial pressure of O2;
, arterial partial pressure of O2;  , percentage saturation of haemoglobin with oxygen; [Hb], haemoglobin concentration; ABE, acid-base excess; [HCO3−], arterial concentration of bicarbonate; [Lac], arterial concentration of lactate.
, percentage saturation of haemoglobin with oxygen; [Hb], haemoglobin concentration; ABE, acid-base excess; [HCO3−], arterial concentration of bicarbonate; [Lac], arterial concentration of lactate.
Table 3.
Fetal arterial blood gases and metabolites during allopurinol treatment
| Time (min) | |||||
|---|---|---|---|---|---|
| n | 0 | 50 | 120 | ||
| pHa | Control | 6 | 7.36 ± 0.01 | 7.38 ± 0.02 | 7.36 ± 0.01 |
| Allopurinol | 6 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.38 ± 0.01 | |
| Allopurinol with NO clamp | 8 | 7.34 ± 0.01 | 7.34 ± 0.01† | 7.34 ± 0.01 | |
| Allopurinol with atenolol | 6 | 7.33 ± 0.01 | 7.33 ± 0.01† | 7.32 ± 0.01 | |
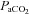 (mmHg) (mmHg) |
Control | 6 | 53.44 ± 1.47 | 51.89 ± 1.29 | 52.11 ± 1.07 |
| Allopurinol | 6 | 52.17 ± 0.87 | 51.50 ± 1.84 | 52.83 ± 1.45 | |
| Allopurinol with NO clamp | 8 | 54.25 ± 1.13 | 53.00 ± 0.89 | 51.63 ± 1.81 | |
| Allopurinol with atenolol | 6 | 56.00 ± 1.51 | 57.83 ± 0.70† | 56.33 ± 2.33 | |
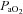 (mmHg) (mmHg) |
Control | 6 | 20.00 ± 0.65 | 19.29 ± 1.19 | 19.29 ± 0.75 |
| Allopurinol | 6 | 20.40 ± 1.21 | 19.00 ± 1.67 | 19.20 ± 1.56 | |
| Allopurinol with NO clamp | 8 | 21.63 ± 1.08 | 18.38 ± 0.63* | 18.94 ± 0.79* | |
| Allopurinol with atenolol | 6 | 20.83 ± 1.08 | 18.00 ± 0.68* | 19.00 ± 1.13 | |
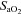 (%) (%) |
Control | 6 | 58.17 ± 2.15 | 54.94 ± 2.53 | 53.99 ± 2.16 |
| Allopurinol | 6 | 53.28 ± 4.45 | 47.25 ± 4.22 | 51.53 ± 4.73 | |
| Allopurinol with NO clamp | 8 | 60.60 ± 3.23 | 50.01 ± 3.41 | 52.40 ± 3.17 | |
| Allopurinol with atenolol | 6 | 52.35 ± 1.93 | 42.00 ± 3.55 | 42.83 ± 2.05 | |
| [Hb] (g dl-1) | Control | 6 | 9.56 ± 0.25 | 9.74 ± 0.23 | 9.83 ± 0.28 |
| Allopurinol | 6 | 9.15 ± 0.43 | 8.85 ± 0.43 | 8.70 ± 0.29 | |
| Allopurinol with NO clamp | 8 | 8.64 ± 0.53 | 8.65 ± 0.55 | 8.53 ± 0.58 | |
| Allopurinol with atenolol | 6 | 9.22 ± 0.20 | 9.53 ± 0.25 | 9.40 ± 0.43 | |
| ABE (meq l−1) | Control | 6 | 4.06 ± 0.46 | 3.78 ± 0.36 | 3.89 ± 0.48 |
| Allopurinol | 6 | 3.00 ± 0.89 | 3.60 ± 0.60 | 4.00 ± 0.45 | |
| Allopurinol with NO clamp | 8 | 2.67 ± 0.56 | 2.33 ± 0.42 | 1.50 ± 0.72 | |
| Allopurinol with atenolol | 6 | 3.67 ± 1.02 | 3.67 ± 1.20 | 3.50 ± 1.09 | |
| [HCO3−] (mm) | Control | 6 | 29.11 ± 0.59 | 30.11 ± 1.09 | 28.78 ± 0.46 |
| Allopurinol | 6 | 28.17 ± 0.75 | 25.17 ± 3.53 | 29.00 ± 0.58 | |
| Allopurinol with NO clamp | 8 | 27.13 ± 0.67 | 26.75 ± 0.56 | 26.25 ± 0.77 | |
| Allopurinol with atenolol | 6 | 27.50 ± 1.43 | 27.50 ± 1.43 | 27.83 ± 1.92 | |
| [Lac] (mm) | Control | 6 | 0.88 ± 0.06 | 0.88 ± 0.09 | 1.06 ± 0.06 |
| Allopurinol | 6 | 0.91 ± 0.07 | 1.07 ± 0.09 | 1.00 ± 0.07 | |
| Allopurinol with NO clamp | 8 | 1.11 ± 0.11 | 1.36 ± 0.16*† | 1.64 ± 0.22*† | |
| Allopurinol with atenolol | 6 | 0.76 ± 0.05 | 0.79 ± 0.04 | 0.94 ± 0.19 | |
Values represent the mean ± SEM at pre-infusion (0), immediate post-infusion (50) and 70 min after the end of infusion (120). Significant differences (P < 0.05) are: *vs. pre-infusion (0), †vs. control (two-way RM ANOVA with post hoc Newman–Keuls test). pHa, arterial pH; 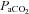 , arterial partial pressure of CO2;
, arterial partial pressure of CO2; 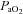 , arterial partial pressure of O2;
, arterial partial pressure of O2; 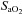 , percentage saturation of haemoglobin with oxygen; [Hb], haemoglobin concentration; ABE, acid-base excess; [HCO3−], arterial concentration of bicarbonate; [Lac], arterial concentration of lactate.
