Abstract
Purinergic and nitrergic co-transmission is the dominant mechanism responsible for neural-mediated smooth muscle relaxation in the gastrointestinal tract. The aim of the present paper was to test whether or not P2Y1 receptors are involved in purinergic neurotransmission using P2Y1−/− knock-out mice. Tension and microelectrode recordings were performed on colonic strips. In wild type (WT) animals, electrical field stimulation (EFS) caused an inhibitory junction potential (IJP) that consisted of a fast IJP (MRS2500 sensitive, 1 μm) followed by a sustained IJP (Nω-nitro-l-arginine (l-NNA) sensitive, 1 mm). The fast component of the IJP was absent in P2Y1−/− mice whereas the sustained IJP (l-NNA sensitive) was recorded. In WT animals, EFS-induced inhibition of spontaneous motility was blocked by the consecutive addition of l-NNA and MRS2500. In P2Y1−/− mice, EFS responses were completely blocked by l-NNA. In WT and P2Y1−/− animals, l-NNA induced a smooth muscle depolarization but ‘spontaneous’ IJP (MRS2500 sensitive) could be recorded in WT but not in P2Y1−/− animals. Finally, in WT animals, 1 μm MRS2365 caused a smooth muscle hyperpolarization that was blocked by 1 μm MRS2500. In contrast, 1 μm MRS2365 did not modify smooth muscle resting membrane potential in P2Y1−/− mice. β-Nicotinamide adenine dinucleotide (β-NAD, 1 mm) partially mimicked the effect of MRS2365. We conclude that P2Y1 receptors mediate purinergic neurotransmission in the gastrointestinal tract and β-NAD partially fulfils the criteria to participate in rodent purinergic neurotransmission. The P2Y1−/− mouse is a useful animal model to study the selective loss of purinergic neurotransmission.
Key points
Neural-mediated relaxation occurs in the gastrointestinal tract. To accomplish this function, two neurotransmitters, ATP or a related purine and nitric oxide, are released by inhibitory motorneurons.
The type of purinergic receptor is still under debate but previous data using a classical pharmacological approach (receptor agonists and antagonists) suggested that P2Y1 receptors are responsible for purinergic neurotransmission in the gastrointestinal tract.
In the present study we used a genetically modified mouse in which P2Y1 receptors had been knocked out.
P2Y1-deficient mice had functional nitrergic neurotransmission but purinergic neurotransmission was absent.
The present work confirms the hypothesis demonstrating that P2Y1 receptors mediate the purinergic component of the smooth muscle relaxation in the gastrointestinal tract.
Introduction
Purinergic and nitrergic co-transmission is the dominant mechanism responsible for neural-mediated smooth muscle relaxation in the gastrointestinal tract. Electrical field stimulation (EFS) of intestinal preparations causes an inhibitory junction potential (IJP) consisting of two components: a fast smooth muscle hyperpolarization followed by a slow and long-lasting hyperpolarization (Crist et al. 1992; He & Goyal, 1993). These electrical responses mediate smooth muscle relaxation. The fast component of the IJP is inhibited after incubating the tissue with P2Y1 antagonists such as MRS2179, MRS2279 or MRS2500, suggesting that P2Y1 receptors are responsible for this mechanism. This pharmacological effect has been demonstrated in several areas of the gastrointestinal tract such as the small intestine (Gallego et al. 2008b), colon (Gallego et al. 2006, 2011; Grasa et al. 2009; Zhang et al. 2010b) and internal anal sphincter (Opazo et al. 2011; Duffy et al. 2012). All these results suggest that P2Y1 receptors are responsible for purinergic neurotransmission in the gastrointestinal tract. The combination of a nitric oxide synthase (NOS) inhibitor and a P2Y1 antagonist completely abolished both electrophysiological and mechanical responses, showing that the inhibitory neurotransmission consists of the release of both a purine and nitric oxide (Gallego et al. 2008a).
Intestinal tissues have an on-going release of inhibitory neurotransmitters. This phenomenon is described as an ‘inhibitory neural tone’. We have recently pharmacologically characterized this mechanism in the rat colon (Gil et al. 2010). Briefly, NOS inhibition depolarizes smooth muscle, increases motility and does not affect on-going spontaneous IJP. In contrast, P2Y1 blockade neither modifies smooth muscle resting membrane potential nor motility but it inhibits spontaneous IJP concentration dependently. These results suggest different functions for each neurotransmitter (Gil et al. 2010).
Preferential P2Y1 agonists such as adenosine 5′-O-2-thiodiphosphate (ADPβS) or specific agonists such as MRS2365 cause smooth muscle hyperpolarization and this effect is blocked by P2Y1 antagonists (Gallego et al. 2006, 2011). Recently, it has been proposed that both ATP/ADP and β-NAD acting on P2Y1 receptors might fulfil part of the criteria to be considered the endogenous purinergic neurotransmitters (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011). However, this is a controversial issue and more data are needed to evaluate the putative role of β-NAD as an endogenous neurotransmitter (Goyal, 2011; Mutafova-Yambolieva et al. 2011).
All these data suggest that P2Y1 receptors are responsible for purinergic smooth muscle hyperpolarization and relaxation. However, the hypothesis has been raised mainly using a pharmacological approach involving both selective P2Y1 agonists and antagonists, and other purinergic receptors might also be involved (Van Crombruggen et al. 2007; Zizzo et al. 2007; Priem & Lefebvre, 2011). In the present paper we confirm the hypothesis using P2Y1 knocked out mice (Fabre et al. 1999).
Methods
Animals and tissue samples
Male C57BL/6J wild type (WT; N= 10) and P2Y1-deficient (B6.129P2-P2ry1tm1Bhk/J) mice (P2Y1−/−; N= 6) 10- to 15-weeks old were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were housed under controlled conditions: temperature 22 ± 2°C, humidity 55%± 10%, 12:12 h light–dark cycle and access to water and food ad libitum. Animals were killed by cervical dislocation. The colon was quickly removed and placed in carbogenated (95% O2 and 5% CO2), physiological saline solution. It was then opened along the mesenteric border and pinned to a Sylgard base (mucosa side up). The mucosal and submucosal layers were removed and six muscle strips were cut (8 mm × 2 mm) distal to the caecum and proximal to the rectum. All procedures were approved by the Ethics Committee of the Universitat Autònoma de Barcelona.
Intracellular microelectrode recording
The tissue was pinned with the circular muscle layer facing upwards in a Sylgard-coated chamber and continuously perfused with carbogenated physiological saline solution at 37 ± 1°C and was allowed to equilibrate for 1 h. Circular smooth muscle cells were impaled with glass microelectrodes filled with 3 M KCl (30–60 MΩ tip resistance). Membrane potential was measured by using a standard Duo773 electrometer (WPI Inc., Sarasota, FL, USA). Tracings were displayed on an oscilloscope 4026 (Racal-Dana Ltd, Windsor, UK) and simultaneously digitized (100 Hz) with PowerLab 4/30 system and Chart 5 software for Windows (both from ADInstruments, Castle Hill, NSW, Australia). In order to stabilize impalements, experiments were performed in the presence of nifedipine (1 μm). The spontaneous inhibitory neural tone was characterized as previously described (Gil et al. 2010). Briefly, the resting membrane potential (expressed in millivolts) was estimated as the most probable bin of the frequency distribution of the membrane potential (0.1 mV bins; 30–60 s recordings). Spontaneous inhibitory junction potentials were evaluated by calculating the mean standard deviation (SD) of the distribution of the membrane potential: SD of the recording inside the cell minus SD of the recording outside the cell (expressed in millivolts). IJPs were also elicited by electrical field stimulation (EFS) using the following parameters: (i) protocol 1: single pulses, pulse duration 0.3 ms and increasing voltage (8, 12, 16, 20, 24, 28, 32, 36 and 40 V) and (ii) train stimuli of 5 s duration (supramaximal amplitude and pulse duration 0.3 ms) were also performed at 1 Hz i.e. 5 pulses (protocol 2) and 5 Hz i.e. 25 pulses (protocol 3). These types of protocols were designed to properly characterise the inhibitory junction potential, which is more purinergic when single stimuli are used and is both purinergic and nitrergic when trains of higher frequency are used. These types of protocols were used in a colonic human preparation (Gallego et al. 2008a). IJP amplitude was measured from the resting membrane potential (Vm) and expressed in millivolts. Train pulses at 1 Hz elicited five consecutive hyperpolarisations, which were defined by their amplitude. For train stimuli at 5 Hz, the fast component of the IJP was assessed by measuring the maximum amplitude of the IJP during the initial pulses and the amplitude of the slow component was assessed at 2.5 and 3.75 s after the beginning of the stimulus.
Muscle bath studies
Muscle strips mounted in a 10 ml organ bath containing carbogenated physiological saline solution were maintained at 37 ± 1°C. A tension of 0.5 g was applied and tissues were allowed to equilibrate for 60–90 min. After this period, strips displayed spontaneous phasic activity. Mechanical activity was measured using an isometric force transducer (Harvard VF-1 Harvard Apparatus Inc., Holliston, MA, USA) connected to a computer through an amplifier. Data were digitalized (25 Hz) using Data 2001 software (Panlab, Barcelona, Spain) coupled to an A/D converter installed in the computer. The release of inhibitory neurotransmitters was studied by using EFS applied for 2 min (pulse duration 0.4 ms; frequency 5 Hz; amplitude 60 V). The area under the curve (AUC) of contractions from the baseline was measured to estimate the mechanical activity and the result was expressed in grams per minute (g min−1).
Solutions and drugs
The composition of the physiological saline solution was (in mm): glucose 10.10; NaCl 115.48; NaHCO3 21.90; KCl 4.61; NaH2PO4 1.14; CaCl2 2.50; and MgSO4 1.16 (pH 7.3–7.4). In all the experiments, phentolamine, propranolol and atropine (1 μm) were added to the physiological saline solution to block α- and β-adrenoceptors and muscarinic receptors. The following drugs were used: tetrodotoxin (Latoxan, Valence, France); apamin, atropine sulphate, β-NAD, l-NNA, phentolamine (Sigma Chemicals, St Louis, MO, USA); MRS2365, MRS2500, propanolol (Tocris, Bristol, UK). Stock solutions were made by dissolving drugs in distilled water except for nifedipine, which was dissolved in 96% ethanol, and l-NNA, which was dissolved in physiological saline solution by sonication.
Data analysis and statistics
Two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison test was used to compare: (1) differences between WT and P2Y1−/− mice after EFS and (2) the effect of the different drugs on EFS-induced inhibition of spontaneous motility. In order to normalize mechanical data, EFS-induced inhibition of spontaneous motility was calculated as percentage of inhibition using the following formula: 1 – (AUC during EFS/AUC previous EFS) × 100. Note that a complete cessation of spontaneous motility means 100% inhibition while 0% is a complete blockade of the inhibitory response observed during EFS. Negative data indicate a larger contractile activity during one EFS than that observed in the prior EFS. To characterise the effect of MRS2500 on EFS-induced inhibition of spontaneous motility, IC50 was calculated using a conventional sigmoid concentration–response curve with variable slope. Paired Student's t test was used to evaluate: (1) differences in the resting membrane potential before and after drug addition and (2) the effect of MRS2500 on the hyperpolarisation and inhibition of spontaneous motility (AUC) induced by MRS2365 and β-NAD. One-way ANOVA was used to evaluate the drug effect on spontaneous IJP and also EFS-induced inhibition of spontaneous motility. Data are expressed as mean ± SEM. A P < 0.05 was considered statistically significant. ‘n’ values indicate the number of samples. Statistical analysis and curve fit were performed with GraphPad Prism version 4.00, (GraphPad Software, San Diego, CA, USA).