, percentage saturation of haemoglobin with oxygen; [Hb], haemoglobin concentration; ABE, acid-base excess; [HCO3−], arterial concentration of bicarbonate; [Lac], arterial concentration of lactate.
Maternal and fetal basal cardiovascular function
Prior to infusion, basal maternal (105 ± 5 mmHg) and fetal (44 ± 2 mmHg) arterial blood pressure, basal maternal (96 ± 4 beats min-1) and fetal (163 ± 3 beats min-1) heart rate, and basal fetal femoral (39.5 ± 2.4 ml min−1) and umbilical (221 ± 22 ml min−1) blood flows were within the normal range for Welsh Mountain sheep at this stage of gestation (Fig. 2). Maternal treatment with allopurinol led to a significant decrease in mean arterial pressure and a marked increase in maternal heart rate (Fig. 2). Treatment of the fetus with the NO clamp or with atenolol did not alter the maternal cardiovascular response to maternal allopurinol infusion. Maternal treatment with allopurinol also led to significant increases in fetal heart rate and umbilical blood flow, without affecting fetal arterial blood pressure or femoral blood flow. Hence, umbilical vascular conductance was markedly enhanced by maternal allopurinol treatment (Fig. 3). Application of fetal NOS blockade with the NO clamp following maternal allopurinol treatment did not attenuate the fetal heart rate or umbilical vascular responses. However, fetal treatment with atenolol following maternal allopurinol treatment completely prevented the effects on fetal heart rate, umbilical blood flow and umbilical vascular conductance (Fig. 3).
Figure 2. Maternal cardiovascular variables during allopurinol treatment.
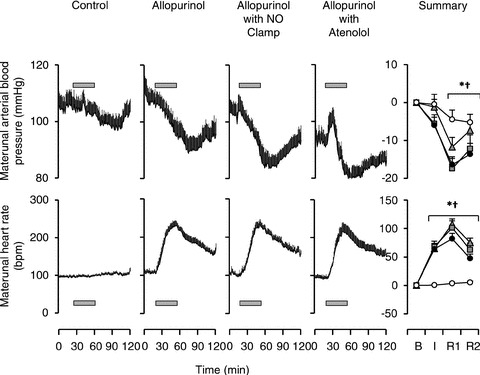
Values represent the mean ± SEM of each minute during maternal vehicle (open circles), maternal allopurinol (black circles), maternal allopurinol with the fetal NO clamp (grey squares) and maternal allopurinol with fetal atenolol (grey triangles). Bar indicates the maternal infusion of allopurinol. A summary is represented on the right-hand column for pre-infusion baseline (B), the infusion period (I), post-infusion recovery 50–90 min (R1) and post-infusion recovery 90–120 min (R2). Significant differences (P < 0.05) are: *vs. pre-infusion (B), †vs. control (two-way RM ANOVA with post hoc Newman–Keuls test).
Figure 3. Fetal cardiovascular variables during allopurinol treatment.
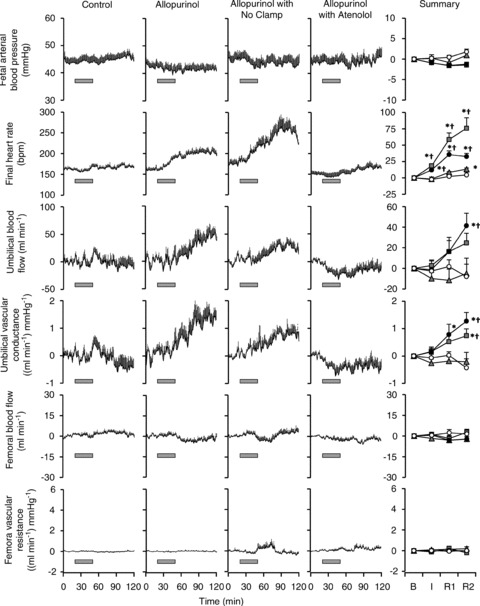
Values represent the mean ± SEM of each minute during maternal vehicle (open circles), maternal allopurinol (black circles), maternal allopurinol with the fetal NO clamp (grey squares) and maternal allopurinol with fetal atenolol (grey triangles). Bars indicate the maternal infusion of allopurinol. A summary is represented on the right-hand column for pre-infusion baseline (B), infusion period (I), post-infusion recovery 50–90 min (R1) and post-infusion recovery 90–120 min (R2). Significant differences (P < 0.05) are: *vs. pre-infusion (B), †vs. control (two-way RM ANOVA with post hoc Newman–Keuls test).
Fetal stimulated cardiovascular function
Treatment of the sheep fetus with phenylephrine produced dose-dependent increments in fetal arterial blood pressure and in fetal femoral vascular resistance following maternal administration with vehicle, allopurinol or allopurinol with the fetal NO clamp (Fig. 4). However, the fetal pressor and vasopressor responses to the higher doses of phenylephrine were significantly diminished during maternal treatment with allopurinol compared with vehicle (Fig. 4). Maternal treatment with allopurinol during fetal treatment with the NO clamp recovered all fetal pressor and vasopressor α1-adrenergic responses back to control levels (Fig. 4).
Figure 4. Fetal pressor and vasopressor responses to phenylephrine.
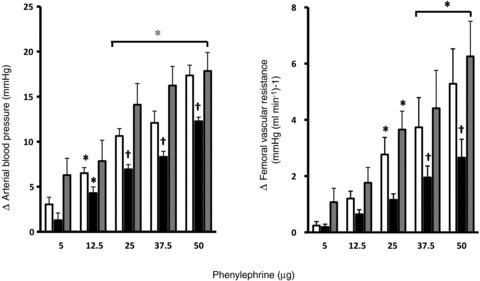
Values represent the mean ± SEM for the change from baseline in fetal arterial blood pressure and in femoral vascular resistance in response to increasing doses of phenylephrine (5, 12.5, 25, 37.5 and 50 μg) during maternal vehicle (open bars), maternal allopurinol (black bars) and maternal allopurinol with the fetal NO clamp (grey bars). Significant differences (P < 0.05) are: *vs. basal, †vs. control (two-way RM ANOVA with post hoc Newman–Keuls test).
Correlation analysis of fetal heart rate and fetal blood pressure responses following bolus doses of phenylephrine revealed significant relationships in each fetus. In all fetuses, progressive increases in fetal arterial blood pressure were accompanied by progressive decreases in fetal heart rate. Fetal cardiac vagal baroreflex function was assessed by calculating the gradient of the arterial blood pressure vs. heart rate relationship in each fetus. During control experiments with maternal vehicle infusion, fetal cardiac baroreflex sensitivity was 2.77 ± 0.30 beats min-1 mmHg−1 (Fig. 5). In contrast, maternal treatment with allopurinol increased the sensitivity of the fetal cardiac baroreflex (5.75 ± 0.78 beats min-1 mmHg−1), an effect which was restored to control levels following maternal allopurinol during fetal treatment with the NO clamp (2.71 ± 0.42 beats min-1 mmHg−1; Fig. 5).
Figure 5. Fetal cardiac baroreflex.
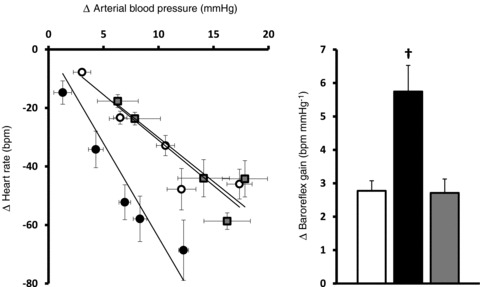
Each point represents the mean ± SEM (x and y) for the change in heart rate and blood pressure following maternal vehicle (open circles), maternal allopurinol (black circles) or maternal allopurinol with the fetal NO clamp (grey squares). The histograms represent the mean ± SEM for cardiac baroreflex sensitivity following saline vehicle (open bars), maternal allopurinol (black bars) and maternal allopurinol with the fetal NO clamp (grey bars). Significant differences (P < 0.05) are: †vs. all (two-way RM ANOVA with post hoc Newman–Keuls test).