Results
Inhibitory junction potential induced by EFS in wild type and P2Y1−/− mice
Smooth muscle Vm did not differ between WT (−39.2 ± 1.7 mV, n= 11) and P2Y1−/− mice (−37.9 ± 0.7 mV, n= 12). The IJPs recorded in P2Y1−/− mice were both qualitatively and quantitatively different from those recorded in WT animals (Fig. 1). Three different protocols of EFS were used to assess the electrophysiological responses: single pulse (Fig. 1A), trains of 5 s at 1 Hz (Fig. 1B) and trains of 5 s at 5 Hz (Fig. 1C). Trains were always elicited at supramaximal voltages based on the results obtained with increasing the voltage with the single pulse protocol (Fig. 1A). In wild type animals, EFS with a single pulse elicited a fast IJP often followed by a slow IJP. At supramaximal voltages, the IJP reached about 30 mV (29.2 ± 1.8 mV) in amplitude and last about 4 s (4.4 ± 0.91 s). In contrast, in P2Y1−/− mice the fast IJP was absent and single pulses elicited a monophasic IJP reaching an amplitude of 6 mV (5.9 ± 0.7 mV) on average and a duration of about 4 s (4.0 ± 0.8 s). In WT animals, trains of 5 s at 1 Hz elicited five consecutive fast IJPs of about 30 mV on average (P1: 27.4 ± 1.9 mV; P2: 26.0 ± 2.5 mV; P3: 27.2 ± 2.2 mV; P4: 25.5 ± 2.4 mV; P5: 24.5 ± 2.5 mV). In contrast, the response in P2Y1−/− mice consisted of a progressive hyperpolarization reaching about 10 mV after the 4th or 5th stimulus (P1: 3.7 ± 0.5 mV; P2: 6.3 ± 1.1 mV; P3: 7.4 ± 1.5 mV; P4: 8.4 ± 1.8 mV; P5: 9.2 ± 1.8 mV). Trains of 5 s at 5 Hz elicited a fast (30 mV) (IJPf: 31.12 ± 1.72 mV) followed by a sustained (20 mV) (IJPs2.5: 21.0 ± 1.9 mV; IJPs3.75: 19.9 ± 2.0 mV) hyperpolarization in WT animals whereas in P2Y1−/− mice only the slow component (about 15 mV) could be recorded (IJPf: 0 mV; IJPs2.5: 14.8 ± 1.9 mV; IJPs3.75: 15.7 ± 2.0 mV). See Figs 2C, 3C and 4C. Comparisons between WT vs. P2Y1−/− animals are depicted by a †.
Figure 1. Representative recordings of inhibitory junction potentials induced by 3 protocols of EFS.
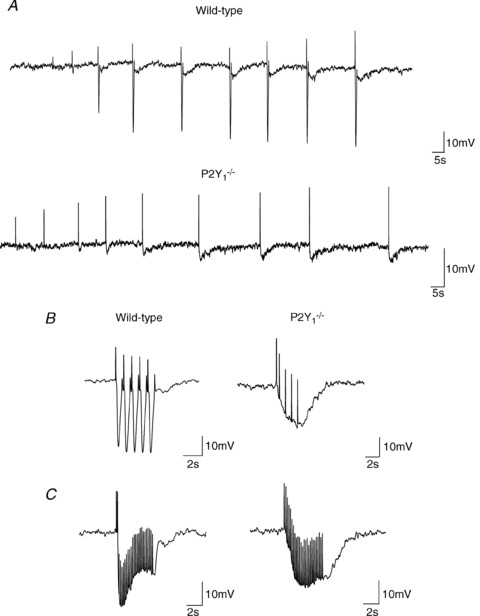
A, protocol 1: single pulses at increasing voltage of EFS (8, 12, 16, 20, 24, 28, 32, 36 and 40 V) in WT (top) and P2Y1−/− mice (bottom). B, protocol 2: train stimuli of 5 s duration and 1 Hz (5 pulses) and C, protocol 3: train stimuli of 5 s duration and 5 Hz (25 pulses). B and C, WT (left) and P2Y1−/− mice (right). (Note that positive deflections correspond to stimulus artefacts elicited at each frequency.)
Figure 2. Effect of l-NNA and MRS2500 in the IJP induced by protocol 1 (single pulse) of EFS.
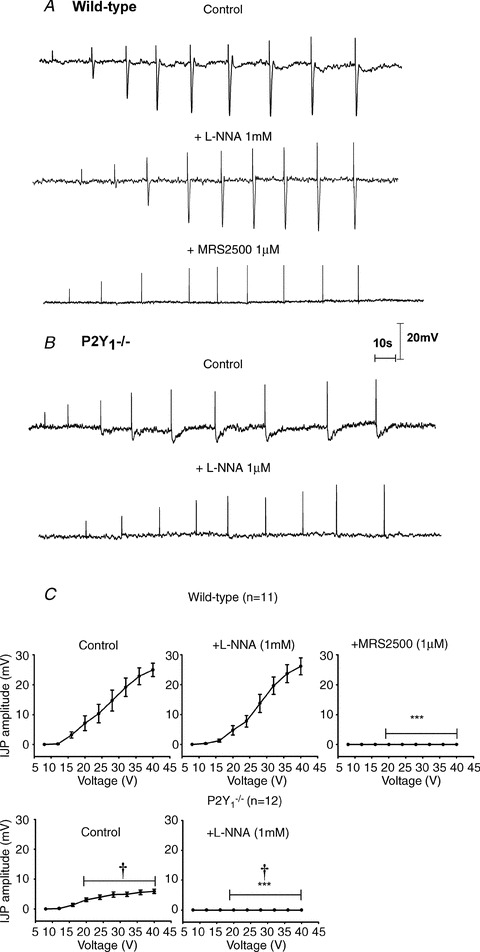
Representative recordings of a wild type animal (A) and P2Y1−/− mouse (B) (notice that positive deflection corresponds to the stimulus artefact). Notice the absence of the fast IJP in P2Y1−/− mice. C, graph showing the amplitude of the response at increasing voltages of stimulation in WT animals (n= 11) and P2Y1−/− mice (n= 12). Comparison between the IJP amplitude from WT and P2Y1−/− mice was significant (two-way ANOVA: P < 0.0001; †P < 0.0001 post hoc Bonferroni test). Comparison between the IJP amplitude after drug addition was also significant (in both cases: two-way ANOVA P < 0.0001) but MRS2500 (1 μm) was needed to abolish the IJP in WT animals; in contrast, no IJP was recorded after incubation with l-NNA in tissue from P2Y1−/− mice. ***P < 0.0001 post hoc Bonferroni test from previous drug addition.
Figure 3. Effect of l-NNA and MRS2500 in the IJP induced by protocol 2 (1 Hz) of EFS.