Discussion
The data show that in late gestation ovine pregnancy, fetal plasma concentrations of uric acid were reduced following maternal treatment with allopurinol. Maternal allopurinol also led to significant increases in fetal heart rate, umbilical blood flow and umbilical vascular conductance, effects which were abolished by fetal treatment with atenolol but not by fetal NO blockade. Maternal allopurinol treatment significantly impaired fetal α1-adrenergic-mediated pressor and femoral vasopressor responses and significantly enhanced the fetal cardiac baroreflex gain. These effects of maternal allopurinol were restored to control levels during fetal NO blockade. Maternal treatment with allopurinol induced maternal hypotension, tachycardia and acid–base disturbance. This was accompanied by stable fetal blood gas and acid–base status. Therefore, the data support the hypothesis that xanthine oxidase has a role in the regulation of fetal cardiovascular function. The data also show that maternal treatment with allopurinol enhances umbilical blood flow via mechanisms involving β1-adrenergic stimulation.
Xanthine oxidase breaks down purine bases forming uric acid (Berry & Hare, 2004). Hypoxanthine, a metabolite of adenosine, is metabolised by xanthine oxidase in a two-stage process to uric acid, producing the free radical •O2− at both stages. In the mother, uric acid is cleared by filtration and renal secretion (Enomoto & Endou, 2005) whilst fetal uric acid diffuses from the fetus to the mother across the placenta (Wallenburg & van Kreel, 1980; Simmonds et al. 1984). In this study, levels of uric acid in the fetal circulation were approximately two-fold greater than those in the maternal compartment under basal conditions, reflecting the feto-maternal diffusion gradient (Simmonds et al. 1984). Further, fetal uric acid levels decreased whilst maternal levels were unchanged following maternal allopurinol administration. Whilst decreases in the levels of uric acid may reflect decreased production and/or enhanced removal from the fetal circulation, the data show that xanthine oxidase activity in the sheep fetus is present under basal conditions and that maternal allopurinol administration can inhibit this basal activity.
The dynamic relationship between •O2− and NO has led to the suggestion that the oxidant milieu is an important modulator of vascular tone, such that increases in the •O2−:NO ratio result in vasoconstriction, and decreases in the ratio promote vasodilatation (Chen & Keaney, 2004). Alterations in this oxidant tone are likely to have a significant influence in circulations that are highly dependent on NO, such as the umbilical vascular bed (Chang et al. 1992; Green et al. 1996; Gardner & Giussani, 2003; Morrison et al. 2003). Accordingly, we have previously reported that fetal treatment with vitamin C or with melatonin led to marked NO-dependent elevations in umbilical vascular conductance (Thakor et al. 2010a). Other studies support the idea that stimulated xanthine oxidase can also affect the vascular oxidant tone, showing that increased xanthine oxidase levels potentiate vasoconstriction in the rat middle cerebral vessels and that allopurinol treatment diminishes the effect (Hernanz et al. 2003). Allopurinol also led to decreased tension in pre-contracted rat aortas (Ellis et al. 1998). In the present study, maternal treatment with allopurinol led to an increase in umbilical vascular conductance. However, fetal NO blockade did not prevent this effect, implying that the mechanism by which maternal allopurinol increases umbilical vascular conductance is not NO dependent and therefore different to the mechanism by which vitamin C or melatonin act on the fetal circulation (Thakor et al. 2010a,b). Conversely, fetal treatment with atenolol, a selective β1-adrenergic antagonist, blocked the increases in both fetal umbilical vascular conductance and fetal heart rate, implying the activation of β1-adrenoreceptors in mediating the umbilical haemodynamic response. An increase in umbilical blood flow following β1-adrenergic stimulation following maternal allopurinol treatment may occur due to more than one reason. For instance, it is known that stimulation of β1-, β2- and β3-adrenoreceptors directly promotes dilatation within the umbilical vascular bed (Dennedy et al. 2002). In addition, it is known that an increase in fetal heart rate will lead to an increase in umbilical blood flow (Morrow et al. 1993). Since atenolol, a selective β1-adrenergic antagonist, abolished both the increases in fetal heart rate and in umbilical blood flow, the data support an indirect effect of allopurinol on umbilical blood flow, secondary to an increase in fetal heart rate due to β1-adrenoreceptor activation.