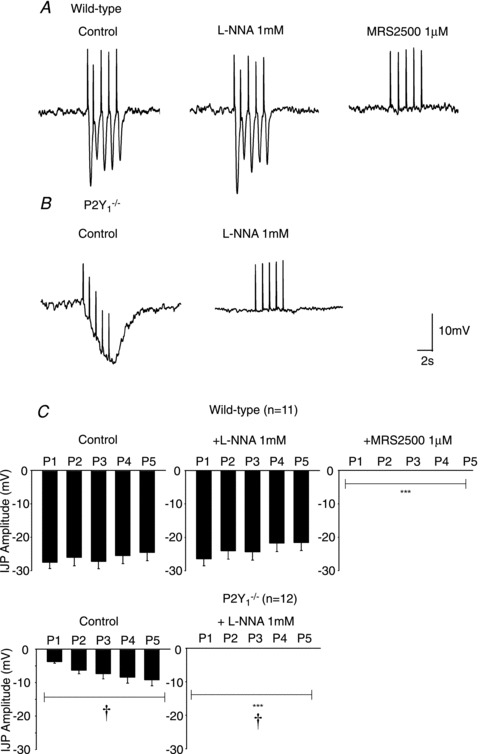
Representative recording of a wild type animal (A) and P2Y1−/− mouse (B). Notice the progressive increase in hyperpolarization in P2Y1−/− mice which was completely abolished by l-NNA. In contrast, 5 consecutive IJP were recorded in tissue from WT animals. (Notice that positive deflections correspond to stimulus artefacts elicited at each frequency.) C, graph showing the amplitude of the response recorded after the 5 consecutive pulses in WT animals (n= 11) and P2Y1−/− mice (n= 12). Comparison between the IJP amplitude from WT and P2Y1−/− mice was significant (two-way ANOVA: P < 0.0001; †P < 0.0001 post hoc Bonferroni test). Comparison between the IJP amplitude after drug addition was also significant (in both cases: two-way ANOVA P < 0.0001) but MRS2500 (1 μm) was needed to abolish the IJP in WT animals. In contrast, no IJP was recorded after incubation with l-NNA in tissue from P2Y1−/− mice. ***P < 0.0001 post hoc Bonferroni test from previous drug addition.
Figure 4. Effect of l-NNA and MRS2500 in the IJP induced by protocol 3 (5 Hz) of EFS.
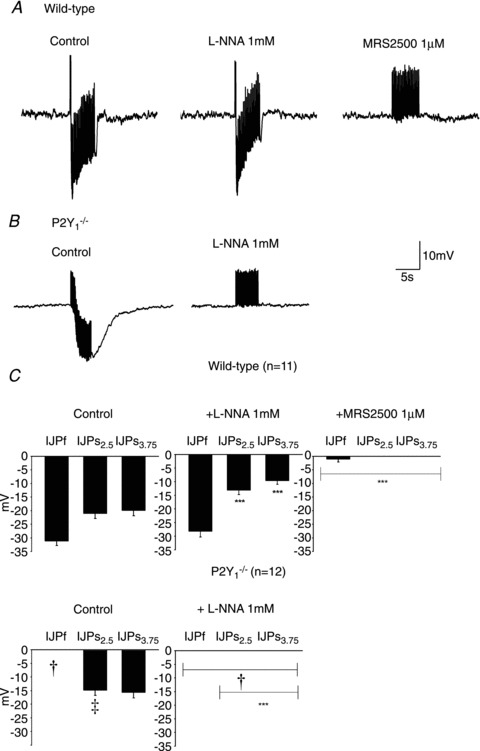
Representative recording of a wild type animal (A) and P2Y1−/− mouse (B). Notice the presence of a fast followed by a sustained component in the IJP recorded from colonic tissue of WT animals and the lack of the first fast IJP in P2Y1−/− mice. (Notice that positive deflections correspond to stimulus artefacts elicited at each frequency.) C, graph showing the amplitude of the response of the fast (IJPf) and slow (IJPs) component of the IJP (measured 2.5 and 3.75 s after the beginning of the stimulus) in WT animals (n= 11) and P2Y1−/− mice (n= 12). Comparison between the IJP amplitude from WT and P2Y1−/− mice was significant (two-way ANOVA: ‡P < 0.0001; †P < 0.0001 post hoc Bonferroni test). Comparison between the IJP amplitude after drug addition was also significant (in both cases: two-way ANOVA P < 0.0001) but MRS2500 (1 μm) was needed to abolish the IJP in WT animals. In contrast, no IJP was recorded after incubation with l-NNA in tissue from P2Y1−/− mice. ***P < 0.0001 post hoc Bonferroni test from previous drug addition.
As previously described both in human and rodent gastrointestinal tissues (Grasa et al. 2009; Gallego et al. 2011), l-NNA (1 mm) and MRS2500 (1 μm) were used to investigate the co-transmission process. In wild type animals, l-NNA did not modify the amplitude of the fast IJP elicited with a single pulse (Fig. 2A and C), elicited with five consecutive pulses (Fig. 3A and C) and elicited with a train of 5 Hz (Fig. 4A and C). In contrast, the slow component of the IJP was absent after incubation with l-NNA (Fig. 2A) and the sustained hyperpolarization was reduced with a train of 5 Hz (Fig. 4A and C). Subsequent addition of 1 μm MRS2500 completely abolished all the electrophysiological responses (Figs 2, 3 and 4). In P2Y1−/− mice l-NNA completely abolished all the electrophysiological responses (Figs 2B, 3B and 4C) showing that the MRS2500-sensitive component was absent in these animals. A detailed quantification of the drug effect is shown in Figs 2C, 3C and 4D. Differences between two consecutive drugs are depicted with asterisks.
Inhibition of spontaneous motility induced by EFS in wild type and P2Y1−/− mice
Both in wild type and P2Y1−/− mice a cyclic spontaneous motility was usually recorded with no major differences in the frequency (WT: 1.6 ± 0.1 contractions min−1, n= 13; P2Y1−/−: 1.4 ± 0.3 contractions min−1, n= 7, n.s.) or in the amplitude of spontaneous contractions (WT: 0.42 ± 0.10 g, n= 13; P2Y1−/−: 0.20 ± 0.03 g, n= 8, n.s.). After incubation with l-NNA, EFS still inhibited spontaneous motility. Subsequent addition of MRS2500 completely abolished the EFS effect (Fig. 5). The IC50 calculated for the P2Y1 antagonist was 22.7 nm (log IC50=−7.64 ± 0.27, R2= 0.839, 17 degrees of freedom). EFS-induced inhibition of spontaneous motility was recorded in P2Y1−/− mice. In these animals, l-NNA completely blocked EFS-induced inhibition of spontaneous motility and, consistent with the electrophysiological response, the MRS2500-sensitive component of the inhibition of spontaneous motility was absent (Fig. 5).
Figure 5. Effect of l-NNA and MRS2500 in the inhibition of the spontaneous motility induced by EFS.