Data in the current study also show that fetal exposure to allopurinol significantly impaired the arterial pressor and femoral vasopressor responses to phenylephrine. The suppressed response to phenylephrine is probably secondary to allopurinol decreasing xanthine oxidase-derived free radical production, thereby increasing NO bioavailability, which acts to offset the α1-adrenergic-mediated peripheral vasoconstriction. In support of this idea, maternal allopurinol did suppress fetal circulating urate levels, the end-product of the xanthine oxidase pathway. In addition, under fetal NO blockade, the suppressive pressor and vasopressor effects of maternal allopurinol were prevented, thereby implying that the actions of allopurinol on the fetal vasculature are NO dependent.
Maternal treatment with allopurinol also altered the autonomic control of the fetal cardiovascular system as evidenced by an increased gain of the fetal cardiac baroreflex. The mechanism underlying this effect of allopurinol may be through decreasing ROS and/or increasing NO levels at the level of the sensor, integrator and/or target organ of the reflex pathway (Paton et al. 2002). Accordingly, administration of xanthine oxidase to the isolated rabbit carotid sinus decreased baroreceptor sensitivity, an effect that could be recovered with the antioxidant enzyme superoxide dismutase (Li et al. 1996). The effect of maternal allopurinol enhancing the gain of the fetal cardiac baroreflex at the level of the heart itself is also possible, since available literature suggests that NO potentiates, rather than depresses, the magnitude of induced bradycardia (Balligand et al. 1993; Conlon et al. 1996; Sears et al. 1999). For example, in vitro and in vivo studies have shown that NO synthase (NOS) inhibition blocks the negative cardiac chronotropic effect of muscarinic receptor stimulation in spontaneously beating neonatal rat cardiomyocytes (Balligand et al. 1993) or significantly attenuates the bradycardic response to efferent vagal stimulation (Conlon et al. 1996). Conversely, treatment with NO donors such as molsidomine and SNP significantly enhanced the decrease in heart rate in response to vagal nerve stimulation in the anaesthetised rabbit and isolated guinea pig atria (Sears et al. 1999).
Maternal treatment with allopurinol also produced both fetal and maternal tachycardia and marked maternal hypotension. The maternal hypotension supports the idea that tonic production of ROS by xanthine oxidase contributes to an oxidant tone that maintains peripheral vascular resistance in the maternal circulation. At first sight, the maternal tachycardia following maternal allopurinol treatment appears to be triggered by a baroreflex in response to significant hypotension. However, close inspection of the trace reveals that maternal tachycardia starts prior to any change in maternal arterial blood pressure. The dissociation between the timing of the maternal depressor and positive chronotropic responses suggest that baroreflex activation is unlikely to contribute to the mechanism increasing maternal heart rate. Further, maternal allopurinol resulted in fetal tachycardia in the absence of fetal hypotension. Given that fetal treatment with atenolol, but not with the fetal NO clamp, blocked the fetal tachycardia following maternal allopurinol administration, it is highly likely that fetal tachycardia is caused via increased sympathetic drive independent of changes in NO. Although not tested in this study, the same mechanism could be responsible for the maternal tachycardia following maternal allopurinol administration.
Maternal treatment with allopurinol also led to increases in maternal plasma pH, HCO3− concentration and acid–base excess. Further, there were significant decreases in maternal 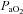 and more moderate decrements in the percentage saturation of haemoglobin, all of which had partially or completely resolved by 120 min of the infusion protocol. The maintenance of saturation of haemoglobin in the face of decreases in
and more moderate decrements in the percentage saturation of haemoglobin, all of which had partially or completely resolved by 120 min of the infusion protocol. The maintenance of saturation of haemoglobin in the face of decreases in 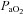 may have been helped by a reverse Bohr shift in the oxygen dissociation curve for haemoglobin induced by the alkalosis in maternal blood (Bohr et al. 1904; Jensen, 2004). It is interesting to speculate on the mechanism mediating the decrease in maternal
may have been helped by a reverse Bohr shift in the oxygen dissociation curve for haemoglobin induced by the alkalosis in maternal blood (Bohr et al. 1904; Jensen, 2004). It is interesting to speculate on the mechanism mediating the decrease in maternal 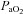 . Central chemoreceptors are activated in response to high acidity and act to drive respiratory rate and increase tidal volume and alveolar ventilation (Guyenet et al. 2010). Conversely, it is possible that increases in maternal pH may therefore act to depress ventilatory drive (Pokorski & Lahiri, 1983). Reductions in alveolar ventilation would lead to increases in alveolar partial pressure of CO2 (
. Central chemoreceptors are activated in response to high acidity and act to drive respiratory rate and increase tidal volume and alveolar ventilation (Guyenet et al. 2010). Conversely, it is possible that increases in maternal pH may therefore act to depress ventilatory drive (Pokorski & Lahiri, 1983). Reductions in alveolar ventilation would lead to increases in alveolar partial pressure of CO2 (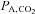 ) and decreases in alveolar and arterial
) and decreases in alveolar and arterial 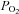 (Ganong, 2003). Opposing this argument is the observation in the present study that maternal arterial partial pressure of CO2 (
(Ganong, 2003). Opposing this argument is the observation in the present study that maternal arterial partial pressure of CO2 (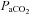 ) did not increase; however, CO2 production may have also been altered by allopurinol administration and/or have become buffered by conversion to HCO3− and/or by being bound to proteins such as carboamino compounds (Ganong, 2003). Irrespective of the mechanism, the reduced maternal
) did not increase; however, CO2 production may have also been altered by allopurinol administration and/or have become buffered by conversion to HCO3− and/or by being bound to proteins such as carboamino compounds (Ganong, 2003). Irrespective of the mechanism, the reduced maternal 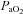 will reduce the trans-placental oxygen diffusion gradient, thereby explaining the mild reduction in fetal
will reduce the trans-placental oxygen diffusion gradient, thereby explaining the mild reduction in fetal 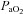 following maternal allopurinol treatment.
following maternal allopurinol treatment.
In conclusion, the data show that maternal treatment with allopurinol affects maternal cardiovascular and metabolic homeostasis and promotes significant changes in fetal cardiovascular function under basal and stimulated conditions. The results are not only of particular significance to the physiological understanding of the control of the fetal cardiovascular system, but they are also of clinical relevance in the context of previous and ongoing trials where allopurinol is being administered to pregnant women when their unborn child shows signs of asphyxic distress (Kaandorp et al. 2010). Collectively, past (Torrance et al. 2009; Derks et al. 2010) and present evidence reports on effects of maternal allopurinol on the fetus of mixed implication, supporting beneficial as well as detrimental consequences. Therefore, the use of allopurinol in clinical practice should be approached with caution.
Acknowledgments
We are grateful to Mr Scott Gentle, Mrs Sue Nicholls and Ms Vanessa Allen for excellent technical assistance at the Barcroft large animal facility. This work was supported by the British Heart Foundation, the BBSRC, the Royal Society and the Frank Edward Elmore Fund and the James Baird Fund.
Glossary
Abbreviations
- dGA
days of gestational age
- •O2−
superoxide
- PE
phenylephrine
- ROS
reactive oxygen species
Author contributions
E.A.H., A.D.K., J.A.H., A.S.T. & D.A.G. contributed to the conception and design of the experiments. All authors contributed to the collection, analysis and interpretation of data; drafting the article or revising it critically and approved the final version of the manuscript.
References
- Balligand J, Kelly R, Marsden P, Smith T, Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993;90:347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benders MJ, Bos AF, Rademaker CM, Rijken M, Torrance HL, Groenendaal F, van Bel F. Early postnatal allopurinol does not improve short term outcome after severe birth asphyxia. Arch Dis Child Fetal Neonatal Ed. 2006;91:F163–F165. doi: 10.1136/adc.2005.086652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, Hare M. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr C, Hasselbalch K, Krogh A. Ueber einen in biologischer beziehung wichtigen einfluss, de die kohlen- saurespannung des blutes auf dessen sauerstoffbindung ubt. Skand Arch Physiol. 1904;16:402–412. [Google Scholar]
- Cao H, Pauff JM, Hille R. Substrate orientation and catalytic specificity in the action of xanthine oxidase: the sequential hydroxylation of hypoxanthine to uric acid. J Biol Chem. 2010;285:28044–28053. doi: 10.1074/jbc.M110.128561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Roman C, Heymann M. Effect of endothelium-derived relaxing factor inhibition on the umbilical-placental circulation in fetal lambs in utero. Am J Obstet Gynecol. 1992;166:727–734. doi: 10.1016/0002-9378(92)91704-e. [DOI] [PubMed] [Google Scholar]
- Chaudhari T, McGuire W. Allopurinol for preventing mortality and morbidity in newborn infants with suspected hypoxic-ischaemic encephalopathy. Cochrane Database Syst Rev. 2008;16:CD006817. doi: 10.1002/14651858.CD006817.pub2. [DOI] [PubMed] [Google Scholar]
- Chen K, Keaney JF. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11:109–121. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- Conlon K, Collins T, Kidd C. Modulation of vagal actions on heart rate produced by inhibition of nitric oxide synthase in the anaesthetized ferret. Exp Physiol. 1996;81:547–550. doi: 10.1113/expphysiol.1996.sp003957. [DOI] [PubMed] [Google Scholar]
- Dawes GS, Johnston BM, Walker DW. Relationship of arterial pressure and heart rate in fetal, new-born and adult sheep. J Physiol. 1980;309:405–417. doi: 10.1113/jphysiol.1980.sp013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennedy MC, Houlihan DD, McMillan H, Morrison JJ. Beta2- and beta3-adrenoreceptor agonists: human myometrial selectivity and effects on umbilical artery tone. Am J Obstet Gynecol. 2002;187:641–647. doi: 10.1067/mob.2002.125277. [DOI] [PubMed] [Google Scholar]