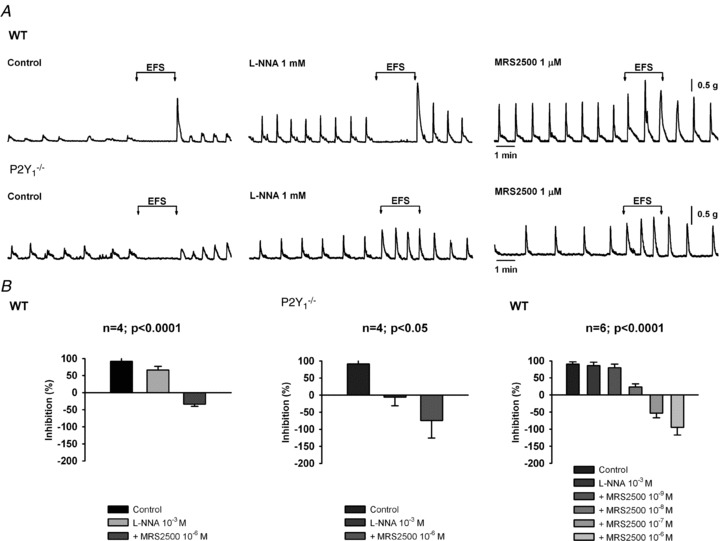
Representative recordings of a wild type animal and P2Y1−/− mouse (A). Histograms (B) showing the inhibitory effect in WT (n= 4; P < 0.0001), P2Y1−/− (n= 4; P < 0.05) and concentration response effect in WT animals (n= 6 P < 0.0001). Notice that 100% is equivalent to 100% of inhibition of spontaneous motility and 0% means that the motility is equivalent to the spontaneous activity. Negative values indicate the presence of an excitatory effect.
Inhibitory neural tone in wild type and P2Y1−/− mice
Both in wild type and P2Y1−/− mice TTX (1 μm, n= 4) and l-NNA (1 mm, n= 4) increased spontaneous contractions (not shown). l-NNA caused a smooth muscle depolarization (WT: 5.3 ± 1.2 mV, n= 10; P2Y1−/−: 5.0 ± 0.3 mV, n= 10). Subsequent addition of MRS2500 (1 μm, n= 11) did not further depolarize or increase motility. In wild type animals ‘spontaneous’ IJPs were often recorded, increasing the ‘noise’ of the recording (Fig. 6A). The SD of the data was comparatively higher in WT than in P2Y1−/− mice (Fig. 6C). In wild type animals, spontaneous IJPs were still present after l-NNA incubation (Fig. 6A and C) and were significantly reduced after incubation with 1 μm MRS2500 (Fig. 6A and C). In order to illustrate this phenomenon (Gil et al. 2010) data frequency distribution of the recording was plotted in bin intervals of 0.5 mV (Fig. 6C). In wild type animals, the frequency distribution was asymmetrical and the presence of a tail towards negative values in the graph was representative of the presence of on-going spontaneous IJPs. The tail was still present in WT animals after incubation with 1 mm l-NNA (notice that the graph is shifted to the right due to the 4–5 mV depolarization). After incubation with MRS2500, the frequency distribution of the data lost the tail and was symmetrical (Fig. 6C). P2Y1−/− animals showed a slightly ‘noisy’ recording (Fig. 6B). The frequency distribution was symmetrical and neither l-NNA nor MRS2500 modified the SD, which is consistent with the absence of the MRS2500-sensitive spontaneous IJP in these animals. In recordings from P2Y1−/− mice the SD was not modified by TTX or apamin (both 1 μm) incubated after l-NNA and MRS2500 (data not shown), suggesting that these oscillations are not neural mediated and probably correspond to unitary potentials (Edwards et al. 1999) previously described in other areas of the gastrointestinal tract.
Figure 6. Spontaneous IJP and Vm. Spontaneous IJP recorded in wild type animals (A) were still recorded in the presence of l-NNA and drastically reduced by 1 μm MRS2500.
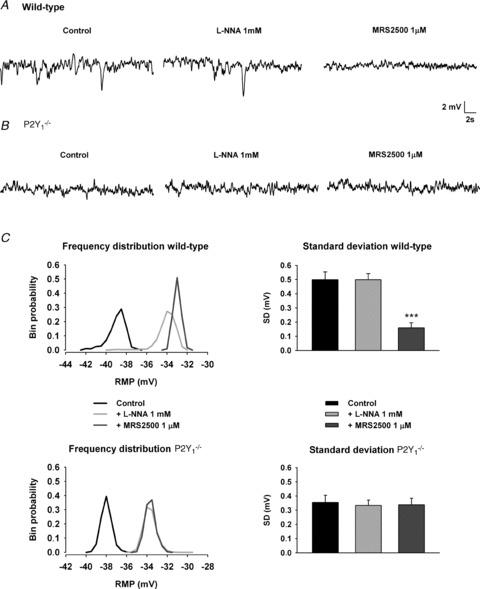
No spontaneous IJPs were recorded in P2Y1−/− mice (B) although the recording was ‘noisy’. To quantify spontaneous IJP the SD was calculated (C, right: comparison with previous drug addition, ***). Frequency distribution of the data from the recording illustrates in WT animals: (1) the presence of a tail towards the most negative values indicative of spontaneous IJP, (2) the shift to the right in the frequency distribution induced by l-NNA indicative of depolarization without a major effect on spontaneous IJP and (3) the absence of the tail after MRS2500 addition. In contrast, in P2Y1−/− mice the frequency distribution was symmetrical in control. l-NNA shifted the distribution to the right but MRS2500 did not change the frequency distribution.
Effect of MRS2365 and β-NAD on smooth muscle Vm and mechanical activity in wild type and P2Y1−/− mice
In wild type mice, the selective P2Y1 agonist MRS2365 (1 μm) induced (1) a smooth muscle hyperpolarization (−10.7 ± 2.6 mV; n= 6) and (2) an increase in the SD of the recording (0.67 ± 0.12, n= 6; P < 0.05) (Fig. 7). Both effects were blocked by the P2Y1 antagonist MRS2500 (1 μm; n= 7). In contrast, in P2Y1−/− animals, 1 μm MRS2365 (n= 5) did not induce any change in Vm or an increase in the SD (Fig. 7). In the presence of the neural blocker TTX (1 μm), 1 μm MRS2365 inhibited spontaneous motility in wild type animals (AUC: control: 3.1 ± 0.6 vs. drug: 1.2 ± 0.3, P < 0.01, n= 4). In the presence of MRS2500 the inhibitory effect induced by 1 μm MRS2365 was not observed (AUC: control: 3.2 ± 0.7 vs. drug: 2.9 ± 0.8; n.s, n= 4). Neither in the absence (n= 4) nor in the presence (n= 4) of MRS2500, MRS2365 did not modify the spontaneous motility in P2Y1−/− animals. The putative endogenous neurotransmitter β-NAD (1 mm) hyperpolarised (−14.9 ± 2.5 mV, n= 6) smooth muscle cells of wild type animals. The hyperpolarization was strongly reduced by pre-incubation with MRS2500 although often a residual effect was observed (−5.4 ± 2.0 mV, n= 5; P < 0.05 from control). In P2Y1−/− animals β-NAD induced a small hyperpolarization (−3.6 ± 1.3 mV; n= 7; P < 0.001 from WT) that was unaffected by pre-incubation with MRS2500 (1 μm) (−3.3 ± 0.8 mV; n= 5) suggesting that other receptors might be responsible for the MRS2500-insensitive response.
Figure 7. Effects of MRS2365 and β-NAD on Vm.
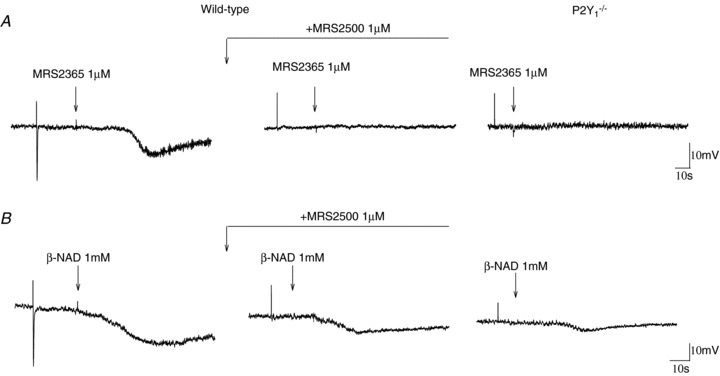
A, representative recordings showing the hyperpolarization induced by 1 μm MRS2365 in wild type animals. The effect was blocked by MRS2500 (1 μm) and was absent in P2Y1−/− animals. B, representative recordings showing the hyperpolarization induced by β-NAD (1 mm) in wild type animals. The effect was partially reversed by MRS2500 (1 μm) and was strongly diminished in P2Y1−/− animals. Notice in both cases the presence of a residual response.
Discussion
Previous data from the human gastrointestinal tissues such as the colon (Gallego et al. 2006, 2008a; Gallego et al. 2011) or small intestine (Gallego D. and Jimenez M., unpublished) and from animal tissues such as the pig or guinea pig small intestine (Wang et al. 2007; Gallego et al. 2008b), rat colon (Grasa et al. 2009) and rat and mouse internal anal sphincter (McDonnell et al. 2008; Opazo et al. 2011) showed that the fast component of the IJP was sensitive to P2Y1 antagonists such as MRS2179, MRS2279 or MRS2500. In the present paper we used P2Y1−/− mice (Fabre et al. 1999) and we combined the study with previously published results from a pharmacological approach using both selective agonists and antagonists of the P2Y1 receptor (Grasa et al. 2009; Gil et al. 2010; Gallego et al. 2011). The present paper confirms that P2Y1 receptors are responsible for purinergic neurotransmission in the gastrointestinal tract.
Three main issues are currently being debated regarding purinergic neurotransmission in the gastrointestinal tract: (1) The nature of the purine released by enteric neurons; (2) The receptor involved in purinergic neurotransmission; and (3) The post-junctional cell (fibroblast-like cells, interstitial cells of Cajal (ICC) or smooth muscle) responsible for inhibitory neurotransmission. The present paper tries to address the second point but our data might also be important for the discussion of the first and third issues. Finally, it is important to add a comment about the value of the model and its relative differences with human tissue.
In the present paper we demonstrate that the P2Y1 receptor mediates purinergic neurotransmission: (1) the fast component of the IJP was totally absent in P2Y1−/− mice using three protocols of EFS, (2) purinergic EFS-mediated inhibition of spontaneous motility was absent in P2Y1−/− mice, (3) P2Y1−/− mice did not have spontaneous IJP and (4) MRS2365, a selective P2Y1 agonist, did not cause smooth muscle hyperpolarization. In contrast, control animals had a prominent fast IJP induced by EFS, a purinergic-mediated inhibition of spontaneous motility, spontaneous IJP and MRS2365-induced hyperpolarization. All these effects were blocked with MRS2500 as we described previously in the rat and human colon (Grasa et al. 2009; Gallego et al. 2011) and confirms the involvement of P2Y1 receptors in the purinergic neurotransmission. Nitrergic neurotransmission is not impaired in P2Y1−/− mice. The junction potential in P2Y1−/− mice consists of a sustained hyperpolarization (see data with single pulse, 1 Hz and 5 Hz) that was totally blocked by l-NNA, showing that NO is properly released by enteric inhibitory neurons and the post-junction mechanism involving guanylate cyclase is able to cause the nitrergic hyperpolarization. Moreover l-NNA was able to increase spontaneous motility and depolarise smooth muscle cells, which suggests a competent inhibitory neural nitrergic tone (Gil et al. 2010). All these results suggest that the purinergic component of the co-transmission process is impaired in P2Y1−/− mice whereas the nitrergic component is not.
Recently it has been proposed that β-NAD is released by enteric neurons and might mediate purinergic neurotransmission (Mutafova-Yambolieva et al. 2007; Hwang et al. 2011). In the guinea-pig taenia coli, Burnstock & Hoyle (1985) demonstrated that β-NAD is broken down rapidly to adenosine and the inhibitory responses to β-NAD are blunted by methylxanthine antagonists yet potentiated by the adenosine uptake blocker, dipyridamole. β-NAD-induced hyperpolarization might differ between species. β-NAD causes a modest (4–8 mV) hyperpolarization in human tissues (Gallego et al. 2011; Hwang et al. 2011) but, as we report in the present paper, 1 mmβ-NAD causes a prominent hyperpolarization in the mouse colon (Hwang et al. 2011). Differences between species might explain these different results. In the present paper we confirm that in the mouse colon an important part of the hyperpolarization induced by β-NAD is mediated by activation of P2Y1 receptors: the effect of β-NAD is reduced in P2Y1−/− mice and it is partially blocked by MRS2500 in WT animals as in human colon (Gallego et al. 2011). These results suggest that an important part of the response is P2Y1 mediated and consequently β-NAD might fulfil part of the criteria, at least in the mouse colon, to be considered an endogenous neurotransmitter. It is important to notice that a residual hyperpolarization can still be recorded in P2Y1−/− mice even in the presence of MRS2500. These results suggest that β-NAD can activate other receptors (including adenosine or P2Y11 receptors, see below) that can contribute to the inhibitory effect but they are unlikely to be junctionally located. Further studies are needed to evaluate the relative contribution of β-NAD and its metabolites compared with other putative purinergic neurotransmitters such as ATP/ADP.