- Derks JB, Oudijk MA, Torrance HL, Rademaker CM, Benders MJ, Rosen KG, Cindrova-Davies T, Thakor AS, Visser GH, Burton GJ, van Bel F, Giussani DA. Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system following repeated episodes of ischemia-reperfusion. Pediatr Res. 2010;68:374–380. doi: 10.1203/PDR.0b013e3181ef7780. [DOI] [PubMed] [Google Scholar]
- Ellis A, Li CG, Rand M. Effect of xanthine oxidase inhibition on endothelium-dependent and nitrergic relaxations. Eur J Pharmacol. 1998;356:41–47. doi: 10.1016/s0014-2999(98)00510-x. [DOI] [PubMed] [Google Scholar]
- Enomoto A, Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin Exp Nephrol. 2005;9:195–205. doi: 10.1007/s10157-005-0368-5. [DOI] [PubMed] [Google Scholar]
- Fletcher AJ, Edwards CM, Gardner DS, Fowden AL, Giussani DA. Neuropeptide Y in the sheep fetus: effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology. 2000;141:3976–3982. doi: 10.1210/endo.141.11.7770. [DOI] [PubMed] [Google Scholar]
- Ganong W. Review of Medical Physiology (Lange Basic Science) USA: McGraw-Hill; 2003. [Google Scholar]
- Gardner DS, Giussani DA. Enhanced umbilical blood flow during acute hypoxemia after chronic umbilical cord compression: a role for nitric oxide. Circulation. 2003;108:331–335. doi: 10.1161/01.CIR.0000080323.40820.A1. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Powlson AS, Giussani DA. An in vivo nitric oxide clamp to investigate the influence of nitric oxide on continuous umbilical blood flow during acute hypoxaemia in the sheep fetus. J Physiol. 2001;537:587–596. doi: 10.1111/j.1469-7793.2001.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Unno N, Jenkins SL, Wentworth RA, Derks JB, Collins JH, Nathanielsz PW. Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late-gestation sheep fetus. Am J Physiol Heart Circ Physiol. 1997;273:H2351–H2360. doi: 10.1152/ajpheart.1997.273.5.H2351. [DOI] [PubMed] [Google Scholar]
- Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol. 1996;497:271–277. doi: 10.1113/jphysiol.1996.sp021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernanz R, Alonso M, Briones A, Vila E, Simonsen U, Salaices M. Mechanisms involved in the early increase of serotonin contraction evoked by endotoxin in rat middle cerebral arteries. Br J Pharmacol. 2003;140:671–680. doi: 10.1038/sj.bjp.0705501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama K, Yoshiura M, Iwamoto T, Ozaki Y. Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984;141:238–243. doi: 10.1016/0003-2697(84)90451-2. [DOI] [PubMed] [Google Scholar]
- Itskovitz J, Rudolph AM. Denervation of arterial chemoreceptors and baroreceptors in fetal lambs in utero. Am J Physiol Heart Circ Physiol. 1982;242:H916–H920. doi: 10.1152/ajpheart.1982.242.5.H916. [DOI] [PubMed] [Google Scholar]
- Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand. 2004;182:215–227. doi: 10.1111/j.1365-201X.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- Kaandorp JJ, Benders MJ, Rademaker CM, Torrance HL, Oudijk MA, de Haan TR, et al. Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study. BMC Pregnancy Childbirth. 2010;10:8. doi: 10.1186/1471-2393-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res. 1996;79:802–811. doi: 10.1161/01.res.79.4.802. [DOI] [PubMed] [Google Scholar]
- Lilja J, Raaska K, Neuvonen P. Effects of orange juice on the pharmacokinetics of atenolol. Eur J Clin Pharmacol. 2005;61:337–340. doi: 10.1007/s00228-005-0930-9. [DOI] [PubMed] [Google Scholar]
- Low JA. Reflections on the occurrence and significance of antepartum fetal asphyxia. Best Pract Res Clin Obstet Gynaecol. 2004;18:375–382. doi: 10.1016/j.bpobgyn.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Masaoka N, Nakajima Y, Hayakawa Y, Ohgame S, Hamano S, Nagaishi M, Yamamoto T. Transplacental effects of allopurinol on suppression of oxygen free radical production in chronically instrumented fetal lamb brains during intermittent umbilical cord occlusion. J Matern Fetal Neonatal Med. 2005;18:1–7. doi: 10.1080/14767050500127716. [DOI] [PubMed] [Google Scholar]
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Gardner DS, Fletcher AJ, Bloomfield MR, Giussani DA. Enhanced nitric oxide activity offsets peripheral vasoconstriction during acute hypoxaemia via chemoreflex and adrenomedullary actions in the sheep fetus. J Physiol. 2003;547:283–291. doi: 10.1113/jphysiol.2002.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow RJ, Bull SB, Adamson SL. Experimentally induced changes in heart rate alter umbilicoplacental hemodynamics in fetal sheep. Ultrasound Med Biol. 1993;19:309–318. doi: 10.1016/0301-5629(93)90103-u. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- Peeters-Scholte C, Braun K, Koster J, Kops N, Blomgren K, Buonocore G, et al. Effects of allopurinol and deferoxamine on reperfusion injury of the brain in newborn piglets after neonatal hypoxia-ischemia. Pediatr Res. 2003;54:516–522. doi: 10.1203/01.PDR.0000081297.53793.C6. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Lahiri S. Relative peripheral and central chemosensory responses to metabolic alkalosis. Am J Physiol Regul Integr Comp Physiol. 1983;245:R873–R880. doi: 10.1152/ajpregu.1983.245.6.R873. [DOI] [PubMed] [Google Scholar]
- Sears CE, Choate JK, Paterson DJ. NO-cGMP pathway accentuates the decrease in heart rate caused by cardiac vagal nerve stimulation. J Appl Physiol. 1999;86:510–516. doi: 10.1152/jappl.1999.86.2.510. [DOI] [PubMed] [Google Scholar]
- Simmonds HA, Stutchbury JH, Webster DR, Spencer RE, Fisher RA, Wooder M, Buckley BM. Pregnancy in xanthinuria: Demonstration of fetal uric acid production? J Inherit Metab Dis. 1984;7:77–79. doi: 10.1007/BF01805810. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Giussani DA. Role of nitric oxide in mediating in vivo vascular responses to calcitonin gene-related peptide in essential and peripheral circulations in the fetus. Circulation. 2005;112:2510–2516. doi: 10.1161/CIRCULATIONAHA.105.562546. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Giussani DA. Nitric oxide reduces vagal baroreflex sensitivity in the late gestation fetus. Pediatr Res. 2009;65:269–273. doi: 10.1203/PDR.0b013e318193f134. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Serón-Ferré M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010a;49:399–406. doi: 10.1111/j.1600-079X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L, Giussani DA. Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol. 2010b;588:4235–4247. doi: 10.1113/jphysiol.2010.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrance HL, Benders MJ, Derks JB, Rademaker CM, Bos AF, Van Den Berg P, et al. Maternal allopurinol during fetal hypoxia lowers cord blood levels of the brain injury marker S-100B. Pediatrics. 2009;124:350–357. doi: 10.1542/peds.2008-2228. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Van Bel F, Shadid M, Moison RM, Dorrepaal CA, Fontijn J, Monteiro L, Van De Bor M, Berger HM. Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics. 1998;101:185–193. doi: 10.1542/peds.101.2.185. [DOI] [PubMed] [Google Scholar]
- van Dijk AJ, Parvizi N, Taverne M, Fink-Gremmels J. Placental transfer and pharmacokinetics of allopurinol in late pregnant sows and their fetuses. J Vet Pharmacol Ther. 2008;31:489–495. doi: 10.1111/j.1365-2885.2008.00976.x. [DOI] [PubMed] [Google Scholar]
- van Kesteren C, Benders M, Groenendaal F, van Bel F, Ververs F, Rademaker C. Population pharmacokinetics of allopurinol in full-term neonates with perinatal asphyxia. Ther Drug Monit. 2006;28:339–344. doi: 10.1097/01.ftd.0000211808.74192.86. [DOI] [PubMed] [Google Scholar]
- Wallenburg H, van Kreel B. Maternal and umbilical plasma concentrations of uric acid and oxypurines at delivery in normal and hypertensive pregnancy. Arch Gynecol. 1980;229:7–11. doi: 10.1007/BF02109823. [DOI] [PubMed] [Google Scholar]