P2Y1 receptors are expressed in smooth muscle cells and in PDGRFα+ cells (fibroblast-like cells) (Iino et al. 2009). Recently it has been reported that PDGRFα+ cells can transduce purinergic signals and have the apparatus to do so (Cobine et al. 2011; Kurahashi et al. 2011) as shown by: (1) the presence of the receptor in these cells (Kurahashi et al. 2011), (2) the abundance of SK3 channels (Vanderwinden et al. 2002; Fujita et al. 2003; Iino et al. 2009) that might contribute to the hyperpolarization and (3) the fact that ATP, ADP or β-NAD activate large-amplitude apamin-sensitive currents that were blocked by MRS2500 (Kurahashi et al. 2011). Data from the present paper using P2Y1−/− mice demonstrate that P2Y1 receptors are needed for purinergic transmission but we cannot discriminate between P2Y1 expressed in different cell types with this animal model. What is clear is that interstitial cells of Cajal, which might participate in transduction of nitrergic neurotransmission (Burns et al. 1996; Iino et al. 2008; Ward & Sanders, 2001), are not needed to transduce purinergic signals. Mutant animals without certain subclasses of ICC have intact purinergic neurotransmission (Alberti et al. 2007; Zhang et al. 2010a). The colon of KitW-sh/W-sh and KitW/W-v mice has intact MRS2500-sensitive neurotransmission including a fast IJP and spontaneous IJP (not shown). Whether PDGRFα+ cells in coordination with ICC can participate in the co-transmission process (NO and ATP) and how the stimulus can be translated to the smooth muscle cells is still unknown. Direct and indirect communication between neurons and muscle and neurons and interstitial cells has also been reported (Mitsui & Komuro, 2002). More studies with selective deletion in the transduction apparatus involving NO and purinergic pathways of specific post-junction cells (smooth muscle, ICC and PDGRFα+ cells) will be extremely valuable in the study of the co-transmission process (Groneberg et al. 2011).
It is essential to check the validity of the mouse model (present study) when comparing it with the inhibitory innervation of the human colon, the main goal of our research. Electrophysiological responses are very similar to those found in human colonic tissues i.e. single pulses elicit a purinergic IJP (not always followed by a nitrergic one) whereas a train of 5 Hz elicits a purinergic IJP always followed by a nitrergic one (Gallego et al. 2008a). An important difference is that trains of 1 Hz show a ‘rundown’ in human colon i.e. the second and following IJPs are clearly diminished compared with the first response (Gallego et al. 2008a). This is not the case in the mouse colon where trains of 1 Hz show a moderate rundown (see data and recordings from Fig. 3) and might explain why a continuous inhibition of spontaneous motility can be recorded during sustained stimulation. A similar result has been reported in the mouse caecum (Zizzo et al. 2007). However it is important to check whether this is just a shift in the mechanism towards higher frequencies or alternatively a pre- or post-junction difference in purinergic neurotransmission.
A second important similarity is the IC50 value for MRS2500 obtained in the present study (22.7 nm) in muscle bath experiments to that we obtained in human colonic tissue (88 nm) (Gallego et al. 2011), suggesting that the receptors involved in the response have similar affinities for the antagonist. The IC50 values are higher than those obtained in isolated cells that express P2Y1 receptors such as platelets (Cattaneo et al. 2004) or HEK cells (Gallego et al. 2011). Lower concentrations are needed in experiments with dispersed cells than those where tissue is used and the response induced by the endogenous neurotransmitter is measured. MRS2500-insensitive P2Y11 receptors have been linked to purinergic relaxation in the guinea pig taenia coli, where the receptor is expressed in smooth muscle cells (King & Townsend-Nicholson, 2008). This is an interesting receptor to look at it because β-NAD binds to both P2Y1 (Kurahashi et al. 2011) and P2Y11 (Moreschi et al. 2006) receptors. Although some studies report that ortholog P2Y11 receptors are not present in rodent tissues (Abbracchio et al. 2006), recent reports identify with inmunohistochemistry “P2Y11-like’ receptors in glial cells of the enteric (Van Crombruggen et al. 2007) and central nervous system (Brandenburg et al. 2010). It is important to know whether P2Y11 receptors are present in smooth muscle cells or alternatively in PDGRFα+ cells where MRS2500-sensitive P2Y1 receptors have been indentified. However, in both human and mouse tissue, the electrophysiological response is absent (not different from 0 mV) after addition of MRS2500 and l-NNA showing that the MRS2500-insensitive response does not mediate junctional responses.
In conclusion, data presented in the present paper identified that P2Y1 receptors mediate purinergic neurotransmission in the gastrointestinal tract. These results confirm what we previously published with human and animal tissues using a conventional pharmacological approach with selective agonists and antagonists. P2Y1-deficient animals might be an interesting biological tool to investigate what happens when a selective impairment of purinergic neurotransmission occurs under pathological circumstances (Strong et al. 2010).
Acknowledgments
The authors would like to thank Claudia Arenas for her technical assistance. This work has been funded by the following grants: BFU2009-11118. D.G. is supported by the Instituto de Salud Carlos III, Centro de Investigación Biomédica en red de enfermedades hepáticas y digestivas (CIBEREHD). V.G. is supported by the Ministerio de Ciencia e Innovación (Spain) (AP2007-01583). M.M.-C. is supported by the Agencia de Gestió d'Ajuts Universitaris i de Recerca (AGAUR), Generalitat de Catalunya FI-DGR 2011.
Glossary
Abbreviations
- AUC
area under curve
- EFS
electrical field stimulation
- ICC
interstitial cells of Cajal
- IJP
inhibitory junction potential
- NOS
nitric oxide synthase
- PDGRFα+ cells
fibroblast-like cells
- Vm
resting membrane potential
- WT
wild type
Author contributions
D.G: Conception and design of the experiments, collection, analysis and interpretation of data and drafting the article or revising it critically for important intellectual content. V.G: Collection, analysis and interpretation of data. M.M.-C: Collection, analysis and interpretation of data. N.M: Collection, analysis and interpretation of data. M.T.M: Drafting the article or revising it critically for important intellectual content. M.J: Conception and design of the experiments, collection and drafting the article or revising it critically for important intellectual content. All authors approved the final version.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, Jimenez M. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–G1510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- Brandenburg LO, Jansen S, Wruck CJ, Lucius R, Pufe T. Antimicrobial peptide rCRAMP induced glial cell activation through P2Y receptor signalling pathways. Mol Immunol. 2010;47:1905–1913. doi: 10.1016/j.molimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Hoyle CH. Actions of adenine dinucleotides in the guinea-pig taenia coli: NAD acts indirectly on P1-purinoceptors; NADP acts like a P2-purinoceptor agonist. Br J Pharmacol. 1985;84:825–831. doi: 10.1111/j.1476-5381.1985.tb17376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y 1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea-pig ileum circular muscle. J Physiol. 1992;447:119–131. doi: 10.1113/jphysiol.1992.sp018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy AM, Cobine CA, Keef KD. Changes in neuromuscular transmission in the W/Wv mouse internal anal sphincter. Neurogastroenterol Motil. 2012;24:e41-e55. doi: 10.1111/j.1365-2982.2011.01806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci. 2003;92:35–42. doi: 10.1254/jphs.92.35. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Auli M, Clave P, Jimenez M. Purinergic and nitrergic junction potential in the human colon. Am J Physiol Gastrointest Liver Physiol. 2008a;295:G522–G533. doi: 10.1152/ajpgi.00510.2007. [DOI] [PubMed] [Google Scholar]
- Gallego D, Gil V, Aleu J, Martinez-Cutillas M, Clave P, Jimenez M. Pharmacological characterization of purinergic inhibitory neuromuscular transmission in the human colon. Neurogastroenterol Motil. 2011;23:792-e338. doi: 10.1111/j.1365-2982.2011.01725.x. [DOI] [PubMed] [Google Scholar]
- Gallego D, Hernandez P, Clave P, Jimenez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G584–G594. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gallego D, Vanden BergheP, Farre R, Tack J, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil. 2008b;20:159–168. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- Gil V, Gallego D, Grasa L, Martin MT, Jimenez M. Purinergic and nitrergic neuromuscular transmission mediates spontaneous neuronal activity in the rat colon. Am J Physiol Gastrointest Liver Physiol. 2010;299:G158–G169. doi: 10.1152/ajpgi.00448.2009. [DOI] [PubMed] [Google Scholar]
- Goyal RK. Evidence for β-nicotinamide adenine dinucleotide as a purinergic, inhibitory neurotransmitter in doubt. Gastroenterology. 2011;141:e27. doi: 10.1053/j.gastro.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Grasa L, Gil V, Gallego D, Martin MT, Jimenez M. P2Y1 receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg D, Konig P, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology. 2011;140:1608–1617. doi: 10.1053/j.gastro.2011.01.038. [DOI] [PubMed] [Google Scholar]
- He XD, Goyal RK. Nitric oxide involvement in the peptide VIP-associated inhibitory junction potential in the guinea-pig ileum. J Physiol. 1993;461:485–499. doi: 10.1113/jphysiol.1993.sp019524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology. 2011;140:608–617. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008;152:437–448. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Involvement of P2Y1 and P2Y11 purinoceptors in parasympathetic inhibition of colonic smooth muscle. J Pharmacol Exp Ther. 2008;324:1055–1063. doi: 10.1124/jpet.107.131169. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell B, Hamilton R, Fong M, Ward SM, Keef KD. Functional evidence for purinergic inhibitory neuromuscular transmission in the mouse internal anal sphincter. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1041–G1051. doi: 10.1152/ajpgi.00356.2007. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 2002;309:219–227. doi: 10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, Usai C, Meis S, Kassack MU, Zocchi E, De FA. Extracellular NAD+ is an agonist of the human P2Y11 purinergic receptor in human granulocytes. J Biol Chem. 2006;281:31419–31429. doi: 10.1074/jbc.M606625200. [DOI] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. β-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci U S A. 2007;104:16359–16364. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutafova-Yambolieva VN, Sanders KM, Hwang SJ. Reply to Evidence for β-nicotinamide adenine dinucleotide as a purinergic, inhibitory neurotransmitter in doubt. Gastroenterology. 2011;141:e27-e28. doi: 10.1053/j.gastro.2011.07.047. [DOI] [PubMed] [Google Scholar]
- Opazo A, Lecea B, Gil V, Jimenez M, Clave P, Gallego D. Specific and complementary roles for nitric oxide and ATP in the inhibitory motor pathways to rat internal anal sphincter. Neurogastroenterol Motil. 2011;23:e11-e25. doi: 10.1111/j.1365-2982.2010.01602.x. [DOI] [PubMed] [Google Scholar]
- Priem EK, Lefebvre RA. Investigation of neurogenic excitatory and inhibitory motor responses and their control by 5-HT4 receptors in circular smooth muscle of pig descending colon. Eur J Pharmacol. 2011;667:365–374. doi: 10.1016/j.ejphar.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol. 2010;588:847–859. doi: 10.1113/jphysiol.2009.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Crombruggen K, Van Nassaw L, Timmermans JP, Lefebvre RA. Inhibitory purinergic P2 receptor characterisation in rat distal colon. Neuropharmacology. 2007;53:257–271. doi: 10.1016/j.neuropharm.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Rumessen JJ, de Kerchove d’ Exaerde A, Jr, Gillard K, Panthier JJ, De Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and disease. Cell Tissue Res. 2002;310:349–358. doi: 10.1007/s00441-002-0638-4. [DOI] [PubMed] [Google Scholar]
- Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1483–G1489. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Carmichael SA, Wang XY, Huizinga JD, Paterson WG. Neurotransmission in lower esophageal sphincter of W/Wv mutant mice. Am J Physiol Gastrointest Liver Physiol. 2010a;298:G14–G24. doi: 10.1152/ajpgi.00266.2009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther. 2010b;333:602–611. doi: 10.1124/jpet.109.160978. [DOI] [PubMed] [Google Scholar]
- Zizzo MG, Mule F, Serio R. Inhibitory purinergic transmission in mouse caecum: role for P2Y1 receptors as prejunctional modulators of ATP release. Neuroscience. 2007;150:658–664. doi: 10.1016/j.neuroscience.2007.09.055. [DOI] [PubMed] [Google Scholar]


